Abstract
Developing AIDS vaccines has been frustrating. A new vaccine triggers immune responses that effectively control early infection with the simian counterpart of HIV in macaques.
HIV is a highly mutable virus that has evolved over millennia to escape host control1. It is not surprising therefore that researchers have faced numerous challenges in inducing effective responses by the T cells or B cells of the immune system against this virus2. Over the past decade, considerable effort has gone into developing AIDS vaccines designed to induce T-cell responses that slow disease progression; such vaccines, however, are unlikely to prevent the explosive burst of viral replication that occurs during primary infection2. On page XXX of this issue, Hansen et al.3 describe an alternative approach. They report that in rhesus macaques induction of a distinct type of T cell (effector memory T cell) may contain early stages of replication of SIV— a virus related to HIV that infects monkeys.
The immune system can recognize pathogens years after an initial exposure using a specialized population of T cells called memory T cells. These cells can be divided into two subsets: effector memory T (TEM) cells and central memory T (TCM) cells4 (Fig. 1). TEM cells patrol effector sites such as mucosal tissues — the main port of entry for most infectious pathogens, including HIV — and can rapidly kill the infected cells. Efficient maintenance of TEM cells requires continuous antigen stimulation. For example, the herpesvirus cytomegalovirus (CMV) has been well-documented to induce high-frequency TEM cell responses5. By contrast, TCM cells are primarily found in secondary lymphoid tissues such as lymph nodes and spleen, and are elicited by non-persisting pathogens or vaccines that provide only transient antigenic stimulation4,6.
Figure.
How efficiently TEM and TCM cells can prevent or control an infection varies depending on the pathogen. For HIV and SIV infections, there is no clear consensus on whether TEM or TCM cells are likely to be more effective. Nonetheless, most candidate AIDS vaccines designed to induce T cell responses tested so far are non-persisting and induce killer (CD8+) TCM cells. These cells can decrease viral load but are relatively ineffective in protecting against initial SIV infection2.
Based on the distinctive characteristics of TEM cells, including their preferential localization to mucosal sites, Hansen and colleagues hypothesized that virus-specific TEM cells may contain SIV replication during a window of vulnerability, in the initial days of infection when relatively few helper (CD4+) T cells are infected7. Indeed, previously this group demonstrated8 that RhCMV/SIV — a recombinant rhesus CMV vaccine expressing multiple SIV proteins — can reduce the risk of progressive infection following repeated rectal SIV administration, most likely by inducing relatively high levels of CD4+ and CD8+ TEM cells.
In their present work3, Hansen et al. extend this approach to a larger cohort of animals. In addition, they examine the effects of a conventional DNA/recombinant adenovirus vaccine that predominantly induces a TCM cell response. And they give a third group of animals the RhCMV/SIV vaccine first and then boost them with the adenovirus vaccine.
Following repeated rectal challenges of vaccinated and control animals with SIV, 13 of 24 animals that received the RhCMV/SIV vaccine showed a distinctive pattern of transient increase in SIV levels in their blood (viraemia) followed by a rapid decay in the virus levels and periodic blips of viraemia that decayed over the year after challenge. This pattern of transient viraemia(s) stands in stark contrast to the typical course of persistent, high-level viral replication generally observed after SIV infection of macaques. The animals that controlled SIV infection also developed new or increased T-cell responses to the viral protein Vif, which confirmed that a limited take of SIV infection had occurred in these animals. The authors, however, could not detect SIV in a subset of animals with transient SIV infection through post-mortem examination, despite intensive efforts. Together these findings suggest that induction of virus-specific TEM cells may control productive SIV replication in a presently undefined reservoir of infected cells before the development of progressive, systemic infection.
How can one tell that TEM cells were responsible for the control of the initial take of SIV infection in these animals? For as-yet unclear reasons, RhCMV vectors do not efficiently induce antibody responses to SIV proteins, including viral envelope proteins. So B cells are unlikely to play a part. Moreover, the SIV-specific CD4+ and CD8+ T cells in RhCMV/SIV-vaccinated animals had a characteristic TEM phenotype and were enriched in effector sites such as the gut mucosa.
Hansen et al. report that, compared with animals that had progressive SIV infection, those that did not develop such infection had higher peak frequencies of SIV-specific CD8+ T cells after immunization. This suggests that the extent to which mucosal sites are ‘seeded’ with TEM cells during immunization may be a crucial factor in subsequent control of infection. It is essential to determine whether this unusual pattern of viral control can be observed in an independent cohort of animals vaccinated with RhCMV/SIV and to determine which T-cell populations mediate the protective effect. Whether protection occurs when vaccinated macaques are exposed to SIV vaginally or intravenously also remains to be seen.
It is noteworthy that just over half of the animals Hansen et al. vaccinated with RhCMV/SIV displayed a pattern of controlled infection, and once infected, the RhCMV/SIV-vaccinated animals showed no better control of viraemia than unvaccinated controls. So if TEM cells pan out to be an important component of an AIDS vaccine strategy, optimally they would be combined with vaccines that induce both neutralizing antibodies (assuming the considerable challenges in inducing these antibodies can be addressed2) and TCM cells capable of controlling viraemia in the event of systemic infection. No matter how desirable, the combined induction of TCM and TEM cells is not trivial: continual antigenic stimulation may deplete the TCM cell pool over time.
Can induction of TEM cells by CMV-based vectors serve as a viable HIV vaccine for humans? In healthy hosts, CMV can cause a disease similar to mononucleosis. And in immunocompromised patients as well as infants with congenital infection it can lead to severe disease. Clinical use of CMV vectors therefore is likely to encounter significant scrutiny.
A clinically acceptable CMV vaccine ought to be non-pathogenic in seronegative hosts. It must also present minimal risk of mother-to-child transmission and genital shedding (to minimize inadvertent transmission to others), while maintaining immunogenicity and persistence. The expected trade-offs between attenuation of pathogenicity and immunogenicity have already been documented9 in limited clinical trials of live recombinant CMV vaccines. But work is underway to develop recombinant viruses that are attenuated in pathogenicity but retain the immunogenicity of the unmodified CMV vaccines. For the future of CMV vectors, as well as for the overall AIDS vaccine enterprise, ultimately persistence is likely to pay off.
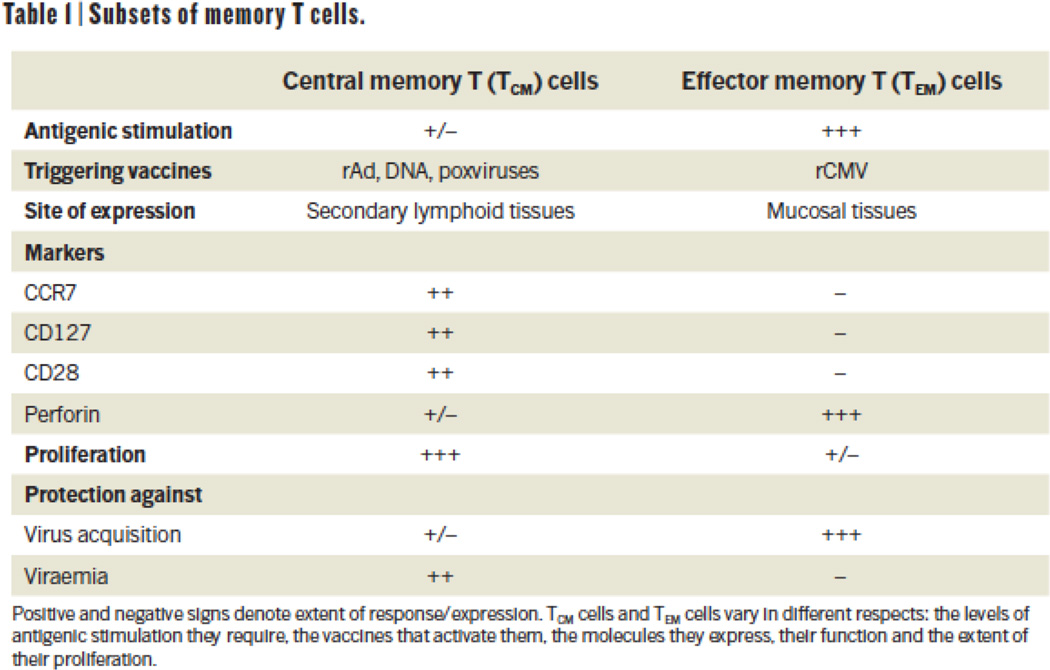
Box 1 Subsets of memory T cells.
TCM cells are characteristically induced by non-persisting vaccines such as recombinant adenoviruses (rAd), DNA and poxviruses. The cells are distinguished by their surface molecules such as CCR7, CD28 and CD127, by their relatively low immediate effector function, and by expression of the cytolytic molecule perforin. TCM cells have a high proliferative capacity and mainly provide protection against viraemia.
For induction of TEM cell responses, continuous antigenic stimulation by recombinant CMV (rCMV) vectors is required. These cells are primed for immediate effector function and cytolysis, but have relatively limited proliferative capacity. The preferential homing of TEM cells to mucosal sites, coupled with their capacity for rapid antiviral effector functions, may enable them to contain lentiviral (such as HIV and SIV) infections at the mucosal portals of entry3.
References
- 1.Johnson WE, Desrosiers RC. Annu. Rev. Med. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- 2.Barouch DH. Nature. 2008;455:613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen SG, et al. Nature. 2011;XXX:XXX–XXX. [Google Scholar]
- 4.Sallusto F, Mackay CR, Lanzavecchia A. Annu. Rev. Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 5.Moss P, Khan N. Hum. Immunol. 2004;65:456–464. doi: 10.1016/j.humimm.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Robinson HL, Amara RR. Nature Med. 2005;11:S25–S32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 7.Haase AT. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 8.Hansen SG, et al. Nature Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heineman TC, et al. J. Infect. Dis. 2006;193:1350–1360. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]