Abstract
Background
Antibodies against transcriptional intermediary factor (TIF)-1γ are associated with malignancy in dermatomyositis (DM). Identification of clinical findings associated with anti-TIF-1γ antibodies in DM is a high priority for both patient diagnosis and risk assessment.
Objective
We sought to define the clinical phenotype of patients with anti-TIF-1γ DM.
Methods
Using a novel, sensitive, and specific assay for anti-TIF-1γ antibodies, we retrospectively tested plasma from 134 adult patients with DM and examined associations between anti-TIF-1γ antibodies and particular clinical and laboratory features.
Results
In all, 55 (41%) patients had autoantibodies to TIF-1γ. Anti-TIF-1γ positive patients were less likely to have systemic features including interstitial lung disease, Raynaud phenomenon, and arthritis/arthralgia. Patients with TIF-1γ autoantibodies had more extensive skin involvement, and some patients manifested characteristic findings including palmar hyperkeratotic papules, psoriasis-like lesions and a novel finding of hypopigmented and telangiectatic (“red on white”) patches.
Limitations
This was a retrospective study from a single tertiary referral center.
Conclusion
TIF-1γ is the most commonly targeted DM-specific autoantigen in adults in a large US cohort. Although these patients tend to have less systemic involvement, their skin disease is often extensive and characteristic. Recognition of cutaneous findings in anti-TIF-1γ positive patients may allow more accurate and timely diagnosis and effective treatment of patients with DM.
Keywords: autoantibodies, Cutaneous Dermatomyositis Assessment and Severity Index, dermatomyositis, malignancy, phenotype, transcriptional intermediary factor-1γ
Dermatomyositis (DM) is a systemic autoimmune disease characterized by inflammation in multiple organ systems, most commonly the skin and muscle. Patients with DM have circulating autoantibodies; for many of these, the antigenic targets have been characterized.1,2 At least 6 myositis-specific autoantibody targets have been defined in patients with DM.3 Because patients with DM and the same autoantibody specificity frequently share similar clinical characteristics, it is likely that precise definition of antibody-associated clinical phenotype will facilitate diagnosis and assessment of systemic risk.
One autoantigen in DM that has recently become the focus of significant interest is transcriptional intermediary factor (TIF)-1γ. TIF-1γ (also called TRIM33, p155/140) belongs to the larger tripartite motif (TRIM) family of proteins that are implicated in a number of important biological processes, including cell proliferation, development, apoptosis, and innate immunity. Anti-TIF-1γ antibodies have been reported in 13% to 21% of adults with DM.4 Several studies have now shown that patients with DM and anti-TIF-1γ antibodies are at higher risk of internal malignancy.4–6 In addition, several studies suggest that these patients are at relatively low risk of interstitial lung disease (ILD).4,5,7–9 However, there are few studies describing the cutaneous manifestations of this antibody subgroup in adults with DM, and the data are conflicting regarding relative frequencies of skin findings.4,5,8,10 Providing additional information regarding this phenotype may aid in understanding disease pathogenesis and help clinicians identify these anti-TIF-1γ positive patients at the bedside.
METHODS
Patients
Patients with DM (age >18 years) were seen in the outpatient clinics at the Stanford University Department of Dermatology between July 2004 and April 2013. Patients were followed up for multiple visits (on average every 3–6 months) with a median time of follow-up of 361 days. The Stanford Institutional Review Board approved the collection of plasma and clinical information. Patients were considered to have DM if they met “probable” or “definite” criteria for DM based on Bohan and Peter11,12 criteria or, for clinically amyopathic patients, if they fulfilled the proposed cutaneous criteria of Euwer and Sontheimer.13 Patients were considered clinically amyopathic if they had cutaneous disease for at least 6 months and had no evidence of muscle weakness and no elevation of muscle enzymes. Skin disease was recorded biannually using the Cutaneous DM Assessment and Severity Index (CDASI).14,15 Unless otherwise noted, each clinical feature was dichotomized as present or absent, the former based on if the patient ever displayed that feature during the course of follow-up. All systemic symptoms were retrospectively reviewed from charts. ILD was defined as the presence of ground-glass opacities and/or fibrotic changes on high-resolution computed tomography scanning of the chest. Patients were considered to have cancer-associated DM if their first sign or symptom of cancer was within 3 years of their first DM symptom. All patients received a chest/abdomen/pelvic computed tomography scan at least once within the first 3 years of their disease for malignancy screening.
Antibody detection
Plasma was collected at the time of their first visit, and many patients were already on topical and/or systemic immunosuppressive therapy at the time of plasma collection. Antibodies against TIF-1γ, Mi-2, nuclear matrix protein 2 (NXP2), small ubiquitin-like modifier (SUMO-1) activating enzyme 1, Jo-1, and melanoma differentiation-associated gene 5 (MDA5) were determined as previously described.16
Statistics
Wilcoxon rank sum test was used to compare continuous variables and 2-sided Fisher exact test was used to compare categorical variables. P values less than .05 were considered statistically significant. Analyses were conducted using SAS (Version 9.3, SAS Institute Inc, Cary, NC).
RESULTS
Patient characteristics and autoantibody frequencies
Major demographic and systemic features of the cohort are shown in Table I. The cohort was mostly (72%) female with a median age of 48.4 years (range 4.6–86.9 years) at age of diagnosis and had an average of 5.3 ± 5.1 years of follow-up. A total of 28 (21%) patients were clinically amyopathic, 22 (16%) had ILD, and 28 (21%) had a cancer-associated DM.
Table I.
Patient characteristics
| Variable | TIF-1γ* 46 (34.3%) |
Mi-2* 8 (6.0%) |
NXP2* 13 (9.7%) |
SAE1 9 (6.7%) |
Jo-1* 6 (4.5%) |
MDA5 17 (12.7%) |
None 23 (17.2%) |
All n = 134 |
P value† |
|---|---|---|---|---|---|---|---|---|---|
| Sex, n (%) | .055 | ||||||||
| Male | 7 (15) | 3 (38) | 8 (62) | 2 (22) | 1 (17) | 4 (24) | 8 (35) | 37 (28) | |
| Female | 39 (85) | 5 (63) | 5 (38) | 7 (78) | 5 (83) | 13 (76) | 15 (65) | 97 (72) | |
| Age at diagnosis, y, mean (SD) | 49.5 (15.9) | 55.2 (11.0) | 51.8 (18.4) | 48.0 (15.9) | 45.7 (12.8) | 46.4 (15.4) | 43.1 (19.0) | 48.4 (16.4) | .67 |
| Race, n (%) | .0081 | ||||||||
| African American | 1 (2) | 1 (12.5) | 0 (0) | 0 (0) | 1 (16.7) | 1 (5.9) | 2 (8.7) | 6 (5) | |
| Asian | 3 (7) | 1 (12.5) | 2 (15.4) | 0 (0) | 1 (16.7) | 6 (35.3) | 2 (8.7) | 15 (11) | |
| Caucasian | 33 (72) | 3 (37.5) | 9 (69.2) | 6 (66.7) | 4 (66.7) | 6 (35.3) | 18 (78.3) | 88 (66) | |
| Latino | 6 (13) | 3 (37.5) | 1 (7.7) | 3 (33.3) | 0 (0) | 0 (0) | 1 (4.4) | 16 (12) | |
| Pacific Islander | 2 (4) | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 4 (23.5) | 0 (0) | 8 (6) | |
| Follow-up, y, mean (SD) | 5.0 (3.9) | 5.6 (11.0) | 8.2 (10.2) | 6.4 (4.9) | 3.5 (2.5) | 4.3 (3.9) | 7.9 (4.7) | 5.3 (5.1) | .068 |
| Tobacco [yes or past], n (%) | 6 (14) | 3 (37.5) | 4 (28.6) | 4 (44.4) | 1 (16.7) | 2 (11.8) | 4 (17.4) | 28 (20.9) | .58 |
MDA5, Melanoma differentiation-associated gene 5; NXP2, nuclear matrix protein 2; SAE1, SUMO1 activating enzyme subunit 1; TIF, transcriptional intermediary factor.
Does not include patients with >1 antibody.
Fisher exact test was used to compare categorical variables and Student t test was used to compare continuous variables.
Of 134 patients, 111 (83%) had at least 1 circulating autoantibody against 1 of the tested antigens. Plasma from 12 (9%) patients reacted with 2 or more antigens, with the specific combinations and frequencies (in parentheses) as follows: TIF-1γ and Mi-2 (7); TIF-1γ and Jo-1 (1); TIF-1γ, Mi-2, and NXP2 (1); Mi-2 and NXP2 (2); and Jo-1 and NXP2 (1). These were excluded in all subsequent analyses.
There was a clear trend for gender distribution to be affected by autoantibody type—for example, patients with anti-NXP2 antibodies were more likely male than other groups. In addition, patients of a given race seemed to preferentially target certain autoantigens—most strikingly we found that Asians and Pacific Islanders were enriched for antimelanoma differentiation-associated gene 5 antibodies.
TIF-1γ was by far the most common autoantibody in the cohort, with 55 (41%) patients having circulating antibodies binding to TIF-1γ and 46 (34%) patients having only anti-TIF-1γ reactivity.
The anti-TIF-1γ phenotype
Extracutaneous manifestations
Patients with anti-TIF-1γ antibodies were significantly more likely to be female (Table II). In addition, there was a trend for an increase in internal malignancy in patients with TIF-1γ autoantibody, although this was not statistically significant. TIF-1γ autoantibody was significantly associated with lower prevalence of Raynaud phenomenon and arthritis/arthralgia. Patients with anti-TIF-1γ antibodies had a lower prevalence of ILD than that seen in the comparator group. Pruritus was more common in anti-TIF-1γ positive patients.
Table II.
Subject characteristics of transcriptional intermediary factor-1γ-positive versus -negative patients
| Variable | TIF-1γ positive* (n = 46) |
TIF-1γ negative (n = 76) |
P value† |
|---|---|---|---|
| Gender, n (%) | .034 | ||
| Male | 7 (15) | 26 (34) | |
| Female | 39 (85) | 50 (66) | |
| Age at diagnosis, y, mean (SD) | 49.5 (15.9) | 49.5 (15.9) | .70 |
| Race, n (%) | .36 | ||
| African American | 1 (2) | 5 (7) | |
| Asian | 3 (7) | 12 (16) | |
| Caucasian | 33 (72) | 46 (61) | |
| Latino | 6 (13) | 8 (11) | |
| Pacific Islander | 2 (4) | 5 (7) | |
| Follow-up, y, mean (SD) | 5.0 (3.9) | 6.4 (5.9) | .27 |
| Tobacco [yes or past], n (%) | 6 (14) | 18 (24) | .51 |
| Internal malignancy, n (%) | 10 (22) | 8 (11) | .12 |
| Interstitial lung disease, n (%) | 2 (5) | 18 (27) | .0040 |
| Clinically amyopathic, n (%) | 12 (26) | 16 (21) | .66 |
| CK (maximum), mean (SD) | 342 (593) | 2317 (6177) | .027 |
| Aldolase (maximum), mean (SD) | 8.2 (4.2) | 16 (15.7) | .00010 |
| Review of systems, n (%) | |||
| Raynaud phenomenon | 4 (11) | 23 (35) | .017 |
| Hand swelling | 8 (31) | 19 (37) | .80 |
| Dysphagia | 16 (37) | 30 (44) | .55 |
| Arthralgia/arthritis | 15 (36) | 41 (59) | .031 |
| Pruritus | 40 (89) | 48 (71) | .036 |
CK, Creatine kinase; TIF, transcriptional intermediary factor.
Patients with >1 antibody were excluded.
Fisher exact test was used to compare categorical variables and Student t test was used to compare continuous variables.
Interestingly, anti-TIF-1γ positive patients had lower mean levels of muscle enzymes (aldolase and creatine kinase) than patients without this antibody. This finding could not be explained by a dilution effect from an increased number of amyopathic patients, as clinically amyopathic patients were no more common in the TIF-1γ group than the comparator (Table II). To further investigate this, we excluded all clinically amyopathic patients and found that anti-TIF-1γ positive patients still had significantly lower maximum creatine kinase (421.5 vs 2885.2; P = .05) and aldolase (8.9 vs 18.4; P = .003) values than anti-TIF-1γ-negative patients.
Cutaneous manifestations
Patients with TIF-1γ autoantibodies were characterized by several significant cutaneous findings (Table III). Over half (54%) of the anti-TIF-1γ-positive patients had a distribution of their rash in a strikingly photoexposed pattern. Consistent with this, a significantly greater percentage of the anti-TIF-1γ-positive cohort had a scalp rash, facial rash, V-neck rash, and back rash, respectively (Table III).
Table III.
Physical examination findings of transcriptional intermediary factor-1γ-positive versus -negative patients
| Variable | TIF-1γ positive* (n = 46), n (%) |
TIF-1γ negative (n = 76), n (%) |
P value† |
|---|---|---|---|
| Diffuse photoerythema | 21 (54) | 11 (17) | .00010 |
| Scalp rash | 40 (87) | 51 (69) | .029 |
| Facial rash | 43 (96) | 61 (81) | .028 |
| V-neck rash | 44 (98) | 56 (75) | .00070 |
| Back rash | 39 (87) | 60 (44) | .0033 |
| Lateral hip (holster) | 29 (71) | 24 (39) | .0020 |
| Psoriasis-like lesions | 6 (15) | 1 (1) | .0081 |
| Calcinosis | 1 (2) | 13 (18) | .0091 |
| “Red on white” | 5 (12) | 1 (1) | .023 |
| Palmar keratotic papules | 5 (12) | 1 (1) | .023 |
| Gottron papules | 31 (67) | 37 (49) | .060 |
| Periungual telangiectasias | 42 (91) | 58 (81) | .13 |
| Heliotrope | 36 (82) | 52 (70) | .19 |
| Alopecia | 25 (63) | 33 (49) | .23 |
| Palmar erythematous papules | 4 (9) | 11 (15) | .41 |
| Ulcers | 19 (41) | 25 (33) | .44 |
| Gottron sign | 35 (78) | 51 (72) | .52 |
TIF, Transcriptional intermediary factor.
TIF-1γ double-positive patients were excluded.
Fisher exact test.
We quantified skin disease severity by recording skin scores using the CDASI instrument. A CDASI score above 14 to 19 represents moderate to severe disease and a difference of 5 points is considered clinically meaningful (Anyanwu et al, unpublished data, June 2014). The average CDASI activity skin score during the period of patient follow-up was 24.0 for anti-TIF-1γ positive patients (n = 38) and 16.9 for all other patients with DM (n = 53; P = .0009). Despite having more severe skin disease, the anti-TIF-1γ positive patients had a striking reduction in the prevalence of calcinosis (Table III).
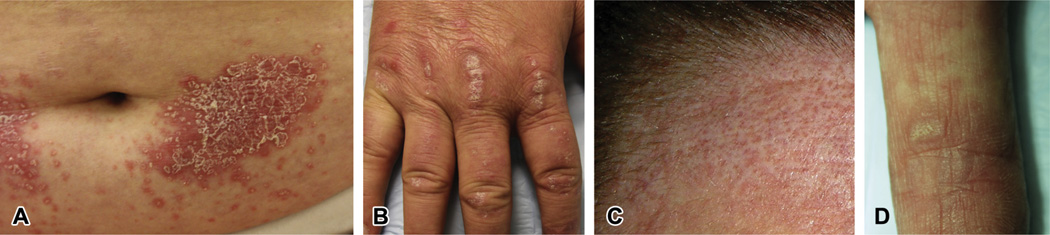
We also noted that many anti-TIF-1γ-positive patients had skin lesions that were clinically psoriasiform. These included classic psoriasis-like lesions (Fig 1, A), and, more commonly, Gottron papules on the back of hands that were distinctly hyperkeratotic and/or scaly (Fig 1, B). These lesions were present in 15% of anti-TIF-1γ positive patients and only found in 1% of the comparator group (P = .008) (Table III).
Fig. 1.
A, Psoriasiform plaque from a patient with dermatomyositis (DM) and anti-transcriptional intermediary factor (TIF)-1γ antibodies but no malignancy. Well-demarcated, erythematous plaque with hyperkeratotic scale on the abdomen. B, Hyperkeratotic Gottron papules from a patient with DM and anti-TIF-1γ antibodies. Thick plaques with micaceous scale over the joints of the back of hand. C, “Red on white” patch seen in a patient with DM. Hypopigmented patch on the forehead with intervening, follicular, erythematous, and telangiectatic macules. This patient was found to have anti-TIF-1γ antibodies but no malignancy. D, Hyperkeratotic papules on the palms from a patient with DM and anti-TIF-1γ antibodies. Hyperkeratotic and verrucous papules in a background of blanchable reticulated erythematous macules on the index finger without associated internal malignancy.
We found that some patients had a characteristic, asymptomatic skin finding consisting of hypopigmented patches admixed with punctate telangiectatic or erythematous macules (Fig 1, C). We term this finding “red on white” to distinguish it from the more classic poikiloderma that can be seen in many patients with DM and in sun-damaged skin of healthy individuals. These red on white lesions were found in 12% of anti-TIF-1γ positive patients and in only 1% of DM control subjects (P = .02) (Table III).
We also noted that some patients presented with small, round, hyperkeratotic papules on the palms and flexor surface of the digits that were asymptomatic and would sometimes resolve over time (Fig 1, D). These papules were found in 12% of anti-TIF-1γ positive patients as compared with on 1% of DM control subjects (P = .02) (Table III).
Risk factors for malignancy in anti-TIF-1γ-positive patients
Anti-TIF-1γ antibodies are associated with an increased risk of malignancy in adult patients with DM. Despite this, our data and those of others4,6,8,16,17 demonstrate that a substantial proportion of patients with DM and anti-TIF-1γ antibodies do not have a malignancy, and so we sought to determine if there might be other risk modifiers for malignancy in this antibody group (Table IV). Increased age was significantly associated with increased risk for cancer, and there was a trend for the association of smoking with cancer. Interestingly, Caucasians only comprised 40% of the cancer-associated DM group, compared with 83% in the noncancer group. No cutaneous findings were significantly associated with malignancy in the anti-TIF-1γ positive patients (not shown).
Table IV.
Features of transcriptional intermediary factor-1γ-positive cancer-associated dermatomyositis
| Variable | Cancer absent (n = 36) |
Cancer present (n = 10) |
P value* |
|---|---|---|---|
| Age at diagnosis, y, mean (SD) | 45.6 (13) | 63.5 (18) | .0050 |
| Race, n (%) | .029 | ||
| African American | 0 (0) | 1 (10) | |
| Asian | 2 (6) | 1 (10) | |
| Caucasian | 29 (83) | 4 (40) | |
| Latino | 3 (9) | 3 (30) | |
| Pacific Islander | 1 (3) | 1 (10) | |
| Tobacco history [yes or past], n (%) | 3 (9) | 3 (33) | .095 |
Fisher exact test was used to compare categorical variables and Student t test was used to compare continuous variables.
DISCUSSION
We have applied a sensitive and specific serologic assay to characterize the frequency and clinical significance of anti-TIF-1γ antibodies in a large US cohort of adult patients with DM. Anti-TIF-1γ was the most common autoantibody in our cohort, seen in 41% of patients. Previously reported frequencies for anti-TIF-1γ in adult DM range from 7% to 24%,6,8,10,18 and our higher prevalence could be a result of the increased sensitivity of our assay and the patient population. It is possible that this may be an underestimate, as it is unknown if the levels of TIF-1γ antibodies vary with DM activity, and thus patients who were tested at a time of low disease activity might have escaped detection. Within the TIF family, there are 3 known isoforms, denoted α, β, and γ, and all are targeted in adult DM.18 We did not test for antibodies recognizing the α and β isoforms; the significance of those antibodies with regards to clinical phenotype is unclear at present.7,18
Most studies demonstrate an association between TIF-1γ antibodies and malignancy in adult patients with DM.4–6,8,10,19 Our study did not reveal a significant association (only a trend) between anti-TIF-1γ antibody and malignancy, although we have previously reported that this trend becomes significant when anti-NXP2 patients are also included in the analysis.16 We and others have previously shown that age is a risk factor for malignancy, independent of anti-TIF-1γ status.16,20 Interestingly, our data also suggest that smoking might increase cancer risk in anti-TIF-1γ patients. We also found that Caucasians were relatively underrepresented in the cancer group, although there were no significant differences in age or smoking status between Caucasians and non-Caucasian patients with anti-TIF-1γ antibodies (not shown). Larger studies will be needed to formally test these associations within the anti-TIF-1γ antibody group.
With regards to systemic conditions, anti-TIF-1γ positive patients appear to have relatively less frequent rheumatic symptoms then the comparator patients with DM. Similar to other studies,5,7–9,21 TIF-1γ antibodies seem to confer a relatively low risk for ILD in adult patients with DM. We also found a lower risk of Raynaud phenomenon and arthritis/arthralgia. Thus, anti-TIF-1γ-positive patients are less likely to demonstrate features associated with the antisynthetase syndrome.
Our study also demonstrates that many other typical skin findings in adult DM (eg, scalp rash, V-neck erythema, holster sign) are more commonly found in the anti-TIF-1γ-positive patients than in other patients with DM, consistent with the findings in juvenile DM.22 Our quantitative data derived from CDASI scores further support that anti-TIF-1γ positive patients have more severe and widespread skin disease.23 We found that anti-TIF-1γ positive patients can also present with a more diffuse photodistributed erythema, which can be confused with other forms of erythroderma. Interestingly, erythroderma is documented to be associated with anti-TIF-1γ antibodies in the juvenile DM population.22 This clinical finding would be consistent with the hypothesis that ultraviolet (UV) light is a critical component in propagating skin disease in patients with anti-TIF-1γ antibodies. Interestingly, a recent study showed that US juvenile patients with DM and relatively high historical UV exposure are at higher risk of having anti-TIF-1γ antibodies.24 It is known that UV light increases the levels of Mi-2 in keratinocytes (the other antigen targeted more commonly in areas of high UV exposure),25,26 and it will be important to test if UV light similarly affects TIF-1γ expression, structure, or subcellular localization in the skin.
It is intriguing that, even though anti-TIF-1γ positive patients tend to have very extensive skin disease,23 they are relatively spared from calcinosis (Table III). It is believed that calcinosis is the result of poorly controlled inflammatory activity. Given the chronic course22 and extensive skin disease found in anti-TIF-1γ positive patients, it is surprising that few patients have calcinosis. The largest study to look at this in juvenile patients with DM suggests that calcinosis is common (30%) in anti-TIF-1γ positive patients, and thus the relative risk for calcinosis may be different between the juvenile and adult anti-TIF-1γ populations.22 Mechanisms that drive calcinosis are not well understood, and it is likely that they are not related to the classic inflammatory activity that we traditionally recognize and may be related to the vasculopathy seen in DM disease.27
We also describe several cutaneous findings that, if present, may help identify adult patients with DM and anti-TIF-1γ antibodies. Psoriasiform lesions in anti-TIF-1γ positive patients could result in errors in patient diagnosis, which is of clinical importance as UV therapy could be prescribed for presumed psoriasis with catastrophic consequences for patients with DM. In addition, we also highlight the red on white skin finding, consisting of hypopigmented macules and patches that are associated with focal, often follicular, telangiectatic erythema (Fig 1, D) that differs from the classic poikiloderma of DM, the latter being indistinguishable from chronic actinic damage. Finally, the clinician should examine the palms of all patients with DM, as the presence of hyperkeratotic, verruca-like papules can be seen in the anti-TIF-1γ population. These lesions can be transient and should be distinguished from the erythematous, often tender, palmar papules that are associated with anti-MDA5 antibodies and ILD.28
Limitations of our study include its retrospective design and the fact that it is based on patient observations at a tertiary referral center. In addition, regarding specific cutaneous manifestations, we only recorded what was observed during the period of follow-up at Stanford, and it is possible that the frequency of many findings is actually higher than those we could document. Despite this, it is hoped that recognition of characteristic features in anti- TIF-1γ positive patients will enhance the ability of the clinician to diagnose and assess systemic risk in patients with DM.
CAPSULE SUMMARY.
Transcriptional intermediary factor-1γ is an autoantigen targeted in patients with dermatomyositis.
Patients with dermatomyositis and transcriptional intermediary factor-1γ autoantibodies have more extensive skin disease and can have characteristic cutaneous findings including palmar hyperkeratotic papules, psoriasis-like lesions, and hypopigmented and telangiectatic patches.
Careful skin examination can help identify patients with dermatomyositis and antitranscriptional intermediary factor-1γ antibodies.
Acknowledgments
Drs Fiorentino and Rosen are supported by National Institutes of Health (NIH) AR062615-01A1. Dr Rosen is supported by NIH RO1 AR-44684 and the Donald and Dorothy Stabler Foundation. The Johns Hopkins Rheumatic Diseases Research Core Center, where the assays were performed, is supported by NIH P30-AR-053503.
Abbreviations used
- CDASI
Cutaneous Dermatomyositis Assessment and Severity Index
- DM
dermatomyositis
- ILD
interstitial lung disease
- NXP2
nuclear matrix protein 2
- TIF
transcriptional intermediary factor
- UV
ultraviolet
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Targoff IN. Myositis specific autoantibodies. Curr Rheumatol Rep. 2006;8:196–203. doi: 10.1007/s11926-996-0025-3. [DOI] [PubMed] [Google Scholar]
- 2.Ghirardello A, Bassi N, Palma L, et al. Autoantibodies in polymyositis and dermatomyositis. Curr Rheumatol Rep. 2013;15:335. doi: 10.1007/s11926-013-0335-1. [DOI] [PubMed] [Google Scholar]
- 3.Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209. doi: 10.1186/ar3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682–3689. doi: 10.1002/art.22164. [DOI] [PubMed] [Google Scholar]
- 5.Kaji K, Fujimoto M, Hasegawa M, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology. 2007;46:25–28. doi: 10.1093/rheumatology/kel161. [DOI] [PubMed] [Google Scholar]
- 6.Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O’Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64:523–532. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 7.Fujikawa K, Kawakami A, Kaji K, et al. Association of distinct clinical subsets with myositis-specific autoantibodies towards anti-155/140-kDa polypeptides, anti-140-kDa polypeptides, and anti-aminoacyl tRNA synthetases in Japanese patients with dermatomyositis: a single-center, cross-sectional study. Scand J Rheumatol. 2009;38:263–267. doi: 10.1080/03009740802687455. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology. 2010;49:1726–1733. doi: 10.1093/rheumatology/keq153. [DOI] [PubMed] [Google Scholar]
- 9.Trallero-Araguas E, Labrador-Horrillo M, Selva-O’Callaghan A, et al. Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine (Baltimore) 2010;89:47–52. doi: 10.1097/MD.0b013e3181ca14ff. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda N, Takahashi K, Yamaguchi Y, Inasaka M, Kuwana M, Ikezawa Z. Analysis of dermatomyositis-specific autoantibodies and clinical characteristics in Japanese patients. J Dermatol. 2011;38:973–979. doi: 10.1111/j.1346-8138.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 11.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 12.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 13.Euwer RL, Sontheimer RD. Amyopathic dermatomyositis: a review. J Invest Dermatol. 1993;100:124S–127S. doi: 10.1111/1523-1747.ep12356896. [DOI] [PubMed] [Google Scholar]
- 14.Klein RQ, Bangert CA, Costner M, et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol. 2008;159:887–894. doi: 10.1111/j.1365-2133.2008.08711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yassaee M, Fiorentino D, Okawa J, et al. Modification of the cutaneous dermatomyositis disease area and severity index, an outcome instrument. Br J Dermatol. 2010;162:669–673. doi: 10.1111/j.1365-2133.2009.09521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentino DF, Chung LS, Christopher-Stine L, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclearmatrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65:2954–2962. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinoy H, Fertig N, Oddis CV, Ollier WE, Cooper RG. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis. 2007;66:1345–1349. doi: 10.1136/ard.2006.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 2012;64:513–522. doi: 10.1002/art.33403. [DOI] [PubMed] [Google Scholar]
- 19.Kang EH, Nakashima R, Mimori T, et al. Myositis autoantibodies in Korean patients with inflammatory myositis: anti-140-kDa polypeptide antibody is primarily associated with rapidly progressive interstitial lung disease independent of clinically amyopathic dermatomyositis. BMC Musculoskelet Disord. 2010;11:223. doi: 10.1186/1471-2474-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Guo G, Chen G, Wu B, Lu L, Bao L. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838–847. doi: 10.1111/bjd.12564. [DOI] [PubMed] [Google Scholar]
- 21.Kang EH, Kuwana M, Okazaki Y, et al. Comparison of radioimmunoprecipitation versus antigen-specific assays for identification of myositis-specific autoantibodies in dermatomyositis patients. Mod Rheumatol. 2014;24:945–948. doi: 10.3109/14397595.2014.896494. [DOI] [PubMed] [Google Scholar]
- 22.Rider LG, Shah M, Mamyrova G, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92(4):223–243. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324–328. doi: 10.1093/rheumatology/kem359. [DOI] [PubMed] [Google Scholar]
- 24.Shah M, Targoff IN, Rice MM, Miller FW, Rider LG. Brief report: ultraviolet radiation exposure is associated with clinical and autoantibody phenotypes in juvenile myositis. Arthritis Rheum. 2013;65:1934–1941. doi: 10.1002/art.37985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burd CJ, Kinyamu HK, Miller FW, Archer TK. UV radiation regulates Mi-2 through protein translation and stability. J Biol Chem. 2008;283:34976–34982. doi: 10.1074/jbc.M805383200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri MH, Satoh M, Martin-Marquez BT, et al. Implications in the difference of anti-Mi-2 and -p155/140 autoantibody prevalence in two dermatomyositis cohorts from Mexico City and Guadalajara. Arthritis Res Ther. 2013;15(2):R48. doi: 10.1186/ar4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 2014;150:724–729. doi: 10.1001/jamadermatol.2013.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]