Adenosine exerts various biological effects via an action on cell surface adenosine receptors (Daly 1983). Thes adenosine receptors are of two types: A1 and A2 (Fredholm 1982, Daly 1983). At both types of adenosine receptors, alkylxanthines (theophylline and caffeine being the prototypes) are competitive antagonists. These classical xanthines show affinities in the 10−5—10−4 m range and show little or no selectivity for either receptor type (Fredholm & Persson 1982). We have recently described a series of novel functionalized congeners of 1,3-dipropyl-8-phenylxanthine (Jacobson et al. 1985a), some of which show a high degree of selectivity towards A1-receptors.
Adenosine produces a marked decrease in blood pressure dependent upon an A2-receptor mediated decrease in peripheral resistance (Jonzon et al. 1985) and a decrease in heart rate mediated via adenosine receptors of the A1-subtype (Jonzon et al. 1985). We have examined the ability of two recently synthesized xanthines to antagonize the hypotensive and negative chronotropic effects of a stable adenosine analogue N-5′-ethyl-carboxamidoadenosine(NECA) under in vivo conditions. One of these compounds, an amino congener, showed selectivity for A1-receptors in vitro, whereas the other, a carboxylic acid congener, did not (Jacobsen et al. 1985).
The experiments were conducted on sodium pentobarbital anaesthetized male Sprague—Dawley rats (Alab Strain) weighing 250–300 g. The rats were tracheotomized and polyethylene catheters were inserted in the common carotid artery for continuous blood pressure and heart rate recording. Thirty minutes after the i.p. administration of the xanthine derivative increasing doses of NECA were given intravenously and the changes in blood pressure and heart rate were recorded. Full dose–response curves were constructed in at least three animals at each dose of the xanthine. Based on the shift in the NECA dose–response curves we calculated the dose of the xanthine derivatives that produced a doubling of the dose of NECA to achieve the same biological effect by a Shild plot.
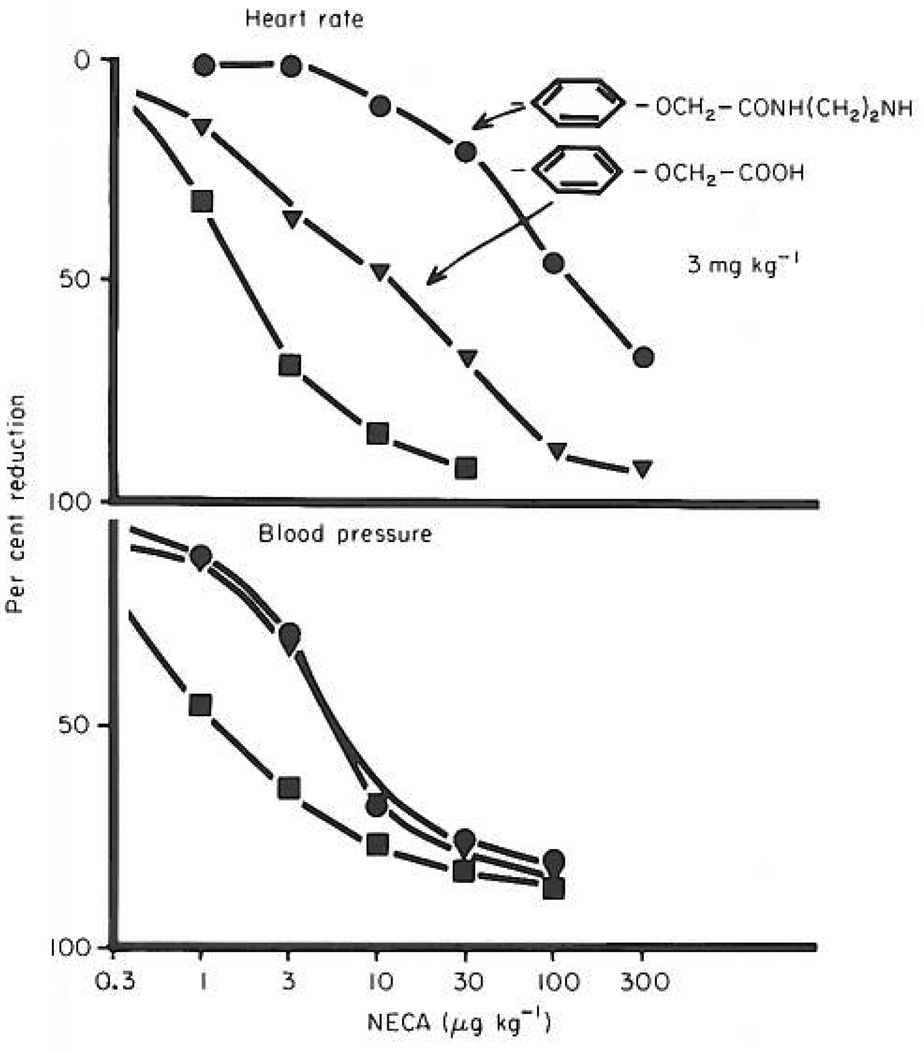
As seen in Figure 1, NECA caused a dose-dependent decrease in heart rate and in blood pressure in the anaesthetized rats. The amino congener of 1,3,-dipropyl-8-phenylxanthine produced a much larger shift of the dose–response curve for heart rate than for blood pressure at the same dose. By contrast, the carboxylic acid congener produced equivalent shifts to the right of the two dose–response curves.
Fig. 1.
The antagonism by two analogues of 1,3-dipropyl-8-phenylxanthine (structures of the 8-phenyl substituents are given in the upper right hand corner of the figure) of the negative chronotropic (upper panel) and hypotensive (lower panel) effect of NECA. Lack dose–response curve is based on four to six animals. ( ) Dose–response curve for NECA alone. (
) Dose–response curve for NECA alone. ( ) Dose–response curve for NECA after i.p. administration of the carboxylic acid congener (second structure) in a dose of 3 mg kg−1 (
) Dose–response curve for NECA after i.p. administration of the carboxylic acid congener (second structure) in a dose of 3 mg kg−1 ( ) Dose–response curve for NECA after i.p. administration of the amino congener (upper structure) in a dose of 3 mg kg−1
) Dose–response curve for NECA after i.p. administration of the amino congener (upper structure) in a dose of 3 mg kg−1
The major finding of the present investigation is that an amino congener of 1,3-dipropyl-8-phenylxanthine is much more potent in antagonizing the effects of adenosine analogues on heart rate than on blood presssure (16-fold difference; 0.043 vs 0.68 mg kg−1. Since the adenosine effects on the heart are mediated via A1-receptors and those on blood pressure via A2-receptors, these results suggest that this novel xanthine derivative is more active as an antagonist at A1-receptors than at A2-receptors under in vivo conditions. By contrast, the carboxylic acid (congener) did not show such selectivity (0.165 vs 0.21 mg kg−1 were required to inhibit the negative chronotropic and hypotensive actions, respectively). The amino congener thus has potential as a very valuable research tool in such applications as elucidating the relative importance of A1- and A2-adenosine receptors in various peripheral tissues, including kidney and lung. There is also the intriguing possibility that a compound of this type could have clinical potential as an inotropic and diuretic agent.
REFERENCES
- Daly JW. Adenosine receptors: characterization with radioactive ligands. In: Daly, Kuroda, Phillis, Shimizu, Ui, editors. Physiology and Pharmacology of Adenosin Derivatives. New York: Raven Press; 1983. pp. 69–70. [Google Scholar]
- Fredholm BB. Adenosine receptors. Med Biol. 1982a;60:289–293. [PubMed] [Google Scholar]
- Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur J. Pharmacol. 1982;81:673–676. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985 doi: 10.1021/jm00147a038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]