Abstract
The prevalence of diabetes mellitus and obesity continues to increase globally. Diabetic vascular complications are the main chronic diabetic complications and associated with mortality and disability. Angiogenesis is a key pathological characteristic of diabetic microvascular complications. However, there are two tissue-specific paradoxical changes in the angiogenesis in diabetic microvascular complications: an excessive uncontrolled formation of premature blood vessels in some tissues, such as the retina, and a deficiency in the formation of small blood vessels in peripheral tissues, such as the skin. This review will discuss the paradoxical phenomena of angiogenesis and its underlying mechanism in obesity, diabetes and diabetic complications.
1. Introduction
Diabetes mellitus (DM) is a metabolic disease and is characterized by high serum glucose levels with the symptoms of polyuria, polydipsia and polyphagia. Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are the main types of diabetes mellitus. T1DM is caused by the loss of pancreatic beta cells, which leads to insulin deficiency. T2DM is characterized by insulin resistance or accompanied by relative insufficient insulin [1]. With the changes of lifestyle and increased obesity, DM case numbers worldwide rose from 153 (127–182) million in 1980, to 347 (314–382) million in 2008, and 90% of the cases were T2DM [2]. It is conservatively predicted that, as the result of overweight, obesity and increasing life span, approximately 429–552 million people globally will have diabetes by 2030 [3, 4]. When the body mass index exceeds 30 kg/m2, people are considered having obesity [5]. The epidemiology study showed that people with obesity have significantly increased risk of diabetes [6]. Therefore, T2DM has been termed as the complication of obesity [7].
DM can result in disorders of different organs, which are defined as diabetic complications. Diabetic ketoacidosis and coma are lethal acute complications [1]. Diabetic vascular complications due to chronical exposure to hyperglycemia are the main chronic diabetic complications and associated with mortality and disability. Macrovascular abnormalities and microvascular abnormalities are two main groups of diabetic vascular complications. Macrovascular abnormalities, including myocardial infarction and cerebrovascular disease are associated with the damage in arteries. Microvascular abnormalities affects small blood vessels, and include diabetic retinopathy (DR), nephropathy and neuropathy [8].
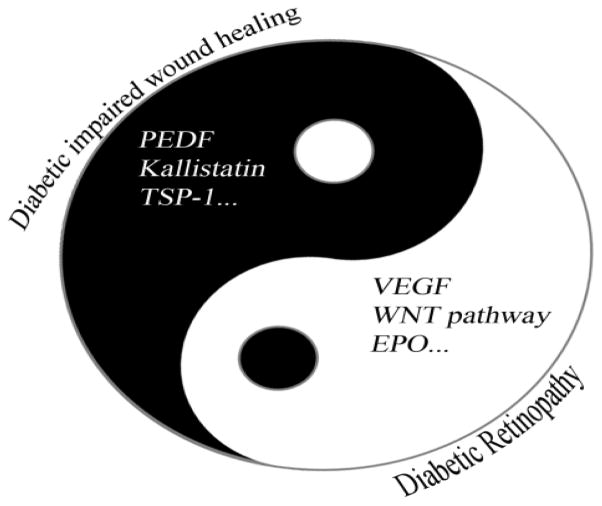
Formation of new blood vessels includes angiogenesis [9], which refers to the formation of new capillaries from proliferation of existing endothelial cells, and vasculogenesis [10], which refers to de novo blood vessel formation from endothelial progenitor cells. Physiological angiogenesis is only triggered in reproduction of endometrium, wound healing and the placenta morphogenesis during pregnancy in adult. Persistent, uncontrolled angiogenesis is a key pathological characteristic of cancer and microvascular complications of diabetes. It is known that angiogenesis is regulated by a counter balance between endogenous angiogenic stimulators and angiogenic inhibitors [9] (Fig. 1). When the angiogenic stimulators predominate, such as vascular endothelial growth factors (VEGFs) [11, 12] and erythropoietin (EPO) [13, 14], endothelial cells proliferate and migrate, leading to angiogenesis. When angiogenic inhibitors are dominant, such as pigment epithelium-derived factor (PEDF) [15, 16], kallistatin [17] and thrombospondin-1 (TSP-1) [18], angiogenesis is suppressed and eventually arrested. The angiogenic balance is a regulator of angiogenesis by directly targeting vascular endothelial cells and is modulated by multiple factors, such as hyperglycemia-induced oxidative stress, cytokines and inflammatory factors. There are detailed reviews regarding the mechanisms for angiogenesis in diabetic complications [19, 20] and the interaction between adipose tissues and angiogenesis [21, 22]. However, in diabetes, there are two tissue-specific paradoxical changes in small blood vessels in diabetes; one is excessive, uncontrolled formation of premature blood vessels in some tissues such as the retina, which is an important pathological feature in proliferative diabetic retinopathy (PDR). The other is the deficiency in the formation of small blood vessels in peripheral tissues such as the skin, which contributes to the impaired wound healing in the skin. Here we review the paradoxical phenomena of angiogenesis and its underlying mechanism in diabetes, diabetic complications and obesity.
Fig. 1.
The disturbed angiogenic balance in impaired wound healing in diabetes and diabetic retinopathy. Diabetic retinopathy is the result of over-production of angiogenic stimulators and reduced angiogenic inhibitors in the retina; impaired wound healing in diabetes is the consequence of elevated systemic levels of angiogenic inhibitor and reduced angiogenic stimulator levels. VEGF vascular endothelial growth factors; EPO erythropoietin; WNT the wingless-type MMTV integration site; PEDF pigment epitheliumderived factor; TSP-1 thrombospondin-1.
2. Paradoxical angiogenesis in patients with diabetes
DR is a most common microvascular complication of diabetes. DR has two stages, dependent on the absence or presence of retinal neovascularization. One is non-proliferative diabetic retinopathy (NPDR) which lacks abnormal retinal angiogenesis. Microaneurysms and intraretinal hemorrhages are the major pathological changes in patients with NPDR. Based on the severity of microaneurysms and intraretinal hemorrhages, NPDR is classified into mild NPDR, moderate NPDR and severe NPDR [23]. Severe NPDR increases the risk of PDR from 5% in mild NPDR to 52% within 1 year, and to 60% of high risk PDR in 5 years [24]. PDR is characterized by pathological retinal angiogenesis accompanied by vitreous hemorrhage or subhyaloid hemorrhage. PDR is the major cause of retinal detachment and vision loss in patients with diabetes. Although laser photocoagulation has side-effects, it remains a standard major treatment of PDR [23]. However, according to the mechanism underlying retinal angiogenesis in PDR, anti-VEGF therapy obtained achieved encouraging effects recently and may become a major therapy of PDR [25–30].
Impaired wound healing is also referred to as chronic wound including slow healing of wound and non-healing wound. Diabetes is one of the major reasons of impaired wound healing [31, 32]. The prevalence of foot ulcers due to impaired wound healing is predicted to be 25% in diabetic patients [33, 34]. Twelve percent of patients with foot ulcer may eventually need amputation. However, the ulcer recurrence rate is 50% in the contralateral limb within 5 years and the 5-year survival rate is only 50% after the lower extremity amputation [34]. Hyperglycemia-induced reactive oxygen species generation is crucial for impaired wound healing [35, 36]. Re-epithelialization and angiogenesis are two essential steps in wound healing. Angiogenesis starts at the 3rd day after wounding [37–39]. Wound healing is delayed in the presence of high levels angiogenic inhibitors [40, 41], and promoted by local administration of VEGF [42, 43]. Multiple growth factors and cytokines, such as VEGF, Fibroblast growth factor (FGF-2) and Platelet-derived growth factor (PDGF), released by keratinocytes, fibroblasts, endothelial cells, macrophages and platelets are involved in wound healing and reduced in diabetic wound [44]. Nitric oxide (NO) is essential for wound healing, which can trigger the mobilization of bone marrow endothelial progenitor cells [44, 45] and mediating the induction of VEGF by growth factors or cytokines in keratinocytes and in wound repair [46]. However, the detailed mechanism of reduced growth factors and cytokines in diabetic wound remains unclear. Enhanced depositions of basement membrane-like materials and pericyte detachment are observed in the buttock skin of the patients with long standing juvenile diabetes [47], suggesting that abnormal structure of the skin and blood vessel may be associated with the impaired wound healing in DM.
3. The mechanism underlying abnormal angiogenesis in diabetes
VEGF is a well-studied key angiogenic stimulator, which promotes proliferation and migration of endothelial cells, and increases permeability of blood vessels via binding with the VEGF receptors, and then activating extracellular signal-regulated kinases 1 and 2 (ERK1/2), Src and phosphatidylinositoI-3-kinase/protein kinase B (Pl3K/Akt) pathways [48]. VEGF also promotes wound healing in diabetic animal models through mobilizing and recruiting bone marrow-derived cells [42]. Anti-VEGF therapies have shown impressive beneficial effects on PDR, which suggests a central role of VEGF in DR [23, 26–30]. Vitreous levels of VEGF are dramatically increased in patients with DR [49–61], which is generated and secreted mainly by retinal Müller cells [62–64]. Changes of the circulating VEGF levels in DM are controversial. Some groups reported that serum VEGF levels are significantly increased in both T1DM and T2DM patients, compared with the control group, and positively correlated with the severity of diabetic complications [49, 65]. The data showed that, in patients with severe diabetic complications, serum VEGF levels are higher than in diabetic patients without complications. Furthermore, serum VEGF levels are significantly decreased with an intense control of the blood glucose. However, it was reported that there was no significant change in serum VEGF levels between diabetic group and control group [66]. Future well-designed studies with large case numbers are required to confirm the changes of serum VEGF levels in DM. In diabetic mice, VEGF mRNA levels in non-wounded skin are elevated compared to non-diabetic mice, which are significantly decreased to an undetectable level at the 5th day after wounding following a transient increase. However, the 5th day after wounding is the peak of VEGF levels in wound healing in non-diabetic control animals [67]. Diminished protein levels of VEGF and other growth factors were also observed in the wound healing of streptozotocin-induced T1DM mice [68]. Topical VEGF administration promotes the wound healing in diabetic animals [42, 43], indicating that the decreased VEGF levels contribute to the impaired wound healing in diabetes. The mechanism responsible for the decreased VEGF expression in the skin diabetic models is not known.
PEDF is a secreted glycoprotein and a member of the serine proteinase inhibitor (serpin) superfamily, which was originally isolated from culture medium of retinal pigment epithelial cells [69]. PEDF has multiple functions including induction of neuronal differentiation [70], neurotrophy [71], anti-cancer [72], enhancing renewal of stem cells [73, 74], anti-inflammation [75, 76], and anti-angiogenesis [16]. PEDF is also identified to regulate lipid metabolism and insulin resistance through increasing serum levels of free fatty acid [77]. PEDF is a potent angiogenesis inhibitor which directly targets endothelial cells by inducing apoptosis of endothelial cells and regulating the angiogenic balance by down-regulating VEGF and blocking the binding of VEGF with the VEGF receptors. As an important component of the angiogenic balance, vitreous levels of PEDF in patients with PDR are significantly decreased compared with those of NPDR group or non-diabetic control group [61, 78, 79]. In an ischemia-induced retinal angiogenesis model, the retina develops more sever retinal angiogenesis in PEDF knockout mice, and ameliorated angiogenesis in PEDF transgenic mice over-expressing PEDF [80], indicating the potential therapeutic effect of PEDF in PDR. Interestingly, circulating levels of PEDF demonstrate opposite changes. Several groups including us have reported that serum PEDF levels are significantly increased in patients with T1DM [81, 82] and T2DM [83, 84]. The elevated PEDF levels are positively associated with body mass index, lipid levels and vascular dysfunction [83]. The elevated serum levels of PEDF are also found in patients with obesity [85] and metabolic syndrome [86] and in T2DM animal models [40, 87]. We have demonstrated that blocking PEDF activity by a neutralizing antibody or knockout of PEDF significantly improved the skin wound healing in T2DM animal models through promoting angiogenesis in the wound [40]. PEDF was shown to down-regulate VEGF expression through inhibiting the nuclear translocation of HIF-1α and mitogen activated protein kinase phosphorylation [88]. Therefore, the elevated serum PEDF levels in DM may contribute to the down-regulation of VEGF in the skin, leading to delayed wound healing. However, the mechanism responsible for the decreased PEDF levels in the retina and elevated PEDF levels in the circulation is presently unknown.
Kallistatin is another secreted member of the serpin superfamily and is expressed in most tissues in humans [89, 90]. It has displayed potent anti-angiogenic activities [17, 91–93]. Ischemia-induced retinal neovascularization is significantly ameliorated in mice over-expressing kallistatin compared with that in wild-type mice [91]. Over-expression of kallistatin significantly ameliorates retinal vascular leakage, leukostasis, and over-expression of VEGF in a T1DM model [91], which demonstrates anti-angiogenic and anti-inflammatory effects of kallistatin in DR. Our previous studies have reported that kallistatin levels are decreased in the retina of a T1DM model and in the vitreous from patients with PDR [94]. However, serum kallistatin levels were recently found to be increased in diabetic patients with complications [95]. There is no difference in the serum kallistatin levels between non-diabetic controls and diabetic patients without complications, indicating that elevated systemic levels of kallistatin are associated with diabetic complications [95]. To establish the role of elevated circulating kallistatin levels in peripheral angiogenesis deficiency, murine skin wound healing assay was used. As expected, kallistatin over-expression alone delays wound closure and reduces angiogenesis in the wound area, compared with wild-type mice. Consistently, the expression and secretion of VEGF are significantly inhibited in kallistatin transgenic mice [41]. Meanwhile, over-expression of kallistatin leads to changes in skin structure and histology, characterized by thinner panniculus adiposus layer, lower microvascular density and decreased density of hair follicles, similar to the skin changes in diabetic patients [41]. It is likely that the skin wound healing delay and skin histology changes in mice over-expressing kallistatin may be through inhibition of the Wnt signaling pathway [41].
Hyperglycemia-upregulated expression of VEGF is mediated by a number of signaling pathways or proteins. VEGF levels are regulated at three levels: the transcriptional level, translational level [96] and posttranslational level [97, 98]. Here, we mainly discuss the regulation of VEGF transcription. The 5′-flanking region of the VEGF gene contains a number of transcription factor-binding sites for specific protein-1/3 (Sp1/3), signal transducer and activator of transcription-3 (Stat3), hypoxia-inducible factor-1 (HIF-1), activator protein-1 (AP-l), activator protein-2 (AP-2), early growth response protein 1 (Egr-1), T-cell factor (TCF) and others [99–103] (Fig. 2). Although, only the mouse VEGF promoter contains a consensus NF-κB-binding site [104], NF-κB is essential for up-regulating the expression and secretion of VEGF in human cells especially in cytokines-induced VEGF generation, possibly by binding to consensus NF-κB-binding sites or regulating activities of AP-1 or p53 [105–109]. NF-κB is a crucial intracellular inflammatory regulator and plays key roles in both impaired wound healing and retinopathy in diabetes [110–112]. HIF-1 is a well-studied transcription factor in driving VEGF expression under hypoxia. The role of HIF-1 in angiogenesis regulation is well reviewed recently and will not be discussed here [48, 113]. Another signaling pathway regulating VEGF under hyperglycemia is the canonical Wnt pathway which contributes to the development of retinal neovascularization. Wnts are secreted glycoproteins rich of cysteine. Wnts regulate gene expression and participate in regulating metabolism, development, carcinogenesis and inflammation by binding to a receptor complex comprised of frizzled (Fz) receptors and low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) [114]. Wnt binding with Fz-LRP5/6 leads to the stabilization of β-catenin, a down-stream effector of the canonical Wnt pathway. β-catenin is translocated into the nucleus and regulates the transcription of target genes such as VEGF [115, 116]. The canonic Wnt pathway is aberrantly activated in DR as shown by increased protein levels in both total and nuclear levels of β-catenin and phosphorylated LRP5/6, compared with those in non-diabetic group [117, 118]. Inhibition of the Wnt pathway by a specific inhibitor or a neutralization antibody against LRP6 dramatically ameliorates retinal neovascularization in DR animal models, suggesting that the over-activation of the canonic Wnt pathway plays a crucial role in DR [118, 119]. The canonic Wnt pathway is essential for maintaining the morphogenesis of hair follicles, and regulating wound healing through inducing the differentiation of epithelial cells and promoting the skin stem cell migration [120–122]. Our recent study has shown that suppressed Wnt signaling in wounded skin is responsible, at least in part, for the deficient wound healing in a diabetes model. Several endogenous anti-angiogenic factors such as endostatin [123], PEDF [80] and kallistatin [91] have been shown to function as inhibitors of Wnt signaling. Over-expression of kallistatin alone is sufficient to inhibit Wnt signaling and wound healing. The wound healing delay can be reversed by topical administration of lithium chloride, a down-stream activator of Wnt signaling [41]. Similarly, PEDF deficiency results in more prominent activation of the canonic Wnt pathway in ischemic retina [80]. These observations suggest that Wnt signaling plays a key regulatory role in angiogenesis in diabetes. Modulation of Wnt signaling likely represents a unifying mechanism for the anti-angiogenic activities of some endogenous angiogenic inhibitors.
Fig. 2.
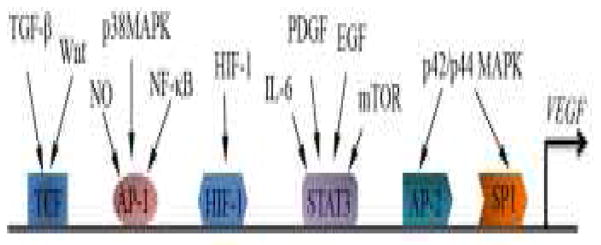
Signaling pathways in the regulation of VEGF transcription. Signaling pathways, related transcription factors of VEGF and the corresponding response elements in the VEGF promoter are summarized. Sp1 specific protein-1; Stat3 signal transducer and activator of transcription-3; HIF-1 hypoxia-inducible factor-1; AP-1 activator protein-1; AP-2 activator protein-2; NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells; TGF-β transforming growth factor beta; MAPK Mitogenactivated protein kinases; NO Nitric oxide; EGF Epidermal growth factor; PDGF Platelet-derived growth factor; IL-6 Interleukin 6; WNT the wingless-type MMTV integration site; TCF T-cell factor.
4. Angiogenesis in adipose tissue of obesity
Adipose tissue is composed of adipocytes, preadipocytes, vascular cells, fibroblasts and immune cells. In healthy adults, white adipose tissue (WAT) constitutes 20% (in man) or 25% (in woman) of body weight. Angiogenesis promotes adipogenesis through multiple mechanisms, such as transporting the nutrients, cytokines, and stem cells to adipose tissues and removing waste generated by the adipocytes (for the detailed mechanisms, please refer to another review [124]). Angiogenic inhibitors, such as angiostatin, endostatin, TNP-470 and thalidomide were identified to reduce body weight of ob/ob mice, a genetic model of obesity, without adverse effects, and specifically target capillary endothelial cells but not preadipocytes [125]. Meanwhile, TNP-470, a blocking antibody against VEGFR2, and MMP inhibitors significantly reduced body weight in the mice with diet-induced obesity or ob/ob mice [126–130]. The molecular mechanisms are unknown. These preclinical studies indicated that angiogenesis could be a therapeutic target of obesity. The safety of inhibition of angiogenesis in obesity therapy has been well discussed [131]. However, adipose tissue is classified into brown adipose tissue (BAT) and WAT. Different from WAT which functions as a storage of lipid and energy, BAT is essential for regulating temperature of important tissues or organs and non-shivering thermogenesis [132]. Higher capillary density is found in BAT, compared with WAT [124, 133]. Angiogenesis promotes the energy expenditure in BAT, which results in the fat loss [131]. The function or activity of BAT is reduced in obesity [134–136]. Scientists expected to treat obesity through activating BAT, transplanting BAT or stimulating stem cells to differentiate into brown adipocytes [137]. Although BAT-related studies in obesity are in the early stage, the effect of anti-angiogenic agents on BAT activity cannot be ignored in the treatment of obesity. Further studies are needed to solve the paradox between BAT and WAT in the anti-angiogenic therapy of obesity.
Many studies have identified that WAT is not only a tissue in energy storage, bu also an important endocrine organ to produce and secret hormones (such as leptin and estrogen), cytokines (such as TNFα) and growth factors (such as VEGF and PDGF) [138]. The inflammation in WAT characterized by macrophage filtration contributes to the chronic inflammatory state of obesity [139]. Further study has identified that the hypoxia of adipose tissue (ATH) results in adipose inflammation [140]. The mechanisms of ATH are related to the reduction of capillary density and blood flow. Reduced capillary density could be a reason for the decreased blood flow in obese adipose tissues. Moreover, there are several mechanisms underlying the reduced blood flow of adipose tissues (please refer to reviews [141, 142]). Here we mainly discuss the reduced capillary density in WAT in obesity.
Reduced capillary density was reported in adipose tissues of obese humans and obese animal models, compared with lean subjects or animals [143–149]. In obese subjects, capillary density is decreased in both visceral and subcutaneous adipose tissues, and there is no difference between those depots [143, 144]. Accompanied with the reduced capillary density, numbers of larger vessels (with α-smooth muscle actin expression) and levels of collagen V expression are significantly increased in adipose tissues, indicating that extracellular matrix might be involved in the angiogenesis defect of adipose tissues [149]. A decreased mRNA level of VEGF was reported in subcutaneous adipose tissues in overweight/obese subjects versus lean subjects, which is strongly correlated with capillary density [146]. Adipose-specific ablation of VEGF contributed to lower adipose vascular density, increased adipose hypoxia and inflammation. Mice with adipose-specific overexpression of VEGF showed increased adipose vascular intensity, ameliorated adipose hypoxia and inflammation, suggesting that reduced angiogenic stimulators contributes to the deficiency of angiogenesis in obesity and adipose inflammation [150]. However, most results of the in vitro studies are not consistent with the in vivo study. VEGF is upregulated in cultured adipocytes or cultured adipose tissues from obese subjects in 1% oxygen [143, 151–153]. One possibility is that the hypoxia in adipose tissues corresponds to 3.8% oxygen, which is not as low as 1% oxygen, and might not be enough to drive the responses to hypoxia [146]. Adipose tissues also generate endogenous angiogenic inhibitors [77, 154]. PEDF, angiostatin, endostatin, and TSP-1 are increased in overweight/obese subjects or animal models of obesity [40, 84–87, 155]. There is no difference in adipose tissue development between TSP-1 deficient mice and control wild-type mice after high-calorie diet feed [156]. It is presently unclear whether TSP-1 contributes to the reduced capillary density and the hypoxia of adipose tissue in obesity. As a potent angiogenic inhibitor with multiple functions, elevated PEDF contributes to the obesity and insulin resistance. Adipocyte-specific PEDF transgenic mice (PEDF-aP2 mice) showed an increased adipocyte lipolysis compared with wild-type mice, confirming the effect of PEDF in regulating lipid metabolism. However, WAT-derived PEDF overexpression has no effect on the adipose vascularization, hypoxia and adipose inflammation in normal condition or after high-calorie diet [157]. There is no difference in glucose and insulin tolerance between PEDF-aP2 mice and control mice [157]. Overexpression of PEDF in adipocytes cannot increase serum levels of PEDF [157], indicating that adipocytes-derived PEDF is unlikely the source of the increased serum PEDF in obesity. This study raised questions to be addressed: 1) WHERE: which tissue is the source of the increased serum PEDF in obesity? 2) HOW: what is the molecular mechanism for the increased levels of PEDF in obese adipose tissues? 3) WHEN: when is the elevated serum PEDF generated and secreted, and do the PEDF increases in adipose tissues contribute to the angiogenic defect in obesity or the development of obesity complications, such as impaired wound healing in T2DM? The role of angiogenic inhibitors in angiogenic defect of obesity warrants further investigation.
5. Conclusion
Diabetic patients with PDR have a disturbed balance between angiogenic stimulators and angiogenic inhibitors. These patients display excess and uncontrolled angiogenesis in the retina correlating with the increased pro-angiogenic factors:anti-angiogenic factors ratios in the retina and vitreous. However, these patients have deficient angiogenesis in the peripheral tissues including the skin, which is ascribed, at least in part, to the elevated levels of angiogenic inhibitors in the circulation and in the skin. The mechanism responsible for the differential changes of angiogenic inhibitor levels remains to be studied. Understanding of this regulation may contribute to the development of new treatment strategies for diabetic complications. Circulation levels of angiogenic inhibitors may also become biomarkers to predict DR or its progression. Appropriate angiogenesis is essential for homeostasis of adipose tissues. Angiogenic defect contributes to hypoxia of adipose tissues, which results in adipose inflammation in obesity. Angiogenic stimulators could promote angiogenesis and ameliorate tissue hypoxia and inflammation in obese adipose tissue. Angiogenesis is required for the growth or expansion of WAT in obesity, and thus, might be a therapeutic target for obesity.
Acknowledgments
This study is supported by grants from National Institutes of Health EY012231, EY018659, EY019309, and GM104934, a grant from IRRF, a grant from Oklahoma Center for the Advancement of Science & Technology (OCAST) HR13-076 and grants from Juvenile Diabetic Research Foundation (JDRF).
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
References
- 1.Assoc AD. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 6.Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13:155–65. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 8.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–35. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 10.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–66. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 13.Ribatti D, Vacca A, Roccaro AM, et al. Erythropoietin as an angiogenic factor. European Journal of Clinical Investigation. 2003;33:891–6. doi: 10.1046/j.1365-2362.2003.01245.x. [DOI] [PubMed] [Google Scholar]
- 14.Arcasoy MO, Hardee ME, Jiang XH, et al. Role of erythropoietin as an angiogenic factor and target in cancer. Blood. 2006;108:127a–a. [Google Scholar]
- 15.Gao GQ, Li Y, Zhang DC, et al. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. Febs Letters. 2001;489:270–6. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 17.Miao RQ, Agata J, Chao L, et al. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–52. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 18.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. Journal of Cellular and Molecular Medicine. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–45. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–45. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 21.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318:2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosberger DF. Diabetic Retinopathy Current Concepts and Emerging Therapy. Endocrinology and Metabolism Clinics of North America. 2013;42:721-+. doi: 10.1016/j.ecl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 24.The Relationship of Glycemic Exposure (Hba(1c)) to the Risk of Development and Progression of Retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 25.Diabetic Retinopathy Clinical Research N. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131:283–93. doi: 10.1001/jamaophthalmol.2013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidinger G, Maar N, Bolz M, et al. Repeated intravitreal bevacizumab (Avastin((R))) treatment of persistent new vessels in proliferative diabetic retinopathy after complete panretinal photocoagulation. Acta Ophthalmol. 2011;89:76–81. doi: 10.1111/j.1755-3768.2009.01622.x. [DOI] [PubMed] [Google Scholar]
- 27.Bin Cho W, Moon JW, Kim HC. Intravitreal triamcinolone and bevacizumab as adjunctive treatments to panretinal photocoagulation in diabetic retinopathy. British Journal of Ophthalmology. 2010;94:858–63. doi: 10.1136/bjo.2009.168997. [DOI] [PubMed] [Google Scholar]
- 28.Shin YW, Lee YJ, Lee BR, et al. Effects of an intravitreal bevacizumab injection combined with panretinal photocoagulation on high-risk proliferative diabetic retinopathy. Korean J Ophthalmol. 2009;23:266–72. doi: 10.3341/kjo.2009.23.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho WB, Oh SB, Moon JW, et al. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009;29:516–22. doi: 10.1097/IAE.0b013e31819a5fc2. [DOI] [PubMed] [Google Scholar]
- 30.Diabetic Retinopathy Clinical Research N. Scott IU, Edwards AR, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanagh PR, Buse JB, Frykberg RG, et al. Consensus Development Conference on Diabetic Foot Wound Care - 7–8 April 1999, Boston, Massachusetts. Journal of the American Podiatric Medical Association. 1999;89:475–83. doi: 10.7547/87507315-89-9-475. [DOI] [PubMed] [Google Scholar]
- 32.Calle-Pascual AL, Garcia-Torre N, Moraga I, et al. Epidemiology of nontraumatic lower-extremity amputation in area 7, Madrid, between 1989 and 1999 - A population-based study. Diabetes Care. 2001;24:1686–9. doi: 10.2337/diacare.24.9.1686. [DOI] [PubMed] [Google Scholar]
- 33.Lavery LA, Armstrong DG, Wunderlich RP, et al. Diabetic foot syndrome - Evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26:1435–8. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- 34.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. Jama-Journal of the American Medical Association. 2005;293:217–28. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 35.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tie L, Li XJ, Wang X, et al. Endothelium-specific GTP cyclohydrolase I overexpression accelerates refractory wound healing by suppressing oxidative stress in diabetes. Am J Physiol Endocrinol Metab. 2009;296:E1423–9. doi: 10.1152/ajpendo.00150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao P, Kodra A, Tomic-Canic M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machado MJ, Watson MG, Devlin AH, et al. Dynamics of angiogenesis during wound healing: a coupled in vivo and in silico study. Microcirculation. 2011;18:183–97. doi: 10.1111/j.1549-8719.2010.00076.x. [DOI] [PubMed] [Google Scholar]
- 39.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–6. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 40.Qi W, Yang C, Dai Z, et al. High Levels of Pigment Epithelium-derived Factor in Diabetes Impair Wound Healing through Suppression of Wnt Signaling. Diabetes. 2014 doi: 10.2337/db14-1111. [DOI] [PubMed] [Google Scholar]
- 41.McBride JD, Jenkins AJ, Liu XC, et al. Elevated Circulation Levels of an Antiangiogenic SERPIN in Patients with Diabetic Microvascular Complications Impair Wound Healing through Suppression of Wnt Signaling. Journal of Investigative Dermatology. 2014;134:1725–34. doi: 10.1038/jid.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brem H, Kodra A, Golinko MS, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–87. doi: 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- 44.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. Journal of Clinical Investigation. 2007;117:1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–59. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank S, Stallmeyer B, Kampfer H, et al. Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J. 1999;13:2002–14. [PubMed] [Google Scholar]
- 47.Braverman IM, Sibley J, Keh A. Ultrastructural Analysis of the Endothelial Pericyte Relationship in Diabetic Cutaneous Vessels. Journal of Investigative Dermatology. 1990;95:147–53. doi: 10.1111/1523-1747.ep12477903. [DOI] [PubMed] [Google Scholar]
- 48.Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacological Reviews. 2004;56:549–80. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 49.Chiarelli F, Spagnoli A, Basciani F, et al. Vascular endothelial growth factor (VEGF) in children, adolescents and young adults with Type 1 diabetes mellitus: relation to glycaemic control and microvascular complications. Diabetic Medicine. 2000;17:650–6. doi: 10.1046/j.1464-5491.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 50.Kocak N, Alacacioglu I, Kaynak S, et al. Comparison of vitreous and plasma levels of vascular endothelial growth factor, interleukin-6 and hepatocyte growth factor in diabetic and non-diabetic retinal detachment cases. Ann Ophthalmol (Skokie) 2010;42(Spec No):10–4. [PubMed] [Google Scholar]
- 51.Praidou A, Papakonstantinou E, Androudi S, et al. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with non-proliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89:248–54. doi: 10.1111/j.1755-3768.2009.01661.x. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe D, Suzuma K, Suzuma I, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–81. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–6. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 54.Funatsu H, Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006;113:294–301. doi: 10.1016/j.ophtha.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 55.Funatsu H, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005;112:806–16. doi: 10.1016/j.ophtha.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 56.Noma H, Funatsu H, Mimura T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87–93. doi: 10.1016/j.ophtha.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 57.Hernandez C, Burgos R, Canton A, et al. Vitreous levels of vascular cell adhesion molecule and vascular endothelial growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2001;24:516–21. doi: 10.2337/diacare.24.3.516. [DOI] [PubMed] [Google Scholar]
- 58.Burgos R, Simo R, Audi L, et al. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia. 1997;40:1107–9. doi: 10.1007/s001250050794. [DOI] [PubMed] [Google Scholar]
- 59.Sato H, Abe T, Wakusawa R, et al. Vitreous levels of vasohibin-1 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetologia. 2009;52:359–61. doi: 10.1007/s00125-008-1229-z. [DOI] [PubMed] [Google Scholar]
- 60.Sato T, Kusaka S, Shimojo H, et al. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology. 2009;116:1599–603. doi: 10.1016/j.ophtha.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Ogata N, Nishikawa M, Nishimura T, et al. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002;134:348–53. doi: 10.1016/s0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 62.Bai Y, Ma JX, Guo J, et al. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–54. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 63.Rodrigues M, Xin X, Jee K, et al. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–73. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Xu X, Elliott MH, et al. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahdy RA, Nada WM, Hadhoud KM, et al. The role of vascular endothelial growth factor in the progression of diabetic vascular complications. Eye. 2010;24:1576–84. doi: 10.1038/eye.2010.86. [DOI] [PubMed] [Google Scholar]
- 66.Semeraro F, Cancarini A, Morescalchi F, et al. Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes Metab. 2014 doi: 10.1016/j.diabet.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Frank S, Hubner G, Breier G, et al. Regulation of Vascular Endothelial Growth-Factor Expression in Cultured Keratinocytes - Implications for Normal and Impaired Wound-Healing. Journal of Biological Chemistry. 1995;270:12607–13. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 68.Shukla A, Dubey MP, Srivastava R, et al. Differential expression of proteins during healing of cutaneous wounds in experimental normal and chronic models. Biochemical and Biophysical Research Communications. 1998;244:434–9. doi: 10.1006/bbrc.1998.8286. [DOI] [PubMed] [Google Scholar]
- 69.Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci. 1989;30:1700–7. [PubMed] [Google Scholar]
- 70.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–4. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 71.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–36. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 72.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer. 2013;13:258–71. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chojnacki A, Weiss S. Pigment epithelium-derived growth factor: modulating adult neural stem cell self-renewal. Nat Neurosci. 2009;12:1481–3. doi: 10.1038/nn1209-1481. [DOI] [PubMed] [Google Scholar]
- 74.Ho TC, Chen SL, Wu JY, et al. PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells. 2013;31:1775–84. doi: 10.1002/stem.1393. [DOI] [PubMed] [Google Scholar]
- 75.Park K, Jin J, Hu Y, et al. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011;178:688–98. doi: 10.1016/j.ajpath.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang SX, Wang JJ, Dashti A, et al. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol. 2008;41:135–43. doi: 10.1677/JME-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crowe S, Wu LE, Economou C, et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10:40–7. doi: 10.1016/j.cmet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Mohan N, Monickaraj F, Balasubramanyam M, et al. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26:435–41. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Zheng BQ, Li T, Chen HB, et al. Correlation between Ficolin-3 and Vascular Endothelial Growth Factor-to-Pigment Epithelium-Derived Factor Ratio in the Vitreous of Eyes with Proliferative Diabetic Retinopathy. American Journal of Ophthalmology. 2011;152:1039–43. doi: 10.1016/j.ajo.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 80.Park K, Lee K, Zhang B, et al. Identification of a Novel Inhibitor of the Canonical Wnt Pathway. Molecular and Cellular Biology. 2011;31:3038–51. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkins AJ, Zhang S, Rowley K, et al. Increased serum pigment epithelium-derived growth factor levels in type 1 diabetes with microvascular complications. Diabetes. 2006;55:A504–A. [Google Scholar]
- 82.Jenkins AJ, Zhang SX, Rowley KG, et al. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med. 2007;24:1345–51. doi: 10.1111/j.1464-5491.2007.02281.x. [DOI] [PubMed] [Google Scholar]
- 83.Jenkins AJ, Fu D, Azar M, et al. Clinical correlates of serum pigment epithelium-derived factor in type 2 diabetes patients. J Diabetes Complications. 2014;28:353–9. doi: 10.1016/j.jdiacomp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tryggestad JB, Wang JJ, Zhang SX, et al. Elevated plasma pigment epithelium-derived factor in children with type 2 diabetes mellitus is attributable to obesity. Pediatr Diabetes. 2014 doi: 10.1111/pedi.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, Smit E, Brouwers MCGJ, et al. Plasma pigment epithelium-derived factor is positively associated with obesity in Caucasian subjects, in particular with the visceral fat depot. European Journal of Endocrinology. 2008;159:713–8. doi: 10.1530/EJE-08-0521. [DOI] [PubMed] [Google Scholar]
- 86.Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–50. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 87.Dai Z, Qi W, Li C, et al. Dual regulation of adipose triglyceride lipase by pigment epithelium-derived factor: a novel mechanistic insight into progressive obesity. Mol Cell Endocrinol. 2013;377:123–34. doi: 10.1016/j.mce.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Zhang SX, Wang JJ, Gao G, et al. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 89.Chao J, Tillman DM, Wang M, et al. Identification of a New Tissue-Kallikrein-Binding Protein. Biochemical Journal. 1986;239:325–31. doi: 10.1042/bj2390325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolf WC, Harley RA, Sluce D, et al. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. Journal of Histochemistry & Cytochemistry. 1999;47:221–8. doi: 10.1177/002215549904700210. [DOI] [PubMed] [Google Scholar]
- 91.Liu X, Zhang B, McBride JD, et al. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013;62:4228–38. doi: 10.2337/db12-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu L, Yang Z, Zhu B, et al. Kallikrein-binding protein suppresses growth of hepatocellular carcinoma by anti-angiogenic activity. Cancer Lett. 2007;257:97–106. doi: 10.1016/j.canlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 93.Zhu B, Lu L, Cai W, et al. Kallikrein-binding protein inhibits growth of gastric carcinoma by reducing vascular endothelial growth factor production and angiogenesis. Mol Cancer Ther. 2007;6:3297–306. doi: 10.1158/1535-7163.MCT-06-0798. [DOI] [PubMed] [Google Scholar]
- 94.Ma JX, King LP, Yang Z, et al. Kallistatin in human ocular tissues: reduced levels in vitreous fluids from patients with diabetic retinopathy. Curr Eye Res. 1996;15:1117–23. doi: 10.3109/02713689608995143. [DOI] [PubMed] [Google Scholar]
- 95.Jenkins A, Januszewski A, Mcbride J, et al. Increased Serum Kallistatin Levels in Patients with Type 1 Diabetes and Its Vascular Complications. Diabetes. 2009;58:A214–A5. doi: 10.1186/2040-2384-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schrufer TL, Antonetti DA, Sonenberg N, et al. Ablation of 4E-BP1/2 prevents hyperglycemia-mediated induction of VEGF expression in the rodent retina and in Muller cells in culture. Diabetes. 2010;59:2107–16. doi: 10.2337/db10-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abcouwer SF, Marjon PL, Loper RK, et al. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Investigative Ophthalmology & Visual Science. 2002;43:2791–8. [PubMed] [Google Scholar]
- 98.Kase S, He SK, Sonoda S, et al. alpha B-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 100.Akiri G, Nahari D, Finkelstein Y, et al. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998;17:227–36. doi: 10.1038/sj.onc.1202019. [DOI] [PubMed] [Google Scholar]
- 101.Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res. 2005;65:564–73. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 102.Clifford RL, Deacon K, Knox AJ. Novel regulation of vascular endothelial growth factor-A (VEGF-A) by transforming growth factor (beta)1: requirement for Smads, (beta)-CATENIN, AND GSK3(beta) J Biol Chem. 2008;283:35337–53. doi: 10.1074/jbc.M803342200. [DOI] [PubMed] [Google Scholar]
- 103.Xie K, Wei D, Shi Q, et al. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004;15:297–324. doi: 10.1016/j.cytogfr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Shima DT, Kuroki M, Deutsch U, et al. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–83. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- 105.Ko HM, Jung HH, Seo KH, et al. Platelet-activating factor-induced NF-kappa B activation enhances VEGF expression through a decrease in p53 activity. Febs Letters. 2006;580:3006–12. doi: 10.1016/j.febslet.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 106.Xie TX, Xia Z, Zhang N, et al. Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep. 2010;23:725–32. [PubMed] [Google Scholar]
- 107.Kiriakidis S, Andreakos E, Monaco C, et al. VEGF expression in human macrophages is NF-kappa B-dependent: studies using adenoviruses expressing the endogenous NF-kappa B inhibitor I kappa B alpha and a kinase-defective form of the I kappa B kinase 2. Journal of Cell Science. 2003;116:665–74. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- 108.Nagineni CN, Kommineni VK, William A, et al. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol. 2012;227:116–26. doi: 10.1002/jcp.22708. [DOI] [PubMed] [Google Scholar]
- 109.Fujioka S, Niu JG, Schmidt C, et al. NF-KB and AP-1 connection: Mechanism of NF-kappa B-Dependent regulation of AP-1 activity. Molecular and Cellular Biology. 2004;24:7806–19. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced Cellular Oxidant Stress by the Interaction of Advanced Glycation End-Products with Their Receptors Binding-Proteins. Journal of Biological Chemistry. 1994;269:9889–97. [PubMed] [Google Scholar]
- 111.Neumann A, Schinzel R, Palm D, et al. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappa B activation and cytokine expression. Febs Letters. 1999;453:283–7. doi: 10.1016/s0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- 112.Zhu P, Ren M, Yang C, et al. Involvement of RAGE, MAPK and NF-kappaB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol. 2012;21:123–9. doi: 10.1111/j.1600-0625.2011.01408.x. [DOI] [PubMed] [Google Scholar]
- 113.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 115.Zhang XB, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Research. 2001;61:6050–4. [PubMed] [Google Scholar]
- 116.Moon RT, Bowerman B, Boutros M, et al. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 117.Ma JX, Hu Y, Zhou T, et al. Activation of the Wnt Pathway in the Retina, a New Pathogenic Mechanism for Diabetic Retinopathy. Diabetes. 2009;58:A228–A. [Google Scholar]
- 118.Chen Y, Hu Y, Zhou T, et al. Activation of the Wnt Pathway Plays a Pathogenic Role in Diabetic Retinopathy in Humans and Animal Models. American Journal of Pathology. 2009;175:2676–85. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee K, Hu Y, Ding LX, et al. Therapeutic Potential of a Monoclonal Antibody Blocking the Wnt Pathway in Diabetic Retinopathy. Diabetes. 2012;61:2948–57. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fathke C, Wilson L, Shah K, et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Bmc Cell Biology. 2006;7 doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu X, Shen QT, Oristian DS, et al. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell. 2011;144:341–52. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whyte JL, Smith AA, Helms JA. Wnt Signaling and Injury Repair. Cold Spring Harbor Perspectives in Biology. 2012;4 doi: 10.1101/cshperspect.a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hanai J, Gloy J, Karumanchi SA, et al. Endostatin is a potential inhibitor of Wnt signaling. J Cell Biol. 2002;158:529–39. doi: 10.1083/jcb.200203064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao Y. Angiogenesis modulates adipogenesis and obesity. Journal of Clinical Investigation. 2007;117:2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10730–5. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tam J, Duda DG, Perentes JY, et al. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLoS One. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brakenhielm E, Cao R, Gao B, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–88. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 128.Demeulemeester D, Collen D, Lijnen HR. Effect of matrix metalloproteinase inhibition on adipose tissue development. Biochemical and Biophysical Research Communications. 2005;329:105–10. doi: 10.1016/j.bbrc.2005.01.103. [DOI] [PubMed] [Google Scholar]
- 129.Lijnen HR, Maquoi E, Hansen LB, et al. Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arteriosclerosis Thrombosis and Vascular Biology. 2002;22:374–9. doi: 10.1161/hq0302.104522. [DOI] [PubMed] [Google Scholar]
- 130.Van Hul M, Lijnen HR. Matrix metalloproteinase inhibition impairs murine adipose tissue development independently of leptin. Endocrine Journal. 2011;58:101–7. doi: 10.1507/endocrj.k10e-267. [DOI] [PubMed] [Google Scholar]
- 131.Cao YH. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nature Reviews Drug Discovery. 2010;9:107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 132.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 133.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. Faseb Journal. 2009;23:3113–20. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 134.Vijgen GH, Bouvy ND, Teule GJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 135.Vijgen GH, Bouvy ND, Teule GJ, et al. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6:e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 137.Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation. 2012;125:2782–91. doi: 10.1161/CIRCULATIONAHA.111.042929. [DOI] [PubMed] [Google Scholar]
- 138.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. International Journal of Obesity. 1998;22:1145–58. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 139.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 141.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 142.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. International Journal of Obesity. 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O’Rourke RW, White AE, Metcalf MD, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–90. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gealekman O, Guseva N, Hartigan C, et al. Depot-Specific Differences and Insufficient Subcutaneous Adipose Tissue Angiogenesis in Human Obesity. Circulation. 2011;123:186–U73. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gavin TP, Stallings HW, Zwetsloot KA, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. Journal of Applied Physiology. 2005;98:315–21. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 146.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lash JM, Sherman WM, Hamlin RL. Capillary Basement-Membrane Thickness and Capillary Density in Sedentary and Trained Obese Zucker Rats. Diabetes. 1989;38:854–60. doi: 10.2337/diab.38.7.854. [DOI] [PubMed] [Google Scholar]
- 148.Pang C, Gao ZG, Yin J, et al. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. American Journal of Physiology-Endocrinology and Metabolism. 2008;295:E313–E22. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spencer M, Unal R, Zhu BB, et al. Adipose Tissue Extracellular Matrix and Vascular Abnormalities in Obesity and Insulin Resistance. Journal of Clinical Endocrinology & Metabolism. 2011;96:E1990–E8. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sung HK, Doh KO, Son JE, et al. Adipose Vascular Endothelial Growth Factor Regulates Metabolic Homeostasis through Angiogenesis. Cell Metabolism. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 151.Ye JP, Gao ZG, Yin J, et al. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E1118–E28. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 152.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 153.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–92. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alvarez-Llamas G, Szalowska E, de Vries MP, et al. Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics. 2007;6:589–600. doi: 10.1074/mcp.M600265-MCP200. [DOI] [PubMed] [Google Scholar]
- 155.Silha JV, Krsek M, Sucharda P, et al. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–14. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 156.Voros G, Lijnen HR. Deficiency of thrombospondin-1 in mice does not affect adipose tissue development. Journal of Thrombosis and Haemostasis. 2006;4:277–8. doi: 10.1111/j.1538-7836.2005.01696.x. [DOI] [PubMed] [Google Scholar]
- 157.Lakeland TV, Borg ML, Matzaris M, et al. Augmented expression and secretion of adipose-derived pigment epithelium-derived factor does not alter local angiogenesis or contribute to the development of systemic metabolic derangements. American Journal of Physiology-Endocrinology and Metabolism. 2014;306:E1367–E77. doi: 10.1152/ajpendo.00046.2014. [DOI] [PubMed] [Google Scholar]