Abstract
All botulinum toxins (BoNTs, types A–G) inhibit synaptic transmitter release from motoneurons, and thus result in respiratory arrest and death. Rapid treatment with anti-BoNT antibodies can prevent progression, but recovery still requires weeks on a ventilator. Even after recovery, there is a potential for persistent fatigue in some cases of botulism even years after the insult, possibly because of motoneuron dropout for previously unknown reasons. Unique among BoNTs, the C-type (BoNT/C) cleaves two proteins involved in neurotransmitter release, syntaxin and SNAP-25, and induces apoptotic cell death in cultured cerebellar neurons. It is not clear, however, whether BoNT/C also affects neurons that encounter toxin in vivo, namely motoneurons. Here, we provide experimental evidence that BoNT/C causes a slow degeneration of motoneurons both in vitro and in vivo. This novel form of BoNT/C-induced cell death may require new treatment strategies.
Keywords: neuronal death, neurotoxin, spinal cord
Introduction
Botulinum toxins (BoNTs, types A–G) represent a Class A bioterrorism threat as defined by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. All BoNTs inhibit motoneuron synaptic activity, and thus cause respiratory arrest and death (reviewed in Refs [1,2]). Transmission by inhalation shows similar symptoms to food-borne botulism, with early symptoms of dysphagia, dry mouth, diplopia, and eventually flaccid paralysis. Rapid treatment with anti-BoNT antibodies can prevent disease progression [3]. As clinical experience with inhaled botulism is limited, as with food-borne botulism, patients often require 2 to 8 weeks on a ventilator for recovery; but even after recovery, there is a potential for persistent fatigue years in some cases of botulism even years after the insult [3]. Neurological damage to motoneurons caused by some forms of BoNT may underlie this persistent, disabling fatigue syndrome.
BoNT/A is FDA approved for the treatment of motor spasticity and the amelioration of skin wrinkles, and works by cleaving a nine amino acid fragments of the synaptic protein, SNAP-25, which inhibits neurotransmitter release and neurotransmission [4,5]. In contrast, in addition to SNAP-25, BoNT/C cleaves a single Lys–Ala bond within the carboxyl terminus of syntaxin to impair functional pairing of synaptic vesicles with the presynaptic membrane [6]. BoNT/B/D/F/G cleave VAMP/synaptobrevin, a type II membrane protein also involved with vesicular release [7]. Unlike BoNT/A, BoNT/C has been reported to cause neurite loss and ultimately apoptosis in cultured cerebellar neurons [8,9]. Thus, disruption of both syntaxin and SNAP-25 seem to be necessary to initiate neuronal injury and death. After exposure to BoNT/C, destruction of the axo-dendritic network is followed by mitochondrial release of cytochrome c and caspase-dependent apoptosis. Interestingly, the initial neuritic injury seems to be caspase-independent [8]. It is not clear, however, whether BoNT/C is toxic in vivo to the neurons that encounter the toxin, namely motoneurons.
Here, we provide experimental evidence that BoNT/C causes a slow degeneration of motoneurons both in vitro and in vivo. This new form of BoNT/C-induced cell death may explain the persistent fatigue encountered in some cases of botulism even years after the insult.
Materials and methods
Animals
E12.5 and adult (12-week-old) C57BL/6 mice were used for in-vitro and in-vivo studies, respectively. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Society for Neuroscience Guidelines for the Use of Animals in Neuroscience Research, using protocols approved by the Animal Research Committee of the Burnham Institute for Medical Research in La Jolla.
In-vitro neurotoxicity
Spinal cord motoneuron cultures
Spinal cord motor neuron cultures were derived from 12.5-day-old C57 BL/6 mouse embryos. Spinal cords with meninges removed were dissected out in dissection buffer (HBSS, Ca2 + free, Mg2 + free, 4 g/l glucose). Cells were dissociated by digestion (0.25% papain in HBSS for 15 min at 37°C), followed by triturating and passing through a 70-μM nylon mesh. Cells were plated at 5 × 105 cells/cm2 on glass coverslips coated with poly-l-ornithine and laminin. Neurons were maintained in culture medium consisting of Neurobasal media (Invitrogen, Carlsbad, California, USA), 2% B-27 supplement (Invitrogen), 2% horse serum (Invitrogen), 0.5 mM l-glutamine (Invitrogen), 1% penicillin/streptomycin (Invitrogen), 1 ng/ml BDNF (Millipore, Billerica, Massachusetts, USA), 100 pg/ml GDNF (Millipore), and 10 ng/ml CNTF (Millipore). Cultures were kept in a 5% CO2-humidified incubator at 37°C. Culture medium was changed twice per week.
BoNT/C exposure
After 7 days in culture, BoNT/C (EMD Chemicals, Gibbstown, New Jersey, USA) (0.02–20 ng/ml, final concentration) was added to the culture medium. Neuronal viability was assessed 72 h after BoNT/C exposure by directly counting all surviving motor neurons, as described below. BoNT/C tests were carried out in triplicate and repeated at least three times in independent experiments.
Motoneuron counting
For labeling motoneurons, spinal cord cultures were fixed with 4% paraformaldehyde (PFA) (containing 0.5% Triton X-100) for 30 min at room temperature, washed with PBS, and incubated with blocking solution (5% BSA, 0.5% Triton X-100 in PBS) for 1 h. Incubation of the primary antibody against SMI-32, a motoneuron-specific marker (Sternberger Monoclonals, Baltimore, Maryland, USA), was performed for 2 h at room temperature. SMI-32 staining was visualized under fluorescence microscopy after exposure to a fluorescent-labeled conjugated anti-mouse secondary antibody (Jackson Research, Westgrove, Pennsylvania, USA) for 1 h. DAPI (1: 2000 dilution) was added for nuclear staining. Cells meeting the following criteria were counted as motoneurons: positive SMI-32 staining, large cell body (> 20 μm in the longest axis), prominent neuritic arborization, and single long axon-like neurite extending over several millimeters [10].
In-vivo neurotoxicity
BoNT/C injection
Three-month-old adult mice were injected with BoNT/C (1 μl, 0.01 ng/g mouse weight dissolved in 1 μl of saline) into either the left or right gastrocnemius muscle. An equal volume of saline was injected into the opposite leg as the vehicle control. We had shown in preliminary experiments that this dose of BoNT/C induced paralysis of the hind limb on the injected side only.
Tissue preparation
Two weeks after injection, mice were anesthetized and perfused transcardially with chilled PBS, followed by cold 4% PFA. The lumbar spinal cord and associated nerve roots were dissected en bloc. The L2–L5 spinal cord segments were identified, isolated, and postfixed in 4% PFA overnight. The spinal cord tissues were cryo-protected in 30% sucrose before being frozen. Frozen tissues were cut into 12-μm-thick sections and mounted onto glass slides.
Motoneuron staining and counting in tissue sections
Serial sections were compiled by collecting two consecutive sections of every 10 sections through the spinal cord L2–L5 segment. All sections were stained by standard methods with cresyl violet. Motoneurons were defined using the aforementioned criteria and consisted of cells in layers VIII and IX of the ventral horn having a cell body diameter greater than 25 μm. Motoneurons were counted using a stereological method for each pair of sections [11]. The total number of motoneurons on each side was counted in 20 pairs of sections from each animal.
Statistical analysis
Values are presented as means ± SEM. A one-way analysis of variance was used for the in-vitro experiments comparing multiple parameters, and a paired t-test was used for the in-vivo experiments comparing motoneuron survival innervating the two lower limbs of the same mouse (SPSS software, Chicago, Illinois, USA). Statistical comparisons were considered significant at P < 0.05.
Results
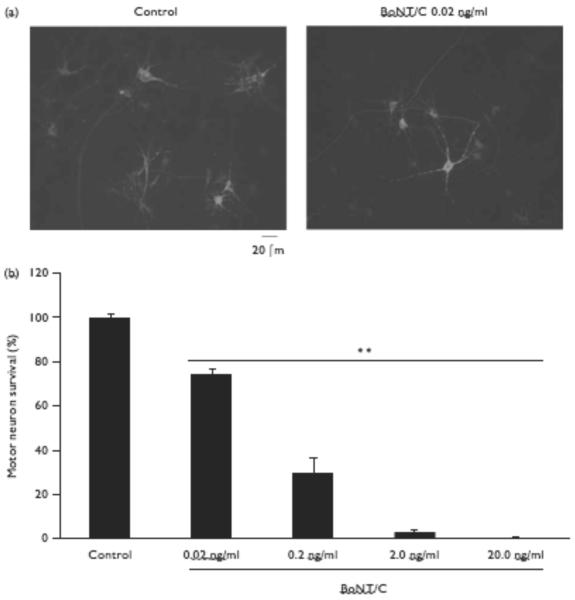
First, we tested whether BoNT/C was neurotoxic to spinal cord motoneurons in vitro. After 1 week in culture, we exposed motoneurons to BoNT/C (0.02–20 ng/ml) for 72 h and counted the surviving neurons. Motoneurons were identified as SMI-32-positive cells with typical morphological features (see Methods; Fig. 1a). The survival rate was calculated as the ratio of surviving motoneurons after BoNT/C exposure to the control group. BoNT/C exposure dose-dependently decreased motoneuron numbers at the lowest dose tested (0.02 ng/ml: 74.17 ± 2.69% survival, P < 0.01) and plateaued at higher concentrations (2 ng/ml: 3.25 ± 0.96%, P < 0.01).
Fig. 1.
Type C botulinum toxin (BoNT/C) neurotoxicity of motoneurons in culture. (a) Representative images taken from control cultures and those receiving BoNT/C. BoNT/C exposure for 72 h decreased the number of motoneurons. Cultures were stained with anti-SMI-32 antibody to identify motoneurons. Scale bar, 20 μm. (b) Motoneuron viability 72 h after exposure to BoNT/C. Data represent the mean±SEM of surviving motoneurons normalized to control (n=6–9 for each condition; **P<0.01).
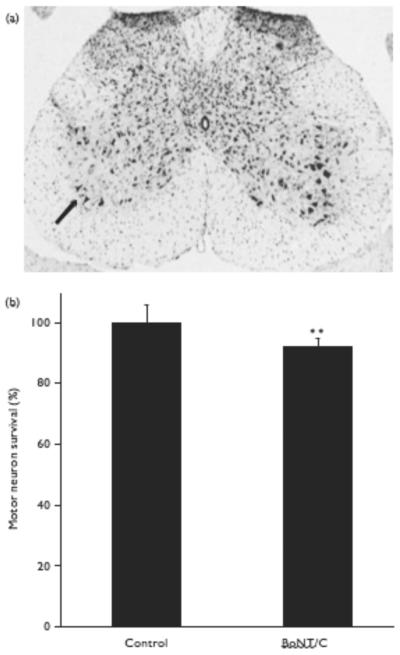
Next, we examined the effect of BoNT/C on spinal cord motoneurons in vivo. In preliminary experiments, we performed a dose-escalation study to determine the amount of BoNT/C that would cause paralysis of a limb, but not result in the death of the animal. To achieve 100% survival of the mice, a very low dose of BoNT/C had to be injected. Along these lines, we found that injection of BoNT/C (1 μl, 0.01 ng/g) into one gastrocnemius muscle in adult mice produced virtually instant ipsilateral limb paralysis and lack of paw movement for at least 5 days, but did not curtail survival. Using this protocol, motoneuron survival was assessed 2 weeks after BoNT/C injection of the L2–L5 spinal cord segments. Exposure to BoNT/C decreased the total number of motoneurons in the ipsilateral anterior horn (P < 0.01) compared with the contralateral side (Fig. 2a and b).
Fig. 2.
Motoneuron toxicity of type C botulinum toxin (BoNT/C) in vivo. (a) Representative cresyl violet-stained spinal cord showing decreased motoneuron number on the ipsilateral side of BoNT/C injection (arrow). (b) Motoneuron number ipsilateral to BoNT/C injection, normalized to the contralateral side receiving control saline injection. Data are expressed as mean±SEM (n=11; **P<0.01).
Discussion
The cleavage of two synaptic proteins (SNAP-25 and syntaxin) by BoNT/C can block neurotransmitter release; recently, it was also reported that low concentrations of BoNT/C (but not BoNT/A, which cleaves only SNAP-25) could lead to the degeneration of cerebellar granule cells [8,9]. However, cerebellar granule cells are not normally exposed when an organism encounters botulinum toxins; only motoneurons are exposed and thus affected. The question therefore arises whether a subpopulation of motoneurons recovering from BoNT/C undergo degeneration? This is an important question with regard to the possible long-term effects of BoNT/C versus BoNT/A in the human population given the use of botulinum toxins for various disease states as well as the potential for exposure because of bioterrorism. Here, we show that cultured motoneurons are sensitive to extremely low concentrations of BoNT/C (but not BoNT/A) because they die within 24 h of exposure. Very low doses of BoNT/C also induce motoneuron degeneration in vivo after injection into ipsilaterally innervated muscles. As the in-vivo effect may appear small, we were only able to administer tiny doses of BoNT/C to the whole animal, if we were to avoid organismal (as opposed to motoneuron) death. Moreover, because we could not control the diffusion of BoNT/C from the injection site to neighboring muscles, we included the large motoneuron pool in the entire L2–L5 spinal segment for the analysis. Therefore, we may have seriously underestimated the degree of motoneuron death after BoNT/C exposure, because we expressed the degeneration as a percentage of the total motoneuron pool in this wide area. Despite these limitations and extremely conservative analysis, we found a highly significant decrement in motoneuron survival after exposure to low-dose BoNT/C in vivo.
Botulinum toxins inhibit neurotransmitter release by cleaving specific synaptic proteins involved in neuro-transmitter release (reviewed in Refs [1,2]). By inhibiting neurotransmitter release from motoneurons, these toxins paralyze the diaphragm and lead to asphyxia. However, some toxin serotypes cleave more synaptic proteins than others, and thus may result in death by toxin-triggered axonal and synaptic injury, similar to neurodegenerative diseases caused by other toxins, such as glutamate and nitric oxide/peroxynitrite [8,12–15]. Alternatively, retrograde transport of BoNT/C may directly activate death mechanisms in the cell body [8]. Our results are consistent with the notion that synaptic damage caused by BoNT/C initiates two distinct processes: blockade of neuronal exocytosis and neurodegeneration. The neurodegeneration is potentially independent of exocytosis, as BoNT/A, unlike BoNT/C, does not result in motoneuron death.
This dissociation between blockade of neuroexocytosis and neurodegeneration may have several explanations: For example, (i) the targets of BoNT/A and BoNT/C may play roles in addition to membrane fusion during exocytosis, with SNAP-25 critical for neuronal development, and syntaxin for axonal maintenance and cell viability, as suggested by knockout experiments in Drosophila [16,17]; (ii) the extent of damage to the neuroexocytotic machinery may account for the different neurotoxic effects. The removal of only nine amino acid residues from SNAP-25 by BoNT/A is sufficient to interfere with neuroexocytosis, but not to induce neurodegeneration, as shown earlier [8,9] and in this study. In contrast, cleavage of SNAP-25 and the removal of almost the entire syntaxin molecule from the plasma membrane by BoNT/C may dramatically influence synaptic and axonal trafficking; (iii) the large syntaxin fragment generated by BoNT/C cleavage may be directly involved in neurotoxicity, perhaps by forming peptide multimers or aggregates, similar to other neurodegenerative diseases (reviewed in Ref. [18]).
Conclusion
BoNT/C, in addition to blocking neurotransmission at the neuromuscular junction, causes slow degeneration of motoneurons both in culture and in vivo. This form of BoNT/C injury may require the development of new treatment options to protect motoneurons from cell death in the organism recovering from exposure to botulinum toxins.
Acknowledgement
This study was supported, in part, by the NIH/NIAID grant P01 AI055789 (PI R. Liddington, Project 3 Leader S.A.L).
Footnotes
Conflicts of interest: none declared.
References
- 1.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 2.Meunier FA, Schiavo G, Molgo J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris. 2002;96:105–113. doi: 10.1016/s0928-4257(01)00086-9. [DOI] [PubMed] [Google Scholar]
- 3.Robinson R. On biochemical terrorism. Neurol Today. 2002;2:1–11. [Google Scholar]
- 4.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 5.Rossetto O, Schiavo G, Montecucco C, Poulain B, Deloye F, Lozzi L, Shone CC. SNARE motif and neurotoxins. Nature. 1994;372:415–416. doi: 10.1038/372415a0. [DOI] [PubMed] [Google Scholar]
- 6.Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, et al. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J Biol Chem. 1994;269:12764–12772. [PubMed] [Google Scholar]
- 8.Berliocchi L, Fava E, Leist M, Horvat V, Dinsdale D, Read D, Nicotera P. Botulinum neurotoxin C initiates two different programs for neurite degeneration and neuronal apoptosis. J Cell Biol. 2005;168:607–618. doi: 10.1083/jcb.200406126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson LC, Neale EA. Syntaxin and 25-kDa synaptosomal-associated protein: differential effects of botulinum neurotoxins C1 and A on neuronal survival. J Neurosci Res. 1998;52:569–583. doi: 10.1002/(SICI)1097-4547(19980601)52:5<569::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Lipton SA, Nicotera P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium. 1998;23:165–171. doi: 10.1016/s0143-4160(98)90115-4. [DOI] [PubMed] [Google Scholar]
- 13.Bonfoco E, Leist M, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Cytoskeletal breakdown and apoptosis elicited by NO donors in cerebellar granule cells require NMDA receptor activation. J Neurochem. 1996;67:2484–2493. doi: 10.1046/j.1471-4159.1996.67062484.x. [DOI] [PubMed] [Google Scholar]
- 14.Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 15.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 16.Schulze KL, Bellen HJ. Drosophila syntaxin is required for cell viability and may function in membrane formation and stabilization. Genetics. 1996;144:1713–1724. doi: 10.1093/genetics/144.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 18.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]