Abstract
Objective(s)
Our goal is to systematically and quantitatively assess treatment response between Asian patients with HCV-6 and HCV-1 treated for 48 weeks with PEG IFN+RBV.
Methods
We performed a literature search in Medline and EMBASE for ‘genotype 6’ in August 2013. Additional abstracts from major international scientific conferences from 2012–2013 were reviewed. Included studies were original articles with ≥10 treatment-naïve Asian HCV-6 patients. Exclusion criteria were co-infections with HBV, HIV and/or other liver diseases. Heterogeneity was defined as Cochrane Q-test with p-value of 0.10 and I-squared statistic (>50%). Results are reported from random-effects model.
Results
A total of 1046 (503 HCV-6; 543 HCV-1) patients from 12 studies were included in the analysis. Pooled SVR rate was 80.2% (CI 74.3–85.0%) (Q-statistic=20.87, p<0.035; I2=47.3%) for HCV-6 and 62.5% (CI 41.9–79.4%) (Q-statistic=52.41, p<0.001; I2=92.37) for HCV-1 patients, respectively. HCV-6 patients had significantly higher SVR rate compared to HCV-1 patients, OR 2.73 (CI 1.69–4.41, p<0.001). Approximately one-fourth of patients without EVR achieved SVR, regardless of genotype (n = 6/23 HCV-1; n = 4/21 HCV-6).
Conclusions
Asian patients with HCV-6 can expect higher SVR rates (~80%) than HCV-1 patients (~63%). EVR as a stopping rule is less clear in Asian patients with HCV-6 and HCV-1.
Keywords: HCV, hepatitis C, sustained virologic response, genotype 6, genotype 1
INTRODUCTION
Chronic infection with hepatitis C virus (HCV) is a major global health problem infecting approximately 170 million people [1]. Chronic infection causes significant sequelae and frequently leads to end-stage liver disease and hepatocellular carcinoma [2]. In Southeast Asia, HCV prevalence in some countries (6–7% in Vietnam and Thailand) is higher than the U.S. prevalence of 1.8%[1]. HCV genotype 1 (HCV-1) and the lesser known genotype 6 (HCV-6) are also the two most common genotypes in parts of Southeast Asia, Hong Kong, and Southern China [1].
HCV genotype is a major independent predictor of sustained virologic response (SVR, defined as undetectable HCV RNA at 24 weeks after end of therapy) in patients treated with pegylated interferon and ribavirin (PEG IFN+RBV) [1]. While there are six known HCV genotypes worldwide, HCV genotypes 1–3 are predominant in Western countries and large registration trials with PEG IFN+RBV have been conducted and focused on these genotypes. HCV genotype 1 (HCV-1) (40–50%) has lower SVR rates than the more treatment-favorable genotypes 2 and 3 (70–80%)[2]. However, results from these studies are often in non-Asian patients and may not be generalizable to Asian patients.
Although there have been several studies published on Asian patients with HCV-1, there is limited treatment information on Asian patients with HCV-6 and even less data directly comparing SVR rates in patients with these two genotypes [3–12]. Some studies suggest that SVR rate in HCV-6 patients is superior to HCV-1, but they are largely limited by their small sample sizes [4,10–21].
Most recently, triple therapy with PEG IFN+RBV and sofosbuvir has been approved by the Food and Drug Administration in the U.S. for the treatment of HCV-1 [22]. However, given the high retail cost of sofosbuvir (Sovaldi®, Gilead Sciences, Foster City, CA, USA)($84,000 for 12 weeks of treatment in the U.S.), this therapy may be cost-prohibitive [22,23]. While Gilead Sciences has proposed price reductions for low- to middle-income countries ($5,000 in Thailand, $2,000 in India), comments from directors from Médecins Sans Frontières’ Access Campaign suggest that “at these prices, access in middle-income countries – where 75% of the world’s poor actually live – is likely to be extremely limited [24],” given the income disparity in such areas. Data on new direct acting antiviral therapy for HCV genotype 6 is also very limited with only 6 patients with HCV genotype 6 included in the landmark NEUTRINO study with sofosbuvir-based therapy [25]. Therefore, PEG-IFN + RBV will likely remain the main therapeutic option in Asian patients residing in these regions for the near future.
Our goals are to quantitatively and qualitatively compare treatment responses to PEG-IFN and RBV between Asian patients infected with HCV-6 versus HCV-1.
MATERIALS AND METHODS
Data Sources and Searches
We performed a comprehensive literature search in PubMed in October 2013 with the following search term: (‘genotype 6’). The search was limited to MEDLINE-indexed articles only and included studies in non-English languages. We also performed a manual search of abstracts using the term ‘genotype 6’ from annual international scientific meetings between 2012 and 2013 by the American Association for the Study of Liver Diseases (AASLD), Digestive Disease Week (DDW), Asian Pacific Study of the Liver (APASL), and European Association for the Study of the Liver (EASL). An EMBASE search was also conducted for the same period using search criteria: (‘genotype 6’/exp).
Study Selection
Studies fulfilling the following specific inclusion criteria were considered for analysis: i) original studies ii) inclusion of treatment-naïve Asian patients iii) minimum sample size of ≥10 HCV-6 patients iv) inclusion of patients treated with PEG IFN+RBV. Studies were excluded if study cohorts included patients with co-infection with hepatitis B, hepatitis D, or human immunodeficiency virus. In studies that included both HCV-6 and HCV-1 patients, we included these HCV-1 patients as the HCV-1 comparison group for our study. Articles were reviewed independently by two of the authors (N. Nguyen and S. McCormack) and checked by a third author (M. Nguyen) with discrepancies resolved by consensus.
Data Extraction
For each study, we collected information on study characteristics (country of origin, practice setting, collaboration), study design, study type (randomized controlled trial (RCTs) versus observational), intention-to-treat (ITT) analysis, and baseline patient characteristics (ethnicity, age, gender, ALT, fibrosis, and HCV RNA levels). We also collected baseline treatment information and treatment response (rapid virologic response [RVR, defined as undetectable HCV RNA after 4 weeks of treatment], early virological response [EVR, defined as <50 IU/mL or >2 log drop from baseline HCV RNA after 12 weeks of treatment] and SVR).
Statistical Analysis
Statistical analyzes were performed to produce pooled event rates (SVR rates in HCV-6 and HCV-1 patients treated for 48 weeks) with corresponding 95% confidence intervals using random-effects models and inverse variance method [26]. Odds ratios (OR) and corresponding 95% confidence intervals were produced for subgroup analyzes. Study heterogeneity was assessed through χ2-based Cochrane Q-statistic with p ≤0.10 and I2 ≥50% considered substantial heterogeneity [26]. Influence analysis to ensure robustness of pooled estimate was conducted for the primary outcome. We also assessed for potential publication bias graphically with a funnel plot of ln[OR] against its standard error (SE) where appropriate. In studies with zero-cell counts, a fixed value of ‘0.5’ was added to all cells of study results tables [26]. All statistical tests were two-sided. All calculations were performed using Comprehensive Meta-Analysis, version 2 (Biostat, Englewood, New Jersey, USA).
RESULTS
Study Search Results
In total, we identified 161 and 251 articles through PubMed and EMBASE, respectively, and 14,648 abstracts from AASLD, DDW, APASL, and EASL. Forty-one studies were identified and assessed for more detailed evaluation and inclusion for analysis [4,10–12,14–20,27–56]. Ten studies did not contain original or extractable data for analysis [13,48–56]. Eight studies were identified as redundant and excluded: duplicates found during our search or earlier abstracts of full articles that were later published [15,19,30–33,42,43]. Six studies included less than ten HCV-6 patients [34–36,44–46]. Four studies did not include patients treated with PEG IFN+RBV [37–40]. One study included patients with co-infections [41]. Ultimately, twelve (eight full articles and four abstracts) met inclusion criteria and were included in this meta-analysis (Figure 1)[4,10–12,14–21]. All twelve studies included HCV-6 patients treated for 48 weeks [4,10–12,14–21]. Characteristics of the twelve studies are described in Table 1.
FIG. 1.
Flow diagram of studies identified and selected for inclusion into meta-analysis.
Table 1.
Characteristics of studies included in the treatment analysis of Asian patients with HCV-6 and HCV-1
| Author | Population | HCV-6
|
HCV-1
|
||||
|---|---|---|---|---|---|---|---|
| males, n | age, years | n | males (%) | age, years | n | ||
| Tangkijvanich et al. [11], 2012 | Thailand | 23 (68) | 41.2±8.4 | 34 | 9 (56) | 46.4±12.5 | 16 |
| Thu Thuy et al. [20], 2012 | Vietnam | 65 (62) | 48.6±8.4 | 70 | n.a. | n.a. | n.a. |
| Lam et al. [21], 2010 | USA | 28 (47) | 52.8±8.0 | 33 | n.a. | n.a. | n.a. |
| Tsang et al. [12], 2010 | Hong Kong | 47 (67) | 50 | 70 | 44 (63) | 48 | 70 |
| Nguyen et al, [60], 2012 | USA | 34 (56) | 49.4±10.8 | 34 | 51 (73) | 50±9.7 | 70 |
| Fung et al. [4], 2008 | Hong Kong | 11 (52) | 49.5 (14–64) | 21 | 12 (57) | 52 (30–63) | 21 |
| Nguyen et al. [14], 2008 | USA | 45 (68) | 50±10 | 12a | n.a. | n.a. | n.a. |
| Seto et al. [18], 2013 | Hong Kong | 41 (68) | 49 | 60 | n.a. | n.a. | n.a. |
| Shao et al. [19], 2012 | China | unknown | unknown | 28 | n.a. | n.a. | n.a. |
| Cai et al. [17], 2011 | China | unknown | unknown | 84 | n.a. | n.a. | n.a. |
| Pham et al. [16], 2009 | Vietnam | unknown | unknown | 42 | n.a. | n.a. | n.a. |
| Rao et al. [10], 2013 | China | unknown | unknown | 14 | unknown | unknown | 365 |
Results are reported as means ± SD, numbers with percentages given in parentheses or medians of study populations with ranges given in parentheses and do not necessarily reflect only patients with HCV genotypes 6 and 1. n.a. = No data available.
Thirty-one patients were treated with standard IFN therapy and were not included in the analysis.
Study and Patient Characteristics
In the primary analysis, a total of 1046 (503 HCV-6; 543 HCV-1) patients from 12 studies were included (Table 1). Six studies were prospective, four were retrospective and two were unknown [4,10–12,14–21]. Of the twelve studies, two were RCTs [20,21], while the remaining studies were observational or nonrandomized trials [4,10–12,14–19]. Study origins included three from North America (USA) [14,15,27], three from Southeast Asia (one in Thailand and two from Vietnam) [11,16,20], and six from China [4,10,12,16–18]. The majority of studies occurred in a university or tertiary referral setting. All studies evaluated SVR overall and in Asian patients with HCV-6 [4,10–12,14–21]. Five studies directly compared Asian patients with HCV-6 versus HCV-1 [4,10–12,15]. All subjects were Asian and mostly male. Only patients treated with PEG IFN+RBV for 48 weeks were included in the analysis.
SVR in HCV-6 and HCV-1 patients treated for 48 weeks
Pooled event rate of SVR in 503 HCV-6 patients was 80.2% (95% CI 74.3–85.0%)(Q-statistic=20.87, p<0.035; I2=47.3%) (Figure 2). On funnel plot analysis for publication bias, the study by Rao H et al had the largest standard error, 1.5 (data not shown). This observation could be explained by the small sample of HCV-6 patients (n=14) who achieved 100% success rate of SVR in this study [10]. On influence analysis with one-study removal method, there was only a small change (~1%) in the pooled event rate when this study was omitted demonstrating the robustness of our estimate.
FIG. 2.
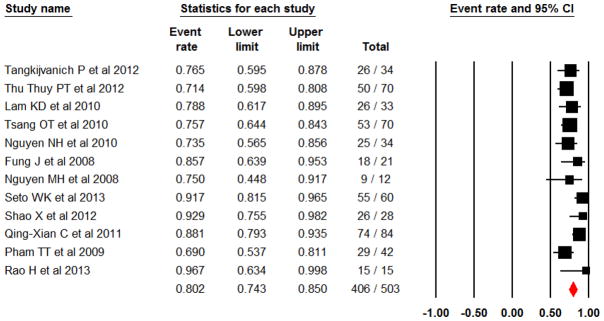
Pooled SVR rate in Asian patients with HCV genotype 6
In the nine studies with ITT analysis, the pooled event rate was 80.4% (95% CI 73.2–86.1%), which was similar to the SVR rate observed in the three studies with non-ITT 80.2% (95% CI 66.3–89.2%). Significant heterogeneity was not indicated in the sub-group analysis with ITT study (Q-statistic = 13.03, p=0.11; I2=38.624). SVR rate was lower in RCTs, 74.6% (CI 58.7–85.8%), than in observational and nonrandomized studies, 81.6% (95% CI 75.2–86.7%). We observed significant heterogeneity in the subgroup analysis with non-RCTs (Q-statistic=18.08, p=0.034; I2=50.208).
In the five studies with a total of 543 Asian HCV-1 patients, pooled SVR rate was 62.5% (95% CI 41.9–79.4%) (Q-statistic=52.41, p<0.001; I2=92.37). All studies were observational in nature and analyzed SVR rates according to ITT [4,10–12,15].
Direct comparison between SVR rates in Asian patients with HCV-6 and HCV-1
In the five studies with direct comparison between Asian patients with HCV-6 and HCV-1, there were a total of 174 HCV-6 and 543 HCV-1 patients. Pooled SVR rates were 77.1% (95% CI 70.0–82.9%) and 62.5% (95% CI 41.9–79.4%), respectively. There was a statistically significant difference between SVR rates in HCV-6 versus HCV-1 patients, OR 2.73 (95% CI 1.69–4.41, p<0.001). These studies were all observational or nonrandomized in nature and analyzed SVR rates according to ITT [4,10–12,15]. No significant heterogeneity was indicated (Q-statistics=1.56, p=0.82; I2=0%). Since I2 was 0%, effect sizes and corresponding CIs were the same between both random- and fixed-effects models.
Rates of SVR in HCV-6 and HCV-1 patients with EVR
In the four studies with EVR and SVR data in HCV-6 patients, there were a total of 207 HCV-6 patients [11,12,20,21]. Pooled SVR rate overall in these studies was 74.8% (95% CI 68.4–80.2%), while pooled EVR rate was 88% (95% CI 82.6–91.9%). In patients with EVR, 81.5% (95% CI 75.2–86.5%) achieved SVR. Of the 21 patients who did not have EVR, 23.8% (95% CI 7.0–56.5%) subsequently achieved SVR. In the three studies with direct comparison between HCV-6 patients with and without EVR, patients with EVR were significantly more likely to have SVR compared to those without EVR, OR 13.7 (95% CI 3.83–49.2, p<0.001).
In the two studies that contained EVR data in HCV-1 patients, there were a total of 91 HCV-1 patients [4,12]. Pooled SVR rate in these studies was 56.0% (95% CI 45.7–65.9%), while pooled EVR rate was 74.7% (95% CI 64.8–82.6%). In patients with EVR, 66.1% (95% CI 54.2–76.4%) achieved SVR [4,12]. Of the 23 patients without EVR, 26.2% (95% CI 12.3–47.5%) subsequently achieved SVR. Patients with EVR had a significantly higher odds of achieving SVR compared to patients without EVR, OR 5.59 (95% CI 1.93–16.2, p=0.002).
DISCUSSION
In the current meta-analysis, we included a total of 12 studies with 503 HCV-6 patients and 543 HCV-1. Pooled SVR rate was 80.2% (95% CI 74.3–85.0%) in HCV-6 patients and 62.5% (95% CI 41.9–79.4%) in HCV-1 patients. HCV-6 patients were significantly more likely to achieve SVR compared to HCV-1 patients, OR 2.73 (95% CI 1.69–4.41, p<0.001). Our result represents the first large and comprehensive study to date to evaluate SVR rates between these patients.
In patients with HCV-1, our pooled SVR rate (~60%) is higher than findings in non-Asian HCV-1 patients from large registration trials (~40–50%) and comparable to studies that specifically evaluated treatment data in Asian HCV-1 patients [3,5–9]. The high rates of SVR in our Asian patients with HCV-1 could be explained by multiple factors: specific host factors, genetic markers and more-treatment favorable polymorphism (CC genotype) near the gene IL-28 on chromosome 19 that influences SVR rates in patients treated with interferon-based therapies [57,58]. The CC genotype is known to be more common in areas where most of these Asian patients with HCV-6 and HCV-1 reside [17,18,57,58].
In our study, HCV-1 and HCV-6 patients with EVR were more likely to achieve SVR compared to those without EVR, which is consistent with the established literature on the positive predictive value of EVR [59]. However, approximately one-fourth of patients without EVR also subsequently achieved SVR, regardless of genotypes. While previous studies have recommended that the absence of EVR is a good stopping rule in patients with HCV-1, data from this recommendation was derived from studies with largely non-Asian patients and/or small numbers of patients without EVR [2,9,59]. Two large RCTs in treatment-naïve Asian patients with HCV-1 by Liu CH et al (n = 308) and Yu ML et al (n = 200) also found that 0% of patients without EVR achieved SVR and also suggested that treatment can be stopped in those without EVR, their recommendations were based on data of a very small number of patients without EVR (n=4 and n=7, respectively)[9,60]. On the other hand, based on data from 23 HCV-1 patients without EVR from this meta-analysis, we cannot recommend stopping treatment in those without the presence of EVR, since a quarter of these patients eventually achieved SVR.
Additionally, the economic burden of not achieving SVR and/or retreatment is much higher in patients that do not achieve SVR versus those that achieve SVR [61]. In a study by Backx et al, the authors demonstrated that failure to achieve SVR was associated with a 13-fold increase in healthcare-related costs, which was related to a higher likelihood of a patient transitioning to a more severe disease state that required more healthcare, while the costs were 56-fold higher for retreated patients [61]. Given that PEG-IFN+RBV is associated with significant side effects and requires a full year of treatment, the risk, benefits, and cost-savings of treatment should be discussed with the patient and the decision to continue treatment if a patient does not achieve EVR should be individualized.
While our data did not allow us to examine response-guided therapy for shortened treatment duration, data from two RCTs by Liu CH et al and Yu ML et al, suggest that HCV-1 patients with RVR may be treated for 24 weeks with PEG-IFN+RBV [9,60]. In contrast, for patients who continue to experience detectable HCV RNA after 24 weeks with PEG-IFN+RBV, studies have suggested that these patients may also stop treatment [2]. In Asian patients with HCV-6, an ongoing RCT with PEG-IFN+RBV in patients with RVR have produced results that suggest there is no significant differences between 24 versus 48 weeks of treatment [62]. Until published data becomes available from this trial, patients with HCV-6 should be treated for 48 weeks with PEG-IFN+RBV.
One of the limitations of our meta-analysis was the small number of studies available, which affected our ability to detect significant publication bias and perform additional sub-group analyses. To account for the limited data available, we sought to be as inclusive as possible and included studies of relatively different characteristics. We also reported results from random-effects models in an attempt to provide a more conservative estimate. Another limitation is the potential selection bias of patients, as most of our data were derived from observational studies. However, our findings are more likely to be generalizable to patients in routine clinical settings, since observational studies have broader inclusion criteria for study patients and more likely representative of the population at large.
In summary, Asian patients with HCV-6 can expect a higher SVR rate (~80%) than Asian patients with HCV-1 when treated for 48 weeks with PEG-IFN+RBV (~63%). Lack of EVR may not be a good stopping rule for Asian patients with HCV-6 or HCV-1. Compared to the high cost of newer therapies, PEG IFN+RBV may remain an acceptable option for Asian patients residing in resource-limited regions with HCV-6 and perhaps also Asian with HCV-1, especially given the higher SVR rates with both genotypes with PEG IFN+RBV in this ethnic group.
FIG. 3.
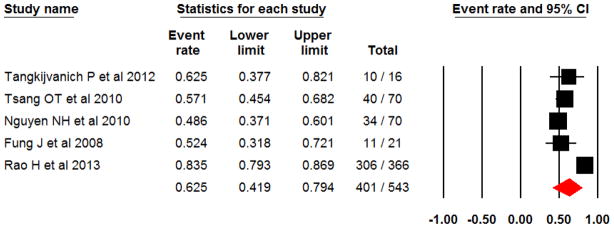
Pooled SVR rate in Asian patients with HCV genotype 1
FIG. 4.
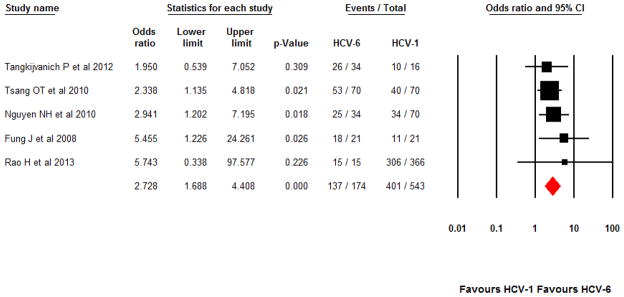
Pooled SVR rate between Asian patients with HCV-6 versus HCV-1 treated for 48 weeks
Acknowledgments
Financial Support
This study was funded in part by the NIH National center for Research Resources, TL1 training grants, 1TL1RR03197, to Nghia H. Nguyen and Shelley A. McCormack.
List of Abbreviations
- HCV
Hepatitis C Virus
- SVR
Sustained Virological Response
- PEG IFN+RBV
Pegylated Interferon and Ribavirin
- AASLD
American Association for the Study of Liver Diseases
- DDW
Digestive Disease Week
- APASL
Asian Pacific Study of the Liver
- EASL
European Association for the Study of the Liver
- RCT
Randomized Controlled Trial
- ITT
Intention-to-treat
- EVR
Early Virological Response
- OR
Odds Ratio
Footnotes
Conflict of Interest
Nghia H. Nguyen, Shelley A. McCormack, Philip Vutien, Brittany E. Yee, Pardha Devaki, David Jencks: None declared.
Mindie H. Nguyen has served as a consultant and an advisory board member for Gilead Sciences Inc, Bristol-Myers Squibb, Novartis, and Bayer.
Author’s contributions
Guarantor of article: Dr. Mindie Nguyen
Nghia Nguyen: study design, acquisition of data, data analysis, statistical analysis, and interpretation and drafting of the manuscript, obtained funding
Shelley McCormack: acquisition of data, data interpretation, and critical review of the manuscript, obtained funding
Philip Vutien: data collection and critical review of the manuscript.
Brittany Yee: data analysis and critical review of the manuscript.
Pardha Devaki: study design and critical review of the manuscript.
David Jencks: study design and critical review of the manuscript.
Mindie Nguyen: concept development, study design, data collection, data analysis and interpretation, and critical revision of the manuscript.
All authors identified above have critically reviewed the paper and approve the final version of this paper, including the authorship statement.
References
- 1.Wantuck JM, Ahmed A, Nguyen MH. Review article: The epidemiology and therapy of chronic hepatitis c genotypes 4, 5 and 6. Aliment Pharmacol Ther. 2014;39:137–147. doi: 10.1111/apt.12551. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis c: An update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang WL, Yu ML, Dai CY, Chang WY. Treatment of chronic hepatitis c in southern taiwan. Intervirology. 2006;49:99–106. doi: 10.1159/000087271. [DOI] [PubMed] [Google Scholar]
- 4.Fung J, Lai CL, Hung I, Young J, Cheng C, Wong D, Yuen MF. Chronic hepatitis c virus genotype 6 infection: Response to pegylated interferon and ribavirin. J Infect Dis. 2008;198:808–812. doi: 10.1086/591252. [DOI] [PubMed] [Google Scholar]
- 5.Hepburn MJ, Hepburn LM, Cantu NS, Lapeer MG, Lawitz EJ. Differences in treatment outcome for hepatitis c among ethnic groups. Am J Med. 2004;117:163–168. doi: 10.1016/j.amjmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 6.Kuboki M, Iino S, Okuno T, Omata M, Kiyosawa K, Kumada H, Hayashi N, Sakai T. Peginterferon alpha-2a (40 kd) plus ribavirin for the treatment of chronic hepatitis c in japanese patients. J Gastroenterol Hepatol. 2007;22:645–652. doi: 10.1111/j.1440-1746.2007.04834.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Choi MS, Paik SW, Kim JH, Kim DY, Lee JH, Koh KC, Yoo BC, Rhee JC, Song SM. peginterferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis c in korea. Korean J Hepatol. 2006;12:31–40. [PubMed] [Google Scholar]
- 8.Lee SD, Yu ML, Cheng PN, Lai MY, Chao YC, Hwang SJ, Chang WY, Chang TT, Hsieh TY, Liu CJ, Chen DS. Comparison of a 6-month course peginterferon alpha-2b plus ribavirin and interferon alpha-2b plus ribavirin in treating chinese patients with chronic hepatitis c in taiwan. J Viral Hepat. 2005;12:283–291. doi: 10.1111/j.1365-2893.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu CH, Liu CJ, Lin CL, Liang CC, Hsu SJ, Yang SS, Hsu CS, Tseng TC, Wang CC, Lai MY, Chen JH, Chen PJ, Chen DS, Kao JH. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive asian patients with hepatitis c virus genotype 1 infection: A multicenter, randomized controlled trial. Clin Infect Dis. 2008;47:1260–1269. doi: 10.1086/592579. [DOI] [PubMed] [Google Scholar]
- 10.Rao H, Wei L, Yang R, Chen XY, Jia S, Gao ZL, Xie Q, Dou X, Xu X, Gong G, Chen G, Li J, Zhang D, Niu J, Chen H, Feng Y, Hou J, You H, Wu Y, Zhao P. IL28B genotype and IFNL4 ss469415590 ΔG variant are both associated with response to pegylated ifn-α and ribavirin therapy in chinese patients with chronic hepatitis C. Hepatology. 2013;58:1123A. [Google Scholar]
- 11.Tangkijvanich P, Komolmit P, Mahachai V, Poovorawan K, Akkarathamrongsin S, Poovorawan Y. Response-guided therapy for patients with hepatitis c virus genotype 6 infection: A pilot study. J Viral Hepat. 2012;19:423–430. doi: 10.1111/j.1365-2893.2011.01566.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsang OT, Zee JS, Chan JM, Li RS, Kan YM, Li FT, Lo FH, Chow DA, Cheung KW, Chan KH, Yeung YW, Ng FH, Li MK, Kwan WK, Lai TS. Chronic hepatitis c genotype 6 responds better to pegylated interferon and ribavirin combination therapy than genotype 1. J Gastroenterol Hepatol. 2010;25:766–771. doi: 10.1111/j.1440-1746.2009.06163.x. [DOI] [PubMed] [Google Scholar]
- 13.Mauss S, Berger F, Vogel M, Pfeiffer-Vornkahl H, Alshuth U, Rockstroh JK, Niederau C, DH Treatment results of chronic hepatitis c genotype 5 and 6 infections in germany. Z Gastroenterol. 2012;50:441–444. doi: 10.1055/s-0031-1282072. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen MH, Trinh HN, Garcia RT, Nguyen G, Lam KD, Keeffe EB. Higher rate of sustained virologic response in chronic hepatitis c genotype 6 treated with 48 weeks versus 24 weeks of peginterferon plus ribavirin. Am J Gastroenterol. 2008;103:1131–1135. doi: 10.1111/j.1572-0241.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen NH, Vutien P, Trinh HN, Garcia RT, Wan K, Nguyen HA, Nguyen KK, Levitt BS, MHN Treatment response and tolerability to pegylated interferon (PEG INF) and ribavirin (RBV) in treatment-naive asian american patients with chronic hepatitis c and genotype 1, 2/3 and 6. Gastroenterology. 2009;136:A791. [Google Scholar]
- 16.Pham TT, Ho DT. Pegylated interferon alfa-2a plus ribavirin in chronic hepatitis c patients with genotype 6. Gastroenterology. 2009;136:A840. [Google Scholar]
- 17.Qing-Xian C, Zhixin Z, XiaoHong Z, Gao ZL, Lin C, Chunxia H. The high IL-28B CC genotype contribute to the good response of chronic hepatitis C genotype 6 in China. Hepatology. 2011;54:829A. [Google Scholar]
- 18.Seto WK, Tsang OT, Liu K, Chan JM, Wong DK, Fung J, Lai CL, Yuen MF. Role of il28b and inosine triphosphatase polymorphisms in the treatment of chronic hepatitis c virus genotype 6 infection. J Viral Hepat. 2013;20:470–477. doi: 10.1111/jvh.12047. [DOI] [PubMed] [Google Scholar]
- 19.Shao X, Zhao Z, Cai Q, Zhang X, Gao Z. The dynamic analysis of the th1/th2 ratio during the interferon alpha/ribavirin combination therapy for hcv genotype 6 infected patients. Journal of Hepatology. 2012;56:S66. [Google Scholar]
- 20.Thu Thuy PT, Bunchorntavakul C, Tan Dat H, Rajender Reddy K. A randomized trial of 48 versus 24 weeks of combination pegylated interferon and ribavirin therapy in genotype 6 chronic hepatitis c. J Hepatol. 2012;56:1012–1018. doi: 10.1016/j.jhep.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Lam KD, Trinh HN, Do ST, Nguyen TT, Garcia RT, Nguyen T, Phan QQ, Nguyen HA, Nguyen KK, Nguyen LH, Nguyen MH. Randomized controlled trial of pegylated interferon-alfa 2a and ribavirin in treatment-naive chronic hepatitis c genotype 6. Hepatology. 2010;52:1573–1580. doi: 10.1002/hep.23889. [DOI] [PubMed] [Google Scholar]
- 22.Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis c virus infection. JAMA Intern Med. 2014;174:1170. doi: 10.1001/jamainternmed.2014.2151. [DOI] [PubMed] [Google Scholar]
- 23.Sovaldi® (sofosbuvir) [package insert] Gilead Sciences; Foster City, CA, USA: 2013. [Google Scholar]
- 24.Médecins Sans Frontières. Global response to hepatitis C hangs on access to new oral drugs. Médecins Sans Frontières; 2014. [Accessed on May 4, 2014]. Press release, Médecins Sans Frontières Website: http://www.msf.org.za/msf-publications/global-response-to-hepatitis-c-hangs-on-access-to-new-oral-drugs. [Google Scholar]
- 25.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis c infection. The New England journal of medicine. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S, editors. Cochrane Handbook For Systematic Reviews of Interventions Version 5.1.0 [updated march 2011] The Cochrane Collaboration, The Cochrane Collaboration; 2011. [Google Scholar]
- 27.Lam KDTH, Do ST, Nguyen TT, Garcia RT, Nguyen T, Phan QQ, Nguyen HA, Nguyen KK, Nguyen LH, Nguyen MH. Randomized controlled trial of pegylated interferon-alfa 2a and ribavirin in treatment-naive chronic hepatitis c genotype 6. Hepatology. 2010;52:1573–1580. doi: 10.1002/hep.23889. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen NH, VuTien P, Garcia RT, Trinh H, Nguyen H, Nguyen K, Levitt B, Nguyen MH. Response to pegylated interferon and ribavirin in asian american patients with chronic hepatitis c genotypes 1 vs 2/3 vs 6. J Viral Hepat. 2010;17:691–697. doi: 10.1111/j.1365-2893.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 29.Fung J, Lai C, Hung I, Young J, Cheng C, Wong D, Yuen M. Chronic hepatitis c virus genotype 6 infection: Response to pegylated interferon and ribavirin. J Infect Dis. 2008;198:808–812. doi: 10.1086/591252. [DOI] [PubMed] [Google Scholar]
- 30.Yuen MF, Lai CL. Response to combined interferon and ribavirin is better in patients infected with hepatitis c virus genotype 6 than genotype 1 in hong kong. Intervirology. 2006;49:96–98. doi: 10.1159/000087270. [DOI] [PubMed] [Google Scholar]
- 31.Pham TT, Ho DT. An optimal duration of treatment for chronic hepatitis c genotype 6 patients. Hepatology. 2011;54:810A. [Google Scholar]
- 32.Lam KD, Trinh HN, Do ST, Nguyen TT, Garcia RT, Nguyen T, Phan QQ, Nguyen HA, Nguyen KK, Nguyen LH, Nguyen MH. Randomized controlled trial of pegylated interferon-alpha 2a and ribavirin in patients with treatment-naive chronic hepatitis c genotype 6. Gastroenterology. 2010;138:S783. doi: 10.1002/hep.23889. [DOI] [PubMed] [Google Scholar]
- 33.Thi TTP, Ho TD. Pegylated interferon alfa-2a plus ribavirin in chronic hepatitis c patient with genotype 6. Hepatology International. 2009;3:49. [Google Scholar]
- 34.Seong MH, Kil H, Kim JY, Lee SS, Jang ES, Kim JW, Jeong SH, Kim YS, Bae SH, Lee YJ, Lee HC, Yun H, Kang BH, Kim K. Clinical and epidemiological characteristics of korean patients with hepatitis c virus genotype 6. Clin Mol Hepatol. 2013;19:45–50. doi: 10.3350/cmh.2013.19.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thanak P, Kitiyakara T, Intaraprasong P, Sobhonslidsuk A, Achalanan N, Kamalaporn P, Kositchaiwat C. Real-world outcomes of hepatitis c treatment in thai patients. Journal of Gastroenterology and Hepatology. 2012;27:247. [Google Scholar]
- 36.Weinstein T, Levine J. Early virologic response in children with hepatitis c genotype 6e. Journal of Pediatric Gastroenterology and Nutrition. 2009;49:1. [Google Scholar]
- 37.Lenz O, Vijgen L, Berke JM, Cummings MD, Fevery B, Peeters M, Smedt GD, Moreno C, Picchio G. Virologic response and characterisation of hcv genotype 2–6 in patients receiving tmc435 monotherapy (study tmc435-c202) Journal of Hepatology. 2013;58:445–451. doi: 10.1016/j.jhep.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Hui CK, Yuen MF, Sablon E, Chan AO, Wong BC, Lai CL. Interferon and ribavirin therapy for chronic hepatitis c virus genotype 6: A comparison with genotype 1. J Infect Dis. 2003;187:1071–1074. doi: 10.1086/368217. [DOI] [PubMed] [Google Scholar]
- 39.Bunchorntavakul C, Siripun A, Chavalitdhamrong D. Fluvastatin monotherapy for the treatment of patients with chronic hepatitis c: A randomized double-blinded placebo-controlled study. American Journal of Gastroenterology. 2011;106:S102–S103. [Google Scholar]
- 40.Chu T, Kulkarni R, Gane EJ, Roberts SK, Stedman C, Angus P, Ritchie B, Lu XY, Ipe D, Lopatin U, Germer S, Iglesias V, Elston R, Berry MM, Smith PF, Shulman NS. The effect of host il28b genotype on early viral kinetics during interferon-free treatment in patients with chronic hepatitis c (chc) Journal of Hepatology. 2011;54:S521. doi: 10.1053/j.gastro.2011.12.057. [DOI] [PubMed] [Google Scholar]
- 41.Cheng JT, Hsien C, Sun HE, Tong MJ. The emerging importance of chronic hepatitis c infection in asian americans. American Journal of Gastroenterology. 2006;101:2737–2743. doi: 10.1111/j.1572-0241.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 42.Pham TTT, Ho DT. Impact of single nucleotide polymorphisms of il28b on svr in treatment chronic hepatitis c genotype 6 in vietnamese. Hepatology International. 2013;7:S366. [Google Scholar]
- 43.Chen XY, Zhang Y, Rao H, Wei L, Shang J, Gao Z, Xie Q, Dou X, Xu X, Gong G, Chen G, Li J, Zhang D, Niu J, Chen H, Feng Y, Hou J, You H, Wu Y, Zhao P. A prospective, randomized, open-label, multicenter study of response-guided therapy (RGT) for hcv treatment-naïve chinese patients with chronic hepatitis c. Hepatology International. 2013;7:S724. [Google Scholar]
- 44.Han B, Mo H, Wong KA. In vitro analyses of hcv ns5b s282t mutants in multiple hcv genotypes show low levels of reduced susceptibility to sofosbuvir (gs-7977), no cross resistance to other classes of direct-acting antivirals, and hypersensitivity to ribavirin. Hepatology. 2012;56:711A. [Google Scholar]
- 45.Lim JH. Changes in serum lipid profile before and after interferon therapy in patients with chronic hepatitis c. Hepatol Int. 2012;6:170. [Google Scholar]
- 46.Loh PY. Outcome of hepatitis c treatment and effect of treatment dose adjustment in treatment naïve asian populations. Hepatol Int. 2013;7:S411. [Google Scholar]
- 47.Mauss S, Berger F, Vogel M, Pfeiffer-Vornkahl H, Alshuth U, Rockstroh J, Niederau C, Huppe D. Treatment results of chronic hepatitis c genotype 5 and 6 infections in germany. Z Gastroenterol. 2012;50:441–444. doi: 10.1055/s-0031-1282072. [DOI] [PubMed] [Google Scholar]
- 48.Chao DT, Abe K, Nguyen MH. Systematic review: Epidemiology of hepatitis c genotype 6 and its management. Aliment Pharmacol Ther. 2011;34:286–296. doi: 10.1111/j.1365-2036.2011.04714.x. [DOI] [PubMed] [Google Scholar]
- 49.Seto WK, Tanaka Y, Liu K, Lai CL, Yuen MF. The effects of IL-28B and ITPA polymorphisms on treatment of hepatitis c virus genotype 6. American Journal of Gastroenterology. 2011;106:1007. doi: 10.1038/ajg.2011.40. [DOI] [PubMed] [Google Scholar]
- 50.Antaki N, Marcellin P. What is the safe duration of therapy for patients infected with hcv genotype 6? Nature Clinical Practice Gastroenterology and Hepatology. 2009;6:78–79. doi: 10.1038/ncpgasthep1335. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis c virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005;3:S97–S101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 52.Chow WC, Ng HS. Hepatitis c, e and g virus--three new viruses identified by molecular biology technique in the last decade. Annals of the Academy of Medicine, Singapore. 1997;26:682–686. [PubMed] [Google Scholar]
- 53.Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis c infection. Aliment Pharmacol Ther. 2013;37:921–936. doi: 10.1111/apt.12300. [DOI] [PubMed] [Google Scholar]
- 54.Al Naamani K, Al Sinani S, Deschenes M. Epidemiology and treatment of hepatitis c genotypes 5 and 6. Canadian Journal of Gastroenterology. 2013;27:e8–12. doi: 10.1155/2013/624986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai W. Viral hepatitis c-asia-pacific perspectives and guidelines. Journal of Gastroenterology and Hepatology. 2012;27:27. [Google Scholar]
- 56.Chutaputti A. Managing hcv genotype 6 patients. Hepatology International. 2011;5:47. [Google Scholar]
- 57.McHutchison JG. The role of genetic markers in hepatitis c virus therapy: A major step for individualized care. Liver Int. 2011;31 (Suppl 1):29–35. doi: 10.1111/j.1478-3231.2010.02389.x. [DOI] [PubMed] [Google Scholar]
- 58.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in il28b and spontaneous clearance of hepatitis c virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis c virus infection. The New England journal of medicine. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 60.Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, Lee LP, Hsieh MY, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL. Rapid virological response and treatment duration for chronic hepatitis c genotype 1 patients: A randomized trial. Hepatology. 2008;47:1884–1893. doi: 10.1002/hep.22319. [DOI] [PubMed] [Google Scholar]
- 61.Backx M, Lewszuk A, White JR, Cole J, Sreedharan A, van Sanden S, Diels J, Lawson A, Neal KR, Wiselka MJ, Ito T, Irving WL. The cost of treatment failure: Resource use and costs incurred by hepatitis c virus genotype 1-infected patients who do or do not achieve sustained virological response to therapy. J Viral Hepat. 2014;21:208–215. doi: 10.1111/jvh.12132. [DOI] [PubMed] [Google Scholar]
- 62.Qing-Xian C, Zhixin Z, Lin C, Min X, Min W, Mingshou H. Shortened treatment duration in treatment-naive genotype 6 chronic hepatitis c patients with rapid virological response: A randomized controlled trial. Hepatology. 2013;58:1098A. [Google Scholar]