Abstract
Obsessive-compulsive disorder (OCD) is a serious and often chronically disabling condition. The current dominant model of OCD focuses on abnormalities in prefrontal-striatal circuits that support executive function (EF). While there is growing evidence for EF impairments associated with OCD, results have been inconsistent, making the nature and magnitude of these impairments controversial. The current meta-analysis uses random-effects models to synthesize 110 previous studies that compared participants with OCD to healthy control participants on at least one neuropsychological measure of EF. The results indicate that individuals with OCD are impaired on tasks measuring most aspects of EF, consistent with broad impairment in EF. EF deficits were not explained by general motor slowness or depression. Effect sizes were largely stable across variation in demographic and clinical characteristics of samples, although medication use, age, and gender moderated some effects.
Keywords: obsessive-compulsive disorder, executive function, meta-analysis
Obsessive-compulsive disorder (OCD) is a serious and often chronically debilitating condition, which affects 2–3% of the population (e.g., Kessler et al., 2005). OCD is characterized by obsessions (intrusive, distressing, and persistent thoughts and images) that are often accompanied by compulsions (ritualized, repetitive behaviors or mental acts) performed in an attempt to avoid or neutralize the distress resulting from obsessions, or according to rules that must be applied rigidly (American Psychiatric Association, 2000). Neuroimaging research has emphasized neurobiological abnormalities that may underlie the clinical and neuropsychological symptoms of OCD. Indeed, the current dominant model of OCD focuses on abnormalities in prefrontal-striatal circuits (see Menzies, Chamberlain, et al., 2008a for review) that support executive function (EF). Executive functions are a set of general-purpose cognitive control abilities, mainly supported by the prefrontal cortex (PFC), which allow individuals to regulate their thoughts and behaviors (e.g., Miyake & Friedman, 2012). EF regulates lower level cognitive processes (e.g., perception, motor responses) and thereby enables self-directed behavior towards a goal (e.g., Banich, 2009), allowing individuals to break out of habits, make decisions and evaluate risks, plan for the future, prioritize and sequence actions, and cope with novel situations. EF deficits thus have important consequences for daily life functioning, and may be major contributors to the lack of cognitive flexibility and perseverative, repetitive behaviors that are cardinal symptoms of OCD.
EF is best characterized as a set of separable but related cognitive processes that have both unique and shared individual differences, genetic influences, and neural substrates (e.g., Collette et al., 2005; Friedman et al., 2008; Miyake et al., 2000). One influential model of EF is the unity/diversity model (Friedman et al., 2008; Miyake et al., 2000; Miyake & Friedman, 2012), in which three fundamental aspects of EF are identified: (1) updating working memory, (2) shifting (e.g. between tasks), and (3) inhibition, as well as a common EF ability (which is related to both updating and shifting, and may subsume inhibition, Friedman et al., 2008; Miyake & Friedman, 2012). Updating is defined as monitoring and coding incoming information for task-relevance, and replacing no longer relevant information with newer, more relevant information. Shifting is defined as switching between task sets or response rules. Inhibition is defined as suppressing or resisting a prepotent (automatic) response in order to make a less automatic but task-relevant response. Common EF is posited to be the ability to monitor for and maintain goal and context information (Miyake et al., 2000; Miyake & Friedman, 2012). This hypothesis regarding the nature of common EF is compatible with the view that the central role of the frontal lobes is active maintenance of goals, plans and other task-relevant information, which may be essential for all aspects of EF (e.g., Miller & Cohen, 2001). Critically, the unity/diversity model of EF may be a useful vantage for the investigation of cognitive deficits and biases in psychopathology, as disorders such as OCD may be characterized by general (e.g., difficulty maintaining goals) or specific (e.g., difficulty shifting to a new set of behaviors) deficits in EF.
While updating, shifting and inhibition are important aspects of EF, this model in no way posits that these are the only components of EF. For example, working memory (WM) is often considered a component of EF. WM is defined as maintaining or manipulating information across a short delay when that information is not available in the environment. WM maintenance tasks (e.g., simple forward span tasks) require only keeping information in mind temporarily (i.e., ‘holding on line’), and involves subsystems for active rehearsal and storage, whereas WM manipulation tasks (e.g., complex and backward span tasks) additionally require the reorganization of the information being maintained (e.g., Fletcher & Henson, 2001)1. WM manipulation is strongly linked to other aspects of EF, while WM maintenance (sometimes called short term memory) is less closely linked to other aspects of EF (e.g., Engle, Tuholski, Laughlin, & Conway, 1999). WM can also be divided into verbal and visuospatial components (e.g., Baddeley, 1992; 1996; Repovs & Baddeley, 2006). Given evidence for impaired visuospatial ability (e.g., block design and design copying tasks; Abramovitch et al., 2013) in individuals with OCD, which might affect visuospatial WM, it is thus important to evaluate visuospatial and verbal WM separately. In sum, when applying the unity/diversity model to a clinical population, it is important to keep in mind additional domains of EF that may be impacted in a particular disorder.
One challenge for the investigation of EF in clinical (or, indeed, any) populations stems from the fact that many complex tasks may tap multiple aspects of EF. For example, verbal fluency tasks (generating words starting with a certain letter or from a category) likely tap several cognitive processes (Rende, Ramsberger, & Miyake, 2002). However, they have been shown to form a distinct component separable from other EF components (Fisk & Sharp, 2004) and to depend on prefrontal function (e.g., Alvarez & Emory, 2006). Planning tasks are also complex, involving multiple cognitive demands (e.g., Goel & Grafman, 1995), and so may not represent a single EF ability. Notably, verbal fluency and planning tasks are frequently used in clinical studies, including studies of individuals with OCD. Such tasks may be commonly implemented in clinical research because they are viewed as more ecologically sensitive: the complexity of verbal fluency and planning tasks may make them more relatable to real-world tasks that require similar skills. Thus, there are both disadvantages (in terms of interpretability) and advantages (in terms of ecological validity) in the use of such complex EF tasks.
While there is growing evidence for EF impairments associated with OCD, results have been inconsistent, causing controversy about the nature and magnitude of these impairments (for review see Chamberlain, Blackwell, Fineberg, Robbins, & Sahakian, 2005; Kuelz, Hohagen, & Voderholzer, 2004b; Menzies, Chamberlain, et al., 2008a; Olley et al., 2007). Two recent meta-analyses targeting cognitive function more broadly in OCD found some evidence of impaired EF, but inconsistent effect sizes, likely due to the differences in the way they operationalized EF. Specifically, Abromovitch, Aromowitz, & Mittelman (2013) grouped tasks into composite measures of planning (d=0.44), response inhibition (d = 0.49), set shifting/cognitive flexibility (which included verbal and design fluency and WAIS similarities in addition to traditional measures of shifting, d=0.52), and verbal (d =0.34) and spatial (d =0.37) WM (which included measures of updating, in addition to WM maintenance and manipulation). Individual tasks and measures were not analyzed separately. Shin, Lee, Kim, & Kwon (2013) included some individual EF tasks, and in comparison to Abromovitch et al. (2013) found a much larger effect for planning (d =0.73), smaller effects on shifting tasks (d=0.31–0.51) and verbal working memory (d=0.11), and somewhat comparable effects on inhibition (d=0.55) and spatial WM (d =0.45) tasks. In addition, both meta-analyses included a relatively small number of studies in most EF analyses (e.g., 12 and 6 studies respectively for planning, compared to 28 in the current meta-analysis), potentially accounting for the variability in effect sizes. Such inconsistencies suggest the need for a larger-scale meta-analysis than has previously been performed, to improve the reliability of EF estimates. The present analysis also uses the well-articulated model of Miyake and Friedman, in conjunction with other perspectives on EF, to provide a more specific rationale for decomposing EF tasks.
Specifically, in addition to variable effect sizes, previous meta-analyses have reported considerable variability in the specific pattern of impairment across different components of EF. Such differences may derive from discrepancies in how EF was operationalized, e.g., how domains of EF were defined. In one case, measures were combined into composites which do not conform to established models of EF such as the unity/diversity model (e.g., fluency tasks, which tap multiple aspects of EF, were grouped with shifting tasks, and updating tasks were grouped with WM tasks), and in the other case only a handful of individual EF tasks were included, and composite measures were not analyzed. In sum, while these previous meta-analyses are valuable in providing a survey of cognitive function in OCD more broadly, they do not permit testing specific hypotheses about EF impairment in OCD.
The current meta-analysis thus addresses these limitations in the extant literature by taking a theoretically-driven approach, applying well-established models of EF to comprehensive analyses, to test competing hypotheses about the nature of EF impairments associated with OCD. At least four hypotheses regarding executive dysfunction in OCD have been proposed, positing that individuals with OCD have (1) a broad impairment in EF, (2) specific impairments in the shifting and/or inhibition components of EF, (3) general slowing of motor responses that accounts for apparent EF deficits, or (4) co-occurring depression that accounts for EF deficits.
Hypothesis 1: Broad impairment in EF
Evidence exists that individuals with OCD have abnormalities in a prefrontal-striatal network that is critical for EF, suggesting that EF may be broadly impaired in individuals with OCD. A meta-analysis of fMRI studies reporting case-control comparisons during a variety of cognitive tasks found evidence for activation abnormalities in a wide PFC network including anterior cingulate cortex (ACC), lateral PFC, and orbitofrontal cortex, as well as in the striatum (caudate and putamen; Menzies, Chamberlain, et al., 2008a). For many different EF tasks, there is joint recruitment of these regions (e.g., Duncan & Owen, 2000). Meta-analyses of neuroimaging studies have found reliable activation of dorsal and ventral lateral PFC and ACC for inhibition (Nee, Wager, & Jonides, 2007), shifting (Wager, Jonides, & Reading, 2004), WM (Wager & Smith, 2003), and verbal fluency (Costafreda, David, & Brammer, 2009), and a narrative review concluded that these regions were also active for planning (Collette, Hogge, Salmon, & Van der Linden, 2006). Orbitofrontal cortex has been implicated in evaluating the reward probabilities associated with different response options (e.g., see Krain et al., 2006 for a meta-analysis), and thus could affect performance across EF tasks, especially when response feedback or reward is involved.
Thus, the frontal-striatal model of OCD would predict broad impairment across multiple aspects of EF that are all supported by the prefrontal areas which are altered in individuals with OCD. Supporting this hypothesis, meta-analyses have reported deficits on a wide variety of EF tasks in individuals with OCD (Abramovitch et al., 2013; Shin et al., 2013). However, as noted above, the magnitude of these effects is inconsistent across previous meta-analyses, and composite measure analyses conforming to established models of EF were not conducted, making it impossible to effectively compare the magnitude of deficits across different aspects of EF. Thus, the breadth and magnitude of EF impairments associated with OCD has not been clearly established, and others have argued that such impairments are an artifact of co-occurring depressing or general motor slowing (see Hypotheses 2–4).
Hypothesis 2: Specific impairment in shifting and/or inhibition
Since highly repetitive behaviors and thoughts are the hallmarks of OCD, it has been proposed that individuals with OCD have particular difficulty shifting attention between different cognitive representations and behaviors, and/or inhibiting inappropriate responses (e.g., Bannon, Gonsalvez, Croft, & Boyce, 2002; Chamberlain et al., 2005; Olley et al., 2007). Hypothesis 2 is distinct from Hypothesis 1 in that it does not predict equivalent impairment on other aspects of EF, which would show deficits only to the extent that tasks designed to assess other aspects of EF also tap inhibition or shifting (e.g., planning tasks may require inhibiting incorrect moves and verbal fluency tasks may require shifting between subcategories). Thus, Hypothesis 2 is inconsistent with the general EF impairment posited by Hypothesis 1. Although impairments on shifting and inhibition tasks have been reported (Abramovitch et al., 2013; Shin et al., 2013), it is unclear whether the magnitude of these deficits is larger than deficits detected in other aspects of EF, a pattern would more clearly suggest the presence of impairments specific to shifting and inhibition.
Hypothesis 3: Apparent EF deficits are actually due to general motor response slowing
Individuals with OCD are often significantly slower than healthy individuals in completing everyday tasks such as eating and dressing (e.g., Hymas, Lees, Bolton, & Head, 1991), and may perform more poorly on timed than untimed tasks (e.g., Alarcón, Libb, & Boll, 1994). It has thus been proposed that individuals with OCD show a general slowing of motor responses, potentially because of abnormalities in the neuromotor system (e.g., Hymas et al., 1991). However, others have argued that response slowing in individuals with OCD is limited to EF tasks, and is not secondary to OCD symptoms such as checking (e.g., Bucci et al., 2007). In addition, there has been no systematic evaluation of whether individuals with OCD are significantly impaired on untimed, accuracy-based measures of EF, which would suggest that EF deficits cannot be attributed to general motor slowing. Critically, previous meta-analyses did not conduct separate analyses of accuracy measures (Abramovitch et al., 2013) or included only a few accuracy-based measures, which were not compared to RT-based measures (Shin et al., 2013).
A broader impairment in general processing speed has also been proposed to account for impaired task performance associated with psychopathology i.e., that the rate of processing limits performance on higher level operations because if processing steps are carried out too slowly, the products of earlier operations may be lost or no longer relevant by the time later operations occur (Nebes et al., 2000). However, in its current form this hypothesis is not empirically falsifiable. Since cognitive slowing is posited to affect even un- timed and unspeeded tasks, impairments on self-paced accuracy measures of EF would not be considered evidence against this hypothesis. Nor would greater impairment on EF tasks than processing speed tasks, since it is always possible to argue that more complex tasks may require more processing steps and are thus more affected by cognitive slowing. Thus, although the motor speed hypothesis can be empirically evaluated (including by meta-analyses), evaluation of the general processing speed hypothesis must await more complete specification of the theory in a way that makes it empirically falsifiable.
Hypothesis 4: Apparent EF deficits are actually due to co-occurring depression
There is a high rate of comorbidity between OCD and depression, with more than 70% of individuals with a primary diagnosis of OCD also experiencing a mood disorder during their lifetime (61% MDD; Brown, Campbell, Lehman, Grisham, & Mancill, 2001). Depression is also associated with broad impairments in EF (see Snyder, 2012 for a meta-analysis). Thus, some have argued that EF deficits in individuals with OCD are actually due to co-occurring depression rather than OCD per se (e.g., Basso, Bornstein, Carona, & Morton, 2001). Indeed, several studies have found that the effects of OCD on EF were no longer significant after controlling for co-occurring depressive symptoms (Aycicegi, Dinn, Harris, & Erkmen, 2003; Basso et al., 2001; Moritz, Kloss, Jahn, Schick, & Hand, 2003). However, other studies have found that co-occurring depressive symptoms do not account for EF deficits in individuals with OCD (Abramovitch, Dar, Schweiger, & Hermesh, 2011; Nedeljkovic et al., 2009), and most studies have not investigated the effects of co-occurring depression.
Current Meta-Analysis
The current meta-analysis synthesizes previous research findings and applies well-established models of EF to test the four hypotheses outlined above. Additionally, we examined the potential moderating effects of demographic (age and gender) and clinical (OCD symptom severity, psychotropic medication use, and co-occurring depression) variables on EF effect sizes. Findings are discussed in light of the barriers that may limit interpretation of the prior literature, and suggestions for potential solutions and future directions are presented.
Method
Inclusion and Exclusion Criteria
To be eligible for inclusion, studies were required to include a group of individuals with a diagnosis of OCD, and a healthy control group with no diagnosed psychopathology. Studies were included if they tested participants on at least one EF task and reported sufficient information to calculate effect sizes. EF tasks were defined as detailed in the Coding Procedures section. Studies were excluded if they investigated OCD in samples of participants with organic brain damage (e.g. following head-injury).
Search Strategies
Searches were conducted in PubMed and ISI Web of Science for papers published through October 2013 using the keywords obsessive compulsive paired with executive function, working memory, response inhibition, inhibitory control, shifting, task switching, planning, verbal fluency, cognitive, or neuropsychological. In addition, a search for unpublished studies was conducted by emailing the corresponding authors of papers included in the meta-analysis, and searching ProQuest for unpublished dissertations and masters theses. The first author, who is experienced in EF research, conducted the search and screening procedures. An initial screen for study eligibility was conducted by examining titles to eliminate studies that clearly did not meet the inclusion criteria. Next, the abstracts of all remaining articles were examined, and if an article appeared likely to meet the inclusion criteria the full text was obtained and checked for inclusion criteria. In addition, the reference lists of included articles, and articles citing included articles, were screened for any studies missed in the database search process. Publication bias was assessed using the trim and fill method (Duval & Tweedie, 2000).
Coding Procedures
Tasks
The types of tasks used in the included studies determined the specific aspects of EF covered in the present meta-analysis. Tasks were coded as tapping one of the following EF components, as detailed below: shifting, inhibition, updating, verbal and visuospatial WM, planning, and verbal fluency. This list is not meant to be exhaustive of all EF abilities, but rather includes all the EF tasks commonly included in the OCD literature. The first author, who is highly experienced with EF research, coded all studies. In addition, for 25% of studies, the coauthors, who are also highly experienced with EF research, coded the EF component measured by each EF task, blind to the first author’s coding. Intercoder agreement for the included EF tasks was high (99%)2; thus, the first author’s coding was used in all analyses. Descriptions of the included tasks tapping each EF construct, their dependent measures, and the number of studies reporting each, are provided in Table 1. For each construct, all tasks were included in a composite measure analysis. All tasks and measures reported by at least three studies were also analyzed individually in separate analyses.
Table 1.
EF Tasks Included in the Meta-Analysis
| Construct | Task | Description | Outcome Measure(s) | # Of Studies |
|---|---|---|---|---|
| Shifting | Intradimensional/Extradimensional Shift (ID/ED) | Learn from feedback to select a stimulus based on one dimension, switch to the previously non-rewarded stimulus (intradimensional shift), then to a different stimulus dimension (extradimensional shift). |
*1. Perseverative errors in intradimensional & extradimensional shifts. 2. # of shifts achieved. |
7^ |
|
| ||||
| Trail Making B (TMT-B) | Alternately connect letters and numbers in sequence (A-1-B-2 etc.) Often compared to Trail Making A (connect letters or numbers only, does not require shifting). |
*1. Trail Making B–Trail Making A time. 2. Time to complete B. |
37^ | |
|
| ||||
| Object Alternation Test/Delayed Object Alternation Test (OAT/DAT) | Find object hidden alternately under two different cups, immediately or after short delay. | Errors | 17^ | |
|
| ||||
| Wisconsin Card Sorting Task (WCST) | Learn from feedback to sort cards by one dimension (e.g. color), and then switch to a different dimension (e.g. shape) when given negative feedback on the first dimension (repeats with multiple sorting rules). |
*1. Perseverative errors. 2. # of rules achieved. |
42^ | |
|
| ||||
| Cued task switching | Perform one of two tasks depending on cue before each trail (e.g. color/shape). | Switch cost (switch–repeat RT). | 3^ | |
|
| ||||
| Inhibition | Color-word Stroop | Identify the color ink a color word is printed in. Trials are incongruent (e.g. “red” written in blue ink) or neutral (non-color word or color patches). |
*1. Stroop interference (incongruent– neutral RT) 2. Incongruent condition time. 3. Accuracy |
28^ |
|
| ||||
| Stop-signal | Quickly categorize and respond to stimuli (e.g. left and right pointing arrows), while withholding responses when a stop signal is presented. | Stop signal RT (time needed to stop a response). | 6^ | |
|
| ||||
| Go-No-Go | Quickly categorize and respond to stimuli (e.g. left and right pointing arrows), while withholding responses to another stimulus. | Commission (no-go) errors | 11^ | |
|
| ||||
| Antisaccade | Look in the opposite direction of visual cue. | Errors (prosaccades) | 2 | |
|
| ||||
| Hayling | Read sentences where the final word is omitted but highly predictable. First complete sentences correctly (Part A), then with an unrelated word (part B). | Part B – Part A RT. | 2 | |
|
| ||||
| Updating | n-Back | Indicate if the stimulus (usually letter) matches the stimulus n (e.g. 3) items back. | Accuracy | 4^ |
|
| ||||
| Visuospatial Working Memory | Block Span (Spatial span, Corsi block tapping) | Tap irregularly arranged blocks/squares in the same order as experimenter (Corsi blocks) or computer (Spatial Span). | Span (Max. length of sequence correctly performed). | 14^ |
|
| ||||
| Self-ordered pointing | Search an array of boxes for hidden tokens. Token is only in each location once. |
*1. Within-search errors (return to previous location). 2. Strategy score (how often search is initiated from same starting box). |
11^ | |
|
| ||||
| Delayed match-to-sample (DMTS) | Maintain a complex spatial pattern across a delay and indicate whether a probe matches it. | Accuracy | 3^ | |
|
| ||||
| Verbal Working Memory | Digit span forward | Repeat sequence of numbers in forward order. | Span (Max. length of sequence correctly performed). | 19^ |
| Digit span backward | Repeat sequence of numbers in reverse order. | Span (Max. length of sequence correctly performed). | 11^ | |
| Letter-number sequencing | Repeat list of alternating letters and numbers, re-sequenced into numbers first, then letters. | Span (Max. length of sequence correctly performed). | 2 | |
| Reading span | Read a series of sentences while remembering the last word in each sentence. Some versions also require verifying if each sentence is true or false. | Number of correctly recalled words. | 2 | |
|
| ||||
| Operation span | Solve a series of math operations while trying to remember a series of unrelated words. | Number of correctly recalled words. | 1 | |
| Self-ordered pointing with words | Point to a different word in an array on each trial. | Between-search errors. | 1 | |
|
| ||||
| Verbal Fluency | Semantic verbal fluency | Say as many words from a semantic category (e.g. animals) as possible in 1 (or 3) min. | # of words | 17^ |
|
| ||||
| Phonemic verbal fluency/Controlled Oral Word Association (COWA) | Say as many items starting with a certain letter (usually F, A, S) as possible in 1 (or 3) min. | # of words | 35^ | |
|
| ||||
| Planning | Tower of London/Tower of Hanoi (TOL/TOH) | Move rings on pegs from a starting position to a target position in as few moves as possible, following a set of rules. |
*1. Number of moves in excess if minimum. 2. Number of problems solved in minimum moves. |
28^ |
Note.
Preferred measure if reported.
Analyzed individually as well as part of composite measure.
In addition, two types of motor speed measures reported by studies in the meta-analysis were included, to determine if there is general motor slowing associated with OCD, as proposed by Hypothesis 3. The Trail Making Test Part A (TMT-A, k=32) shares the motor speed and sequencing demands of the Trial Making Test Part B (TMT-B), but does not require shifting like the TMT-B. In addition, 10 studies reported one or more general motor speed measures, including simple reaction time (k=4), choice reaction time (k =2), finger tapping (tap fingers as quickly as possible; k =1), and grooved pegboard (put pegs into holes in a board as quickly as possible; k = 4). These tasks were included in a general motor speed composite score.
Moderator Variables
Information was coded on current OCD symptom severity, age, gender, psychotropic medication use, and co-occurring depression.
Symptom severity
Total Yale-Brown Obsessive Compulsive Scale (Y-BOCS; Goodman, Price, Rasmussen, Mazure, Fleischmann, et al., 1989b) scores were reported by 81% of studies. The Y-BOCS is the most frequently used questionnaire to assess OCD symptom severity, and has good reliability and validity (Goodman, Price, Rasmussen, Mazure, Delgado, et al., 1989a; Goodman, Price, Rasmussen, Mazure, Fleischmann, et al., 1989b). It is a clinician-rated 10-items scale, with each item rated from 0 (no symptoms) to 4 (severe symptoms), providing a total range of 0–40.
Age
The mean age of the OCD group was included as a continuous variable in meta-regression analyses. Age was reported by all studies.
Gender
The percentage of females in the OCD group was included as a continuous variable in meta-regression analyses. Gender was reported by 96% of studies.
Medication
The percentage of the OCD group currently taking psychotropic medications was coded for each sample. Medication usage was reported by 81% of studies. Many studies only reported the total number of participants using medication; thus, a more detailed analysis of the types or duration of medication could not be conducted. However, when type was reported, medications were generally antidepressants.
Co-occurring depression
Because of the diversity of depression measures reported and the lack of detailed depression reporting in many studies, continuous measures of co-occurring depressive symptoms could not analyzed. Instead, the presence of co-occurring depression or depressive symptoms in the sample was coded as a categorical variable. The sample was coded as containing individuals with co-occurring depression or depressive symptoms if (a) any OCD participants were reported to have a comorbid diagnosis of a depressive disorder and/or (b) mean depressive symptoms on a standard depression questionnaire were reported in the clinical range. The sample was coded as containing participants without co-occurring depression or depressive symptoms only if (a) and (b) were not met (since the absence of diagnosed depression does not preclude clinically significant levels of depressive symptoms). The clinical range was defined as follows, using published cut-point norms: Hamilton Depression Rating Scale >7 (Kearns et al., 1982), Montgomery–Asberg Depression Rating Scale >7 (Kearns et al., 1982), Beck Depression Inventory > 9 (Beck, 1978), Beck Depression Inventory – II > 13 (Beck, Steer, & Brown, 1996), Children’s Depression Inventory > 12 (Kovacs, 1983). Applying both criteria, 55% of samples were coded as having co-occurring depression/depressive symptoms, 18% as having no co-occurring depression/depressive symptoms, and 27% did not report enough information to code. Co-occurring depression was included as a categorical variable in meta-analyses of variance whenever there were at least three studies in the smaller category. In addition, samples with no co-occurring depression/depressive symptoms were analyzed separately to provide a conservative test of the hypothesis that EF deficits in OCD are driven by co-occurring depression.
Statistical Methods
For each study, effect sizes comparing the performance of the OCD and control groups on each EF measure were calculated as Cohen’s d. The sign of d was set such that a positive value always indicated poorer performance for the OCD group relative to the control group (e.g. lower accuracy, higher error rates, or longer RTs). Before analyses were conducted, effect sizes were adjusted, weighted, and screened for outliers. First, since it has been demonstrated that d is slightly over-estimated when sample sizes are small, Hedges’ (1980) small sample bias correction was applied (dadj=d(1−(3/4N)−9, where N= number of participants in both samples combined). Second, since sampling error is also higher for smaller sample sizes, effect sizes were weighted by sample size using inverse variance weights (w = (2(n1+ n2)n1n2)/(2(n1+ n2)2 + n1n2d2), where n1 and n2 are the number of participants in the OCD and control groups respectively). Finally, analyses were screened for outliers with effect sizes +/− 3 SD from the mean effect size in each analysis; only two such outliers were detected: one was excluded from the shifting composite analysis and one from the digit span forward and verbal WM composite analyses (see Table 2 footnotes a–c).
Table 2.
Mean Effect Size Analyses
| Measure | N | k | d | 95% CI | SE | Z | p | Homogeneity Test | Publication Bias | IQ Matching | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| LL | UL | v | Q | df | p | d Trimmed & Filled (k trimmed) | d Published Only (k) | d IQ Matched Only (k) | |||||||
| Inhibition: | |||||||||||||||
| Inhibition Comp. | 2753 | 50 | 0.37* | 0.24 | 0.51 | 0.07 | 5.35 | <.001 | 0.15 | 141.01 | 49 | <.001 | 0.37* (0) | 0.40* (46) | 0.43*(24) |
| Stroop (Incongruent Time) | 861 | 16 | 0.55* | 0.21 | 0.90 | 0.18 | 3.14 | .002 | 0.39 | 85.36 | 15 | <.001 | 0.55* (0) | 0.55* (16) | 0.99 (4) |
| Stroop (Interference) | 1326 | 18 | 0.36* | 0.17 | 0.56 | 0.10 | 3.72 | .002 | 0.10 | 44.89 | 17 | <.001 | 0.36* (0) | 0.40* (17) | 0.53* (7) |
| Stroop (Accuracy) | 240 | 6 | 0.39* | 0.11 | 0.67 | 0.14 | 2.73 | .006 | 0.00 | 3.62 | 5 | .606 | 0.39* (0) | 0.39* (6) | 0.38 (3) |
| Stop Signal (SSRT) | 248 | 6 | 0.62* | 0.23 | 1.01 | 0.20 | 3.14 | .002 | 0.13 | 10.80 | 5 | .055 | 0.62* (0) | 0.60* (5) | 0.62* (6) |
| Go/No-Go (No-Go Accuracy) | 438 | 11 | 0.24 | −0.07 | 0.55 | 0.16 | 1.51 | .132 | 0.16 | 25.14 | 10 | .005 | 0.24 (0) | 0.18 (10) | 0.41 (5) |
|
| |||||||||||||||
| Shifting: | |||||||||||||||
| Shifting Comp.a | 4762 | 74 | 0.50* | 0.42 | 0.58 | 0.04 | 12.23 | <.001 | 0.05 | 122.35 | 73 | <.001 | 0.36* (19) | 0.49* (68) | 0.53* (36) |
| ID/ED Shift (Accuracy) | 435 | 7 | 0.50* | 0.31 | 0.70 | 0.10 | 5.13 | <.001 | 0.00 | 1.99 | 6 | .921 | 0.48* (1) | 0.49* (6) | 0.53* (5) |
| OAT/DAT (Accuracy) | 1131 | 17 | 0.32* | 0.15 | 0.49 | 0.09 | 3.72 | <.001 | 0.05 | 29.07 | 16 | .023 | 0.23* (4) | 0.29* (15) | 0.30* (9) |
| TMT-B (Time) | 2659 | 37 | 0.54* | 0.42 | 0.66 | 0.06 | 8.67 | <.001 | 0.07 | 76.69 | 36 | <.001 | 0.54* (0) | 0.53* (33) | 0.59*(16) |
| WCST (Perseverative Errors)b | 2706 | 42 | 0.44* | 0.33 | 0.55 | 0.06 | 7.82 | <.001 | 0.06 | 75.80 | 41 | <.001 | 0.29*(12) | 0.45* (39) | 0.46*(20) |
| Cued Task Switching (Switch Cost) | 102 | 3 | 0.35 | −0.05 | 0.76 | 0.21 | 1.70 | .089 | 0.00 | 0.53 | 2 | .767 | 0.35 (0) | 0.35 (3) | – |
|
| |||||||||||||||
| Updating: | |||||||||||||||
| n-Back | 375 | 4 | 0.71* | 0.26 | 1.15 | 0.23 | 3.13 | .002 | 0.12 | 8.08 | 3 | .044 | 0.61* (1) | 0.71* (4) | – |
|
| |||||||||||||||
| Verbal Working Memory: | |||||||||||||||
| Verbal WM Comp. | 1673 | 24 | 0.22* | 0.08 | 0.36 | 0.07 | 3.06 | .002 | 0.06 | 46.30 | 24 | .004 | 0.22* (0) | 0.22* (22) | 0.21 (11) |
| Verbal WM Manipulation Comp. | 942 | 14 | 0.31* | 0.10 | 0.52 | 0.11 | 2.87 | .004 | 0.09 | 31.22 | 13 | .003 | 0.26* (1) | 0.31* (12) | 0.22 (8) |
| Digit Span Backward (Span) | 771 | 11 | 0.21 | 0.00 | 0.42 | 0.11 | 1.95 | .052 | 0.06 | 20.01 | 10 | .029 | 0.21 (0) | 0.20 (9) | 0.11 (7) |
| Verbal WM Maintenance Comp.c | 1206 | 19 | 0.07 | −0.06 | 0.20 | 0.07 | 1.02 | .310 | 0.02 | 25.74 | 18 | .106 | 0.06 (1) | 0.07 (17) | 0.01 (8) |
| Digit Span Forward (Span)c | 1206 | 19 | 0.08 | −0.05 | 0.20 | 0.06 | 1.21 | .225 | 0.02 | 24.29 | 18 | .146 | −0.07 (7) | 0.07 (17) | 0.03 (8) |
|
| |||||||||||||||
| Visuospatial Working Memory: | |||||||||||||||
| Visuospatial WM Comp. | 1483 | 24 | 0.47* | 0.31 | 0.62 | 0.08 | 6.01 | <.001 | 0.07 | 44.10 | 23 | .005 | 0.47* (0) | 0.48* (21) | 0.47*(12) |
| Block Span (Span) | 1107 | 14 | 0.43* | 0.27 | 0.59 | 0.08 | 5.43 | <.001 | 0.03 | 18.89 | 13 | .13 | 0.33* (3) | 0.43* (12) | 0.41* (8) |
| Self-Ordered Pointing (Accuracy) | 596 | 11 | 0.62* | 0.37 | 0.87 | 0.13 | 4.93 | <.001 | 0.09 | 20.89 | 10 | .022 | 0.56* (1) | 0.72* (9) | 0.74* (8) |
| DMTS (Accuracy) | 122 | 3 | 0.49 | −0.10 | 1.09 | 0.30 | 1.62 | .106 | 0.16 | 4.68 | 2 | .096 | 0.49 (0) | 0.49 (3) | – |
|
| |||||||||||||||
| Verbal Fluency: | |||||||||||||||
| VF Comp. | 2681 | 40 | 0.36* | 0.26 | 0.47 | 0.05 | 6.79 | <.001 | 0.04 | 62.91 | 39 | .009 | 0.29* (7) | 0.37* (37) | 0.42*(19) |
| Phonemic VF (Words)d | 2462 | 35 | 0.39* | 0.31 | 0.47 | 0.04 | 9.17 | <.001 | 0.00 | 30.39 | 34 | .645 | 0.39* (0) | 0.38* (33) | 0.41*(18) |
| Semantic VF (Words) | 1088 | 17 | 0.34* | 0.11 | 0.57 | 0.12 | 2.96 | <.001 | 0.15 | 50.77 | 16 | <.001 | 0.26* (2) | 0.34* (17) | 0.42* (8) |
|
| |||||||||||||||
| Planning: | |||||||||||||||
| Planning Comp. | 2017 | 28 | 0.44* | 0.31 | 0.57 | 0.07 | 6.42 | <.001 | 0.06 | 55.05 | 27 | .001 | 0.35* (6) | 0.42* (26) | 0.50*(17) |
| TOL/TOH (Accuracy) | 1853 | 25 | 0.44* | 0.27 | 0.61 | 0.09 | 5.11 | <.001 | 0.12 | 71.85 | 24 | <.001 | 0.36* (4) | 0.41* (23) | 0.53*(17) |
| TOL/TOH (Movement Latency) | 850 | 12 | 0.42* | 0.23 | 0.62 | 0.10 | 4.17 | <.001 | 0.05 | 20.03 | 11 | .045 | 0.29* (3) | 0.44* (11) | 0.50* (6) |
|
| |||||||||||||||
| Motor Speed Tasks: | |||||||||||||||
| TMT-A (Time) | 2297 | 32 | 0.57* | 0.45 | 0.68 | 0.06 | 9.74 | <.001 | 0.04 | 48.79 | 31 | .022 | 0.49* (5) | 0.55* (30) | 0.60*(22) |
| General motor Speed Comp. | 514 | 10 | 0.34* | 0.05 | 0.63 | 0.15 | 2.30 | .021 | 0.13 | 23.12 | 9 | .006 | 0.34* (0) | 0.39* (8) | 0.08 (3) |
Notes. Dashes indicate too few IQ matched studies to conduct analysis (k< 3). N= number of participants; k = number of studies; d= weighted mean Cohen’s d effect size; CI= confidence interval; LL= lower limit; UL = upper limit; v = random-effects variance component; Q = heterogeneity; Comp. = composite score; WCST = Wisconsin Card Sorting Test; OAT/DAT = Object alternation task/delayed alternation task; TMT–B = Trail Making Test Part B; TMT–A =Trail Making Test Part A; ID/ED = Intradimensional/Extradimensional; WM = working memory; DMTS = delayed-match-to-sample; VF = verbal fluency; TOL/TOH = Tower of London, Tower of Hanoi.
One outlier excluded (d=2.66). With this outlier included the weighted mean effect size is d=0.53.
a One outlier excluded (d=2.66). With this outlier included the weighted mean effect size is d=0.47.
One outlier excluded (d=1.96). With this outlier included the weighted mean effect size is d=0.14.
One outlier excluded (d =−0.58). With this outlier included the weighted mean effect size is d=.0.37
significant (p < .05)
Only one effect size from each sample comparison was included in each analysis to avoid statistical dependence. When there were three or more studies reporting a particular task, individual tasks were analyzed separately, as described in Coding Procedures. In addition, composite effect sizes were calculated by averaging effect sizes within a construct (e.g. all inhibition measures). In addition, when individuals were tested more than once (e.g., at different points in treatment), only task performance at the first testing timing was analyzed, as practice effects may diminish the EF demands of tasks.
Random effects meta-analytic models were used for all analyses. Although many meta-analyses have used fixed-effects models in the past, random effects models are now considered more appropriate, as there are likely to be many sources of variability between study samples beyond sampling error, violating the assumptions of fixed-effects models (Raudenbush, 2009). Importantly, random effects models allow inferences to be drawn about a broader population of studies, rather than just about the samples tested.
Analyses were conducted using the SPSS meta-analysis macro developed by David B. Wilson (Wilson, 2006). For each analysis, weighted mean effect sizes with 95% confidence intervals were calculated. The null hypothesis that the mean effect size is zero was tested with the z statistic at the alpha = .05 significance level. Heterogeneity in effect sizes was tested with the Qt statistic (Hedges & Olkin, 1985). Qt quantifies the degree to which the studies contributing to each weighted mean effect size can be considered homogeneous. If Qt is significant, it suggests that there are substantive differences between the studies in that analysis. Publication bias was assessed with the trim-and-fill method (Duval & Tweedie, 2000). In addition, separate analyses were conducted including only published studies to determine whether the inclusion of unpublished studies affected the results. To ensure that effects were not driven by failure to match OCD and control samples on IQ, separate analyses were also conducted including only those studies that reported that IQ did not significantly differ between groups.
Moderator analyses were conducted via mixed-effects models with method of moments estimation. Current symptom severity (Y-BOCS), OCD group age, and medication status (% receiving psychotropic medications) were included as continuous variables in separate and combined meta-regression analyses. Co-occurring depression (individuals with clinically-significant levels of depressive symptoms and/or depressive disorder diagnosis) was included as a categorical variable in meta-analyses of variance whenever there were at least three studies in the smaller category. Moderator analyses were conducted only for measures with 20 or more effect sizes, as analyses with fewer studies have inadequate power and may produce unstable estimates (Marín-Martínez & Sánchez-Meca, 1998; Sánchez-Meca & Marín-Martínez, 1998).
Results
The search process identified 110 studies for inclusion, 104 of which were published in peer-reviewed journals and 6 of which were unpublished. An additional 26 papers were screened but were excluded because they did not report sufficient information to calculate effect sizes (k=11), did not include a healthy control group (k=7), included the same data as another paper in the meta-analysis (k=4), pre-matched/pre-trained subjects on task performance (k=2), or used tasks not clearly classifiable into an EF component (k=2; see Supplementary Information, Table S1). Included studies are marked with an * in the reference list. The full data sets used in the analyses are available from the authors upon request.
In total, the included studies comprised 6,315 participants (3,162 individuals with OCD and 3,153 healthy control participants). On average, individuals with OCD in the included studies were 31.36 years old (SD=7.16, range 12–493), and there were equal numbers of males and females with OCD (50.14% female, SD=20.40, range 0–100%). Across studies, the average Y-BOCS (Goodman et al., 1989) score was 23.07 (SD=4.32, range 3–30), corresponding to a moderate level of symptom severity, and 38.90% of individuals with OCD were currently taking psychotropic medications (SD=37.35, range 0–100%).
Weighted mean effect sizes
Individuals with OCD performed more poorly than healthy control participants on most EF tasks, but the magnitude of these impairments varied somewhat depending on the aspect of EF and the task. Table 2 reports the effect size (d) for each measure, along with their 95% confidence intervals and significance test; these effect sizes, with 95% confidence intervals, are also plotted in Figure 1. Table 2 also provides the test for homogeneity of effect sizes across samples (Q), and tests for sensitivity of the results to publication bias, inclusion of unpublished studies, and IQ matching, as detailed in following sections.
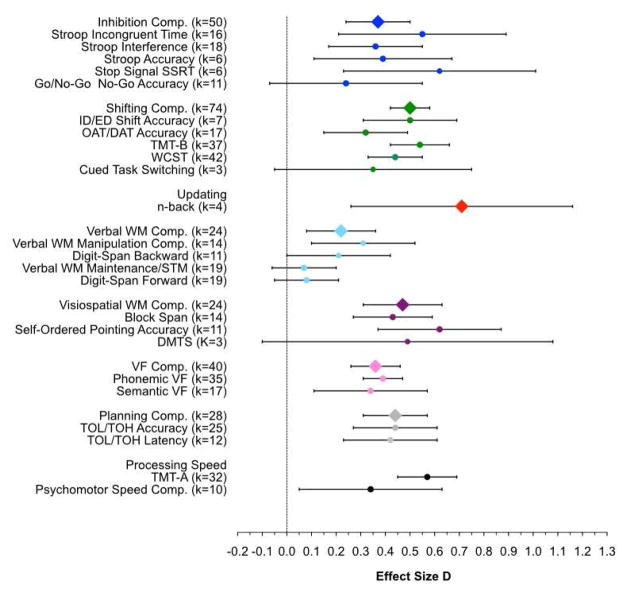
Figure 1.
Weighted mean effect sizes for all analyses. Error bars are 95% confidence intervals. Measures for which the lower error bar does not pass the vertical line are significantly greater than 0. Compared to healthy control participants, individuals with OCD are significantly impaired on most EF tasks. Executive function (EF) composite measures are indicated with diamond symbols, and individual measures within each EF component are indicated by circle symbols in the same color. Black circles indicate non-EF comparison measures. Comp. = Composite score; WCST = Wisconsin Card Sorting Test; OAT/DAT = Object alternation task/delayed alternation task; TMT–B = Trail Making Test Part B; TMT–A =Trail Making Test Part A; ID/ED = Intradimensional/Extradimensional; WM = working memory; DMTS = delayed-match-to-sample; VF = verbal fluency; TOL/TOH = Tower of London, Tower of Hanoi.
Inhibition
There were significant small to medium effects of OCD groups compared to healthy groups for the inhibition composite measure (d = .37), Stroop incongruent condition time (d = .55), interference (d = .36) and accuracy (d = .39), and stop signal task reaction times (SSRT; d = .62), but only a small and non-significant effect for accuracy on the go/no-go task (d = .24).
Shifting
There was a moderate and significant effect of group for shifting composite scores (d = .50). Examining the individual shifting tasks, the largest effect size was for TMT-B (d = .54). However, the effect size was as large for the TMT-A, which does not require shifting (d = .57), suggesting that this effect may be primarily driven by slowed general motor speed or sequencing, and not shifting per se. Thus, it may be more informative to focus on shifting tasks that are not confounded by general motor speed demands because they are self-paced and have accuracy, rather than RT, outcome measures (see Table 1). These all have somewhat smaller, but significant, effects (Intradimensional/Extradimensional (ID/ED) Shift, d = .50; Object Alternation/Delayed Object Alternation Test (OAT/DAT), d = .32; Wisconsin Card Sorting Task (WCST; d = .44). The only shifting task which did not show a significant effect was cued task switching (d = .35), which was marginal; however, this should be interpreted with caution as there were few studies using this task (k = 3).
Updating
Only four studies tested updating, all with an n-back test. Nonetheless, there was a large effect size (d = .71), although confidence intervals are wide due to the small number of studies.
Verbal WM
There was a small but significant effect of group on overall verbal WM composite scores (d = .22). Separating measures into those requiring manipulation of information in WM versus simple WM maintenance, there was a small but significant effect of group on WM manipulation composite scores (d = .31), with a marginal effect for digit span backward (d = .21). There was no evidence for meaningful verbal WM maintenance impairments in individuals with OCD. There were very small and non-significant effects for verbal WM maintenance composite scores (d = .07), and digit span forward (d = .08).
Visuospatial WM
There were significant effects of OCD groups compared to healthy control groups for visuospatial WM composite scores (d = .47), block span (d = .43), and self-ordered pointing (d = .62). The effect size for delayed-match-to-sample (DMTS) was comparable in magnitude to those for block span and composite scores (d = .49), but did not reach significance due to the small number of studies using this task (k = 3).
Verbal Fluency
There was a small but significant effect of group for verbal fluency composite scores (d = .36), with comparable and significant effects for phonemic (d = .39) and semantic (d = .34) verbal fluency.
Planning
There was a significant effect for planning composite scores (d = .44). Since some studies used latency measures (i.e., time until first move), which may be influenced by overall slowing, latency and accuracy measures were also analyzed separately. There were similar and significant effects for accuracy measures (d = .44) and latency measures (d = .42).
General Motor Speed
There was a significant effect of OCD groups compared to healthy groups for general motor speed measures (d = .34), although a great deal of variability between studies led to a wide confidence interval (d = .05–.63). As noted above, there was also a significant effect for the TMT-A, the comparison task for the TMT-B, which requires both general motor speed and sequencing (d = .57).
Heterogeneity Analyses
There was significant heterogeneity among effect sizes for all measures except Stroop accuracy, stop signal (marginal), ID/ED shift, cued task switching, verbal WM maintenance, digit span forward, block span, DMTS (marginal), and phonemic verbal fluency. There may be multiple sources of this variability. First, some variability is likely due to differences in methodology across studies. In composite score analyses, tasks are likely to vary in sensitivity (e.g., standard neuropsychological tests are less sensitive to subtle impairments than those designed to assess individual differences in the normal range). Even in analyses of single tasks and measures, task versions may vary in sensitivity (e.g., the standard neuropsychological version of the Stroop task, with separate blocks of neutral and incongruent stimuli, is easier than versions in which trial types are intermixed). Given the myriad variations in tasks, it is not possible to account for this variation. Second, some variability is due to differences in the demographic characteristics of the patient groups included in each study (see Moderator Analyses). Finally, there are likely additional unmeasured moderators, given the known heterogeneity of clinical profiles, genetics, and neurobiology in all diagnostic categories, including OCD.
Sensitivity Analyses
Publication Bias
Effect sizes for Duval & Tweedie’s (2000) trim-and-fill analyses are reported in Table 2. Overall, there was little evidence of publication bias. Across analyses, effects were very robust to the trim-and-fill procedure: on average, weighted mean d was only 0.05 lower than for untrimmed analyses, and no significant measures became non-significant.
IQ Matching
Effect sizes for IQ matched samples only are reported in Table 2. Across analyses, effects were very robust to IQ matching: on average, for studies that matched groups on IQ, weighted mean d actually was .04 higher than those for all samples combined. Nearly all analyses that were significant for all samples remained significant when restricted to IQ-matched samples. Two significant effects, for the verbal WM overall and manipulation composite scores, had slightly reduced effect sizes and became non-significant due to low power, as there were few IQ-matched samples (k =11, k=8). In addition, the effect size for Stroop accuracy was higher for IQ-matched samples, but the effect became non-significant due to low power (k =4). IQ-matched samples could not be analyzed for cued task switching, n-back, and DMTS because there were fewer than three IQ-matched samples.
Moderator Analyses
Effect sizes were largely stable across variation in demographic and clinical characteristics of the samples, although age and gender did moderate some effects. Specifically, was some evidence for increasing effect sizes with increasing age and increasing percentage of female participants, while symptom severity did not moderate effect sizes. Importantly, moderator analyses indicate that deficits in EF associated with OCD are not driven by co-occurring depressing or medication use.
Depression
Comparisons of samples with and without co-occurring diagnosis of a depressive disorder or elevated depressive symptoms are reported in Table 3. Table 3 gives effect sizes for samples without co-occurring depression (absent) and those with possible co-occurring depression (possible), along with the confidence interval and significance test for each group, and the test for the significance of the difference in effect sizes between the group (Q Between). Across analyses, effects were very robust to depression. Depression was only a significant moderator for one task measure, Stroop interference, with larger effect sizes for samples without co-occurring depression diagnosis or elevated depressive symptoms. However, this finding should be interpreted with caution since there were few Stroop interference studies in participants without depression (k=5). In addition, for the TMT-A comparison measure, effects were marginally larger for samples with possible co-occurring depression.
Table 3.
Depression Moderator ANOVA Analyses
| DV | Depression1 | d | 95% CL
|
SE | z | p | K | v | Q Between (df) | p | Q Within (df) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | ||||||||||||
| Inhibition Comp. | Absent | 0.47* | 0.18 | 0.76 | 0.15 | 3.14 | .002 | 10 | 0.14 | 0.02 (1) | .894 | 31.41 (30) | .396 |
| Possible | 0.49* | 0.29 | 0.69 | 0.10 | 4.86 | <.001 | 22 | ||||||
|
| |||||||||||||
| Stroop Interference | Absent | 0.72* | 0.34 | 1.09 | 0.19 | 3.71 | <.001 | 5 | 0.11 | 3.88 (1) | .048* | 11.84 (11) | .376 |
| Possible | 0.23 | −0.06 | 0.53 | 0.15 | 1.57 | .117 | 8 | ||||||
|
| |||||||||||||
| Stroop Incongruent Time | Absent | 1.24* | 0.46 | 2.02 | 0.40 | 3.12 | .002 | 3 | 0.36 | 1.56 (1) | .211 | 7.36 (5) | .195 |
| Possible | 0.59 | −0.06 | 1.24 | 0.33 | 1.79 | .073 | 4 | ||||||
|
| |||||||||||||
| Stop Signal (SSRT) | Absent | 0.39 | −0.17 | 0.95 | 0.28 | 1.38 | .169 | 3 | 0.13 | 1.28 (1) | .257 | 3.80 (4) | .434 |
| Possible | 0.84* | 0.30 | 1.39 | 0.28 | 3.03 | .003 | 3 | ||||||
|
| |||||||||||||
| Go/No-Go (Accuracy) | Absent | 0.43 | −0.21 | 1.06 | 0.32 | 1.32 | .186 | 3 | 0.23 | 0.16 (1) | .691 | 4.75 (5) | .447 |
| Possible | 0.26 | −0.32 | 0.83 | 0.26 | 0.87 | .384 | 3 | ||||||
|
| |||||||||||||
| Shifting Comp. | Absent | 0.55* | 0.34 | 0.76 | 0.11 | 5.14 | <.001 | 10 | 0.02 | 0.48 (1) | .487 | 52.03 (52) | .473 |
| Possible | 0.47* | 0.38 | 0.56 | 0.05 | 10.19 | <.001 | 44 | ||||||
|
| |||||||||||||
| OAT/DAT | Absent | 0.47* | 0.13 | 0.82 | 0.18 | 2.70 | .007 | 3 | 0.00 | 1.88 (1) | .170 | 8.03 (9) | .531 |
| Possible | 0.21* | 0.05 | 0.37 | 0.08 | 2.63 | .009 | 8 | ||||||
|
| |||||||||||||
| TMT-B | Absent | 0.58* | 0.25 | 0.91 | 0.17 | 3.44 | .001 | 4 | 0.05 | 0.02 (1) | .884 | 25.64 (25) | .427 |
| Possible | 0.55* | 0.41 | 0.69 | 0.08 | 7.74 | <.001 | 23 | ||||||
|
| |||||||||||||
| WCST | Absent | 0.29* | 0.09 | 0.49 | 0.10 | 2.86 | .004 | 9 | 0.01 | 0.73 (1) | .392 | 25.70 (26) | .480 |
| Possible | 0.39* | 0.27 | 0.51 | 0.06 | 6.61 | <.001 | 19 | ||||||
|
| |||||||||||||
| Verbal Working Memory Comp. | Absent | 0.31^ | −0.01 | 0.62 | 0.16 | 1.90 | .058 | 5 | 0.05 | 0.30 (1) | .584 | 17.05 (15) | .316 |
| Possible | 0.20 | 0.01 | 0.40 | 0.10 | 2.05 | .040 | 12 | ||||||
|
| |||||||||||||
| Verbal Working Memory Manipulation Comp. | Absent | 0.24 | −0.22 | 0.70 | 0.23 | 1.01 | .310 | 3 | 0.10 | 0.58 (1) | .448 | 6.74 (7) | .456 |
| Possible | 0.46* | 0.12 | 0.79 | 0.17 | 2.69 | .007 | 6 | ||||||
|
| |||||||||||||
| Verbal Working Memory Maintenance Comp. | Absent | 0.20 | −0.16 | 0.56 | 0.18 | 1.10 | .273 | 5 | 0.11 | 0.05 (1) | 0.822 | 15.70 (12) | .206 |
| Possible | 0.25^ | −0.02 | 0.53 | 0.14 | 1.79 | .074 | 9 | ||||||
|
| |||||||||||||
| Digit Span Forward | Absent | 0.20 | −0.16 | 0.56 | 0.18 | 1.09 | .274 | 5 | 0.11 | 0.05 (1) | .825 | 15.71 (12) | .205 |
| Possible | 0.25^ | −0.03 | 0.53 | 0.14 | 1.78 | .076 | 9 | ||||||
|
| |||||||||||||
| Visuospatial Working Memory Comp. | Absent | 0.41* | 0.06 | 0.76 | 0.18 | 2.27 | .023 | 6 | 0.08 | 0.10 (1) | .747 | 20.76 (18) | .292 |
| Possible | 0.48* | 0.27 | 0.68 | 0.11 | 4.51 | <.001 | 14 | ||||||
|
| |||||||||||||
| Block Span | Absent | 0.57* | 0.16 | 0.99 | 0.21 | 2.72 | .001 | 3 | 0.05 | 0.47 (1) | .493 | 10.04 (9) | .347 |
| Possible | 0.41* | 0.19 | 0.63 | 0.11 | 3.62 | <.001 | 8 | ||||||
|
| |||||||||||||
| Verbal Fluency Comp. | Absent | 0.37* | 0.12 | 0.61 | 0.13 | 2.87 | .004 | 6 | 0.02 | 0.02 (1) | .875 | 26.17 (25) | .400 |
| Possible | 0.39* | 0.27 | 0.51 | 0.06 | 6.26 | <.001 | 21 | ||||||
|
| |||||||||||||
| Phonemic Verbal Fluency | Absent | 0.37* | 0.13 | 0.61 | 0.12 | 2.99 | .003 | 6 | .01 | 0.03 (1) | .870 | 25.83 (25) | .417 |
| Possible | 0.39* | 0.27 | 0.51 | 0.06 | 6.58 | <.001 | 21 | ||||||
|
| |||||||||||||
| Semantic Verbal Fluency | Absent | 0.28 | −0.23 | 0.79 | 0.26 | 1.09 | .278 | 3 | 0.12 | 0.46 (1) | .498 | 9.05 (9) | .432 |
| Possible | 0.49* | 0.18 | 0.79 | 0.16 | 3.11 | .002 | 8 | ||||||
|
| |||||||||||||
| Planning Comp. | Absent | 0.40* | 0.09 | 0.72 | 0.16 | 2.56 | .010 | 6 | 0.07 | 0.02 (1) | .898 | 18.72 (20) | .540 |
| Possible | 0.42* | 0.24 | 0.61 | 0.09 | 4.55 | <.001 | 16 | ||||||
|
| |||||||||||||
| TOL/TOH Accuracy | Absent | 0.41* | 0.07 | 0.75 | 0.18 | 2.34 | .019 | 6 | 0.11 | 0.03 (1) | .874 | 14.30 (18) | .709 |
| Possible | 0.38* | 0.16 | 0.59 | 0.11 | 3.40 | <.001 | 14 | ||||||
|
| |||||||||||||
| TMT-A | Absent | 0.45* | 0.21 | 0.69 | 0.12 | 3.69 | <.001 | 4 | 0.00 | 2.75 (1) | .097^ | 21.19 (21) | .448 |
| Possible | 0.68* | 0.56 | 0.80 | 0.06 | 11.36 | <.001 | 19 | ||||||
Note. d= weighted mean Cohen’s d effect size; CI= confidence interval; LL= lower limit; UL = upper limit; v = random-effects variance component; Q within= within-group (residual) heterogeneity; Q between = between-group (moderator) heterogeneity; Comp. = composite score; WCST = Wisconsin Card Sorting Test; OAT/DAT = Object alternation task/delayed alternation task; TMT–B = Trail Making Test Part B; TMT–A =Trail Making Test Part A; ID/ED = Intradimensional/Extradimensional; WM = working memory; DMTS = delayed-match-to-sample; VF = verbal fluency; TOL/TOH = Tower of London, Tower of Hanoi.
Depression possible = average depressive symptom questionnaire scores in clinical range and/or individuals with diagnosis of any depressive disorder. Depression absent = average depressive symptom questionnaire scores below clinical range and no patients with a diagnosed depressive disorder.
Significant (p<.05).
Marginal (p<.10).
On average, across the 20 EF analyses for which there were enough studies for meta-ANOVA analysis, effect sizes were very similar for those with and without co-occurring diagnosis of a depressive disorder or elevated depressive symptoms (Δd = 0.04). Nearly all EF analyses that were significant with all samples included remained significant when restricted to samples without co-occurring depression diagnosis or elevated depressive symptoms, with the exception of stop signal SSRT, verbal WM overall and manipulation composite scores, and semantic verbal fluency, all of which had low power due to having few samples without co-occurring depression (k=3–5).
Continuous moderators
Meta-regression analyses for continuous moderators are reported in Table 4. For each measure with sufficient studies to conduct meta-regression analyses, Table 4 provides the regression coefficients for each moderator, with their associated 95% confidence intervals and significance test. Age significantly moderated visuospatial WM composite scores, and marginally moderated shifting composite scores, verbal fluency composite scores, phonemic verbal fluency, and planning composite scores, such that effect sizes were larger for older samples. These effects remained significant or marginal when controlling for gender (visuospatial WM z = 2.15, p =.032; shifting composite scores z = 2.53, p =.011; verbal fluency composite z = 2.62, p = .009; phonemic verbal fluency z = 1.97, p = .049; planning composite scores z = 1.91, p = .056). Controlling for medication use, the effect of age remained significant or marginal for visuospatial WM composite scores (z = 1.82, p = .069), verbal fluency composite scores (z = 2.00, p =.046), and planning composite scores (z =1.71, p =.087), while the effect of age became non-significant on shifting composite scores (z=1.31, p=.189) and phonemic verbal fluency (z=1.15, p=.250). Controlling for symptom severity, the effect of age remained significant or marginal on visuospatial WM (z = 2.09, p =.037), shifting composite scores (z = 1.70, p = .089), and planning composite scores (z = 1.97, p =.049), while the effects on verbal fluency composite scores (z = 1.09, p =.275) and phonemic verbal fluency (z=1.40, p=.162) became non-significant.
Table 4.
Moderator regression analyses
| DV | IV | Beta | B | 95% CI
|
SE | z | p | K | v | Q model (df) | p | Q within(df) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||||||||
| Inhibition Comp. | Severity (Y-BOCS) | 0.132 | 0.000 | −0.001 | 0.001 | 0.001 | 0.83 | .407 | 39 | 0.157 | 0.69 (1) | .407 | 38.95 (37) | .382 |
| Age | 0.099 | 0.007 | −0.012 | 0.025 | 0.010 | 0.70 | .486 | 49 | 0.153 | 0.49 (1) | .486 | 48.93 (47) | .340 | |
| Gender | −0.109 | −0.003 | −0.002 | 0.004 | 0.003 | −0.75 | .451 | 48 | 0.142 | 0.57 (1) | .451 | 47.77 (46) | .401 | |
| Medication | 0.288 | 0.004 | −0.000 | 0.008 | 0.002 | 1.90 | .058^ | 40 | 0.130 | 3.59 (1) | .058 | 39.72 (38) | .393 | |
|
| ||||||||||||||
| Shifting Comp. | Severity (Y-BOCS) | 0.179 | 0.015 | −0.006 | 0.036 | 0.011 | 1.40 | .162 | 61 | 0.06 | 1.96 (1) | .162 | 59.46 (59) | .459 |
| Age | 0.201 | 0.011 | −0.001 | 0.023 | 0.006 | 1.76 | .078^ | 74 | 0.04 | 3.11 (1) | .078 | 73.49 (72) | .429 | |
| Gender | 0.297 | 0.001 | 0.000 | 0.002 | 0.000 | 2.63 | .009* | 73 | 0.04 | 6.93 (1) | .009 | 71.84 (71) | .450 | |
| Medication | 0.022 | 0.000 | −0.002 | .002 | 0.001 | 0.16 | .869 | 58 | 0.06 | 0.03 (1) | .869 | 56.74 (56) | .447 | |
|
| ||||||||||||||
| TMT-B | Severity (Y-BOCS) | 0.099 | 0.008 | −0.020 | 0.036 | 0.014 | 0.56 | .573 | 33 | .08 | 0.32 (1) | .573 | 32.07 (31) | .414 |
| Age | 0.226 | 0.016 | −0.006 | 0.037 | 0.011 | 1.40 | .160 | 37 | 0.07 | 1.97 (1) | .160 | 36.76 (35) | .387 | |
| Gender | 0.273 | 0.005 | −0.001 | 0.011 | 0.003 | 1.66 | .097^ | 35 | 0.05 | 2.76 (1) | .097 | 34.16 (33) | .412 | |
| Medication | 0.177 | 0.002 | −0.002 | 0.006 | 0.002 | 0.95 | .342 | 30 | 0.08 | 0.90 (1) | .342 | 37.94 (28) | .473 | |
|
| ||||||||||||||
| WCST | Severity (Y-BOCS) | 0.106 | 0.009 | −0.019 | 0.036 | 0.014 | 0.60 | .547 | 34 | 0.07 | 0.36 (1) | .547 | 31.83 (32) | .475 |
| Age | 0.067 | 0.004 | −0.014 | 0.022 | 0.009 | 0.42 | .673 | 42 | 0.06 | 0.18 (1) | .673 | 40.04 (40) | .467 | |
| Gender | 0.026 | 0.001 | −0.006 | 0.007 | 0.003 | 0.17 | .868 | 42 | 0.06 | 0.03 (1) | .868 | 40.19 (40) | .462 | |
| Medication | −0.002 | −0.000 | −0.003 | 0.003 | 0.002 | −0.01 | .990 | 33 | 0.08 | 0.00 (1) | .990 | 31.96 (32) | .469 | |
|
| ||||||||||||||
| Verbal WM Comp. | Severity (Y-BOCS) | 0.227 | 0.014 | −0.013 | 0.041 | 0.014 | 1.01 | .311 | 19 | 0.05 | 1.03 (1) | .311 | 18.87 (17) | .336 |
| Age | 0.180 | 0.010 | −0.011 | 0.031 | 0.011 | 0.92 | .358 | 25 | 0.06 | 0.84 (1) | .358 | 25.24 (23) | .338 | |
| Gender | 0.338 | 0.010 | −0.001 | 0.020 | 0.005 | 1.80 | .072^ | 25 | 0.05 | 3.23 (1) | .072 | 25.13 (23) | .344 | |
|
| ||||||||||||||
| Medication | 0.311 | 0.003 | −0.002 | 0.008 | 0.002 | 1.30 | .194 | 17 | 0.07 | 1.69 (1) | .194 | 15.80 (15) | .396 | |
|
| ||||||||||||||
| Visuospatial WM Comp. | Severity (Y-BOCS) | 0.315 | 0.025 | −0.007 | 0.056 | 0.016 | 1.54 | .122 | 22 | 0.06 | 2.39 (1) | .122 | 21.60 (20) | .263 |
| Age | 0.424 | 0.026 | 0.004 | 0.047 | 0.011 | 2.30 | .021* | 24 | 0.04 | 5.31 (1) | .021 | 24.25 (22) | .334 | |
| Gender | 0.088 | 0.003 | −0.009 | 0.014 | 0.006 | 0.43 | .665 | 23 | 0.07 | 0.19 (1) | .665 | 23.90 (21) | .298 | |
| Medication | 0.334 | 0.003 | −0.001 | 0.008 | 0.002 | 1.57 | .118 | 21 | 0.06 | 2.45 (1) | .118 | 19.58 (19) | .420 | |
|
| ||||||||||||||
| Verbal Fluency Comp. | Severity (Y-BOCS) | 0.083 | 0.006 | −0.019 | 0.031 | 0.013 | 0.46 | .646 | 31 | 0.05 | 0.21 (1) | .646 | 30.72 (29) | .379 |
| Age | 0.287 | 0.014 | −0.001 | 0.030 | 0.008 | 1.87 | .061^ | 40 | 0.04 | 3.51 (1) | .061 | 39.23 (38) | .414 | |
| Gender | 0.123 | 0.002 | −0.004 | 0.008 | 0.003 | 0.76 | .448 | 39 | 0.03 | 0.58 (1) | .448 | 37.54 (37) | .444 | |
| Medication | 0.145 | 0.001 | −0.002 | 0.004 | 0.001 | 0.78 | .434 | 31 | 0.03 | 0.61 (1) | .434 | 28.69 (29) | .481 | |
|
| ||||||||||||||
| Phonemic Verbal Fluency | Severity (Y-BOCS) | 0.321 | 0.014 | −0.004 | 0.033 | 0.010 | 1.49 | .136 | 28 | 0.00 | 2.23 (1) | .136 | 19.29 (26) | .824 |
| Age | 0.334 | 0.013 | −0.001 | 0.026 | 0.007 | 1.84 | .065^ | 35 | 0.00 | 3.39 (1) | .065 | 27.00 (33) | .760 | |
| Gender | −0.025 | −0.000 | −0.006 | 0.005 | 0.003 | −0.14 | .892 | 35 | 0.00 | 0.02 (1) | .892 | 30.37 (33) | .599 | |
| Medication | −0.036 | −0.000 | −0.002 | 0.002 | 0.001 | −0.15 | .878 | 28 | 0.00 | 0.02 (1) | .877 | 18.82 (26) | .844 | |
|
| ||||||||||||||
| Planning Comp. | Severity (Y-BOCS) | −0.134 | −0.010 | −0.039 | 0.020 | 0.015 | −0.64 | .523 | 25 | 0.07 | 0.41 (1) | .523 | 22.40 (23) | .497 |
| Age | 0.358 | 0.017 | −0.002 | 0.034 | 0.009 | 1.93 | .053^ | 28 | 0.05 | 3.73 (1) | .053 | 25.35 (26) | .499 | |
| Gender | 0.174 | 0.004 | −0.005 | 0.012 | 0.004 | 0.87 | .383 | 27 | 0.06 | 0.76 (1) | .383 | 24.55 (25) | .488 | |
| Medication | 0.106 | 0.001 | −0.003 | 0.005 | 0.002 | 0.48 | .640 | 24 | 0.08 | 0.23 (1) | .630 | 20.37 (22) | .560 | |
|
| ||||||||||||||
| Planning Accuracy | Severity (Y-BOCS) | −0.336 | −0.026 | −0.059 | 0.007 | 0.017 | −1.52 | .129 | 23 | 0.10 | 2.30 (1) | .129 | 18.12 (21) | .641 |
| Age | 0.160 | 0.009 | −0.015 | 0.032 | 0.009 | 0.72 | .468 | 25 | 0.11 | 0.53 (1) | .469 | 19.89 (23) | .648 | |
| Gender | 0.137 | 0.003 | −0.006 | 0.011 | 0.004 | 0.61 | .545 | 25 | 0.12 | 0.37 (1) | .545 | 19.10 (23) | .488 | |
| Medication | −0.031 | −0.000 | −0.005 | 0.005 | 0.003 | −0.13 | .899 | 22 | 0.12 | 0.02 (1) | .899 | 16.79 (20) | .666 | |
|
| ||||||||||||||
| TMT-A | Severity (Y-BOCS) | 0.117 | 0.008 | −0.017 | 0.032 | 0.013 | 0.60 | .549 | 27 | 0.04 | 0.36 (1) | .549 | 25.98 (25) | .409 |
| Age | 0.138 | 0.008 | −0.012 | 0.029 | 0.011 | 0.78 | .436 | 32 | 0.04 | 0.61 (1) | .436 | 31.02 (30) | .414 | |
| Gender | 0.133 | 0.002 | −0.004 | 0.004 | 0.003 | 0.71 | .475 | 30 | 0.03 | 0.51 (1) | .475 | 28.44 (28) | .441 | |
| Medication | −0.156 | −0.000 | −0.001 | 0.000 | 0.000 | −0.80 | .426 | 26 | 0.04 | 0.63 (1) | .426 | 24.55 (24) | .430 | |
Note. d= weighted mean Cohen’s d effect size; CI= confidence interval; LL= lower limit; UL = upper limit; v = random-effects variance component; Q within = within-group (residual) heterogeneity; Q model = moderator heterogeneity; Comp. = composite score; WCST = Wisconsin Card Sorting Test; TMT–B = Trail Making Test Part B; TMT–A =Trail Making Test Part A; VF = verbal fluency.
There was a significant effect of gender on shifting composite scores, and marginal effects of gender on TMT-B and verbal WM composite scores, such that samples with more female participants had worse performance. Controlling for age, the effect of gender remained significant on shifting composite scores (z = 2.48, p = .013) and verbal WM composite scores (z = 2.02, p = .044), but became non-significant on TMT-B (z = 1.63, p = .104). Controlling for medication, the effect of gender remained significant for shifting composite scores (z = 2.53, p = .011) but became non-significant on TMT-B (z = 1.54, p = .123) and verbal WM composite scores (z = 1.26, p = .207). Controlling for symptom severity, the effect of gender remained significant for shifting composite scores (z = 2.25, p = .025), but became non-significant for TMT-B (z = 1.21, p = .228) and verbal WM composite scores (z = 1.30, p = .192).
The percentage of individuals with OCD taking psychotropic medication marginally moderated inhibition composite scores, such that samples with a higher percentage of medicated participants exhibited worse performance. This effect remained marginal after controlling for age (z = 1.76, p =.078) and gender (z = 1.68, p =.092), but not symptom severity (z = 1.30, p = .194). OCD symptom severity, as assessed by the Y-BOCs, did not significantly moderate any analyses.
Discussion
Evaluating Hypotheses: EF is Broadly Impaired in OCD
In sum, the current meta-analysis found that in comparison to their healthy peers, individuals with OCD exhibited significantly impaired performance on tasks measuring most aspects of EF, with most effect sizes in the d = 0.3–0.5 range4. These effects were not due to failure to match groups on IQ, or to publication bias. The results are consistent with Hypothesis 1, which posits a broad impairment across multiple aspects of EF that may be driven by dysfunction in prefrontal-striatal circuits (e.g., Kuelz et al., 2004b; Menzies, Chamberlain, et al., 2008a). The exception was verbal WM maintenance, where task performance for individuals with OCD was comparable to controls. However, this finding is not incompatible with Hypothesis 1, since simple maintenance of information in WM (as opposed to manipulation) is not strongly linked to other aspects of EF (e.g., Engle et al., 1999). Hypothesis 2, which posits a specific impairment in shifting and/or inhibition, was not supported, as effect sizes in other EF domains were equivalent to those for shifting and inhibition. The results are thus consistent with the theory that individuals with OCD have impairments in the unitary component of EF (i.e., common EF), posited to be the ability to actively maintain task goals and use this information to effectively bias lower-level processes (Friedman et al., 2008; Miyake & Friedman, 2012). Although other explanations are also possible (e.g., multiple specific aspects of EF could be independently impaired in OCD), impairment in common EF is the most parsimonious interpretation. It is also possible that individuals with OCD have deficits in common EF as well as processing-specific impairments in shifting and/or updating (recall that there is no inhibition-specific component, e.g., Friedman et al., 2008). Indeed, the largest effect size in the meta-analysis was for updating working memory (n-back), which is believed to depend critically on striatal gating of information into prefrontal cortex (e.g., Chatham et al., 2011; Frank, Loughry, & O’Reilly, 2001; Hazy, Frank, & O’Reilly, 2007). This suggests that individuals with OCD might have specific updating impairments in addition to common EF impairments, since both striatal and prefrontal dysfunction may contribute to deficits on updating tasks. Future research using a latent variable approach is needed to address these possibilities, as discussed in Future Directions.
Hypothesis 3, which posits that apparent EF deficits are due to general motor response slowing, was not supported. The current analysis revealed that individuals with OCD do exhibit significant general motor slowing, and are especially slowed on the TMT-A, which requires both motor speed and sequencing. However, significant impairments were also detected on accuracy measures from self-paced EF tasks, with effect sizes as large or larger than many of the response time tasks. Thus, while individuals with OCD do have slowed responses even on simple general motor speed tasks, these deficits cannot fully account for deficits on EF tasks. (However, as discussed in the introduction, it is impossible to rule out a deficit in general processing speed – as opposed to general motor speed—that could potentially reduce accuracy).
Finally, co-occurring depression does not account for EF deficits in OCD as posited by Hypothesis 4. OCD samples with no depression diagnoses and low levels of depressive symptoms were significantly and equivalently impaired on almost all measures of EF. This raises the question of how EF deficits associated with OCD and MDD (e.g., Snyder, 2012) are related to one another. One possibility is that prefrontal abnormalities that lead to impairments in EF may be transdiagnostic risk factors for psychopathology, including OCD and depressive disorders including MDD (e.g., Nolen-Hoeksema & Watkins, 2011; see below, Relating deficits across cognitive domains and disorders). It is also possible that OCD and MDD have independent effects on EF that might be detected with more sensitive continuous analyses of depressive symptoms, which were not possible here because of the wide variety of depression measures reported in the primary literature.
Effect sizes were largely stable across variation in demographic characteristics of the samples, although there was some evidence for larger deficits for older OCD groups (for shifting, visuospatial WM, verbal fluency and planning). This finding warrants further research, as empirical studies have not investigated age effects. In addition, medication use was associated with larger impairments on inhibition composite scores, and a higher percentage of female participants was associated with larger impairments in shifting and verbal WM. Although these effects were not found for any other measures, they may warrant further empirical study, as some medications may have cognitive side-effects, and one previous study found larger EF impairments for women with OCD on some measures (Mataix-Cols et al., 2006). The fact that symptom severity did not moderate effect sizes suggests that EF impairment may be a stable trait associated with OCD rather than fluctuating with current symptoms. However, this finding must be interpreted with caution given the relatively narrow range of severity levels in the included studies.
Limitations
There are several limitations in the conclusions that can be drawn from the current meta-analysis, due to limitations in the primary literature. First, co-occurring depression was coded as a categorical variable. This was necessary because the primary literature reports a wide variety of depression measures, which cannot easily be converted into a single continuous measure. The categorical depression measure (no co-occurring depression, versus any amount of co-occurring depression) provides a conservative test demonstrating that EF deficits are present in non-depressed individuals with OCD. However, this categorical measure limits the ability to detect the extent to which co-occurring depression might contribute to larger EF deficits in individuals with OCD. Future research could address this issue in several ways–individual studies could examine correlations between depression and EF performance in samples with OCD, and increased reporting of a common set of depression measures across studies, or psychometric studies to allow conversion of different measures to a common scale, would allow future meta-analyses to use continuous depression measures.
Second, the current meta-analysis is limited in its ability to determine to what extent EF deficits are related to specific OCD symptoms, versus anxiety more broadly. To address this issue, there is a need for increased reporting and analyzing of more detailed information about co-occurring anxiety disorders and anxiety symptoms, as well as more specific sets of OCD symptoms (e.g., compulsions and obsessions separately). Moving towards this more dimensional approach holds promise for uncovering mechanisms of psychopathology that may be obscured by heterogeneous diagnostic categories (e.g., Insel et al., 2010). Finally, as discussed in Future Directions, the current meta-analysis is limited by the types of EF tasks included in the primary literature. Specifically, many of these tasks are too broad to answer fine-grained questions about specific aspects of EF.
Implications for the Frontal-Striatal Model
Consistent with the EF deficits reviewed here, individuals with OCD have been found to have structural and functional abnormalities in PFC (for reviews see Menzies, Chamberlain, et al., 2008a; Nitschke & Heller, 2005). Earlier versions of the frontal-striatal model posited a specific deficit in orbitofrontal function (e.g., Graybiel & Rauch, 2000). However, both the results of the current meta-analysis (which found deficits in EF tasks known to depend primarily on other areas of PFC; e.g., Nee et al., 2007; Wager et al., 2004), and more recent versions of the frontal-striatal model based on neuroimaging evidence (Menzies, Chamberlain, et al., 2008a), suggest that function is disrupted in a wider PFC network not limited to orbitofrontal cortex.
Functional neuroimaging during EF tasks has revealed activation differences between individuals with OCD and healthy controls across a wide PFC network, including anterior cingulate (e.g., Fitzgerald et al., 2005; Maltby, Tolin, Worhunsky, O’Keefe, & Kiehl, 2005; Yucel et al., 2007) and dorsolateral and ventrolateral PFC (Maltby et al., 2005; Roth et al., 2007; van den Heuvel, Veltman, Groenewegen, Cath, et al., 2005a), in addition to orbitofrontal cortex (Maltby et al., 2005; Roth et al., 2007). However, both hyperactivation and hypoactivation have been found across studies. Thus, while there is strong evidence for differences in PFC function in individuals with OCD compared to controls, the direction of these differences is unclear, and may depend on task or individual characteristics yet to be differentiated.
Future Directions
Given the compelling evidence that individuals with OCD are impaired on most EF tasks, we would argue that there is no longer a need for further case-control studies of performance on standard neuropsychological measures of EF. Rather, there is now the opportunity to build on the foundation of such previous studies to better understand the specific mechanisms and causal processes contributing to EF deficits in OCD, and to move towards translational applications. To do so, we advocate for (1) better assessment of EF using multiple, specific, measures of different EF components, based on well-established EF models, (2) probing deficits at multiple levels of analysis, (3) investigating how EF deficits are related across disorders, and how EF deficits are related to deficits in other cognitive domains, and (4) using longitudinal, mediational, and behavior genetic approaches to probe possible causal links between EF deficits and OCD.
More precise assessment of EF deficits
Future research would benefit from the use of more sensitive and specific measures of each aspect of EF, and by the use of multiple measures. We argue that investigating how specific aspects of OCD are related to specific EF components is critical for elucidating the cognitive, neural, and genetic mechanisms involved. Many previous studies, including many in the current meta-analysis, have used EF measures that are too broad to answer these fine-grained questions. For example, verbal fluency tasks have been a perennial favorite for assessing EF. However, they and other complex neuropsychological tests tap a wide variety of cognitive processes, including not only aspects of EF but also non-executive abilities (Miyake, et al., 2000). As a result, impairments on such measures are difficult to interpret. This concern can be addressed by using tasks designed to specifically place demands on one aspect of EF, while keeping other demands minimal (e.g., Aron, 2008; Miyake, et al., 2000).
In addition, including multiple measures of each aspect of EF would allow construction of latent or composite measures, which greatly increase construct validity and power (e.g., Miyake, et al., 2000). While the current meta-analysis found broad impairment in EF, which is most parsimoniously explained by a deficit in common EF, latent variable approaches are also needed to answer the key question of whether impairments across multiple aspects of EF are due to impairment of the common EF component, or multiple separate impairments, or both. For example, such a design could address the question of whether deficits on EF tasks are fully accounted for by deficits in common EF (i.e., once common EF is accounted for, there is no longer any evidence of process-specific impairments that are applicable only to individual aspects of EF), or whether there are processing-specific deficits not fully accounted for by common EF impairments (e.g., an updating-specific impairment related to striatal dysfunction). Research designs needed to test these models pose logistical challenges for research in clinical populations, as they require longer testing sessions to collect multiple measures and large (200+) sample sizes. However, overcoming these challenges– for example by administering tasks in several shorter sessions to reduce fatigue and collaborating across sites to increase sample size– may pay large dividends for determining the specific cognitive and neural mechanisms affected in individuals with OCD.
Understanding deficits at multiple levels of analysis
While the current meta-analysis demonstrates impairments in EF at the level of behavioral task performance, future research is needed to investigate the specific neural mechanisms contributing to EF deficits in OCD. For example, human genetic studies and animal models have suggested a role for abnormalities in the dopamine, serotonin and glutamate systems in OCD (e.g., Albelda & Joel, 2012; Pauls, 2008; Rolls, Loh, & Deco, 2008). A promising area of research is the integration of what is known about the role of these neurotransmitter systems in PFC networks with behavioral and neuroimaging evidence for EF impairments in individuals with OCD, building a more detailed model of the neurobiology of OCD that spans multiple levels of analysis.
Relating deficits across cognitive domains and disorders
While the current meta-analysis focused on EF, cognitive deficits associated with OCD are not restricted to EF, with meta-analytic evidence for deficits of a similar magnitude in processing speed, episodic memory, and attention (Abromovitch et al., 2013; Shin et al., 2013; Woods, Vevea, Chambless, & Bayen, 2002). An important question is thus how these deficits are related to one another. Some may be independent, for example, general motor slowing may be related to dysfunction in premotor-striatal loops, which are adjacent to, but separate from, the PFC-striatal loops involved in EF (e.g., Haber & Calzavara, 2009). In other cases, a common deficit may lead to impairments across domains. For example, some have argued that poor performance on memory tasks by individuals with OCD is largely attributable to EF deficits, which impact the ability to generate and implement organizational strategies (e.g., grouping words semantically) during encoding and retrieval (e.g., Olley et al., 2007). To test these possibilities, future empirical research is needed to test relations among performance in different domains (e.g., whether memory impairments are fully mediated by EF impairments).
In addition, OCD is far from the only psychiatric disorder associated with EF deficits, with similar effect sizes to those in OCD for MDD (d = 0.3–0.7; e.g., Rock, Roiser, Riedel, & Blackwell, 2013; Snyder, 2013) and attention deficit hyperactivity disorder (d = 0.3–0.7; e.g., Frazier, Demaree, & Youngstrom, 2005; Walshaw, Alloy, & Sabb, 2010) and somewhat larger deficits in schizophrenia (d = 0.7–1.3; e.g., Forbes, Carrick, McIntosh, & Lawrie, 2009; Meshalam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009) and bipolar disorders (d = 0.4–0.8; e.g., Kurtz & Gerraty, 2009; Mann-Wrobel, Carreno, & Dickinson, 2011). Hence a second important question is the extent to which EF deficits are shared, or differ, across disorders.
Importantly, while effect sizes differ somewhat across disorders, the same broad pattern of impairment across multiple aspects of EF is found in each, suggesting that PFC abnormalities that lead to impairments in EF may be transdiagnostic risk factors for psychopathology (e.g., Buckholtz & Meyer-Lindenberg, 2012; Nolen-Hoeksema & Watkins, 2011). This general vulnerability may combine with unique genetic, neurobiological, and environmental factors to produce divergent trajectories, leading to different disorders. However, when more detailed measures at multiple levels of analysis are considered in some cases these shared behavioral deficits may arise from distinct neural mechanisms (e.g., perturbations in different neurotransmitter systems; e.g., Gigante et al., 2012; Luykx et al., 2012). Future studies that systematically investigate relations between EF impairments, risk factors, and psychopathology are needed to refine understanding of how such broad EF deficits arise across disorders, and which aspects of these deficits are transdiagnostic versus disorder specific.
Possible Causal Links between OCD and EF
Previously research has left the question of how OCD and EF impairments are causally related largely unaddressed. For example, the vast majority of studies, including in the current meta-analysis, have used cross-sectional case-control designs, that do not provide any information about temporal precedence that could contribute to understanding causal links. There are (at least) three non-mutually-exclusive possibilities for these causal relationships. First, individual differences in neurobiology could both confer risk for OCD and lead to EF deficits. Specifically, alterations in PFC function may cause impaired EF as well as contribute to OCD symptoms, such as perseverative behaviors and the inability to inhibit compulsions (e.g., Menzies, Chamberlain, et al., 2008a). Supporting this model, differences in PFC and impairments in EF are present in non-affected relatives of individuals with OCD (e.g., Cavedini, Zorzi, Piccinni, Cavallini, & Bellodi, 2010; Menzies, Williams, et al., 2008b; Rajender et al., 2011), suggesting that they may represent endophenotypes for OCD. This possibility can be further tested with future behavior genetic research testing for shared genetic influence on EF and OCD, as well as by longitudinal studies tracking children at risk for OCD (e.g., due to family history) before onset.
The hypothesis that PFC and basal ganglia dysfunction may play a causal role in OCD is also supported by evidence that acquired brain damage to these areas can cause obsessions and compulsions along with EF impairments (e.g., see Coetzer, 2004 for review). Experimentally induced lesions to PFC and basal ganglia structures in animal models thus present an obvious target for testing causal models of OCD. However, such animal models have been difficult to develop and validate, potentially due to the large differences in prefrontal structure and function between rodents and humans (e.g., see Albelda & Joel, 2012 for review). As an alternative approach, further research that carefully evaluates the location of lesions associated with the onset of OCD symptoms (e.g., through voxel based lesion symptom mapping), and associated EF deficits, may help to shed light on possible causal associations between dysfunction in particular brain areas and OCD.
Second, OCD could directly cause EF deficits. For example, OCD-related intrusive thoughts may consume cognitive resources and interfere with the ability to maintain task goals. This possibility could be tested experimentally (e.g., by triggering intrusive thoughts prior to tasks). However, this direct effect is unlikely to be the only causal path, as there is some evidence that EF deficits remain stable after OCD symptoms have remitted (Bannon, Gonsalvez, Croft, & Boyce, 2006; Roh et al., 2005). Further research with individuals in remission is needed to confirm this possibility. If true, this finding suggests that even when treatment is considered successful from the perspective of eliminating affective symptoms and behaviors, persistent EF deficits may continue to undermine daily functioning and quality of life. It may therefore be useful to make deficits in EF as a focus for intervention a topic for future translational research.
Third, poor EF could contribute to the development or maintenance of OCD. Current models of anxiety suggest individuals with poor emotion regulation abilities engage in frequent and excessive worrying (Borkovec & Roemer, 1995; Mennin, Heimberg, & Turk, 2002). Meanwhile, better EF is linked to more effective emotion regulation strategies (e.g., Ochsner & Gross, 2005). Thus, it may be that poor EF leads individuals to develop alternative, but less adaptive or efficient, strategies for coping with emotional challenges that ultimately increase levels of OCD symptoms. For example, the anxiety reduction hypothesis posits that compulsions are a maladaptive strategy for reducing anxiety associated with intrusive thoughts, and are thus reinforced through avoidance learning (e.g., see Clark, 2004 for review). Thus, reduced ability to use EF to engage more adaptive anxiety-regulation strategies (e.g., shifting attention away from the anxiety-provoking thought) could contribute to the development or maintenance of compulsive behaviors. Because this path is speculative, longitudinal research is necessary to explore EF, emotion regulation, and the onset of OCD or escalation of OCD symptoms.
Understanding causal links between EF deficits and OCD will be critical for developing strategies for prevention and remediation. For example, if EF deficits precede and contribute to the development of OCD, those at risk (e.g., due to family history) may benefit from early intervention to train EF or teach compensatory strategies. In addition, regardless of the initial direction of the causal arrow, poor EF may reduce the effectiveness of therapeutic interventions. For example, deficits in EF could interfere with Cognitive Behavioral Therapy (e.g., Mohlman & Gorman, 2005), particularly with techniques such as Exposure and Response Prevention, which requires top-down processes such as shifting (e.g., from elaboration of automatic, catastrophic thoughts to metacognitive awareness of thoughts), inhibitory control (e.g., resisting engaging in compulsive behaviors), and updating (e.g., replacing maladaptive beliefs and behaviors with adaptive ones). EF deficits may therefore present a major barrier to successful completion of treatment.
Conclusions
In sum, OCD is associated with broad EF impairment, and these deficits cannot be accounted for by co-occurring depression or general motor slowing. These results are consistent with theories that posit that prefrontal dysfunction is a contributing factor in OCD, but additional research is needed to determine the causal links between EF impairments and OCD and build a more detailed model of the neurobiology of these impairments. A better understanding of when and how EF impairments arise for individuals with OCD may have important implications for treatment, such as pharmacological interventions targeting specific aspects of prefrontal function, or training programs to improve EF or teach compensatory strategies to mitigate the effects of EF impairments. Given the centrality of EF to our ability to successfully navigate daily life, such research has the potential to improve outcomes for many individuals affected by OCD.
Supplementary Material
Acknowledgments
Preparation of this manuscript was supported in part by the National Institutes of Mental Health (P50-MH079485, R01-MH61358, F32-MH098481, T32-MH15442).
Footnotes
Although updating and working memory manipulation are clearly related, and some may consider updating to be a subtype of WM manipulation processes, updating specifically requires adding and removing information from WM, while WM manipulation often involves reorganizing (e.g., reordering) information already held in WM. Thus, there are unique aspects of updating not shared with other types of WM manipulation. For example, the basal ganglia are thought to play a key role in gating information into and out of working memory during updating (e.g., Chatham et al., 2011; Frank, Loughry, & O’Reilly, 2001), which is of strong interest in regard to OCD given evidence of basal ganglia dysfunction.
In addition, the following tasks were nominated for inclusion by one author each, but were excluded because there was not agreement that they clearly assessed a single, specific aspect of EF: (1) verbal fluency switching (excluded because it mixes verbal fluency and shifting demands), (2) Rey Complex Figures immediate recall (excluded because it mixes working memory and episodic/incidental memory demands), and (3) delayed response task (excluded because working memory demands appear to be minimal).
Six studies had adolescent participants (ages 12–14). Since adult and non-adult studies could potentially differ, supplementary analyses were conducted with studies of adult participants (ages 18+) only. Effect sizes were virtually identical with the adolescent samples excluded as when they were included. Thus, all studies are included in the analyses reported here.
Comparing the results of the current meta-analysis to those of the previous meta-analyses that included some EF measures, effect sizes for individual tasks reported by Shin et al. (2013) were fairly consistent with the current results for analyses in which Shin et al. (2013) included ten or more studies, while analyses with only 5–6 studies both over and under-estimated effects compared to the current study, likely reflecting imprecision in these estimates due to the small number of studies. Comparing the current results to those of Abromovitch et al. (2013) is not straightforward, because they did not report analyses of individual measures, and grouped measures into composites that do not always align with the theoretically-motivated grouping of measures used in the current meta-analysis. For the most comparable composite measures across meta-analyses, planning effect sizes were identical (d=0.44), and inhibition effect sizes were somewhat smaller in the current study (d=0.37 vs. 0.49). For the less comparable composites, the shifting composite measure effect size in the current study was similar to that of the shifting/fluency/WAIS similarities measure in Abromovitch et al. (2013; d=0.50 vs. 0.55), the verbal WM/updating composite (d=0.34) was between those for verbal WM (d=0.22), and updating (d= 0.71) in the current study, and the spatial WM/updating composite (d=0.37) was lower than both the visuospatial WM (d=0.47) and updating effect sizes in the current study (potentially due to the relatively small number of studies included in the Abromovitch et al. (2013) analysis, and differences in the included tasks).
References
- *.Abbruzzese M, Bellodi L, Ferri S, Scarone S. Frontal-lobe dysfunction in schizophrenia and obsessive-compulsive disorder: A neuropsychological study. Brain and Cognition. 1995a;27:202–212. doi: 10.1006/brcg.1995.1017. [DOI] [PubMed] [Google Scholar]
- *.Abbruzzese M, Ferri S, Scarone S. Wisconsin Card Sorting Test performance in obsessive-compulsive disorder: No evidence for involvement of dorsolateral prefrontal cortex. Psychiatry Research. 1995b;58:37–43. doi: 10.1016/0165-1781(95)02670-R. [DOI] [PubMed] [Google Scholar]
- *.Abbruzzese M, Ferri S, Scarone S. The selective breakdown of frontal functions in patients with obsessive-compulsive disorder and in patients with schizophrenia: A double dissociation experimental finding. Neuropsychologia. 1997;35:907–912. doi: 10.1016/s0028-3932(96)00095-4. [DOI] [PubMed] [Google Scholar]
- Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive–compulsive disorder: A meta-analysis. Clinical Psychology Review. 2013;33:1163–1171. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- *.Abramovitch A, Dar R, Schweiger A, Hermesh H. Neuropsychological impairments and their association with obsessive-compulsive symptom severity in obsessive-compulsive disorder. Archives of Clinical Neuropsychology. 2011;26:364–376. doi: 10.1093/arclin/acr022. [DOI] [PubMed] [Google Scholar]
- *.Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Alarcón RD, Libb JW, Boll TJ. Neuropsychological testing in obsessive-compulsive disorder: A clinical review. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:217–228. doi: 10.1176/jnp.6.3.217. [DOI] [PubMed] [Google Scholar]
- Albelda N, Joel D. Current animal models of obsessive compulsive disorder: An update. Neuroscience. 2012;211:83–106. doi: 10.1016/j.neuroscience.2011.08.070. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsycholgy Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Aron AR. Progress in executive-function research. Current Directions in Psychological Science. 2008;17:124–129. doi: 10.1111/j.1467-8721.2008.00561.x. [DOI] [Google Scholar]
- *.Aronowitz BR, Hollander E, DeCaria CM, Cohen L, Saoud JB, Stein DJ, Rosen WD. Neuropsychology of obsessive-compulsive disorder: Preliminary findings. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1994;7:81–86. [Google Scholar]
- *.Aycicegi A, Dinn W, Harris C, Erkmen H. Neuropsychological function in obsessive-compulsive disorder: Effects of comorbid conditions on task performance. European Psychiatry. 2003;18:241–248. doi: 10.1016/S0924-9338(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. doi: 10.1111/j.1467-8721.2009.01615.x. [DOI] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Executive functions in obsessive-compulsive disorder: State or trait deficits? Australian and New Zealand Journal of Psychiatry. 2006;40:1031–1038. doi: 10.1111/j.1440-1614.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez C, Croft R, Boyce PM. Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Research. 2002;110(2):165–174. doi: 10.1016/s0165-1781(02)00104-x. [DOI] [PubMed] [Google Scholar]
- *.Basso MR, Bornstein RA, Carona F, Morton R. Depression accounts for executive function deficits in obsessive-compulsive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2001;14:241–245. [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Philadelphia, PA: Center for Cognitive Therapy; 1978. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- *.Bohne A, Savage CR, Deckersbach T, Keuthen NJ, Wilhelm S. Motor inhibition in trichotillomania and obsessive–compulsive disorder. Journal of Psychiatric Research. 2008;42:141–150. doi: 10.1016/j.jpsychires.2006.11.008. [DOI] [PubMed] [Google Scholar]
- *.Bohne A, Savage C, Deckersbach T. Visuospatial abilities, memory, and executive functioning in trichotillomania and obsessive-compulsive disorder. Journal of Clinical and Experimental Neuropsychology. 2005;27:385–399. doi: 10.1080/13803390490520418. [DOI] [PubMed] [Google Scholar]
- *.Boisseau CL. Doctoral dissertation. 2011. Neuropsychological functioning and temperament in obsessive- compulsive disorder and eating disorders: Investigating the proposed obsessive-compulsive spectrum. Retrieved from ProQuest Dissertations and Theses (UMI 3445732). [Google Scholar]
- *.Boisseau CL, Thompson-Brenner H, Caldwell-Harris C, Pratt E, Farchione T, Harrison Barlow D. Behavioral and cognitive impulsivity in obsessive–compulsive disorder and eating disorders. Psychiatry Research. 2012;200:1062–1066. doi: 10.1016/j.psychres.2012.06.010. [DOI] [PubMed] [Google Scholar]
- *.Boldrini M, Del Pace L, Placidi GPA, Keilp J, Ellis SP, Signori S, Cappa SF. Selective cognitive deficits in obsessive-compulsive disorder compared to panic disorder with agoraphobia. Acta Psychiatrica Scandinavica. 2005;111:150–158. doi: 10.1111/j.1600-0447.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- *.Boone KB, Ananath J, Philpott L, Kaur A, Djenderedjian A. Neuropsychological characteristics of nondepressed adults with obsessive-compulsive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1991;4:96–109. [Google Scholar]
- Boonstra MA, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: A meta-analytic review. Psychological Medicine. 2005;35:1097–1108. doi: 10.1017/S003329170500499X. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: Meta-analytic study. The British journal of Psychiatry. 2009;195:475–482. doi: 10.1192/bjp.bp.108.055731. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Roemer L. Perceived functions of worry among generalized anxiety disorder subjects: Distraction from more emotionally distressing topics? Journal of Behavior Therapy and Experimental Psychiatry. 1995;26:25–30. doi: 10.1016/0005-7916(94)00064-S. [DOI] [PubMed] [Google Scholar]
- *.Borkowska A, Pilaczyñska E, Rybakowski JK. The frontal lobe neuropsychological tests in patients with schizophrenia and/or obsessive-compulsive disorder. The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:359–362. doi: 10.1176/appi.neuropsych.15.3.359. [DOI] [PubMed] [Google Scholar]
- *.Britton JC, Rauch SL, Rosso IM, Killgore WDS, Price LM, Ragan J, Chosak A, et al. Cognitive inflexibility and frontal-cortical activation in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:944–953. doi: 10.1016/j.jaac.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037/0021-843X.110.4.585. [DOI] [PubMed] [Google Scholar]
- *.Bucci P, Galderisi S, Catapano F, Di Benedetto R, Piegari G, Mucci A, Maj M. Neurocognitive indices of executive hypercontrol in obsessive-compulsive disorder. Acta Psychiatrica Scandinavica. 2007;115:380–387. doi: 10.1111/j.1600-0447.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- *.Carona FD. Doctoral dissertation. 2004. Neuropsychological deficits in obsessive compulsive disorder versus unipolar depression. Retrieved from ProQuest Dissertations and Theses (UMI 3120653). [Google Scholar]
- *.Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: Searching for a new intermediate phenotype. Biological Psychiatry. 2010;67:1178–1184. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Blackwell KA, Munakata Y. Speed isn’t everything: Complex processing speed measures mask individual differences and developmental changes in executive control. Developmental Science. 2013;16:269–286. doi: 10.1111/desc.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. The American Journal of Psychiatry. 2007a;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chamberlain SR, Fineberg N, Blackwell AD, Clark L, Robbins T, Sahakian B. A neuropsychological comparison of obsessive–compulsive disorder and trichotillomania. Neuropsychologia. 2007b;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Herd SA, Brant AM, Hazy TE, Miyake A, O’Reilly RC, Friedman NP. From an executive network to executive control: A computational model of the n-back task. Journal of Cognitive Neuroscience. 2011;23:3598–3619. doi: 10.1162/jocn_a_00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Christensen K, Kim S, Dysken M, Hoover K. Neuropsychological performance in obsessive-compulsive disorder. Biological Psychiatry. 1992;31:4–18. doi: 10.1016/0006-3223(92)90003-i. [DOI] [PubMed] [Google Scholar]
- *.Ciesielski KT, Hämäläinen MS, Geller DA, Wilhelm S, Goldsmith TE, Ahlfors SP. Dissociation between MEG alpha modulation and performance accuracy on visual working memory task in obsessive compulsive disorder. Human Brain Mapping. 2007;28:1401–1414. doi: 10.1002/hbm.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ciesielski KT, Rowland LM, Harris RJ, Kerwin AA, Reeve A, Knight JE. Increased anterior brain activation to correct responses on high-conflict Stroop task in obsessive-compulsive disorder. Clinical Neurophysiology. 2011;122:107–113. doi: 10.1016/j.clinph.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Clark DA. Cognitive-Behavioral Therapy for OCD. New York: Guilford Press; 2004. [Google Scholar]
- Coetzer BR. Obsessive-compulsive disorder following brain injury: A review. The International Journal of Psychiatry in Medicine. 2004;34:363–377. doi: 10.2190/XENN-NNWT-7N2K-R26A. [DOI] [PubMed] [Google Scholar]
- *.Cohen L, Hollander E, DeCaria CM, Stein DJ, Simeon D, Liebowitz MR, Aronowitz BR. Specificity of neuropsychological impairment in obsessive-compulsive disorder: A comparison with social phobic and normal control subjects. Journal of Neuropsychiatry and Clinical Neurosciences. 1996;8:82–85. doi: 10.1176/jnp.8.1.82. [DOI] [PubMed] [Google Scholar]
- *.Cohen Y. Anxiety and selective attention in obsessive–compulsive disorder. Behavioral Research and Therapy. 2003;41:1311–1323. doi: 10.1016/S0005-7967(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25:409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, Oxman AD. Should unpublished data be included in metaanalyses–current convictions and controversies. Journal of the American Medical Association. 1993;269:2749–2753. doi: 10.1001/jama.269.21.2749. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, David AS, Brammer MJ. A parametric approach to voxel-based meta-analysis. NeuroImage. 2009;46:115–122. doi: 10.1016/j.neuroimage.2009.01.031. [DOI] [PubMed] [Google Scholar]
- *.de Geus F, Denys DAJP, Sitskoorn MM, Westenberg HGM. Attention and cognition in patients with obsessive-compulsive disorder. Psychiatry and Clinical Neurosciences. 2007;61:45–53. doi: 10.1111/j.1440-1819.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: A meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- *.Dittrich WH, Johansen T. Cognitive deficits of executive functions and decision-making in obsessive-compulsive disorder. Scandinavian Journal of Psychology. 2013;54:393–400. doi: 10.1111/sjop.12066. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment. 2003;7:1–76. [PubMed] [Google Scholar]
- *.Fenger MM, Gade A, Adams KH, Hansen ES, Bolwig TG, Knudsen GM. Cognitive deficits in obsessive-compulsive disorder on tests of frontal lobe functions. Nordic Journal of Psychiatry. 2005;59:39–44. doi: 10.1080/08039480510018814. [DOI] [PubMed] [Google Scholar]
- Fisk JE, Sharp CA. Age-related impairment in executive functioning: Updating, inhibition, shifting, and access. Journal of Clinical and Experimental Neuropsychology. 2004;26:874–890. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: A meta-analysis. Psychological Medicine. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cognitive Affective & Behavioral Neuroscience. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-Analysis of intellectual and neuropsychological test ferformance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:534–555. doi: 10.1037/0894-4105.18.3.543.supp. [DOI] [PubMed] [Google Scholar]
- *.Frevert TA. Masters thesis. 1999. Neuropsychology of obsessive compulsive disorder. Retrieved from ProQuest Dissertations and Theses (UMI 1396364) [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Grafman J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33:623–642. doi: 10.1016/0028-3932(95)90866-P. [DOI] [PubMed] [Google Scholar]
- *.Ghassemzadeh H. Neuropsychological and neurological deficits in obsessive-compulsive disorder: The role of comorbid depression. International Journal of Clinical Medicine. 2012;03:200–210. doi: 10.4236/ijcm.2012.33040. [DOI] [Google Scholar]
- *.Ghisi M, Bottesi G, Sica C, Sanavio E, Freeston MH. Is performance on the go/nogo task related to not just right experiences in patients with obsessive compulsive disorder? Cognitive Therapy and Research. 2013:1–11. doi: 10.1007/s10608-013-9560-1. [DOI] [Google Scholar]
- Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: A meta-analysis. Bipolar Disorders. 2012;14(5):478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Archives of General Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989b;46:1006. doi: 10.1001/archpsyc.1989.01810110048007. 1011.10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/S0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- *.Grisham JR, Williams AD. Responding to intrusions in obsessive-compulsive disorder: The roles of neuropsychological functioning and beliefs about thoughts. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44:343–350. doi: 10.1016/j.jbtep.2013.01.005. [DOI] [PubMed] [Google Scholar]
- *.Gross-Isseroff R, Sasson Y, Voet H, Hendler T, Luca-Haimovici K, Kandel-Sussman H, Zohar J. Alternation learning in obsessive-compulsive disorder. Biological Psychiatry. 1996;39:733–738. doi: 10.1016/0006-3223(95)00179-4. [DOI] [PubMed] [Google Scholar]
- *.Gu BM, Park JY, Kang DH, Lee S, Yoo SY, Jo H, Choi CH, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Research Bulletin. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Han JY, Kang DH, Gu BM, Jung WH, Choi JS, Choi CH, Kwon JS. Altered brain activation in ventral frontal-striatal regions following a 16-week pharmacotherapy in unmedicated obsessive-compulsive disorder. Journal of Korean Medical Science. 2011;26:665–574. doi: 10.3346/jkms.2011.26.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hartston HJ, Swerdlow NR. Visuospatial priming and stroop performance in patients with obsessive compulsive disorder. Neuropsychology. 1999;13:447–457. doi: 10.1037//0894-4105.13.3.447. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV. Unbiased estimation of effect size. Evaluation in Education. 1980;5:25–27. doi: 10.1016/0191-765X(80)90005-1. [DOI] [Google Scholar]
- Hedges LV, Olkin I. Statistical Method for Meta-Analysis. Academic Press; 1985. [Google Scholar]
- Henseler I, Gruber O, Kraft S, Krick C, Reith W, Falkai P. Compensatory hyperactivations as markers of latent working memory dysfunctions in patients with obsessive-compulsive disorders: An fMRI study. Journal of Psychiatry & Neuroscience. 2008;33:209–215. [PMC free article] [PubMed] [Google Scholar]
- *.Herrmann M. Reduced response-inhibition in obsessive–compulsive disorder measured with topographic evoked potential mapping. Psychiatry Research. 2003;120:265–271. doi: 10.1016/S0165-1781(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. 2011 2011. Available from www.cochrane-handbook.org.
- *.Hollander E, Wong C. The relationship between executive function impairment and serotonergic sensitivity in obsessive-compulsive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1996;9:230–233. [Google Scholar]
- *.Hur JW, Shin NY, Kim SN, Jang JH, Choi JS, Shin YC, Kwon JS. Do pathological gambling and obsessive-compulsive disorder overlap? A neurocognitive perspective. CNS Spectrums. 2012;17:207–213. doi: 10.1017/S1092852912000545. [DOI] [PubMed] [Google Scholar]
- *.Hwang S, Kwon JS, Shin YW, Lee K, Kim Y, Kim MS. Neuropsychological profiles of patients with obsessive-compulsive disorder: Early onset versus late onset. Journal of the International Neuropsychological Society. 2007;13:30–37. doi: 10.1017/S1355617707070063. [DOI] [PubMed] [Google Scholar]
- *.Hymas N, Lees A, Bolton D, Head D. The neurobiology of obsessional slowness. Brain. 1991;114:2203–2233. doi: 10.1093/brain/114.5.2203. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS, Quinn KJ, Wang PS. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- *.Isik Taner Y, Erdogan Bakar E, Oner O. Impaired executive functions in paediatric obsessive-compulsive disorder patients. Acta Neuropsychiatrica. 2011;23:272–281. doi: 10.1111/j.1601-5215.2011.00562.x. [DOI] [PubMed] [Google Scholar]
- *.Jaafari N, Frasca M, Rigalleau F, Rachid F, Gil R, Olié JP, et al. Forgetting what you have checked: A link between working memory impairment and checking behaviors in obsessive-compulsive disorder. European Psychiatry. 2013;28:87–93. doi: 10.1016/j.eurpsy.2011.07.001. [DOI] [PubMed] [Google Scholar]
- *.Jurado M, Junqué C, Vallejo J, Salgado P. Impairment of incidental memory for frequency in patients with obsessive-compulsive disorder. Psychiatry Research. 2001;104:213–220. doi: 10.1016/S0165-1781(01)00322-5. [DOI] [PubMed] [Google Scholar]
- *.Kang DH, Jang JH, Han JY, Kim JH, Jung WH, Choi JS, et al. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40:340–346. doi: 10.1016/j.pnpbp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- *.Kashyap H, Kumar JK, Kandavel T, Reddy YCJ. Neuropsychological functioning in obsessive-compulsive disorder: Are executive functions the key deficit? Comprehensive Psychiatry. 2013;54:533–540. doi: 10.1016/j.comppsych.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Kearns NP, Cruickshank CA, McGuigan KJ, Riley SA, Shaw SP, Snaith RP. A comparison of depression rating scales. The British Journal of Psychiatry. 1982;141:45–49. doi: 10.1192/bjp.141.1.45. [DOI] [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- *.Kim M, Park S, Shin MS, Kwon JS. Neuropsychological profile in patients with obsessive-compulsive disorder over a period of 4-month treatment. Journal of Psychiatric Research. 2002;36:257–265. doi: 10.1016/S0022-3956(02)00017-1. [DOI] [PubMed] [Google Scholar]
- *.Kim MS, Kim YY, Yoo SY, Kwon JS. Electrophysiological correlates of behavioral response inhibition in patients with obsessive–compulsive disorder. Depression and Anxiety. 2006;24:22–31. doi: 10.1002/da.20195. [DOI] [PubMed] [Google Scholar]
- *.Kloft L, Kischkel E, Kathmann N, Reuter B. Evidence for a deficit in volitional action generation in patients with obsessive-compulsive disorder. Psychophysiology. 2010;48:755–761. doi: 10.1111/j.1469-8986.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- *.Koçak OM, Nalçaci E, Özgüven HD, Nalçaci EG, Ergenç İ. Evaluation of cognitive slowing in OCD by means of creating incongruence between lexicon and prosody. Psychiatry Research. 2010;179:306–311. doi: 10.1016/j.psychres.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory 1983 [Google Scholar]
- Krain AL, Hefton S, Pine DS, Ernst M, Xavier Castellanos F, Klein RG, Milham MP. An fMRI examination of developmental differences in the neural correlates of uncertainty and decision-making. Journal of Child Psychology and Psychiatry. 2006;47:1023–1030. doi: 10.1111/jcpp.2006.47.issue-10. [DOI] [PubMed] [Google Scholar]
- *.Krikorian R. Inhibitory control in obsessive-compulsive disorder. Brain and Cognition. 2004;54:257–259. doi: 10.1016/j.bandc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- *.Kuelz AK, Riemann D, Halsband U, Vielhaber K, Unterrainer J, Kordon A, Voderholzer U. Neuropsychological impairment in obsessive-compulsive disorder: Improvement over the course of cognitive behavioral treatment. Journal of Clinical and Experimental Neuropsychology. 2006;28:1273–1287. doi: 10.1080/13803390500507246. [DOI] [PubMed] [Google Scholar]
- *.Kuelz AK, Riemann D, Zahn R, Voderholzer U. Object alternation test: Is it sensitive enough to detect cognitive dysfunction in obsessive–compulsive disorder? European Psychiatry. 2004a;19:441–443. doi: 10.1016/j.eurpsy.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Kuelz A, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: A critical review. Biological Psychology. 2004b;65:185–236.10. doi: 10.1016/j.biopsycho.2003.07.007. 1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology. 2009;23:551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lacerda A, Dalgalarrondo P, Caetano D, Haas G, Camargo E, Keshavan M. Neuropsychological performance and regional cerebral blood flow in obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(4):657–665. doi: 10.1016/S0278-5846(03)00076-9. [DOI] [PubMed] [Google Scholar]
- *.Lacey J, Burness C, Costa D, Gacinovic S, Pilowsky L, Ell P, Marks I, et al. Wisconsin Card Sorting Task (WCST) errors and cerebral blood flow in obsessive-compulsive disorder (OCD) British Journal of Medical Psychology. 1997;70:403–411. doi: 10.1111/j.2044-8341.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- *.Lawrence NS, Wooderson S, Mataix-Cols D, David R, Speckens A, Phillips ML. Decision Making and Set Shifting Impairments Are Associated With Distinct Symptom Dimensions in Obsessive-Compulsive Disorder. Neuropsychology. 2006;20:409–419. doi: 10.1037/0894-4105.20.4.409. [DOI] [PubMed] [Google Scholar]
- *.Lee HJ, Yost BP, Telch MJ. Differential performance on the go/no-go task as a function of the autogenous-reactive taxonomy of obsessions: Findings from a non-treatment seeking sample. Behaviour Research and Therapy. 2009;47:294–300. doi: 10.1016/j.brat.2009.01.002. [DOI] [PubMed] [Google Scholar]
- *.Lennertz L, Rampacher F, Vogeley A, Schulze-Rauschenbach S, Pukrop R, Ruhrmann S, et al. Antisaccade performance in patients with obsessive–compulsive disorder and unaffected relatives: Further evidence for impaired response inhibition as a candidate endophenotype. European Archives of Psychiatry and Clinical Neuroscience. 2012;262:625–634. doi: 10.1007/s00406-012-0311-1. [DOI] [PubMed] [Google Scholar]
- *.Li B, Sun JH, Li T, Yang YC. Neuropsychological study of patients with obsessive-compulsive disorder and their parents in China: Searching for potential endophenotypes. Neuroscience Bulletin. 2012;28:475–482. doi: 10.1007/s12264-012-1262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luykx JJ, Laban KG, van den Heuvel MP, Boks MPM, Mandl RCW, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of 1H-MRS findings. Neuroscience and Biobehavioral Reviews. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: An event-related fMRI study. NeuroImage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mann Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: An update and investigation of moderator variables. Bipolar Disorders. 2011;13:334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Marín-Martínez F, Sánchez-Meca J. Testing for dichotomous moderators in meta-analysis. The Journal of Experimental Education. 1998;67:69–81. doi: 10.1080/00220979809598345. [DOI] [Google Scholar]
- *.Martin A, Pigott TA, Lalonde FM, Dalton I, Dubbert B, Murphy DL. Lack of evidence for Huntington’s disease-like cognitive dysfunction in obsessive-compulsive disorder. Biological Psychiatry. 1993;33:345–353. doi: 10.1016/0006-3223(93)90323-6. [DOI] [PubMed] [Google Scholar]
- *.Martin A, Wiggs C, Altemus M, Rubenstein C, Murphy D. Working memory as assessed by subject-ordered tasks in patients with obsessive-compulsive disorder. Journal of Clinical and Experimental Neuropsychology. 1995;17:786–792. doi: 10.1080/01688639508405167. [DOI] [PubMed] [Google Scholar]
- *.Mataix-Cols D, Rahman Q, Spiller M, Alonso MP, Pifarré J, Menchón JM, Vallejo J. Are there sex differences in neuropsychological functions among patients with obsessive-compulsive disorder? Applied Neuropsychology. 2006;13:42–50. doi: 10.1207/s15324826an1301_6. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychology: Science and Practice. 2002;9:85–90. doi: 10.1093/clipsy.9.1.85. [DOI] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, Bullmore ET. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008a;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Williams G, Chamberlain SR, Ooi C, Fineberg N, Suckling J, Sahakian B, et al. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. The American Journal of Psychiatry. 2008b;165:1308–1315. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- *.Millet B, Dondaine T, Reymann JM, Bourguignon A, Naudet F, Le F. Obsessive compulsive disorder networks: Positron emission tomography and neuropsychology provide new insights. PLoS ONE. 2013;8:e53241. doi: 10.1371/journal.pone.0053241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Gorman JM. The role of executive functioning in CBT: A pilot study with anxious older adults. Behavioral Research and Therapy. 2005;43:447–465. doi: 10.1016/j.brat.2004.03.007. [DOI] [PubMed] [Google Scholar]
- *.Morein-Zamir S, Craig KJ, Ersche KD, Abbott S, Muller U, Fineberg NA, Bullmore ET, et al. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology. 2010;212:357–367. doi: 10.1007/s00213-010-1963-z. [DOI] [PubMed] [Google Scholar]
- *.Moritz S, Birkner C, Kloss M, Jacobsen D, Fricke S, Bothern A, Hand I. Impact of comorbid depressive symptoms on neuropsychological performance in obsessive-compulsive disorder. Journal of Abnormal Psychology. 2001;110:653–658. doi: 10.1037//0021-843X.110.4.653. [DOI] [PubMed] [Google Scholar]
- *.Moritz S, Birkner C, Kloss M, Jahn H, Hand I, Haasen C, Krausz M. Executive functioning in obsessive-compulsive disorder, unipolar depression, and schizophrenia. Archives of Clinical Neuropsychology. 2002;17:477–483. doi: 10.1016/S0887-6177(01)00130-5. [DOI] [PubMed] [Google Scholar]
- *.Moritz S, Hottenrott B, Randjbar S, Klinge R, Von Eckstaedt FV, Lincoln TM, Jelinek L. Perseveration and not strategic deficits underlie delayed alternation impairment in obsessive-compulsive disorder (OCD) Psychiatry Research. 2009a;170:66–69. doi: 10.1016/j.psychres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- *.Moritz S, Jelinek L, Hottenrott B, Klinge R, Randjbar S. No evidence for object alternation impairment in obsessive-compulsive disorder (OCD) Brain and Cognition. 2009b;69:176–179. doi: 10.1016/j.bandc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- *.Moritz S, Kloss M, Jahn H, Schick M, Hand I. Impact of comorbid depressive symptoms on nonverbal memory and visuospatial performance in obsessive-compulsive disorder. Cognitive Neuropsychiatry. 2003;8:261–272. doi: 10.1080/135468000344000020. [DOI] [PubMed] [Google Scholar]
- *.Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, Togao O, Tomita M, Masuda Y, Yoshioka K, Kuroki T, Kanba S. Working memory dysfunction in obsessive-compulsive disorder: A neuropsychological and functional MRI study. Journal of Psychiatric Research. 2009a;43:784–791. doi: 10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, Togao O, Tomita M, Masuda Y, Yoshioka K, Kuroki T, Kanba S. Working memory dysfunction in obsessive-compulsive disorder: A neuropsychological and functional MRI study. Journal of Psychiatric Research. 2009b;43:784–791. doi: 10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- *.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Sanematsu H, Togao O, et al. Duration effect of obsessive-compulsive disorder on cognitive function: A functional MRI study. Depression and Anxiety. 2009c;26:814–823. doi: 10.1002/da.20484. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, et al. A functional MRI comparison of patients with obsessive–compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Research: Neuroimaging. 2005;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- *.Nedeljikovic M, Kyrios M, Moulding R, Doron G. Neuropsychological changes following cognitive-behavioral treatment of obsessive-compulsive disorder (OCD) International Journal of Cognitive Therapy. 2011;4:8–20. doi: 10.1080/00048670802653273. [DOI] [Google Scholar]
- *.Nedeljkovic M, Kyrios M, Moulding R, Doron G, Wainwright K, Pantelis C, Purcell R, et al. Differences in neuropsychological performance between subtypes of obsessive-compulsive disorder. The Australian and New Zealand journal of psychiatry. 2009;43:216–226. doi: 10.1080/00048670802653273. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive Affective & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/CABN.7.1.1. [DOI] [PubMed] [Google Scholar]
- *.Nicholson KA. Doctoral dissertation. 1994. A neuropsychological investigation of cortical and subcortical mechanisms of obsessive-compulsive disorder. Retrieved from ProQuest Dissertations and Theses. [Google Scholar]
- Nitschke JB, Heller W. Distinguishing neural substrates of heterogeneity among anxiety disorders. International Review of Neurobiology. 2005;67:1–42. doi: 10.1016/S0074-7742(05)67001-8. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6:589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- *.Okasha A, Rafaat M, Mahallawy N, Nahas ElG, Dawla ElA, Sayed M, Kholi ElS. Cognitive dysfunction in obsessive-compulsive disorder. Acta Psychiatrica Scandinavica. 2000;101:281–285. doi: 10.1111/j.1600-0447.2000.tb10926.x. [DOI] [PubMed] [Google Scholar]
- Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive–compulsive disorder: A selective review. Journal of Affective Disorders. 2007;104:15–23. doi: 10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- *.Ornstein TJ, Arnold P, Manassis K, Mendlowitz S, Schachar R. Neuropsychological performance in childhood OCD: A preliminary study. Depression and Anxiety. 2010;27:372–380. doi: 10.1002/da.20638. [DOI] [PubMed] [Google Scholar]
- *.Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix-Cols D, Giampietro V, et al. A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2009;174:202–209. doi: 10.1016/j.pscychresns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Pauls DL. The genetics of obsessive compulsive disorder: A review of the evidence. American Journal of Medical Genetics. 2008;148C:133–139. doi: 10.1002/ajmg.c.30168. [DOI] [PubMed] [Google Scholar]
- *.Penades R, Catalán R, Andrés S, Salamero M, Gasto C. Executive function and nonverbal memory in obsessive-compulsive disorder. Psychiatry Research. 2005;133:81–90. doi: 10.1016/j.psychres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- *.Penadés R, Catalán R, Rubia K, Andrés S, Salamero M, Gastó C. Impaired response inhibition in obsessive compulsive disorder. European Psychiatry. 2007;22:404–410. doi: 10.1016/j.eurpsy.2006.05.001. [DOI] [PubMed] [Google Scholar]
- *.Purcell R, Maruff P, Kyrios M, Pantelis C. Cognitive deficits in obsessive–compulsive disorder on tests of frontal–striatal function. Biological Psychiatry. 1998a;43:348–357. doi: 10.1016/S0006-3223(97)00201-1. [DOI] [PubMed] [Google Scholar]
- *.Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: A comparison with unipolar depression, panic disorder, and normal controls. Archives of General Psychiatry. 1998b;55:415–423. doi: 10.1001/archpsyc.55.5.415. [DOI] [PubMed] [Google Scholar]
- *.Rajender G, Bhatia MS, Kanwal K, Malhotra S, Singh TB, Chaudhary D. Study of neurocognitive endophenotypes in drug-naïve obsessive–compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatrica Scandinavica. 2011;124(2):152–161. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- *.Rampacher F, Lennertz L, Vogeley A, Schulze-Rauschenbach S, Kathmann N, Falkai P, Wagner M. Evidence for specific cognitive deficits in visual information processing in patients with OCD compared to patients with unipolar depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:984–991. doi: 10.1016/j.pnpbp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- *.Rankins D, Bradshaw JL, Georgiou-Karistianis N. The semantic Simon effect in Tourette’s syndrome and obsessive-compulsive disorder. Brain and Cognition. 2006;61:225–234. doi: 10.1016/j.bandc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- *.Rao N, Reddy Y, Kumar K, Kandavel T, Chandrashekar C. Are neuropsychological deficits trait markers in OCD? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:1574–1579. doi: 10.1016/j.pnpbp.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Raudenbush WS. Analyzing effect sizes: Random-effects models. In: Harris C, Hedges LV, VJC, editors. The Handbook of Research Synthesis and Meta-Analysis. 2. New York: Russell Sage Foundation Publications; 2009. pp. 295–315. [Google Scholar]
- Rende B, Ramsberger G, Miyake A. Commonalities and differences in the working memory components underlying letter and category fluency tasks: A dual-task investigation. Neuropsychology. 2002;16:309–321. doi: 10.1037//0894-4105.16.3.309. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley AD. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: Evidence from unaffected first-degree relatives. American Journal of Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- *.Roh KS, Shin MS, Kim MS, Ha TH, Shin YW, Lee KJ, Kwon JS. Persistent cognitive dysfunction in patients with obsessive-compulsive disorder: A naturalistic study. Psychiatry and Clinical Neurosciences. 2005;59:539–545. doi: 10.1111/j.1440-1819.2005.01411.x. [DOI] [PubMed] [Google Scholar]
- Rolls E, Loh M, Deco G. An attractor hypothesis of obsessive-compulsive disorder. European Journal of Neuroscience. 2008 doi: 10.1111/j.1460-9568.2008.06379.x. [DOI] [PubMed] [Google Scholar]
- *.Roopesh BN, Janardhan Reddy YC, Mukundan CR. Neuropsychological deficits in drug naïve, non-depressed obsessive-compulsive disorder patients. Asian Journal of Psychiatry. 2013;6:162–170. doi: 10.1016/j.ajp.2012.10.003. [DOI] [PubMed] [Google Scholar]
- *.Roth RM. Doctoral dissertation. 1999. Executive Functions in Obsessive-Compulsive Disorder: A Neuropsychological and Event-Related Potential Investigation. Retrieved from ProQuest Dissertations and Theses. [Google Scholar]
- *.Roth RM, Baribeau J, Milovan DL, O’Connor K. Speed and accuracy on tests of executive function in obsessive-compulsive disorder. Brain and Cognition. 2004;54:263–265. doi: 10.1016/j.bandc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- *.Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Human Brain Mapping. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rubia K, Cubillo A, Woolley J, Brammer MJ, Smith A. Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Human Brain Mapping. 2011;32:601–611. doi: 10.1002/hbm.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Meca J, Marín-Martínez F. Testing continuous moderators in meta-analysis: A comparison of procedures. British Journal of Mathematical and Statistical Psychology. 1998;51:311–326. doi: 10.1111/j.2044-8317.1998.tb00683.x. [DOI] [Google Scholar]
- *.Schmidtke K. Cognitive Frontal Lobe Dysfunction in Obsessive-Compulsive Disorder. Biological Psychiatry. 1998;43:666–673. doi: 10.1016/S0006-3223(97)00355-7. [DOI] [PubMed] [Google Scholar]
- Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychological Medicine. 2013:1–10. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- *.Shin NY, Lee AR, Park HY, Yoo SY, Kang DH, Shin MS, Kwon JS. Impact of coexistent schizotypal personality traits on frontal lobe function in obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:472–478. doi: 10.1016/j.pnpbp.2007.09.020. [DOI] [PubMed] [Google Scholar]
- *.Shin YS, Shin NY, Jang JH, Shim G, Park HY, Shin MS, Kwon JS. Switching strategy underlies phonemic verbal fluency impairment in obsessive–compulsive disorder. Journal of Obsessive-Compulsive and Related Disorders. 2012;1:221–227. doi: 10.1016/j.jocrd.2012.07.005. [DOI] [Google Scholar]
- *.Simpson H, Rosen WG, Huppert J, Lin S, Foa E, Liebowitz MR. Are there reliable neuropsychological deficits in obsessive–compulsive disorder? Journal of Psychiatric Research. 2006;40:247–257. doi: 10.1016/j.jpsychires.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A Meta-analysis and review. Psychological Bulletin. 2012 doi: 10.1037/a0028727. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Spitznagel MB, Suhr JA. Executive function deficits associated with symptoms of schizotypy and obsessive-compulsive disorder. Psychiatry Research. 2002;110:151–163. doi: 10.1016/S0165-1781(02)00099-9. [DOI] [PubMed] [Google Scholar]
- Tetzlaff J, Moher D, Pham B, Altman D. Survey of views on including grey literature in systematic reviews. 14th Cochrane Colloquium; Dublin, Ireland. 2006. [Google Scholar]
- *.Thomas SJ, Gonsalvez CJ, Johnstone SJ. How specific are inhibitory deficits to obsessive-compulsive disorder? A neurophysiological comparison with panic disorder. Clinical Neurophysiology, online ahead of print. 2013 doi: 10.1016/j.clinph.2013.08.018. [DOI] [PubMed] [Google Scholar]
- *.Tükel R, Gürvit H, Ertekin BA, Oflaz S, Ertekin E, Baran B, Atalay F. Neuropsychological function in obsessive-compulsive disorder. Comprehensive Psychiatry. 2012;53:167–175. doi: 10.1016/j.comppsych.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman ATF, Boomsma DI. Genetic and environmental influences on obsessive-compulsive symptoms in adults: a population-based twin-family study. Psychological Medicine. 2007;37 doi: 10.1017/S0033291707000980. [DOI] [PubMed] [Google Scholar]
- van den Heuvel O, Veltman D, Groenewegen H, Cath D, van Balkom A, van Hartskamp J, van Dyck R. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Archives of General Psychiatry. 2005a;62:301. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- *.van den Heuvel O, Veltman D, Groenewegen H, Witter M, Merkelbach J, Cath D, van Dyck R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005b;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- *.Van der Linden M, Ceschi G, Zermatten A, Dunker D, Perroud A. Investigation of response inhibition in obsessive-compulsive disorder using the Hayling task. Journal of the International Neuropsychological Society. 2005;11:776–783. doi: 10.1017/S1355617705050927. [DOI] [PubMed] [Google Scholar]
- *.van der Wee NJA, Ramsey NF, Jansma JM, Denys DA, van Megen HJGM, Westenberg HMG, Kahn RS. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. NeuroImage. 2003;20:2271–2280. doi: 10.1016/j.neuroimage.2003.05.001. [DOI] [PubMed] [Google Scholar]
- *.Veale D, Sahakian B, Owen AM, Marks I. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychological Medicine. 1996;26:1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Viard A, Flament M, Artiges E, Dehaene S, Naccache L, Cohen D, Martinot JL. Cognitive control in childhood-onset obsessive-compulsive disorder: A functional MRI study. Psychological Medicine. 2005;35:1007–1017. doi: 10.1017/S0033291704004295. [DOI] [PubMed] [Google Scholar]
- *.Voderholzer U, Schwartz C, Freyer T, Zurowski B, Thiel N, Herbst N, et al. Cognitive functioning in medication-free obsessive-compulsive patients treated with cognitive-behavioural therapy. Journal of Obsessive-Compulsive and Related Disorders. 2013;2:241–248. doi: 10.1016/j.jocrd.2013.03.003. [DOI] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive Affective & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Walshaw PD, Alloy LB, Sabb FW. Executive function in pediatric bipolar disorder and attention-deficit hyperactivity disorder: In search of distinct phenotypic profiles. Neuropsychology Review. 2010;20:103–120. doi: 10.1007/s11065-009-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, Robbins TW. Executive function in Tourette’s syndrome and obsessive–compulsive disorder. Psychological Medicine. 2005;35:571–582. doi: 10.1017/S0033291704003691. [DOI] [PubMed] [Google Scholar]
- Woods CM, Vevea JL, Chambless DL, Bayen UJ. Are compulsive checkers impaired in memory? A meta-analytic review. Clinical Psychology: Science and Practice. 2002;9:353–366. doi: 10.1093/clipsy.9.4.353. [DOI] [Google Scholar]
- Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database of Systematic Reviews. 2011;11:1–20. doi: 10.1002/14651858.MR000027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Harrison B, Wood S, Fornito A, Wellard R, Pujol J, Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Archives of General Psychiatry. 2007;64:946. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- *.Zitterl W, Urban C, Linzmayer L, Aigner M, Demal U, Semler B, Zitterl-Eglseer K. Memory deficits in patients with DSM-IV obsessive-compulsive disorder. Psychopathology. 2001;34:113–117. doi: 10.1159/000049292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.