Abstract
Antigen cross-presentation describes the process through which dendritic cells (DCs) acquire exogenous antigens for presentation on MHC class I molecules. The ability to cross-present has been thought of as a feature of specialized DC subsets. Emerging data, however, suggest that the cross-presenting ability of each DC subset is tuned by and dependent on several factors, such as DC location and activation status, and the type of antigen and inflammatory signals. Thus, we argue that capacity of cross-presentation is not an exclusive trait of one or several distinct DC subtypes, but rather a common feature of the DC family in both mice and humans. Understanding DC subset activation and antigen-presentation pathways might yield improved tools and targets to exploit the unique cross-presenting capacity of DCs in immunotherapy.
Keywords: cross-presentation, dendritic cell subsets, antigen presentation, immunotherapy
DCs and antigen cross-presentation
DCs are professional antigen-presenting cells (APCs) that are uniquely capable of attracting and activating naïve CD4+ and CD8+ T cells. After infection or inflammation, DCs undergo a complex maturation process, and migrate to lymph nodes (LNs) where they present antigens to T cells. Immature DCs acquire exogenous antigens, which they can present on MHC class I molecules via the process of cross-presentation. Cross-presentation is thought to occur through one of two main pathways [1]. The ‘canonical’ endosome-to-cytosol pathway involves transport of exogenous antigens from endosomal vesicles into the cytosol, where they are trimmed and processed by the proteasome and subsequently loaded on MHC class I molecules in the endoplasmic reticulum, similar to endogenous antigens [2]. In the second, proteasome-independent cytosol-independent pathway, DCs use endosomal proteases to process and load captured antigens directly onto MHC class I molecules in endosomal compartments [3]. Antigen cross-presentation should not be confused with cross-priming, which also describes priming of CD8+ T cells and which requires additional signals. Antigen cross-presentation is crucial for the priming of CD8+ cytotoxic T cell responses against pathogens and tumors, but is also required for the maintenance of self-tolerance [4]. For proper differentiation between the two, it is important that experimental studies include crucial controls, for example, peptide controls, and titration of DCs or antigens.
Different DC subtypes are equipped with a diverse repertoire of antigen capture and innate sensing mechanisms. Thus, different DC subsets may differ in their capacity to acquire and process internalized antigens, produce cytokines, and activate T cells in response to distinct microorganisms. Cross-presentation capacity could be intrinsic to the specific DC subset or could be influenced by antigen or other environmental factors. For instance, steady state CD8α+ DCs are equipped with machinery to control endosomal maturation and acidification and are efficient in transporting antigens into the cytosol relative to CD8α− DCs [5-7]. These components may be regulated or induced by environmental stimuli. Furthermore, although plasmacytoid DCs (pDCs) fail to transport cytochrome c to their cytosol, a measure of cross-presenting ability, they are able to cross-present exogenous antigens. Possibly, pDCs and other CD8α− DCs may exploit different mechanisms to cross-present and/or transport antigens to the cytosol for cross-presentation.
Cross-presentation has mostly been studied in human monocyte-derived DCs (MoDCs), and in mouse spleen- or bone-marrow-derived DCs, using model proteins, such as chicken ovalbumin (OVA), which are provided as cell-associated or soluble antigens [8-13]. Such in vitro studies provide the basis for current understanding of antigen cross-presentation mechanisms. However, the cross-presenting ability of other DC subsets, such as human pDCs or CD8α−CD11b− DCs in mice, for different antigen sources should not be ignored [14-17]. In vivo studies in which specific DC subsets are selectively depleted, for example, CD8α+ DCs in Batf3−/− mice or diphtheria-toxin-based depletion studies, have provided pivotal information on the functional role of DC subsets in antigen presentation [18,19]. However, the interpretation of such depletion studies investigating cross-presentation can be complicated by incomplete deletion, depletion-associated side effects, and DC crosstalk (reviewed in [20]). Nevertheless, multiple in vivo studies have demonstrated that the CD8−lineage DCs [18] are indispensable for antigen cross-presentation and not pDCs [21,22] or Langerhans cells (LCs) [23]. By contrast, other in vivo studies with pDC-depleted mice have provided evidence that activated pDCs do play a role in antigen cross-presentation and CD8+ T cell priming [16,24]. Furthermore, in Batf3−/− mice residual cross-presentation capacity is observed and is responsible for protection against tumors. This indicates that other DC subsets cross present, albeit less efficiently than CD8α+ DCs [18]. These studies support the view that, under certain circumstances, specific DC subsets are required for in vivo cross-presentation, and that other DC subsets might be dispensable. They also leave us wondering about whether or not all DCs may be potent cross-presenters in specified conditions, and if yes, what is needed to acquire these cross-presenting abilities.
Here, we review the capacities of mouse DC populations to cross-present directly cell-associated, soluble, immune-complexed and particulate antigens, and antigens derived from nonviral intruders such as bacteria or fungi in different locations and under (non)-inflammatory conditions, and we examine how these findings extrapolate to human DC subsets.
Phenotype and cross-presentation capacity of DC subsets in mice
Genetic profiling has identified a common origin of many DC subsets together with the transcription factors needed for DC lineage commitment (Box 1) [25-29]. An outstanding question is whether efficient cross-presentation is an exclusive trait of some DC subpopulations or a common feature of many or even all DCs.
Box 1. Characterization of DC subsets.
The characterization of DC subsets is an ongoing process. Characterization of migratory DC subsets in peripheral tissues and lymphoid organs is particularly complicated due to tissue-specific and inflammation-dependent expression kinetics of phenotypic markers. The use of a combination of markers (all nonexclusive when used alone) is therefore advised to study the selective characteristics of DC subsets.
Murine conventional DCs: express high levels of CD11c and are further subdivided in blood-derived resident DCs and migratory DCs. The first group resides in the spleen and LNs and is generally subdivided into CD8α+ and CD11b+ or CD4+.
CD8α+-expressing DCs: identified in the spleen and LNs, selectively express the transcription factors basic leucine zipper transcription factor, ATF-like 3 (Batf3) and interferon regulatory factor 8 (IRF8), and high levels of CD24, CD205 (DEC-205), chemokine (C motif) receptor (XCR1), and C-type lectin domain family 9A (CLEC9A). CD103 expression varies between DCs, but is mostly found on migratory CD8α+ DCs and may relate to an activation or developmental state [109]. Analyses of CD24+ DCs in CD8α-deficient mice and FLT3L-stimulated bone-marrow-derived DCs reveals that CD8α is dispensable for the characteristic functional capacities of this subset [30]. As CD8α is expressed relatively late in DC development, is has been suggested that CD24+CD8α− cells may develop into CD8α+ DCs [17].
CD11b+ DCs: The transcription factor reticuloendotheliosis homolog B (RelB) drives the development of cDCs that lack CD8α but express CD11b, CD172a [signal regulatory protein (Sirp-α)], and DC immunoreceptor (DCIR)2, and may show expression of Dectin-1 (Clec7a). Less than 50% of CD11b/CD172a+ cells express CD4, but no clear discrimination has been found in the function between CD4+ and CD4− CD11b+ DCs.
CD8α−CD11b− DCs: a population of spleen DCs that may express CD24, but not CD4, CD8, and CD11b/CD172α.
Migratory DCs: differ in phenotype dependent on the microenvironment in which they reside, such as skin, intestine, or lung tissues. In skin, LCs abundantly express the C-type lectin langerin (CD207). However, later findings indicate that CD207 is also expressed by (CD103+) dermal DCs [34].
MoDCs: isolated from spleen are characterized either by the expression of CD11b+Ly6c+CD11c+MHCII+, or on the expression of DC-SIGN/CD209a in combination with CD11b+CD11c+ for identification.
Human conventional DCs: are CD11c+ and are divided according to the specific and nonoverlapping expression of CD1c (BDCA1) and CD141 (BDCA3). Recently, DCs were characterized in human LNs, tonsil, and spleen in untreated breast cancer patients: pDCs (BDCA4), LCs (Epcam+), CD1a+ DCs, CLEC9a+ DCs, and two populations of BDCA1+ DCs showing differential expression of CD206. Three of these DC subsets (LCs, CD1a+ and CD206+ DCs) are absent from cervical LNs draining the oropharynx, iliac LNs, tonsils, and spleen, suggesting that these cells specifically drain from the skin [91].
pDCs: murine pDCs express intermediate levels of CD11c, and high levels for CD45RA (B220), sialic acid-binding immunoglobulin-like lectins-H (Siglec-H) and/or mouse pDC Ag 1 [mPDCA1; bone marrow stromal cell antigen 2 (BST-2), 120g8], whereas their human equivalents lack the expression of CD11c, but rather are discriminated based on the expression of BDCA2, BDCA4, or CD123.
CD8α+ DCs
CD8α+ DCs (Box 1) [17,30] are generally thought of as the dominant, if not exclusive, cross-presenting DC subset, irrespective of antigen type (Table 1). It has been shown over a decade ago that, in a population of low-density splenocytes isolated from mice primed with OVA-loaded β2-microglobulin-deficient cells, ex vivo depletion of CD8α- high but not CD11b+ cells abrogated cross-presentation to OT-I cells [13]. The cross-presenting ability of splenic CD8α+ DCs has since been confirmed, not only for cell-associated antigens, but also for proteins, OVA-coated latex beads, immune complexes, and many pathogens (Table 1) [31-35]. However, in experiments using Escherichia coli and Saccharomyces cerevisiae, CD8α+ DCs cross-present less well than CD8α− DCs [33,35], suggesting that the immunostimulatory features of distinct pathogens may determine the cross-presenting capacity of DC subsets. This is also suggested by the finding that CD8α+ DCs isolated from skin-draining LNs fail to cross-present intramembrane antigens (i.e., cell-associated antigens) in the K5.mOVA transgenic mouse model [36]. In this model, the transgene OVA is fused to the transmembrane domain of the transferrin receptor under the control of the K5 keratin promoter [36]. By contrast, in a model in which transgenic mice express yellow fluorescent protein linked to cytotoxic T lymphocyte (CTL) epitopes for glycoprotein B (gB) of herpes simplex virus under the rat insulin promoter, the CD8α+ LN DCs were instrumental in cross-tolerization of gB-specific hybridoma T cells [37]. Furthermore, CD8α+ DCs from mesenteric LNs poorly cross-presented intestinal soluble OVA [38]. The cross-priming function of CD8α+ DCs in LNs might also be dictated by immunostimulatory features of the surrounding environment. CD8α+ DCs in skin-draining LNs can potently cross-present OVA–Toll-like receptor (TLR)7 conjugates [39] and saponin-formulated antigens [40], emphasizing the potential of pattern-recognition receptor (PRR) ligands and adjuvants in conventional DC (cDC) function.
Table 1.
Mouse spleen.
| Subsets defined by: | Cell-associated | Soluble protein | IC | Particulate | Bacteria/yeast/yarasite |
|---|---|---|---|---|---|
| CD8α+CD11b− |
Yes
a [9,13,17,33,44,47–50,53,58,105,107,110] b[51] a/b [11] |
Yes
a [5,7,9,47,53,54,38] b [110] a/b [11,57,102] a/c [40] Low a [110] |
Yes
a [55] Low a [54] |
Yes
a [5,30,53] |
Yes
(E. coli) [33,53] (rLM) [34] (Plasmodium) [31] (Salmonella) [32] (yeast) [35] |
| CD8α+CD103− |
No
a [41] |
Low
a [41] |
|||
| CD8α+CD103+ |
Yes
a [41] |
Yes
a [41] |
|||
| CD8α− |
No
a [9,47,48] |
Yes
a [47] Low a [5,7,9,38] |
Yes
a [55] |
No
a [53] Low a [5] |
Yes
(yeast) [35] (Salmonella) [32] |
| CD8α−CD11b+ |
No
a[13,44] b [51] Low a [49,50] |
No
a/b [57] Low a [54] |
Yes
a [54] |
||
| CD4−CD8− |
Low
a [33,53] |
Yes
a/b [102] No a [102] Low a [53] |
No
(Plasmodium) [31] |
||
| CD4+ |
No
a [20,53,107] |
Yes
c [40] No a [40,102] a/b [57] Low a [53] |
No
a [30] |
Yes
(E. coli) [33,53] Low (Plasmodium) [31] |
|
| CD8α−CD11b− |
Yes
a [49,50,58] |
||||
| CD8α−CD11b−CD24+ |
Yes
a [17] b [51] |
Yes
a/b [57] |
|||
| pDCs |
No
a [9,49,50,58] |
Yes
a [16] No a [9] |
Yes
a [16] |
No
(rLM) [34] (Plasmodium) [31] |
Not matured.
Matured with TLR-L or CD40L.
Saponin-based formulation.
A recent study revealed that splenic CD8α+CD103+ DCs presented cell-associated and soluble antigens more efficiently than CD8α+CD103− DCs did [41], suggesting differences within the CD8α+ subset. However, most studies have not distinguished CD103+ from CD103− CD8α+ DCs, making it difficult to interpret these results for DC subsets in LNs or for other types of antigen. Although CD8α is not expressed in in vitro generated Fms-related tyrosine kinase 3 ligand (FLT3-L) DCs cultures, the use of CD24 enables the identification of CD8α+ DC equivalents that potently cross-present cell-associated [42-44] and soluble [42,43,45] antigens, and antigen-coated latex beads [43]. Moreover, the cross-presentation of cellular [46] and soluble [46] antigens resides predominantly in the CD103+ DCs. The expression of CD103 itself seems, however, not equivalent to a cross-presenting phenotype [46]. In conclusion, CD8α+ are able to cross-present a broad spectrum of antigenic formulations at steady state conditions, but environmental factors may affect that function in the peripheral lymphoid organs, in particular in responses to nonsterile infections.
CD11b+ DCs
Splenic DCs that lack CD8α [9,47,48] or that express CD11b [13,37,44,49-51] or CD4 [52,53] are inefficient in cross-presenting cell-associated antigens (Table 1), soluble proteins [5,7,9,38,42,47,53,54] or antigen-coated beads [5,30,53]. Furthermore, CpG-matured CD11b+ DCs loaded with dying cells fail to cross-present their cargo in vivo [51]. Therefore, these DCs seem to lack cross-presenting capabilities. However, CD8α− [55] and CD11b+ DCs do induce potent CD8+ T cell responses when immune complexes are used instead of cell-associated or soluble antigens [54]. Moreover, CD4+ DCs potently cross-present soluble antigens administered together with saponin-based adjuvants [40] and initiate CD8 T cell responses to E. coli [33,53]. DCs lacking CD8α also more efficiently cross-present OVA antigens from Salmonella typhimurium [32] and S. cerevisiae than CD8α+ DCs do [35]. These data emphasize the importance of specific immune activation signals to acquire the ability to cross present. The exact mechanism for the stimulation of cross-presentation by saponin-based adjuvants is not clear yet. This is of interest, because in contrast to most clinically used adjuvants, these adjuvants seem to stimulate CD8+ T cell responses in particular [40,56].
CD8α−CD11b− DCs
A population of splenic DCs that express CD24, but not CD4, CD8 and CD11b/CD172α was described in 2007 that has similar cross-presenting capacity to CD8α+ DCs (Box 1) [57]. Forty-two percent upregulated CD8α after overnight culture [57] and >80% of CD24+CD8α− cells expressed CD8α 4 days after transfer [17], suggesting that these cells were ‘in transit’ towards a CD8α+ DC phenotype. A different study isolated splenic CD11b− DCs into CD8α+ and CD8α− subsets, and found that CD11b−CD8α− DCs were more potent cross-presenters of cell-associated antigens than were CD8α+ DCs under steady-state conditions [58]. However, CD24 was not included in that study, preventing direct comparison with the data of Bedoui et al. [17]. The CD11b−CD8α− DCs preferentially internalized small particles, derived from dying cells (and were accordingly designated merocytic DCs), that are stored in non-acidic compartments with reduced lysosomal degradation for prolonged periods of time. The size of the internalized particles seems favorable for cross-presentation (0.5 and 3 mm) [59]. Indeed, CD11b−CD8α− DCs show sustained antigen presentation to both CD4+ and CD8+ T cells with enhanced effector functions and memory formation when compared with CD8α+ DCs [49,50]. It will be interesting to determine whether these DCs exploit comparable mechanisms as CD8α+ DCs to delay antigen degradation to enhance their antigen-presenting capacity.
Notably, the cross-presenting capacity of merocytic DCs is also most increased after crosstalk with pDCs when compared to CD8α+ and CD11b+ DCs [51]. It will be interesting to dissect the role of merocytic DCs during bacterial and viral infections and whether there is a human equivalent of this DC subset.
Mouse pDCs
Relative to splenic cDCs, cross-presentation by pDCs (Box 1) remains largely understudied. The few available studies suggest that splenic murine pDCs do not cross-present cell-associated antigens [49,50,58], soluble OVA, and peptide-coated beads [16] at steady-state (Table 1). They do not seem to prime CD8+ T cells in response to Listeria monocytogenes [34], Plasmodium berghei [31], or after intragastric administration of protein [38]. In vitro generated FLT3-L pDCs also show low cross-presenting abilities when loaded with cell-associated [58] or soluble [45,60] antigens. By contrast, activation of pDCs by the TLR7/8 ligand R848 and to a lesser extent CpG (TLR9) leads to efficient cross-presentation of soluble antigens and antigen-coated beads [16], again emphasizing the significance of specific stimulation of DC subsets in determining their cross-presenting capacity. Thus, murine pDCs seem inefficient in cross-presenting exogenous antigens but further work is needed.
Migratory DCs
Conventional DCs include lymphoid-organ-resident DCs and migratory DCs. They are present in nonlymphoid organs and migrate to the draining LNs. The phenotype of migratory DCs differs depending on the surrounding microenvironment, for example, skin, intestine, or lung. Characterization of these cells is an ongoing process and studies have used different panels for discrimination, making it difficult to compare findings. LCs are the primary DCs in the epidermis. They express the C-type lectin langerin (CD207) and lack CD103. In the dermis, DCs are generally divided into CD207+ and CD207− cells and further subdivided based on expression of CD103, CD11b, and CD326 [36,61,62]. LCs were initially reported to cross-present OVA in the K5.mOVA transgenic mouse model [63]. However, it was later recognized that CD207 is also expressed by (CD103+) dermal DCs (dDCs), which are in fact responsible for cross-presentation in this model [36,62]. TLR7-conjugated antigens are efficiently presented by LCs, skin-derived CD103−CD205−CD326− DCs, and to a lesser extent by CD11b+CD205−CD326− DCs (claimed to be resident CD11b DCs) and CD103+ DCs [39]. LCs are efficiently targeted in vivo for cross-presentation of anti-DEC205-coupled OVA. By contrast, LCs targeted through langerin fail to stimulate T cell proliferation [64], suggesting that cross-presentation by LCs is dependent on how antigens enter the cell and are routed to the antigen-processing machinery.
In immunological tissues in the intestinal tract (Peyer’s patches, mesenteric LNs, and lamina propria) and lung CD11c+, CD11b−/CD11b+, CD103+ and F4.80− are mostly used to identify DCs [65,66]. CD11b+ DCs in the mesenteric LNs cross-present intragastrically administered antigen better than CD8α+ DCs [38]. In lung draining LNs, one study has shown that CD103+ DCs, but not CD11b+CD205int DCs, present soluble antigens LNs [66] and antigens from infected cells [67]. Although in the latter study productive infection was not observed in CD103+ DCs, direct transfer of genetic material cannot be excluded. Another study has shown that CD103−CD11bhi DCs efficiently capture antigens from the lungs, migrate to the draining LNs, and cross-prime CD8+ T cells [68]. Expression of CD11b on DCs in the periphery is promiscuous, therefore, these CD103−CD11bhi cells may not only refer to migratory lung DCs, but also to inflammatory (monocyte-derived) DCs that are attracted during the course of the ongoing infection at the time of antigen exposure.
In all studies differential cross-presentation capacity is observed in subsets of migratory DCs in the skin, intestinal tract, and lungs. The limited number of these studies and the large experimental diversities (e.g., different subset-specific markers, antigens, and stimulatory/inflammatory conditions) has hampered side-by-side comparisons between migratory DC subsets.
Mouse MoDCs
Bone-marrow-derived DCs generated in vitro using granulocyte–macrophage colony-stimulating factor (GM-CSF) alone or in combination with interleukin (IL)-4 cross-present cell-associated antigens [42,58], immune complexes [69-74], PLGA [poly(lactic-co-glycolic acid)] particles [75], or polystyrene beads [76], and cross-priming is efficiently induced by targeting antigen conjugated to anti-DEC205 [69] or anti-DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-Grabbing nonintegrin) [77]. Responses to soluble protein range from none [73-75] to low [72] and high [42,69,70,77-80]. The suboptimal results with soluble antigen may be overcome by using an adjuvant, such as saponin-based adjuvants [56,81]. The administration of long peptides [82-84] might also be a promising alternative, because it circumvents the need for additional adjuvants. It has been suggested that mouse bone-marrow-derived DCs display similar morphology, phenotype, and immunostimulatory activity as blood MoDCs [85] that may differentiate from monocytes under inflammatory conditions. Inflammatory MoDCs isolated from the spleen (characterized in these studies as CD11b+Ly6c+CD11c+MHCII+) are efficiently cross-presenting soluble antigens [9,11]. These cells may relate to tumor necrosis factor (TNF)/inducible NO synthase (iNOS)-producing DCs, of which the gene expression profiles are similar to those of activated monocytes rather than cDCs [28]. New additional markers, such as Fc receptor phenotypes, might be helpful for better discrimination.
Human DC subsets
Cross-presenting capacity of human blood DC subsets
cDCs and pDCs are the two main subtypes distinguished in the blood. The cDC subset can be further divided into at least two subtypes by the expression of CD11c in combination with CD1c blood dendritic cell antigen (BDCA)1 and CD141 (BDCA3) [86,87]. BDCA1+ DCs are presented as the human counterpart of murine CD11b+ DCs [25,26]. In contrast to murine CD11b+ DCs, blood-derived BDCA1+ DCs cross-present cell-associated [2,8,88-90], long peptides [91], soluble antigens [81,89,90,92,93], as well as immune complexes [8,92] (Table 2). In some studies cross-presentation was rather low in steady state conditions, but was enhanced by addition of either TLR ligands [88] or saponin-based adjuvants [2,8,81]. BDCA1+ DCs are less efficient in cross-presentation of cell-associated and soluble antigens than are BDCA3+ DCs [90].
Table 2.
Human DC subsets.
| Subsets defined by: | Cell-associated | Soluble protein | IC |
|---|---|---|---|
| CD1c (BDCA1) |
Yes
[2] b/c [88] Low b [89] d [90] c [14] |
Yes
a [8] c [14] c/d [89,93] No d [99] c/d [81] Low [92] d [90] c/d [8] a [81] |
Yes
d [8] Low [92] |
| CD141 (BDCA3) |
Yes
d [90] c [14] b [89] b/c [88] |
Yes
[92] d [90] c [14,89,93,99] No/low d [89,93,99] |
Yes
[92] |
| CBDCs CD141/DNGR1e |
Yes
c/d [82] (long peptide) No d [82] |
||
| pDCs |
Yes
c [14] b [94] b/c [2,88] |
Yes
c [14] c/d [93] Low c/d [14] No d [90] 0/c [8] |
No
c/d [8] |
|
MoDCs
(IL-4/GM-CSF) In vitro |
Yes
Blood [12] b [10,95–98] |
Yes
d [108,111] a/c [8] a [81] No d [8,81] Low c/d [82] (long peptide) |
Yes
c/d [8] [112] |
Saponin-based formulation.
Cells loaded with virus (and thus intrinsic PRR activity of virus).
Matured with TLR-L or CD40L.
Not matured.
CBDCs, cord blood dendritic cells; DNGR, dendritic cell natural killer lectin group receptor.
In contrast to murine pDCs, human pDCs cross-present cell-associated antigens [2,14,88,94], soluble antigens [14,93], and a vaccinal lipopeptide preparation [2], but fail to cross-present immune complexes or antigens packed within saponin-based adjuvant formulations to CD8+ T cells [8]. However, some groups have demonstrated that soluble antigens are not efficiently cross-presented by pDCs [8,90].
Human BDCA3+ and murine CD8α+ DCs overlap both in gene expression patterns and ability to cross-present cell-associated antigens [88-90], long peptides [91], soluble proteins [89,90,92,93], and immune complexes [92]. All blood DCs cross-present poorly unless properly activated by TLR agonists [88,89,91]. BDCA3+ DCs generated in vitro from cord blood hematopoietic stem cells (HSCs) are able to cross-present long peptides when matured with the TLR3 ligand poly I:C [82]. The low numbers of BDCA3+ DCs circulating in peripheral blood limits the ex vivo modulation of these cells and requires efficient in vivo targeting strategies. Alternatively, adaptation of the protocol to produce BDCA3+ DCs from cord blood HSCs [82] may lead to generation of cord blood DCs enriched for this specific subset.
Studies with human MoDCs differentiated in vitro with GM-CSF and IL-4 report variable results with regard to effective cross-presentation [8,10,81,82,95-98]. Ex vivo data on cross-presenting function of (activated) monocytes is so far lacking. Furthermore, as for their murine counterparts, only a few studies have investigated the cross-presentation capacity of human LCs [15,99,100].
In conclusion, findings from mice do not always translate to the human setting. For instance, murine pDCs mostly fail to cross-present antigens but human counterparts seem to cross-present antigens and cross-prime CD8+ T cells efficiently. Furthermore, care should be taken when isolated DC subsets are used, for example, BDCA1+ DCs express low levels of CD14, which should be taken into account when using a lineage cocktail or CD14+ monocyte depletion. Studies suggest that most human DC subsets can cross-present exogenous antigens when the antigens are provided in an appropriate fashion.
Factors influencing cross-presenting capacity
The capacity to cross-present exogenous antigens may not be restricted to a specialized DC subset. Rather, it seems that a cross-presentation program can be initiated in most if not all DC subsets. Factors emerging as important for the modulation of the cross-presentation activity of specific DC subsets are: (i) type of antigen; (ii) presence of DC stimulatory factors, which can be altered by pathogens or adjuvants; and (iii) timing and phase of the immune response.
Type of antigen
DCs encounter antigens in many shapes and sizes, derived from various sources. The ability of DCs to handle these different antigen types is largely determined by the repertoire of antigen uptake receptors, and the ability to engulf antigens through receptor-independent processes, such as (micro)pinocytosis (Figure 1). The current paradigm of superior cross-presentation by murine CD8α+ DCs is mainly derived from the preferential use of specific antigen types such as cell-associated antigens, a restricted number of soluble model antigens or bead-bound antigens. CD8α+ DCs have in a few studies been found to perform worse than CD8α− DCs in cross-presentation of immune complexes [54,55]. The same has been observed for presentation of yeast [35] and Salmonella-derived [32] antigens. In some cases, the results may have been affected by the disregard of additional markers to distinguish CD11b+ cells from merocytic cells, which are even more potent cross-presenters of cell-associated materials in direct comparison analyses. This may be especially true for studies in which DCs are isolated from FLT3-ligand-treated mice because CD8α−CD11b− DCs are preferentially enriched in these animals [17,50].
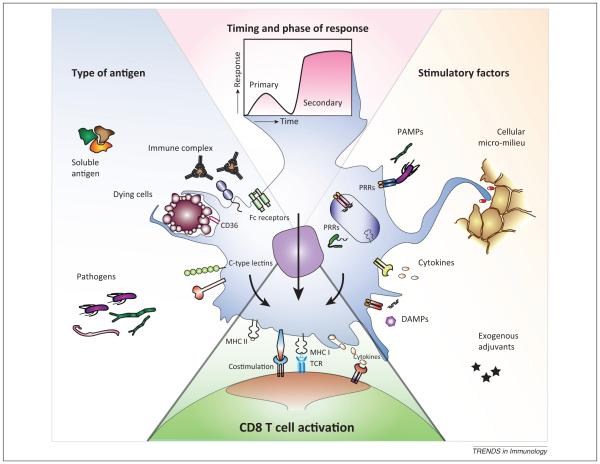
Figure 1.
Decisive factors in dendritic cell (DC) cross-presentation. The ability of DCs to cross-present antigens is not just an intrinsic property of the specific DC subset, but is also determined by: (i) type of antigen; (ii) presence of DC stimulatory factors; and (iii) timing and phase of the immune response. DCs encounter antigens of many origins, shapes, and sizes. The ability of DCs to internalize soluble antigens, immune complexes, dying cells, or whole pathogens is largely determined by the repertoire of antigen uptake receptors (e.g., Fc receptors, CD36, and C-type lectins) and the ability to engulf antigens through receptor-independent processes. The dynamic endocytic receptor expression is, in turn, affected by ligation of pattern-recognition receptors [PRRs; e.g., Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-like helicases (RLHs)] recognizing a particular group of pathogens expressing a unique set of pathogen- or danger-associated molecular patterns (PAMPs/DAMPs). These stimuli are also able to modulate the intracellular mechanisms of cross-presentation, emphasizing the significance of different adjuvants used in different studies. In addition, the surrounding cellular and soluble factors in the micro-milieu can significantly alter the cross-presenting potential of DCs. The effects of all these modulating factors are concordantly dependent on the timing relative to antigen processing by the DCs and thereby affect the outcome for T cell activation in different phases of the immune response.
Different DC subsets express a distinct endocytic receptor repertoire that is dynamically regulated by stimuli. DCs exploit their endocytic receptors, such as the mannose receptor (recognizing OVA), to transport antigens into early endosomes in which subsequently the cross-presentation machinery will be recruited. The neonatal Fc receptor (FcRn) is essential for uptake (and thus presentation) of immune complexes by CD11b+ DCs. The same is true for OVA-coated beads, which are only cross-presented when first opsonized with IgG [54]. Similarly, human blood BDCA3+ DCs induce strong T cell activation upon internalization of soluble protein, indicative for a potent cross-presentation machinery, whereas pDCs hardly induce T cell activation [90]. The absence of cross-presentation of soluble protein by pDCs is not unexpected because an earlier study showed that pDCs inefficiently take up soluble proteins in a receptor-independent fashion. By contrast, pDCs efficiently internalize and present antigens from immune complexes to CD4+ T cells [101]. When antigens are targeted trough the C-type lectin receptor dendritic cell immunoreceptor [DCIR; C-type lectin domain family 4 member A (CLEC4a)], all human DC subsets tested, including ex vivo generated DCs, skin-isolated LCs, and blood myeloid DCs and pDCs, are able to cross-present antigens and activate CD8+ T cells [15]. Thus, virtually all DCs have the machinery to cross-present antigens, provided that the antigen is offered to the DC in a suitable format under the appropriate conditions.
Stimulatory factors regulating DC cross-presentation
Each DC subset contains a restricted set of (inducible) PRRs that may have developed to react to a particular group of pathogens expressing a unique set of pathogen-associated molecular patterns. The recognition of such patterns by PRRs enhances peptide loading onto MHC class I molecules by the recruitment of the cross-presentation machinery to the endosomes [80].
How cross-presentation compares across antigens from diverse pathogens is unknown. The function of distinct human DC subsets in human LNs upon pathogenic infection is particularly unclear. It is uncertain whether the immune stimulatory features or other properties of pathogens are decisive in determining the functional characteristics of the DCs. The answer might not always be found in cell-activating molecules. Coexpression of LLO, a lysosome-disrupting hemolysin from L. monocytogenes, enhances cross-presentation of OVA E. coli [33], illustrating that specific proteins/enzymes enable access of antigens to the cytosol and thereby improve the cross-presenting capacity of DCs. This means that care should be taken in generalizing the capacities of DCs in immune responses against groups (intra- or extracellular) of pathogens.
A large set of TLR ligands are known to act as adjuvants and stimulate cross-presentation [16,45,82,89,102], illustrating that the antigen-presenting capability of different DC subsets is dependent on the stimulatory agent used. The timing of stimulation relative to antigen encounter is another crucial factor. TLR stimulation shuts down the ability to internalize certain antigen uptake receptors [103,104] and may impair cross-presentation [41,105], and thus limit the clinical efficacy of otherwise potent adjuvants [106].
DCs do not work alone in combating infections, because the surrounding environment can alter DC cross-presentation and cross-priming potential. For example, type I interferons improve DC capacity to store and process exogenous antigens, leading to enhanced cross-presentation and activation of antigen-specific CD8+ T cell responses [107,108]. Other soluble factors or immune cells in the microenvironment can affect the immunological outcome. DCs in the gut are exposed to a completely different micro-milieu than DCs in the skin or to DCs that circulate in the blood. Therefore, the location and the micro-milieu largely determine the cross-presenting potential of DCs. BDCA3+ DCs in blood only cross-present when matured by R848 [14] or poly I:C stimulation, whereas BDCA3+ DCs that have differentiated further and migrated to the skin are already cross-presenting without additional stimulation [99]. A challenge is to identify checkpoints that regulate DC function.
Timing of antigen entry and stage of immune response
The timing of antigen entry can affect DC cross-presenting capacity. For example, DCs harvested from mice 18 h after antigen challenge showed cross-presentation activity in the CD8α+ and dDCs subsets, whereas CD8α+ DCs were unable to prime CTLs when isolated 36 h after stimulation [39]. Similarly, the capacity of DCs in brachial LNs at day 2 (primary infection site) and axillary LNs at day 6 (secondary site) to cross-present herpes simplex virus-1 antigens to gBT-I cells ex vivo was examined. Only CD8α+ DCs presented antigens at both time points, whereas migratory CD103+ DCs had cross-presentation capacity only 6 days post-infection [36]. Immunization in the presence of saponin-based adjuvants led to a dominant role for CD8α+ DCs after 12 h, whereas migratory DCs joined in and efficiently cross-presented antigens after 24 h, and even after 48 h [40]. Thus, different DC subsets contribute to cross-priming at different time points during the initiation of an immune response.
The phase of the immune response should also be considered when studying cross-presentation. The finding that pDCs inefficiently take up soluble proteins, but do take up protein immune complexes suggests that pDCs are not first in line for MHC class I presentation of soluble proteins, but play a role in the second line of defense when the immune system has generated specific antibodies against this protein. In the initial phase of the immune response, pDCs produce large amounts of type I interferons that not only signal foreign invaders but also trigger antigen cross-presentation by cDCs [108]. This may be a general phenomenon that does not solely account for pDCs or Fc receptors. Therefore, in addition to the antigen type and stimulatory environment, timing and phase of the immune response are crucial for determining effective cross-presentation.
Concluding remarks
Available data suggest that all the classically characterized DC subsets have the ability to cross-present exogenous antigens. The type of antigen and presence and timing of inflammatory signals and other components of the microenvironment that program DC differentiation and activation are decisive in determining which DC subsets become dominant and sometimes indispensable for cross-presentation. Understanding the functional reprogramming of distinct DC subsets under different inflammatory conditions should provide further insight into the plasticity and exclusivity of functional specializations of DC subsets, including their ability to cross-present exogenous antigens. Knowledge of how to manipulate antigen-presentation pathways will be instrumental for vaccine adjuvant development and should ultimately lead to effective vaccination strategies against cancer and infectious diseases.
Acknowledgments
Limitations of space preclude extensive citation of the literature; we apologize to those whose work is not mentioned in this review. This work was supported by grants from the Dutch Cancer Society (KWF2008-4617), The Netherlands Organization for Scientific Research (NWO-Vici-918.66.615), and the Netherlands Institute for Regenerative Medicine (NIRM, grant No. FES0908).
References
- 1.Joffre OP, et al. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 2.Hoeffel G, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Di Pucchio T, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- 5.Savina A, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Lin ML, et al. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3029–3034. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnurr M, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–2472. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 9.Segura E, et al. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weck MM, et al. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 11.Zhan Y, et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8(+) spleen dendritic cells. Eur. J. Immunol. 2011;41:2585–2595. doi: 10.1002/eji.201141540. [DOI] [PubMed] [Google Scholar]
- 12.Albert ML, et al. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 13.den Haan JM, et al. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tel J, et al. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T-cells, despite lower Ag uptake than myeloid dendritic cell subsets. Blood. 2012;121:459–467. doi: 10.1182/blood-2012-06-435644. [DOI] [PubMed] [Google Scholar]
- 15.Klechevsky E, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2011;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouries J, et al. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedoui S, et al. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J. Immunol. 2009;182:4200–4207. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 18.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith MM, et al. Zinc finger transcription factor zDC is a negative regulator required to prevent activation of classical dendritic cells in the steady state. J. Exp. Med. 2012;209:1583–1593. doi: 10.1084/jem.20121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Blijswijk J, et al. Advantages and limitations of mouse models to deplete dendritic cells. Eur. J. Immunol. 2013;43:22–26. doi: 10.1002/eji.201243022. [DOI] [PubMed] [Google Scholar]
- 21.Sapoznikov A, et al. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervantes-Barragan L, et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igyarto BZ, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi H, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Crozat K, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol. Rev. 2010;234:177–198. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 26.Robbins SH, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meredith MM, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satpathy AT, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JC, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Askew D, Harding CV. Antigen processing and CD24 expression determine antigen presentation by splenic CD4+ and CD8+ dendritic cells. Immunology. 2008;123:447–455. doi: 10.1111/j.1365-2567.2007.02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundie RJ, et al. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14509–14514. doi: 10.1073/pnas.0806727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yrlid U, Wick MJ. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J. Immunol. 2002;169:108–116. doi: 10.4049/jimmunol.169.1.108. [DOI] [PubMed] [Google Scholar]
- 33.Schulz O, et al. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belz GT, et al. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backer R, et al. CD8-dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur. J. Immunol. 2008;38:370–380. doi: 10.1002/eji.200737647. [DOI] [PubMed] [Google Scholar]
- 36.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 37.Belz GT, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung Y, et al. CD8alpha-11b+ dendritic cells but not CD8alpha+ dendritic cells mediate cross-tolerance toward intestinal antigens. Blood. 2005;106:201–206. doi: 10.1182/blood-2004-11-4240. [DOI] [PubMed] [Google Scholar]
- 39.Oh JZ, et al. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118:3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson NS, et al. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol. Cell Biol. 2012;90:540–552. doi: 10.1038/icb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu CH, et al. Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J. Immunol. 2009;182:4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 42.Cheong C, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naik SH, et al. Cutting edge: generation of splenic CD8+ and CD8-dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 45.Kool M, et al. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. J. Leukoc. Biol. 2011;90:1177–1190. doi: 10.1189/jlb.0610342. [DOI] [PubMed] [Google Scholar]
- 46.Sathe P, et al. The acquisition of antigen cross-presentation function by newly formed dendritic cells. J. Immunol. 2011;186:5184–5192. doi: 10.4049/jimmunol.1002683. [DOI] [PubMed] [Google Scholar]
- 47.Iyoda T, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoves S, et al. A critical role for granzymes in antigen cross-presentation through regulating phagocytosis of killed tumor cells. J. Immunol. 2011;187:1166–1175. doi: 10.4049/jimmunol.1001670. [DOI] [PubMed] [Google Scholar]
- 49.Reboulet RA, et al. Prolonged antigen storage endows merocytic dendritic cells with enhanced capacity to prime anti-tumor responses in tumor-bearing mice. J. Immunol. 2010;185:3337–3347. doi: 10.4049/jimmunol.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hennies CM, et al. Selective expansion of merocytic dendritic cells and CD8DCs confers anti-tumour effect of Fms-like tyrosine kinase 3-ligand treatment in vivo. Clin. Exp. Immunol. 2011;163:381–391. doi: 10.1111/j.1365-2249.2010.04305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nierkens S, et al. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71:6428–6437. doi: 10.1158/0008-5472.CAN-11-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz O, et al. CD36 or alphavbeta3 and alphavbeta5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8 alpha+ murine dendritic cells. J. Immunol. 2002;168:6057–6065. doi: 10.4049/jimmunol.168.12.6057. [DOI] [PubMed] [Google Scholar]
- 53.Schnorrer P, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker K, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9927–9932. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.den Haan JMM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J. Exp. Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.den Brok MH, et al. Saponin-based adjuvants create a highly effective anti-tumor vaccine when combined with in situ tumor destruction. Vaccine. 2012;30:737–744. doi: 10.1016/j.vaccine.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 57.Vremec D, et al. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109:1165–1173. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]
- 58.Janssen E, et al. Efficient T cell activation via a Toll-Interleukin 1 Receptor-independent pathway. Immunity. 2006;24:787–799. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Tran KK, Shen H. The role of phagosomal pH on the size-dependent efficiency of cross-presentation by dendritic cells. Biomaterials. 2009;30:1356–1362. doi: 10.1016/j.biomaterials.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinohara ML, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat. Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merad M, et al. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 62.Henri S, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waithman J, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J. Immunol. 2007;179:4535–4541. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 64.Flacher V, et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J. Invest. Dermatol. 2010;130:755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelsall B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol. 2008;1:460–469. doi: 10.1038/mi.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.del Rio M-L, et al. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 67.Helft J, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J. Clin. Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ballesteros-Tato A, et al. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Platt CD, et al. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Regnault A, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West MA, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 72.Rafiq K, et al. Immune complex-mediated antigen presentation induces tumor immunity. J. Clin. Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schuurhuis DH, et al. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J. Immunol. 2006;176:4573–4580. doi: 10.4049/jimmunol.176.8.4573. [DOI] [PubMed] [Google Scholar]
- 74.Schuurhuis DH, et al. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J. Immunol. 2002;168:2240–2246. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 75.Han R, et al. Surface modification of poly(D, L-lactic-co-glycolic acid) nanoparticles with protamine enhanced cross-presentation of encapsulated ovalbumin by bone marrow-derived dendritic cells. J. Biomed. Mater. Res. A. 2011;96:142–149. doi: 10.1002/jbm.a.32860. [DOI] [PubMed] [Google Scholar]
- 76.Merzougui N, et al. A proteasome-dependent, TAP-independent pathway for cross-presentation of phagocytosed antigen. EMBO Rep. 2011;12:1257–1264. doi: 10.1038/embor.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tacken PJ, et al. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood. 2011;118:4111–4119. doi: 10.1182/blood-2011-04-346957. [DOI] [PubMed] [Google Scholar]
- 78.Datta SK, et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J. Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 79.Reinicke AT, et al. Dendritic cell cross-priming is essential for immune responses to Listeria monocytogenes. PLoS ONE. 2009;4:e7210. doi: 10.1371/journal.pone.0007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgdorf S, et al. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 81.Robson NC, et al. Processing and cross-presentation of individual HLA-A, -B, or -C epitopes from NY-ESO-1 or an HLA-A epitope for Melan-A differ according to the mode of antigen delivery. Blood. 2010;116:218–225. doi: 10.1182/blood-2009-10-249458. [DOI] [PubMed] [Google Scholar]
- 82.Poulin LF, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8 + dendritic cells. J. Exp. Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faure F, et al. Long-lasting cross-presentation of tumor antigen in human DC. Eur. J. Immunol. 2009;39:380–390. doi: 10.1002/eji.200838669. [DOI] [PubMed] [Google Scholar]
- 84.Bürdek M, et al. Three-day dendritic cells for vaccine development: antigen uptake, processing and presentation. J. Transl. Med. 2010;8:90. doi: 10.1186/1479-5876-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schreurs MW, et al. Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur. J. Immunol. 1999;29:2835–2841. doi: 10.1002/(SICI)1521-4141(199909)29:09<2835::AID-IMMU2835>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 86.Ziegler-Heitbrock L, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 87.MacDonald KP, et al. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 88.Crozat K, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8 + dendritic cells. J. Exp. Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segura E, et al. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flinsenberg TW, et al. Fcgamma receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood. 2012;120:5163–5172. doi: 10.1182/blood-2012-06-434498. [DOI] [PubMed] [Google Scholar]
- 93.Mittag D, et al. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J. Immunol. 2011;186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 94.Lui G, et al. Plasmacytoid dendritic cells capture and cross-present viral antigens from influenza-virus exposed cells. PLoS ONE. 2009;4:e7111. doi: 10.1371/journal.pone.0007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marañón C, et al. Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6092–6097. doi: 10.1073/pnas.0304860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arrode G, et al. Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8(+) T cells by dendritic cells. J. Virol. 2000;74:10018–10024. doi: 10.1128/jvi.74.21.10018-10024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arrode G, et al. Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells. J. Virol. 2002;76:142–150. doi: 10.1128/JVI.76.1.142-150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weck MM, et al. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- 99.Haniffa M, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benitez-Ribas D, et al. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. J. Exp. Med. 2006;203:1629–1635. doi: 10.1084/jem.20052364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pooley JL, et al. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 103.Benitez-Ribas D, et al. Activation of human plasmacytoid dendritic cells by TLR9 impairs Fc gammaRII-mediated uptake of immune complexes and presentation by MHC class II. J. Immunol. 2008;181:5219–5224. doi: 10.4049/jimmunol.181.8.5219. [DOI] [PubMed] [Google Scholar]
- 104.Tel J, et al. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J. Immunol. 2010;184:4276–4283. doi: 10.4049/jimmunol.0903286. [DOI] [PubMed] [Google Scholar]
- 105.Wilson NS, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 106.Nierkens S, et al. Route of administration of the TLR9 agonist CpG critically determines the efficacy of cancer immunotherapy in mice. PLoS ONE. 2009;4:e8368. doi: 10.1371/journal.pone.0008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spadaro F, et al. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 109.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol. Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 110.Dresch C, et al. Thymic but not splenic CD8(+) DCs can efficiently cross-prime T cells in the absence of licensing factors. Eur. J. Immunol. 2011;41:2544–2555. doi: 10.1002/eji.201041374. [DOI] [PubMed] [Google Scholar]
- 111.Audran R, et al. Encapsulation of peptides in biodegradable microspheres prolongs their MHC class-I presentation by dendritic cells and macrophages in vitro. Vaccine. 2003;21:1250–1255. doi: 10.1016/s0264-410x(02)00521-2. [DOI] [PubMed] [Google Scholar]
- 112.Dhodapkar KM, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]