Abstract
Objectives
Empirical studies of the relationship between depression and cardiovascular disease (CVD) tend to be limited to examination of one-way relationships. This study assessed both cross-sectional association and longitudinal reciprocal relationships between late-life depressive symptoms and CVD.
Methods
The National Health and Aging Trends Study waves 1 (T1) and 2 (T2, one year later) provided the data. The study sample (N = 5,414) represented Medicare beneficiaries aged 65 years or older. We fit structural equation models to examine: 1) cross-sectional association between depression and CVD at each wave; and 2) longitudinal reciprocal relationship between T1 depression and T2 CVD and between T1 CVD and T2 depression.
Results
At T1, 28.6% reported a CVD diagnosis, and at T2, 4.9% reported having had a new diagnosis or new episode of heart attack or heart disease and 2.2% reported having had a stroke since T1. In addition to significant cross-sectional relationships between depression and CVD, T1 CVD had significant impact on T2 depressive symptoms, and T1 depressive symptoms had significant impact on T2 CVD, with a 1-point increase in depressive symptom score increasing the odds of having a new CVD diagnosis or episode by 21%.
Conclusions
The care of older adults with CVD and/or depression needs to include interventions focusing on lifestyle and psychological factors that can reduce risks for both CVD and depression. Depression prevention and treatment also needs to be an integral part of CVD prevention and management.
Keywords: Depression, CVD, older adults
Data from a national probability sample of household resident adults in the United States show that two-thirds of all those aged 50 years and older (and 74.4% of black patients aged 50 years and older) who met the diagnostic criteria for major depressive disorder (MDD) had a diagnosis of heart disease, stroke, hypertension, and/or diabetes.1 The data also show that those with comorbid MDD and cardiovascular disease (CVD) or CVD risk factors had significantly greater functional impairments than did those with MDD alone. MDD or clinically significant late-life depression has been found to be an independent risk factor for CVD, after other CVD risk factors were adjusted for.2–7 One study2 found that depression was associated with an almost 1.5-fold increased risk for coronary heart disease. Another study5 found that depression was associated with almost twofold increased odds of stroke, even after age, socioeconomic status, lifestyle, and psychological factors were adjusted for.
Extant research also found that depression is a frequent complication of CVD. Heart disease and/or stroke increased the risk of older adults’ developing depressive symptoms, independent of other depression risk and demographic factors and of previous history of depression, as CVD increases vulnerability to late-life depression, both physiologically and psychologically, and it may precipitate or perpetuate some geriatric depressive syndromes.8–12 In addition to a bio-physiologic connection between CVD and depression, experiencing symptoms of a potentially life-threatening and/or debilitating disease is likely to subject many older adults to severe psychological distress at a time when their coping mechanisms are also likely to be weakened. Depressive symptom severity may be abated with effective CVD treatment; however, CVD-depression association remains significant, often with high depressive symptoms continuing for several years.13,14 One study15 found that both depression and anxiety increased significantly between 6 months and 5 years after stroke diagnosis.
The link between depression and CVD may be attributable, in part, to a common genetic vulnerability and to pathophysiologic factors, including increased platelet reactivity, decreased heart rate variability, and increased proinflammatory markers such as C-reactive protein.16–21 Unmanaged psychosocial stress and negative lifestyle habits associated with depression can also lead to hypertension, arterial damage, irregular heart rhythms, and a weakened immune system. Studies also found that higher depressive symptom severity was associated with lower antioxidant vegetable and fruit intakes, lower adherence to antihypertensive medication use, reduced physical activity, and withdrawal from telehealth services in persons with heart problems.22–27 Cigarette smoking, heavy alcohol use, low social support, neglect of self-care management, and nonadherence to CVD prevention and treatment regimens among depressed older adults may also be associated with recurrent CVD episodes and increased CVD morbidity and mortality.28–30 Although untreated depression can be a serious risk factor for development of CVD and/or worsening CVD morbidity, there is also emerging evidence that treatment of late-life depression can reduce the risk for cardiovascular disease and vice versa.14 Improvement in depressive symptoms after stroke has also been associated with improvement in motor and cognitive statuses among older adults and in social role functioning in adults of all ages.13,31
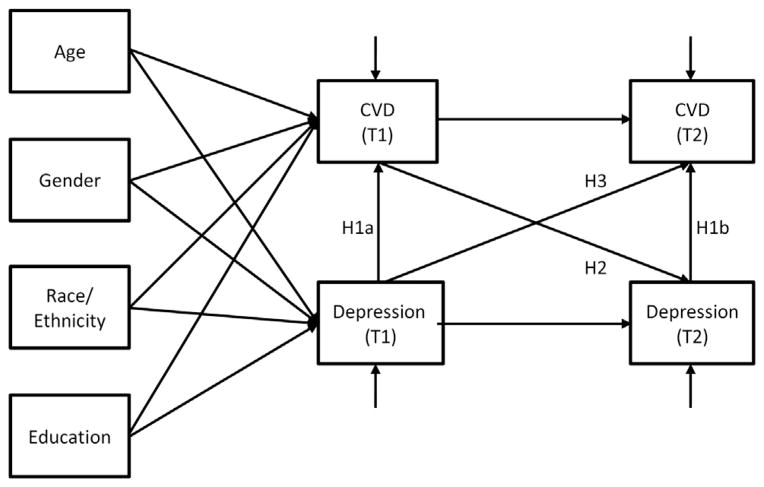
Previous research strongly suggests longitudinal, reciprocal/bidirectional links between depression and CVD in older adults,12,32 but empirical studies tended to be limited to examination of one-way relationships—either the effect of depression on CVD or the effect of CVD on depression over time. The purpose of the present study was to assess both cross-sectional association and longitudinal reciprocal relationships between depressive symptoms, focusing on cognitive-affective dimensions, and CVD (i.e., diagnosis of heart disease and/or stroke) among a nationally representative sample of older adults aged 65 or older. The primary study hypotheses were:
-
1a)
T1 (time 1) CVD will be significantly associated with T1 depressive symptoms, and 1b) T2 (time 2; one year later) CVD will be significantly associated with T2 depressive symptoms.
-
2)
T1 CVD will predict T2 depressive symptoms.
-
3)
T1 depressive symptoms will predict T2 CVD.
Direct effects from T1 depression to T2 depression and from T1 CVD to T2 CVD were also estimated. Covariates were demographics (age, sex, race/ ethnicity, and education), CVD risk factors (hypertension, diabetes, and current smoking), and other potential depression risk factors (diagnosis of any type of cancer, diagnosis of dementia/Alzheimer disease, activities and instrumental activities of daily living [ADL/IADL] impairments, and social isolation).
METHODS
Data and Sample
Data for this study came from the National Health and Aging Trends Study (NHATS) waves 1 (T1) and 2 (T2) conducted in 2011 and 2012, respectively. The sample is representative of U.S. Medicare beneficiaries aged 65 years or older.33 The sample for the present study (N = 5,414: 5,326 in community; 63 in residential care settings that were not nursing homes; and 25 in nursing homes; of the sample, 5,243 were self-interviewed, and 171 were proxy-interviewed). They were older adults who had lived in their own or another’s homes, who were self-interviewed at T1, and who were reinterviewed at T2. The unweighted sample attrition rate from T1 (N = 6,680) to T2 (N = 5,414) was 19%. Of 1,266 T1 sample persons who were not interviewed at T2, 880 (69.5%) refused participation; 240 (19.0%) had died; 66 (5.2%) were too sick to participate; 80 (6.3%) did not participate for other reasons. Compared with those without CVD at T1, those with CVD were less likely to have refused T2 interviews, but they were more likely to have died (11.8% versus 14.6%, χ2(1) = 8.91, p = 0.009 for refusal and 5.1% versus 1.9%, χ2(1) = 49.47, p <0.001 for death). However, the overall attrition rates did not differ between those who had a CVD diagnosis at T1 and those who did not (19.4% versus 18.2%, χ2(1) = 1.13, p = 0.301).
Measures
Cardiovascular disease
At T1, each sample person was asked if a doctor had ever said that he/she had had certain diseases and conditions. Those who responded affirmatively to “a heart attack or myocardial infarction,” “any heart disease including angina or congestive heart failure,” or “a stroke” were categorized as having a CVD. At T2, all sample participants were asked about a new diagnosis (or new episode) since their last interview. For a sample person who was proxy interviewed at T2, the proxy responded whether or not the sample person had had a diagnosis since T1. Those who responded affirmatively to heart attack/myocardial infarction, heart disease, or stroke were categorized as having had a new CVD diagnosis or episode at T2.
Depressive symptoms
At both waves, depressive symptoms were measured with the two-item Patient Health Questionnaire-2 (PHQ-2),34 which captures the cognitive/affective symptoms of anhedonia and depressed mood by asking: “Over the last month, how often [have you/has the sample person] (a) had little interest or pleasure in doing things; and (b) felt down, depressed, or hopeless?” Responses were based on a four-point scale (1=not at all; 2=several days; 3=more than half the days; 4 = nearly every day). The combined score was used as a symptom severity score. Previous research suggests that CVD burden is more likely to affect cognitive-affective than somatic symptom dimensions of depression.35,36 Depressive symptom data were missing for 29 sample persons at T1 and 42 sample persons at T2.
CVD risk factors
These included a diagnosis of hypertension, diagnosis of diabetes, or self-reported current smoking at T1. Each sample person was asked if a doctor had ever said that he/she had had hypertension or diabetes. Each sample person was also asked if he/she currently smoked.
Depression risk factors
Depression risk factors included T1 and T2 diagnosis of cancer; diagnosis of dementia/Alzheimer disease; social isolation; and the number of T1 ADL/IADL impairments (possible ranges of 0 to 14). Social isolation was measured with a question, “Looking back over the last year, who are the people you talked with most often about important things?” Those who said that they had no one were categorized as socially isolated. ADL or IADL impairments have been found to be significantly associated with depression in older adults and vice versa over time.37,38
Demographic covariates
These were age; sex (male versus female); race/ethnicity (all others versus non-Hispanic white); and education (a bachelor’s degree versus no bachelor’s degree).
Analysis Model
We used the svy procedures of Stata/MP 13 (Stata Corp., 2013) for data management and descriptive statistics to specify the NHATS complex sampling design and sampling weights. We used Mplus 7.1139 for the estimation of structural equation models (SEM) to test the primary study hypotheses, incorporating the NHATS sampling design and sampling weights: 1) cross-sectional association between depression and CVD at each wave; and 2) longitudinal reciprocal relationship between T1 depression and T2 CVD and between T1 CVD and T2 depression. Because the CVD outcome was binary, the probit link function was used. Model fit was evaluated using root mean square error of approximation less than 0.05,40 the comparative fit index greater than 0.95,41 and the Tucker-Lewis Index greater than 0.90.42 The initial model contained the following: 1) age, sex, race/ ethnicity, and education as time invariant covariates and T1 number of ADL/IADL impairments that were modeled to affect both T1 CVD and T1 depressive symptoms; 2) T1 depressive symptoms, cancer and dementia diagnosis at each wave, and social isolation at each wave as time-varying covariates were modeled to affect depressive symptoms at each wave. The initial model did not demonstrate adequate fit, however, and modifications were made based on theoretically viable additions to the model and modification indices. The final analysis model, as shown in Figure 1, was selected, with only the demographics (age, sex, race/ ethnicity, and education) as covariates. The exclusion of covariates other than the demographic variables did not affect the results of the primary hypotheses. To examine odds ratios for the binary CVD outcomes, we also fit the full information maximum likelihood SEM model with a logit link function, and provide logit regression results with odds ratios (Ors) and 95% confidence intervals (CIs). Given that a couple of previous studies also found the relationship between depression and CVD/CVD risk factors to be present in women but not in men,43,44 we also estimated sex effects in a multigroup model, with or without the sex equality constraints applied. We did not find any sex difference in the outcomes of interest. Thus we present the results of the sex-inclusive model only.
FIGURE 1. Analytic model.
Notes: H: Hypothesis.
RESULTS
Sample Characteristics, Prevalence of CVD, and Depressive Symptoms
The respondents were, on average, 74.4 years old at T1; 44.2% male and 55.8% female; 81.8% non-Hispanic white; and 28.6% reported a CVD diagnosis at T1 and 71.4% did not. As shown in Table 1, compared with those without a CVD diagnosis, those with a CVD diagnosis were older (75.98 ± 0.18 versus 73.82 ± 0.12, t = 10.09, df = 56, p <0.001); included a higher proportion of men (49.5% versus 42.1%, χ2(1) = 24.74, p <0.001); included a higher proportion of non-Hispanic whites and a lower proportion of Hispanics (83.5% versus 81.1% for non-Hispanic whites and 4.8% versus 7.0 for Hispanics, χ2(3) = 10.27 for race/ethnicity, p = 0.035); and had less education (e.g., 20% versus 28.2% with a college degree, χ2(3) = 54.03 for education, p <0.001).
TABLE 1.
Demographics and Functional Health Status at Time 1 (N = 5,414)
| All (100%) | With T1 CVD (28.55%) | Without T1CVD (71.45%) | p | |
|---|---|---|---|---|
| Age in years, M (SE) | 74.43 (0.10) | 75.98 (0.18) | 73.82 (0.12) | <0.001 |
| Age group (%) | <0.001 | |||
| 65–69 | 30.68 | 22.61 | 33.91 | |
| 70–74 | 26.13 | 23.91 | 27.01 | |
| 75–79 | 19.44 | 21.85 | 18.47 | |
| 80–84 | 14.01 | 18.30 | 12.30 | |
| 85+ | 9.74 | 13.33 | 8.31 | |
| Sex (%) | <0.001 | |||
| Male | 44.23 | 49.54 | 42.11 | |
| Female | 55.77 | 50.46 | 57.89 | |
| Race/ethnicity (%) | 0.035 | |||
| Non-Hispanic white | 81.81 | 83.49 | 81.13 | |
| Black/African American | 8.22 | 8.51 | 8.10 | |
| Hispanic | 6.37 | 4.81 | 7.0 | |
| All other | 3.60 | 3.19 | 3.77 | |
| Education (%) | <0.001 | |||
| <High school diploma | 20.57 | 25.24 | 18.70 | |
| High school diploma | 27.64 | 27.24 | 27.80 | |
| Some college | 25.97 | 27.56 | 25.33 | |
| Bachelor’s degree | 25.82 | 19.96 | 28.17 | |
| No. of ADL/IADL impairment, M (SE) | 0.93 (0.05) | 1.36 (0.10) | 0.76 (0.06) | <0.001 |
Notes: All statistics are weighted. t = 10.09, df = 56, for age; χ2(4) = 113.39 for age group; χ2(1) = 24.74 for sex; χ2(3) = 10.27 for race/ethnicity; χ2(3) = 54.03 for education; and t = 5.87, df = 56, for the ADL/IADL impairment.
Table 2 shows that 28.6% had a CVD diagnosis at T1. With respect to specific CVD conditions at T1, 23.7% reported having had a heart attack or heart disease, and 8.7% reported having had a stroke. In addition, at T1, 63.4% reported having hypertension; 23.4%, diabetes; 18%, a cancer diagnosis; 1.8%, a dementia/Alzheimer disease diagnosis; 5.8%, having no one to talk with; and 8.3%, current smokers. At T2, 6.6% reported a new CVD diagnosis (3.3%) or a new episode (3.3%). With respect to specific CVD conditions, 4.9% reported having had a new diagnosis or new episode of heart attack or heart disease since T1, and 2.2% reported having had a stroke since T1. In addition, small proportions reported new diagnoses since T1 of hypertension (2.4%), diabetes (1.5%), and cancer (5.9%); 4% reported having no one to talk with; and 7.9% were current smokers at T2.
TABLE 2.
Prevalence of CVD and CVD and Depression Risk Factors
| T1 (2011) | T2 (2012, new diagnosis/episode) | |
|---|---|---|
| CVD (%) | 28.55 | 6.56a |
| Heart attack/heart disease | 23.74 | 4.86 |
| Stroke | 8.65 | 2.21 |
| CVD and depression risk factors (%) | ||
| Hypertension | 63.39 | 2.39 |
| Diabetes | 23.38 | 1.45 |
| Cancer | 17.99 | 5.93 |
| Dementia/Alzheimer’s | 1.81 | 1.37 |
| Social isolation | 5.82 | 4.02 |
| Current smoking | 8.30 | 7.88b |
3.29% had no CVD diagnosis at T1 but received a new CVD diagnosis at T2, and 3.27% had a CVD diagnosis at T1 but had a new CVD episode at T2.
Includes those who continued to smoke and those who resumed smoking since T1.
As shown in Table 3 and as expected, depressive symptoms were significantly higher among those with CVD than among those without CVD at T1 (3.19 ± 0.04 versus 2.75 ± 0.02, t = 9.18, p <0.001). T2 depressive symptom severity was also significantly higher among those who had received a new CVD diagnosis or episode since T1 (3.42 ± 0.10) than among those who continued to be free of CVD (2.72 ± 0.03) or had not had any new episode since T1 (3.03 ± 0.04) (F(2,55) = 71.70; p <0.001). Table 3 also shows that the depressive symptom scores of those who continued to be free of CVD did not change between T1 and T2 (2.73 ± 0.02 at T1 and 2.72 ± 0.03 at T1; t = 0.67, df = 56, p = 0.503), and the symptom scores decreased among those who reported a CVD diagnosis at T1 but no new episode at T2 (from 3.12 ± 0.04 at T1 to 3.03 ± 0.04 at T2; t = 2.26, df = 56, p = 0.028). Symptom severity did not significantly change among those who had a new diagnosis or episode (from 3.35 ± 0.10 at T1 to 3.42 ± 0.10 at T2; t = 0.48, df = 53, p = 0.630).
TABLE 3.
Depressive Symptoms by CVD
| T1 M (SE) | T2 M (SE) | |
|---|---|---|
| T1 (N = 5,396) | ||
| Without CVD | 2.75 (0.02) | |
| With CVD | 3.19 (0.04) | |
| t = 9.18; p <0.001 | ||
| T2 (N = 5,371) | ||
| No CVD | 2.73 (0.02)a | 2.72 (0.03)a |
| With T1 diagnosis but no new diagnosis | 3.12 (0.04)b | 3.03 (0.04)b |
| With new diagnosis/ episode of CVD | 3.35 (0.10)c | 3.42 (0.10)c |
| F (2,55) = 71.70; p <0.001 | F (2,55) = 71.70; p <0.001 | |
| Multiple group comparisona | a < b; a < c; b = c | a < b < c |
Bonferroni corrected; α < 0.05.
Results of Structural Equation Modeling
As shown in Table 4, as hypothesized, cross-sectional relationships between T1 depression and T1 CVD (Hypothesis 1a in Fig. 1: B = 0.43, SE = 0.05, Z = 9.42, p <0.001) and between T2 depression and T2 CVD (Hypothesis 1b in Fig. 1: B = 0.34, SE = 0.07, Z = 4.81, p <0.001) were significant. As also hypothesized, T1 CVD had significant impact on T2 depressive symptoms (Hypothesis 2 in Fig. 1: B = 0.17, SE = 0.04, Z = 4.44, p <0.001), and T1 depressive symptoms had a significant impact on T2 CVD (Hypothesis 3 in Fig. 1: B = 0.19, SE = 0.04, Z = 5.61, p <0.001). In the latter relationship, a 1-point increase in the depressive symptom score (e.g., from absence of depressed mood to experience of depressed mood several days during the preceding month, from several days to more than half the days, or from more than half the days to nearly every day) increased the odds of having a new CVD diagnosis or a new CVD episode by 21% (95% CI: 1.13–1.30). As also expected, T1 depressive symptoms significantly predicted T2 depressive symptoms (B = 0.44, SE = 0.02, Z = 22.65, p <0.001), and T1 CVD was a significant predictor for a new CVD event at T2 (B = 0.89, SE = 0.13, Z = 7.00, p <0.001; OR: 2.44, 95% CI: 1.90–3.13). T1 CVD was significantly associated with all demographic covariates, with older age and being male increasing the odds of having a CVD diagnosis, and being nonwhite and having a college degree decreasing such odds. On the other hand, T1 depressive symptoms were not significantly associated with age, but they were significantly positively associated with being nonwhite and negatively associated with being male and having a college degree.
TABLE 4.
Probit and Logistic Regression Results From SEM Model
| Probit Regression Results
|
Logistic Regression Results
|
Odds ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| B (SE) | Z | p | B (SE) | Z | p | ||
| T1 CVD on | |||||||
| Age | 0.03 (0.00) | 10.94 | <0.001 | 0.05 (0.00) | 10.18 | <0.001 | 1.05 (1.04–1.05) |
| Male | 0.22 (0.04) | 4.95 | <0.001 | 0.41 (0.07) | 5.86 | <0.001 | 1.51 (1.31–1.73) |
| Other than non–Hispanic white | −0.09 (0.04) | −2.15 | 0.032 | −0.17 (0.07) | −2.45 | 0.014 | 0.84 (0.73–0.97) |
| Bachelor’s degree or higher | −0.32 (0.06) | −5.27 | <0.001 | −0.48 (0.10) | −4.77 | <0.001 | 0.62 (0.51–0.76) |
| T1depressive symptoms on | |||||||
| T1 CVD | 0.23 (0.03) | 9.30 | <0.001 | 0.43 (0.05) | 9.42 | <0.001 | |
| Age | 0.00 (0.00) | −1.24 | 0.215 | 0.00 (0.00) | −1.37 | 0.171 | |
| Male | −0.19 (0.04) | −4.21 | <0.001 | −0.13 (0.04) | −3.15 | 0.002 | |
| Other than non–Hispanic white | 0.39 (0.07) | 5.97 | <0.001 | 0.31 (0.07) | 4.25 | <0.001 | |
| Bachelor’s degree or higher −0.43 (0.06) | −0.43 (0.06) | −7.00 | <0.001 | −0.40 (0.04) | −9.56 | <0.001 | |
| T2 depression score on | |||||||
| T1 depressions score | 0.44 (0.01) | 34.59 | <0.001 | 0.44 (0.02) | 22.65 | <0.001 | |
| T1 CVD | 0.08 (0.02) | 3.62 | <0.001 | 0.17 (0.04) | 4.44 | <0.001 | |
| T2 CVD | 0.10 (0.02) | 4.26 | <0.001 | 0.34 (0.07) | 4.81 | <0.001 | |
| T2 CVD on | |||||||
| T1 depressive symptoms | 0.08 (0.02) | 4.20 | <0.001 | 0.19 (0.04) | 5.61 | <0.001 | 1.21 (1.13–1.30) |
| T1 CVD | 0.28 (0.04) | 7.19 | <0.001 | 0.89 (0.13) | 7.00 | <0.001 | 2.44 (1.90–3.13) |
Notes: MPlus does not generate the fit indices for logistic regression models.
Probit model:
Goodness of fit: χ2 (8) = 45.32, p <0.001.
Comparative Fit Index = 0.966.
Tucker-Lewis Index = 0.906.
Root Mean Square Error of Approximation = 0.029.
Weighted Root Mean Square Residual = 1.156.
DISCUSSION
The findings of the present study based on two waves of panel data (1 year apart) show an extremely high prevalence of CVD and CVD risk factors (hypertension, diabetes, and/or smoking) among older adults. Depressive symptoms were higher among those with CVD than among those without CVD, and CVD and depressive symptoms had a reciprocal relationship. The extent of the effect of T1 depressive symptoms on the risk of T2 CVD was quite strong with each point increase on depressive symptom scores being associated with 1.21-fold increased odds of a new CVD diagnosis or a new CVD episode. The effect of T1 CVD on T2 depressive symptoms is also notable given that it was significant even after we adjusted for demographic factors and T1 depressive symptoms that tend to explain a large variance of T2 depressive symptoms. The findings provide further support for significant cross-sectional and longitudinal reciprocal relationships between cognitive/affective depressive symptoms and CVD in late life.
The study had the following limitations. First, the 1-year follow-up was a relatively short period in which to examine longer-term effects, as one previous study15 found that depression and anxiety were more frequent at 5 years after stroke than at 6 months. Second, more detailed data on clinical characteristics and history of depression and the PHQ-9 covering somatic as well as cognitive and affective dimensions of depression, rather than PHQ-2, would have allowed diagnosis of probable MDD and more in-depth analysis of depressive symptoms associated with CVD. Previous research suggested that severity, number of episodes, and duration of depression may moderate the relationship between depression and CVD.45 Third, T1 CVD diagnosis referred to that of lifetime (“ever,” without data on specific onset/date of the initial diagnosis). Although all hypothesized relationships between depressive symptoms and CVD were significant, data on the date of initial diagnosis would have allowed additional analysis taking disease duration into account. Fourth, although the overall attrition rates at T2 did not differ between those who had had a CVD diagnosis at T1 and those who had not, the higher mortality rate among those with CVD may have resulted in underestimating the strengths of the longitudinal relationships between CVD and depression as well as those between T1 and T2 CVD and depressive symptoms.
Despite these limitations, the study findings, based on a nationally representative sample of older adults, point to the importance of early identification of depressed older adults who are at risk of developing CVD and both prevention and treatment of depression and CVD at the same time in older adults. Modifiable risk factors for depression and CVD tend to be the same unhealthy life style choices—including smoking, heavy drinking, physical inactivity, and unhealthy eating habits—and psychosocial stressors.22,25,29,30,46,47 By helping older adults engage in healthy lifestyles, risks for both depression and CVD may be alleviated. A meta-analysis found that most cardiac rehabilitation programs delivered in the home for older adults can significantly mitigate depressive symptoms.48 In addition, the present study also points to the need to consider depression as a modifiable risk factor for CVD. Thus, instituting psychotherapeutic interventions (e.g., cognitive behavioral therapy, problem-solving therapy, or interpersonal therapy to alleviate psychosocial stressors and improve coping skills) for depression prevention and treatment might be an important step to alleviating vulnerability to CVD among older adults, and these interventions may need to become important components of CVD prevention and management. Combined pharmacologic and psychotherapy treatments with chronic disease management may be more effective for reducing depressive symptom severity among CVD patients.49 Given the association between higher depressive symptom severity and withdrawal from telehealth treatment of heart failure among home care clients,26 treatment of depression among CVD patients may also improve adherence to CVD treatment. As depression treatment can also increase social role functioning among stoke patients,31 it may also lead to improvement in the overall quality of life among these older adults as well.
In sum, the findings of the present study show longitudinal reciprocal relationships between CVD and depression in late life and underscore that the care for older adults with CVD or depression needs to include interventions focusing on lifestyle factors that can reduce risks for both CVD and depression. Depression prevention and treatment also need to be an integral part of CVD prevention and management.
Acknowledgments
This research was funded from an internal research grant from the University of Texas at Austin.
Footnotes
The authors have no conflicts of interest.
References
- 1.González HM, Tarraf W. Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. Int Psychogeriatr. 2013;25:833–841. doi: 10.1017/S1041610212002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JM, Stewart JC, Stump TE, et al. Risk of coronary heart disease events over 15 years among older adults with depressive symptoms. Am J Geriatr Psychiatry. 2011;19:721–729. doi: 10.1097/JGP.0b013e3181faee19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher D, O’Regan C, Savva GM, et al. Depression, anxiety and cardiovascular disease: which symptoms are associated with increased risk in community dwelling older adults? J Affect Disord. 2012;142:132–138. doi: 10.1016/j.jad.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Glymour MM, Yen JJ, Kosheleva A, et al. Elevated depressive symptoms and incident stroke in Hispanic, African-American, and white older Americans. J Behav Med. 2012;35:211–220. doi: 10.1007/s10865-011-9356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CA, Mishra GD. Depression and risk of stroke in midaged women: a prospective longitudinal study. Stroke. 2013;44:1555–1560. doi: 10.1161/STROKEAHA.113.001147. [DOI] [PubMed] [Google Scholar]
- 6.Scuteri A, Modestino A, Fedullo F, et al. Depression treatment selectively modifies arterial stiffness in older participants. J Gerontol A Biol Sci Med Sci. 2013;68:719–725. doi: 10.1093/gerona/gls230. [DOI] [PubMed] [Google Scholar]
- 7.Windle M, Windle RC. Recurrent depression, cardiovascular disease, and diabetes among middle-aged and older adult women. J Affect Disord. 2013;150:895–902. doi: 10.1016/j.jad.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 9.Hackett ML, Yapa C, Parag V, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 10.Luijendijk HJ, Tiemeier H, van den Berg JF, et al. Heart failure and incident late-life depression. J Am Geriatr Soc. 2010;58:1441–1448. doi: 10.1111/j.1532-5415.2010.02921.x. [DOI] [PubMed] [Google Scholar]
- 11.Luijendijk HJ, Stricker BH, Wieberdink RG, et al. Transient ischemic attack and incident depression. Stroke. 2011;42:1857–1861. doi: 10.1161/STROKEAHA.110.604405. [DOI] [PubMed] [Google Scholar]
- 12.Hare DL, Toukhsati SR, Johansson P, et al. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 13.Ostir GV, Berges IM, Ottenbacher A, et al. Patterns of change in depression after stroke. J Am Geriatr Soc. 2011;59:314–320. doi: 10.1111/j.1532-5415.2010.03266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Göthe F, Enache D, Wahlund LO, et al. Cerebrovascular diseases and depression: epidemiology, mechanisms and treatment. Panminerva Med. 2012;54:161–170. [PubMed] [Google Scholar]
- 15.Lincoln NB, Brinkmann N, Cunningham S, et al. Anxiety and depression after stroke: a 5 year follow-up. Disabil Rehabil. 2013;35:140–145. doi: 10.3109/09638288.2012.691939. [DOI] [PubMed] [Google Scholar]
- 16.Mittag O, Meyer T. The association of depressive symptoms and ischemic heart disease in older adults is not moderated by gender, marital status or education. Int J Public Health. 2012;57:79–85. doi: 10.1007/s00038-011-0256-6. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge P, Rosmalen JG, Kema IP, et al. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: a critical review of the literature. Neurosci Biobehav Rev. 2010;35:84–90. doi: 10.1016/j.neubiorev.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Joynt KE, Whellan DJ, O’connor CM. Why is depression bad for the failing heart? A review of the mechanistic relationship between depression and heart failure. J Card Fail. 2004;10:258–271. doi: 10.1016/j.cardfail.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.McCaffery JM, Frasure-Smith N, Dubé MP, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 20.Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak—the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9:526–539. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 21.Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: the case of depression. Prog Cardiovasc Dis. 2013;55:557–562. doi: 10.1016/j.pcad.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alosco ML, Spitznagel MB, Miller L, et al. Depression is associated with reduced physical activity in persons with heart failure. Health Psychol. 2012;31:754–762. doi: 10.1037/a0028711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnet F, Irving K, Terra JL, et al. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Gentil L, Vasiliadis HM, Préville M, et al. Association between depressive and anxiety disorders and adherence to antihypertensive medication in community-living elderly adults. J Am Geriatr Soc. 2012;60:2297–2301. doi: 10.1111/j.1532-5415.2012.04239.x. [DOI] [PubMed] [Google Scholar]
- 25.Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and anti-oxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112:2022–2027. doi: 10.1016/j.jand.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radhakrishnan K, Jacelon CS, Bigelow C, et al. Association of comorbidities with home care service utilization of patients with heart failure while receiving telehealth. J Cardiovasc Nurs. 2013;28:216–227. doi: 10.1097/JCN.0b013e3182512331. [DOI] [PubMed] [Google Scholar]
- 27.Win S, Parakh K, Eze-Nliam CM, et al. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97:500–505. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carney RM, Freedland KE, Miller GE, et al. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 29.Mukamal KJ, Chung H, Jenny NS, et al. Alcohol consumption and risk of coronary heart disease in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:30–37. doi: 10.1111/j.1532-5415.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 30.van Gool CH, Kempen GI, Penninx BW, et al. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32:81–87. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- 31.Schmid AA, Damush T, Tu W, et al. Depression improvement is related to social role functioning after stroke. Arch Phys Med Rehabil. 2012;93:978–982. doi: 10.1016/j.apmr.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Mosovich SA, Boone RT, Reichenberg A, et al. New insights into the link between cardiovascular disease and depression. Int J Clin Pract. 2008;62:423–432. doi: 10.1111/j.1742-1241.2007.01640.x. [DOI] [PubMed] [Google Scholar]
- 33.Kasper JD, Freedman VA. National Health and Aging Trends Study Round 1 User Guide: Final Release. Baltimore, MD: Johns Hopkins University School of Public Health; 2012. Nov, [Accessed August 20, 2013]. Available at: http://www.nhats.org/scripts/documents/NHATS_Round_1_User_Guide_Final_Release.pdf. [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire 2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 35.Linke SE, Rutledge T, Johnson BD, et al. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: a report from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Arch Gen Psychiatry. 2009;66:499–507. doi: 10.1001/archgenpsychiatry.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michal M, Wiltink J, Kirschner Y, et al. Differential associations of depressive symptom dimensions with cardiovascular disease in the community: results from the Gutenberg health study. PLoS One. 2013;8:e72014. doi: 10.1371/journal.pone.0072014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry LC, Murphy TE, Gill TM. Depressive symptoms and functional transitions over time in older persons. J Gerontol B Psychol Sci Soc Sci. 2011;66:585–594. doi: 10.1097/JGP.0b013e3181ff6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geerlings SW, Beekman ATF, Deeg DJH, et al. Physical health and the onset and persistence of depression in older adults: An eight-wave prospective community-based study. Psychol Med. 2000;30:369–380. doi: 10.1017/s0033291799001890. [DOI] [PubMed] [Google Scholar]
- 39.Muthén LK, Muthén BO. Mplus 7.11. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 40.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1:130–149. [Google Scholar]
- 41.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 42.Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- 43.Rice MC, Katzel LI, Waldstein SR. Sex-specific associations of depressive symptoms and cardiovascular risk factors in older adults. Aging Ment Health. 2010;14:405–410. doi: 10.1080/13607860903586185. [DOI] [PubMed] [Google Scholar]
- 44.Seifert CL, Poppert H, Sander D, et al. Depressive symptoms and the risk of ischemic stroke in the elderly—influence of age and sex. PLoS One. 2012:7e50803. doi: 10.1371/journal.pone.0050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baune BT, Stuart M, Gilmour A, et al. Moderators of the relationship between depression and cardiovascular disorders: a systematic review. Gen Hosp Psychiatry. 2012;34:478–492. doi: 10.1016/j.genhosppsych.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Henderson KM, Clark CJ, Lewis TT, et al. Psychosocial distress and stroke risk in older adults. Stroke. 2013;44:367–372. doi: 10.1161/STROKEAHA.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukamal KJ, Chung H, Jenny NS, et al. Alcohol use and risk of ischemic stroke among older adults: the Cardiovascular Health Study. Stroke. 2005;36:1830–1834. doi: 10.1161/01.STR.0000177587.76846.89. [DOI] [PubMed] [Google Scholar]
- 48.Gellis ZD, Kang-Yi D. Meta-analysis of the effect of cardiac rehabilitation interventions on depression outcomes in adults 64 years of age and older. Am J Cardiol. 2012;110:1219–1224. doi: 10.1016/j.amjcard.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Rustad JK, Stern TA, Hebert KA, et al. Diagnosis and treatment of depression in patients with congestive heart failure: a review of literature. Prim Care Companion CNS Disord. 2013;15(4) doi: 10.4088/PCC.13r01511. PCC. 13r01511. [DOI] [PMC free article] [PubMed] [Google Scholar]