Abstract
Introduction
Osteoarthritis (OA) constitutes a growing public health burden and the most common cause of disability in the United States. Non-pharmacologic modalities and conservative pharmacologic therapies are recommended for the initial treatment of OA, including acetaminophen, and topical and oral non-steroidal anti-inflammatory drugs. However, safety concerns continue to mount regarding the use of these treatments and none have been shown to impact disease progression. Viscosupplementation with injections of hyaluronans (HAs) are indicated when non-pharmacologic and simple analgesics have failed to relieve symptoms (e.g., pain, stiffness) associated with knee OA. This review evaluates literature focusing on the efficacy and/or safety of HA injections in treating OA of the knee and in other joints, including the hip, shoulder, and ankle.
Methods
Relevant literature on intra-articular (IA) HA injections as a treatment for OA pain in the knee and other joints was identified through PubMed database searches from inception until January 2013. Search terms included “hyaluronic acid” or “hylan”, and “osteoarthritis”.
Discussion
Current evidence indicates that HA injections are beneficial and safe for patients with OA of the knee. IA injections of HAs treat the symptoms of knee OA and may also have disease-modifying properties, potentially delaying progression of OA. Although traditionally reserved for second-line treatment, evidence suggests that HAs may have value as a first-line therapy in the treatment of knee OA as they have been shown to be more effective in earlier stages and grades of disease, more recently diagnosed OA, and in less severe radiographic OA.
Conclusion
For primary care physicians who treat and care for patients with OA of the knee, IA injection with HAs constitutes a safe and effective treatment that can be routinely administered in the office setting.
Keywords: Hyaluronic acid, Intra-articular injection, Osteoarthritis, Rheumatology, Viscosupplementation
INTRODUCTION
Osteoarthritis (OA) is a chronic condition that causes pain and substantial disability [1]. It primarily affects the hands, knees, and hips, severely limiting the daily activity and quality of life for many individuals in the United States (US) [1]. Approximately 25% of people with pain from knee OA experience difficulty performing major activities of daily living, including walking a quarter mile, climbing stairs, and kneeling [1]. In addition, 15% use an assistive device in order to walk [2].
OA constitutes a growing public health burden [1] and represents the most common cause of disability in the US [1, 3]. Nearly one million years are lived with disability from knee and hip OA, making it the third leading cause of years lived with a disability in the US [1]. OA is highly prevalent and affects over 27 million Americans [1]. Because OA accounts for 630,000 joint replacements and 11 million outpatient visits annually [1], it generates staggering socioeconomic costs. In addition, OA impedes work productivity, accounting for more than 13 billion dollars in job-related costs [1]. OA is not caused by normal aging, but its prevalence increases with age [1, 4], rising sharply at 45 years old and affecting a large proportion of the working population [1]. With the well documented aging of the US population, the prevalence and burden of OA is expected to increase in the coming years due to longer life expectancies and the first of more than 78 million baby boomers reaching retirement age in 2011 [1]. Half of all US adults will develop symptomatic OA in their lives [1, 5] and as more adults continue to work beyond age 65, OA will likely further impair their work quality and ability to engage in retirement activities without pain and disability [1].
Another major factor contributing to the rise in OA prevalence is the obesity epidemic [1]. Weight increases OA risk and it is, therefore, not surprising that arthritis is highly prevalent among obese adults [1, 3, 6]. Ultimately, two of every three obese adults can be expected to develop OA in their lifetime [1].
Due to the increasing prevalence of OA, an understanding of the pathogenesis of OA and the wide range of available treatment options is necessary to effectively manage this disease. Given that most patients seek pain care from their primary care physicians, primary care providers are ideally positioned to manage patients with pain and functional impairment due to OA. This review first summarizes the pathophysiology and current pharmacologic management of OA. Literature evaluating viscosupplementation (VS) injections with hylans/hyaluronic acids (HAs) involving the knee and other (e.g., hip, ankle, shoulder) joints is subsequently presented and discussed.
METHODS
Relevant literature on intra-articular HA injections as a treatment for OA pain in the knee and other joints was identified through PubMed database searches from inception until January 2013. Information regarding intervention efficacy and safety was abstracted from each article. Representative key randomized, controlled trials were sought for each of the Food and Drug Administration (FDA)-approved HA products, and study efficacy and safety outcomes were reported here. While this is not a systematic review, search terms included “hyaluronic acid” or “hylan”, and “osteoarthritis”. A manual review of the references cited in the publications was also performed to be as inclusive of published studies as possible. Excluded papers were those not published, not indexed in PubMed, or not written in English. This article does not contain any studies with human or animal subjects performed by the author.
OVERVIEW OF OA PATHOPHYSIOLOGY
Osteoarthritis is a disorder of the entire joint that affects all joint tissues that communicate at the cellular level by releasing and responding to inflammatory mediators [4]. Cartilage destruction is the hallmark of OA and the degradation of type II collagen is the pivotal event that leads to irreversible progression of OA [4]. The balance that exists between matrix synthesis and degradation in normal cartilage is disrupted in OA by several factors produced by the synovium and chondrocytes, including cytokines, growth factors, aggrecanases, and matrix metalloproteinases (MMPs) [7].
In OA, the viscosity and elasticity of synovial fluid is much lower due to decreases in endogenous hyaluronan concentration and molecular weight [8, 9]; this impairs the ability of synovial fluid to lubricate joints, absorb loads, and exert anti-inflammatory effects.
Although the mechanism of the inflammatory process in OA is unknown, abnormal mechanical and oxidative stresses are likely involved [4]. Three mechanical risk factors have been found to strongly correlate with cartilage loss: malalignment of the tibiofemoral joint, bone marrow lesions, and meniscal disease manifested either as a tear or as extrusion [10].
Inflammation of the synovium is a trigger for various symptoms of OA by releasing soluble factors that, while increasing and perpetuating cartilage damage, have the potential to be used as biomarkers [3]. Synovial inflammation also triggers changes in the peripheral nervous system, affecting the afferent processing of nociceptive signals from the joint and surrounding tissues [7, 10]. Inflammation may also contribute to perpetuating cartilage degradation by promoting destruction and impairing repair [4]. In addition, impaired proprioceptive acuity may be involved in OA pathogenesis, as proprioceptive deficits are greater in people with knee OA compared with people of a similar age without OA [10].
TREATMENT OPTIONS FOR OA
Many patients with a clinical diagnosis of OA are treated with a combination of non-pharmacologic and pharmacologic modalities [11]. Conservative non-pharmacologic treatment options for the management of knee, hip, and hand OA include exercise and weight loss, as well as the use of thermal modalities and assistive devices [11]. Acetaminophen and oral or topical non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as the initial pharmacologic management of OA [11, 12]. Topical NSAIDs have better safety and tolerability profiles compared with oral NSAIDs [13]. Acetaminophen and NSAIDs are frequently used to treat knee OA because they are inexpensive and readily available over the counter [14]. Research has shown that both acetaminophen and NSAIDs produce small-to-moderate effect sizes (0.13–0.44) with regard to pain relief [12].
The systemic safety of acetaminophen and NSAIDs including cyclooxygenase-2 (COX-2) inhibitors has come into question by the Food and Drug Administration (FDA) and clinicians [15–18]. Excessive doses of acetaminophen can be hepatotoxic [15], and NSAIDs are associated with a wide spectrum of adverse effects, including gastrointestinal (GI) complications, renal toxicity, hepatotoxicity, and cardiovascular (CV) events, the risk of which increases in the presence of chronic co-morbidities that are common among older patients with OA [18]. The risk of adverse side effects with chronic NSAID use increases with age, dose and duration of exposure [3, 16–18]. The FDA requires acetaminophen and all NSAIDs (to include both prescription or over the counter formulations) to carry a boxed warning regarding the potential adverse effects [19, 20].
Other oral narcotics, opioids, and tramadol are also indicated for the treatment of OA [12], but also carry risks of dependence and adverse side effects [21, 22]. A recent study of older adults found a higher incidence of fractures, hospital safety events, and all-cause mortality with opioid use compared with non-selective NSAIDs and coxibs [23]. The FDA has also issued warnings about the potential abuse of opioids [24].
To alleviate OA pain quickly, IA injections of corticosteroids can be used, although the duration of effect wanes by week 4 [25]. Continuous injection of steroids (once every 3 months) showed a marked decrease in the effectiveness after the first year [26], and may produce destructive cartilage changes [27, 28].
THE ROLE OF HYALURONANS AND HYLANS IN THE TREATMENT OF OA
Description of HAs and Hylans
Hyaluronan, a long-chain polymer of repeating disaccharides of sodium-glucuronate-N-acetylglucosamine, is found at high levels in joints, acting as a lubricant and shock absorber [29, 30]. Hylans are derivatives of hyaluronan (sodium hyaluronate) [29, 30].
Clinical use of HAs and Hylans
Hyaluronan products are approved in the US for the treatment of pain associated with OA of the knee in patients who have not responded adequately to conservative non-pharmacologic therapy and to simple analgesics, such as acetaminophen and NSAIDs [29–35]. Currently, seven HAs/hylan products are FDA-approved, including Synvisc® and Synvisc-One® (hylan G-F 20; Genzyme Biosurgery, Ridgefield, NJ, USA), Hyalgan® (sodium hyaluronate; Fidia Pharma USA, Parsippany, NJ, USA), Supartz® (sodium hyaluronate; Anika Therapeutics, Woburn, MA, USA), Orthovisc® (sodium hyaluronate; Ferring Pharmaceuticals, Parsippany, NJ, USA), Euflexxa® (sodium hyaluronate), and Gel-One® (cross-linked hyaluronate; Seikagaku Corporation, Toyko, Japan) [29–35].
The active ingredient in these products is approximately 20 mg sodium hyaluronate per dose. The recommended number of injections per course range from a single injection for Synvisc-One and Gel-One to 5 for Hyalgan or Supartz (Tables 1 and 2) [29–35]. Table 2 illustrates the indicated number of injections for each product with their potential duration of effectiveness.
Table 1.
Characteristics of various hylans/hyaluronic acids
| Product name | Generic name | Soluble HA MW (kDa) |
Number of injections |
Dosage per injection (mg/mL) |
|---|---|---|---|---|
| Synvisc/Synvisc-ONE [29, 30] | Hylan G-F 20 | 6,000 | 1/3 | 16/2 |
| Hyalgan [32] | Sodium hyaluronate | 640 | 3 or 5 | 20/2 |
| Supartz [33] | Sodium hyaluronate (hyaluronan) | 1,200 | 3 or 5 | 25/2.5 |
| Orthovisc [34] | High MW hyaluronan | 2,900 | 3 or 4 | 30/2 |
| Euflexxa [31] | 1% sodium hyaluronate | 2,400–3,600 | 3 | 20/2 |
| Gel-One [35] | Hydrogel composed of cross-linked hyaluronate | Hydrogel (infinite) | 1 | 30/3 |
kDa kilodalton, MW molecular weight
Table 2.
Potential duration of effectiveness with indicated number of injections for hylans/hyaluronic acids
The effect size of HA is comparable to that of other treatments for knee OA pain [12]. As recently reviewed by Zhang et al. [12], the effect size of acetaminophen is 0.14, NSAIDs 0.20, COX-2 inhibitors 0.44, intra-articular corticosteroids 0.58, and intra-articular HA 0.60. Two of the most recent meta-analyses of viscosupplement use found effect sizes of 0.37 [36] and 0.31–0.46 [37], within the effect size range reported by Zhang et al. [12].
Mechanisms of Action
IA injection of HAs may reduce the pain associated with knee OA by several mechanisms, including inhibition of tissue nociceptors, stimulation of endogenous HA, direct anti-inflammatory effects, and inhibition of MMP activity [7]. First, HAs exhibit a synergistic effect that reduces the mechanical, chemical or thermal noxious stimuli to the innervated tissues of the synovial joint, thereby restoring normal homeostasis and reducing pain and stiffness [38]. The administration of IA HA may also improve joint rheology by increasing synovial fluid elasticity and viscosity [8, 39]. To decrease inflammation, HA suppresses the production and activity of pro-inflammatory mediators and proteases as well as alters the function of certain immune cells [7]. Histological evidence also shows that HA prevents the degradation of cartilage and may promote its regeneration [7]. Lastly, IA injections of HAs administered to patients with symptomatic knee OA have been shown to preserve knee cartilage, as measured by both cartilage volume and cartilage defects [40, 41]
EFFICACY OF VISCOSUPPLEMENTATION IN KNEE OA
Current evidence indicates that HA injections are beneficial to alleviate pain in patients with OA of the knee [7]. Many randomized controlled trials (described below) have demonstrated the effectiveness of HAs compared with placebo, although the clinical endpoints of these trials vary.
Randomized, Placebo-Controlled Studies
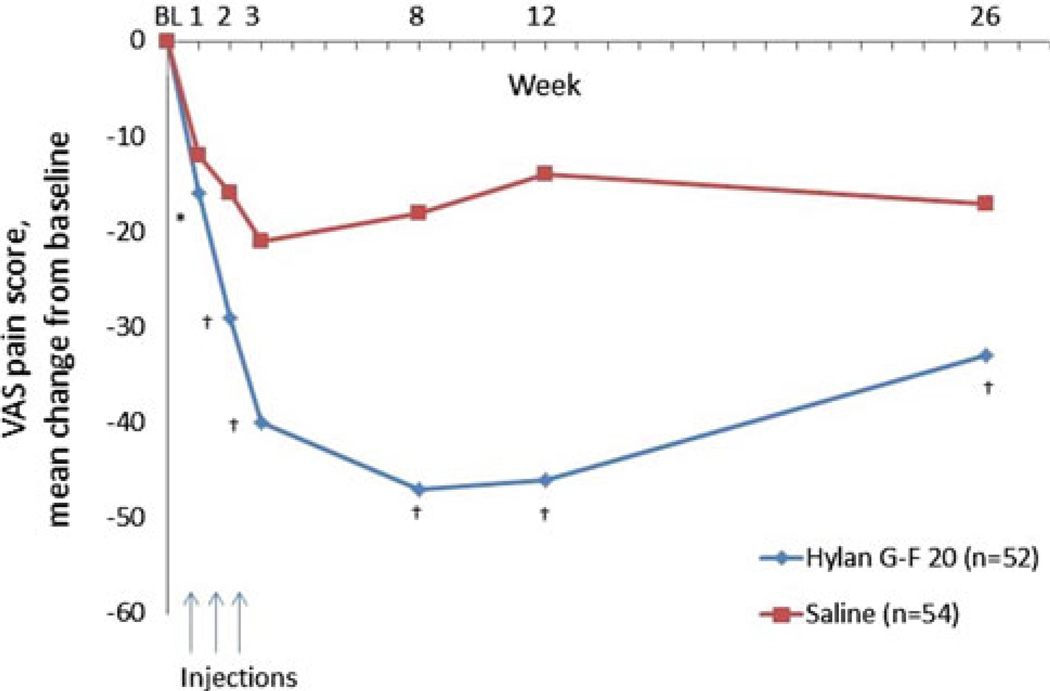
An early double-blind, randomized 12-week study compared the efficacy and tolerability of IA saline versus Synvisc (hylan G-F 20) given in two different administration schedules: three injections given 1 week apart versus two injections given 2 weeks apart [42]. Both hylan G-F 20 regimens showed significantly greater improvements in pain and other outcomes (e.g., reduction in activity while performing daily tasks), while the 3-injection group showed significantly greater improvements in all outcomes measured at the 12-week evaluation [42]. Hylan G-F 20 was later evaluated in a 26-week multicenter, double-masked, clinical study in which patients with chronic idiopathic knee OA received either 3 injections of 2 mL hylan G-F 20 1 week apart or saline solution at the same intervals [43]. Dramatic and early improvement in all outcomes was demonstrated following the first injection and maintained through 26 weeks with hylan G-F 20 versus placebo, including pain during weight-bearing movement (Fig. 1) [43].
Fig. 1.
Mean change from baseline (BL) of the visual analog scale (VAS) for pain during weight-bearing movement of the knee with 3-weekly injections of hylan G-F 20 or saline over 26 weeks [43]. *P < 0.01; †P = 0.0001 versus saline/placebo
In addition, a randomized, double-blind, placebo-controlled study of 3 once-weekly injections of hylan G-F 20 or native high-molecular weight hyaluronan (Supartz) reported a significant reduction from baseline in weight-bearing, resting, and maximum pain with the IA injections after 26 weeks that was similar between all groups [44]. When the 52-week results for both HA arms were pooled, a longer duration of benefit was observed with HA than with placebo [44].
Several key trials have also demonstrated the utility of Hyalgan in the treatment of knee OA [45–48], the most recent of which was a 26-week, randomized, multicenter, masked observer’s clinical trial comparing HA to placebo injection and to the oral NSAID naproxen in 495 patients [48]. This study confirms the results of previous trials demonstrating the benefit of a series of 5 weekly IA injections of HA over placebo in the treatment of OA of the knee. In this study, treatment differences were observed for the primary efficacy measure, which was pain experienced during a 50-foot walk [48]. In addition, HA was shown to be as effective as oral naproxen, with no significance between group differences noted [48].
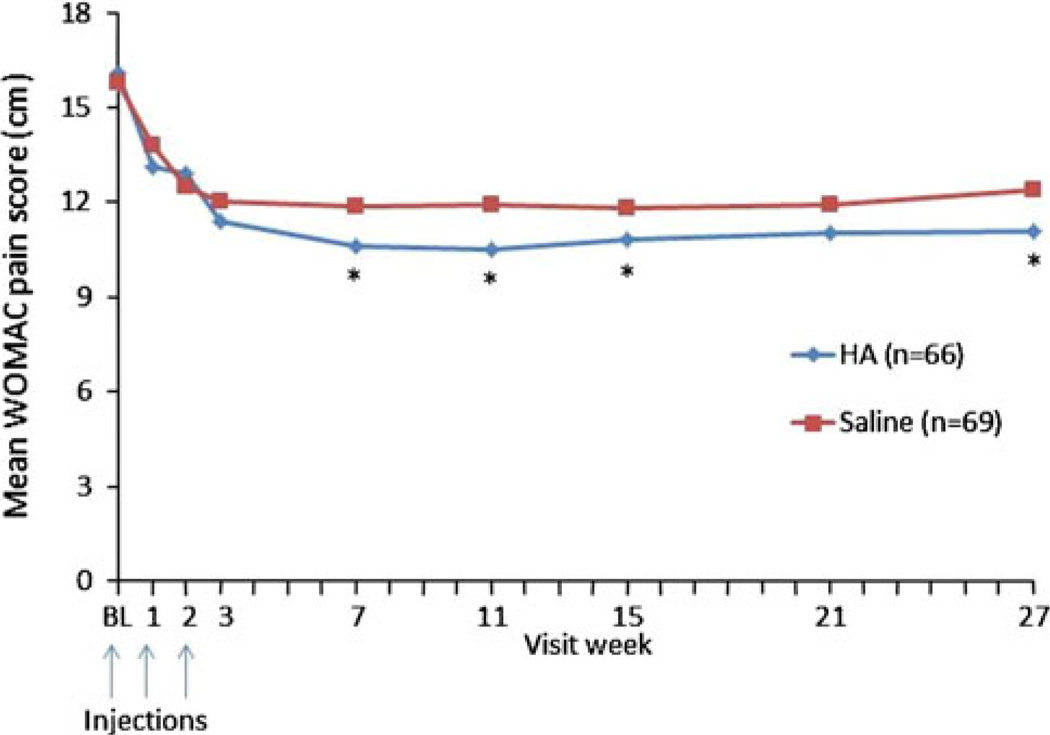
Early trials with Supartz [44, 49, 50] demonstrated its efficacy among patients with knee OA. The results of these trials were confirmed in a more recent 18-week double-blind, randomized, multicenter, placebo-controlled study, in which 223 patients received either 25 mg (2.5 mL) HA or saline [51]. Five injections were given at one-week intervals and patients were followed for an additional 13 weeks, with the Western Ontario and McMaster Universities Arthritis Index (WOMAC) OA instrument used to measure the primary efficacy variable [51]. Scores for the pain and stiffness subscales of the WOMAC index were significantly lower in the HA-treated group, although there was no statistical difference between groups seen in the physical function subscale [51]. Patients treated with HA experienced a benefit up to 13 weeks after the final injection [51].
Three randomized, controlled trials support the efficacy of Orthovisc in knee OA [52–54]. Among these was a prospective, multicenter, randomized, double-blind trial of 226 patients randomized to 3-weekly injections of 30 mg HA or saline and observed for an additional 25 weeks (Fig. 2) [52]. In patients with moderate OA knee pain whose baseline WOMAC pain score for the contralateral knee was less than 12 (scale range 0–20 for pain), HA treatment was significantly more effective than saline, as indicated by improvement in WOMAC pain, stiffness, and function scores, as well as 50-foot walk time [52].
Fig. 2.
Mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores with 3 hylan/hyaluronic acid (HA) injections through 26 weeks [52]. *P<0.05, BL baseline
Two randomized, double-blind trials evaluated the efficacy of Euflexxa in patients with knee OA [55, 56]. An initial randomized, placebo-controlled trial by Tamir and colleagues [56] found that the HA provided similar relief to placebo. The FLEXX trial was a 26-week, randomized, double-blind, multicenter, controlled study of 588 subjects with knee OA randomized to 3-weekly IA injections of buffered saline or Euflexxa (20 mg/2 mL) [55]. The primary outcome in this study was self-reported change from baseline in pain score following a 50-foot walk test [55]. Euflexxa provided significant and sustained (over 6 months) relief of knee pain, while also providing significant improvements in subject function, satisfaction with treatment, and health-related quality of life [55]. An extension of this trial showed that the pain reduction observed from initial injections of Euflexxa was sustained for an additional 23 weeks, suggesting that the efficacy of Euflexxa re-injection was comparable to that reported for re-injection of other HA agents [57].
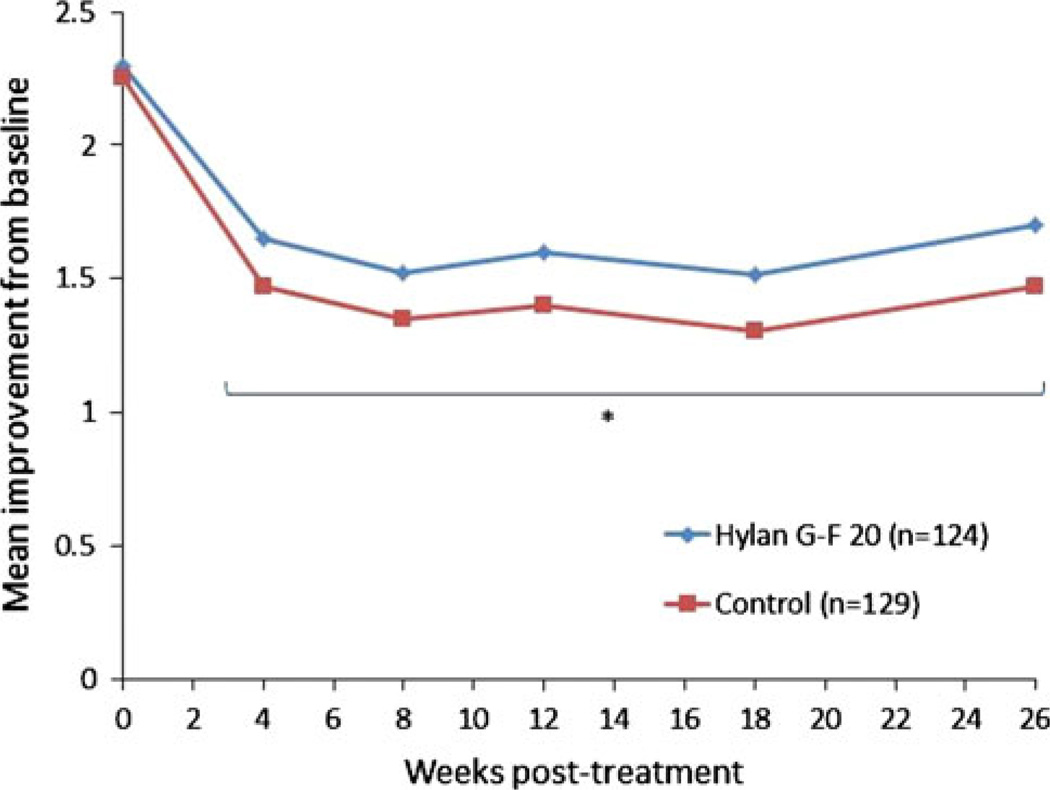
More recently, HA regimens have been reduced by the number of injections, with two HA products consisting of only one injection. The formulation of Synvisc-One, a single, 6-mL injection of hylan G-F 20, was shown to be effective in a 26-week, randomized, double-blind, placebo-controlled trial in patients with symptomatic knee OA [58]. In this study, one injection was effective in providing statistically significant, clinically relevant pain relief, as measured by WOMAC A1 (walking pain) over 26 weeks (Fig. 3) [58].
Fig. 3.
Mean improvements from baseline in Western Ontario and McMaster Universities Arthritis Index pain subscores [58]
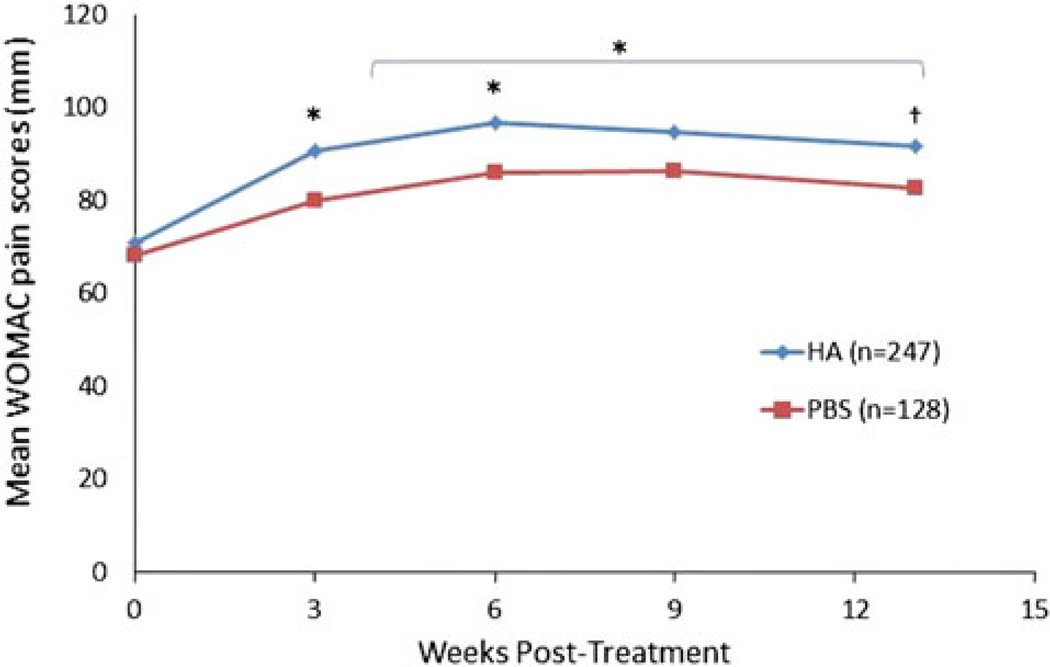
Data from a study enrolling 258 patients demonstrated that a single injection of Gel-One significantly improved WOMAC pain (Fig. 4) as early as 3 weeks through 13 weeks following a single injection [59]. No other endpoints measured were significantly different from placebo [59]. An extension study demonstrated that repeat HA treatment was as effective as initial therapy for an additional 13 weeks [60]; however, no placebo comparison could be made for the repeat treatment as all patients in the extension study received the HA [60].
Fig. 4.
Mean Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores [59]. *P ≤ 0.005; †P = 0.037. HA Hyaluronic acid; PBS Phosphate Buffered Saline
Meta-Analyses
Several meta-analyses and systemic reviews have evaluated the efficacy of HAs in the treatment of pain from knee OA (Table 3). The results of these meta-analyses vary widely. Several found HAs to be an effective treatment for knee OA [37, 61–63], while others reported summary effect sizes [36, 64, 65] suggesting that the intervention is associated with a small, clinically irrelevant effect. One likely explanation for this variation is the varied methodology used in the different reports [36, 37, 61–65].
Table 3.
Summary of meta-analyses/systematic reviews of hylans/hyaluronic acids (Has) in knee osteoarthritis
| References | Lo et al. [63] |
Wang et al. [62] |
Arrich et al. [65] |
Bellamy et al. [61] |
Bannuru et al. [119] |
Colen et al. [64] |
Rutjes et al. [36] |
|---|---|---|---|---|---|---|---|
| Total number of trials | 22 | 20 | 22 | 76 | 49 | 74 | 70 |
| Efficacy outcomes (HA versus placebo) | ES (change from baseline) | SPED, normalized to % change | Mean difference, SPID | WMD in pain during/ after exercise (VAS) | Overall pain, pain at different time points: 4, 8, 16, 20, and 24 weeks | MCID, pain | MCID, pain intensity and safety |
| Summary of pooled results | ES: 0.32 (P< 0.001) | SPID 7.9% (95% CI 4.1–11.7%) | WMD 10–14 week: −4.3 mm (P= 0.013) WMD 22–30 week: −7.1 mm (P= 0.013) | % change 5–13 week: +26% for pain; +23% for function | Peak ES at 8 weeks: 0.46 (95% CI 0.28–0.65) | WMD: −10.20 (95% CI −15.97 to −4.42) | ES: −0.37 (95% CI −0.46 to −0.28) |
| Conclusion | Small treatment effect | Significant improvement in pain | Not effective in measured outcomes | Effective treatment for pain, function, and patient global assessment at 5–13 week | Modest efficacy for knee pain | Further studies needed to determine efficacy of HA | Small, clinically irrelevant benefit |
ES effect size, MCID minimal clinically important difference, SPID sum of pain intensity differences, VAS visual analog scale, WMD weighted mean difference
For example, Campbell and colleagues [66] compared the methodology of 6 systematic reviews examining the efficacy of HA versus placebo in the treatment of knee OA to determine the reasons for discrepant results among meta-analyses. According to this analysis, different trials were selected for inclusion in the meta-analyses due to differences in the electronic databases, search strategies, and selection criteria [66]. While similar methods for data extraction were used, differences were found in both the outcome measures and time points selected for extraction [66]. Moreover, methodological quality was not always formally assessed and different statistical methods for data synthesis resulted in conflicting estimates of efficacy [66]. Collectively, these methodological differences may at least partly account for the discrepant results, especially with regard to the studies included in each analysis.
Comparative Head-to-Head Studies of HAs in Knee OA
Very few, well-controlled head-to-head studies have compared the efficacy of different HAs in the treatment of knee OA. Similar to the metaanalyses and systematic reviews discussed above, existing studies have provided inconsistent results. Several trials have evaluated the use of hylan G-F 20 versus various other HAs [66–70]. In a prospective, randomized trial comparing clinical effectiveness, functional outcome and patient satisfaction following IA injection with hylan G-F-20 (Synvisc) and sodium hyaluronate (Hyalgan), earlier and longer pain relief, as well as greater patient satisfaction was shown with hylan G-F 20 [67].
Other randomized controlled trials have shown that hylan G-F 20 had comparable efficacy compared with Orthovisc [68, 69]. Additional studies found Euflexxa to be non-inferior to hylan G-F 20 [70], while a medium molecular weight HA product (Structovial®; Pierre Fabre Medicaments, Lisbon, Portugal) was determined to be equally effective in comparison to hylan G-F 20 [71]. These results indicate that it is difficult to draw any definitive conclusions regarding the comparative efficacy of the various HA products. Further studies will need to be performed to compare the various HAs/hylans.
Efficacy of Repeat VS Treatments
Since knee injuries are occurring at younger ages, more people are developing OA at earlier ages [72]. Many physicians are now treating knee OA in younger patients (≤50 years old) and using VS earlier in the OA treatment paradigm [73]. Therefore, it is important to determine the effectiveness of repeat treatments of HAs over time to evaluate their long-term effects on OA knee pain.
Although data on this topic are limited, this question was the focus of one open-label multicenter study in which 108 patients received 5-weekly injections of 20 mg HA [74]. Fifty-nine patients were followed for up to 12 months, and 14 patients received a second course of treatment after 4–8 months [74]. Patients who completed the study showed significant improvements in pain and duration of walking lasting 12 months; patients who required a second treatment cycle showed additional improvement [74]. Two other recent open-label extension studies found that repeat courses of HA led to additional reductions in patients’ WOMAC pain scores after the first course of IA HAs. The rates of adverse events did not increase for the repeat courses of HA compared with adverse events reported with the first course of IA HAs [57, 60].
In addition, in a post hoc analysis of results from a randomized trial of patients randomized to “appropriate care with hylan G-F 20,” and “appropriate care without hylan G-F 20,” patients who received hylan G-F 20 were subdivided into two groups: patients who received a single course and patients who received multiple courses [75]. Both subgroups demonstrated significantly greater improvements in pain scores from baseline compared to the appropriate care group, with no differences between subgroups [75]. The number of local adverse events and rates of arthrocentesis were similar between patients who received a single or multiple courses of treatment [75].
Two long-term studies also found benefit with repeat courses of hylan or HA injections over a 2-year period [76, 77]. Waddell and colleagues followed patients for up to 104 weeks and found 20% of patients reported pain relief with one course of hylan G-F 20 [77]. The meantime for the patients who required a second course was 19.6 months, with 75% of patients not needing the second course for a year or longer after the first course of injections [77]. All outcome measures significantly improved with repeat hylan G-F 20 injections, with a slightly higher incidence of local AEs [77]. An earlier study by Scali evaluated patients for a total of 30 months; patients were injected with a HA (Hyalart) weekly for 5 weeks every 6 months [76]. Repeated treatment cycles were associated with sustained improvement, which was more evident in patients with early stage OA [76]. No local or systemic AEs were reported [76].
Finally, in a recent retrospective study of patients that received repeat injections of Supartz (sodium hyaluronate) for symptoms associated with knee OA, data were collected on 220 patients and 303 knee joints from 5 clinical sites [73]. Patients were at least 18 years of age with a confirmed diagnosis of OA and had received a minimum of one repeat treatment series of injections of Supartz in the affected knee [73]. In this study, repeat treatments were shown to delay the need for total knee arthroplasty for up to 4 years [73]. Before receiving sodium hyaluronate injections, approximately 94% of the patients rated their pain level as severe or moderate, but following repeat injections, 92% of patients demonstrated improvement in knee pain over time [73]. These data provide preliminary support for the favorable safety and efficacy profiles of repeat courses of HA injections, but additional studies are needed to confirm these findings.
VISCOSUPPLEMENTATION IN OA: SAFETY
HAs and hylan products have been shown to have an excellent safety and tolerability profile with few serious side effects [38]. Side effects are primarily local [78], such as transient pain and swelling at the injection site, and include a greater frequency of joint pain and swelling compared to placebo in some reports [38]. For example, the majority of published studies on hylan G-F 20 report a low incidence of local AEs that are consistent with those reported in the product information and are typically mild-to-moderate in nature, transient, and resolve spontaneously or with symptomatic treatment [78]. In addition, no differences have been observed between HAs and controls with respect to significant systemic side effects, including GI, CV, and renal events [38].
Several studies have demonstrated that HAs produce local adverse events at rates similar to rates observed for IA injections of saline [43] and of steroids [3, 78, 79], while comparative head-to-head trials have yielded inconsistent results. Several trials have observed a higher incidence of local AEs with hylan G-F 20 compared with other HAs [67–69].
EFFICACY OF VISCOSUPPLEMENTATION IN OTHER JOINTS
Hip
As described earlier, IA HAs are indicated in the US only for knee OA. To date, their off-label use has been investigated in clinical studies in a number of other joints, including the hip. Spitzer et al. [80] conducted a large, prospective, randomized, double-blind trial, where 305 patients were randomized to two injections of hylan G-F 20 administered 2 weeks apart (n = 150), or 1 injection of 40 mg methylprednisolone acetate (MPA) and 1 sham injection 2 weeks later (n = 155). WOMAC total and subscale scores, clinician observations, and patient global assessments were collected at baseline and at 4, 8, 12, 16, 20, and 26 weeks [80]. In this study, both hylan G-F 20 and MPA yielded clinically meaningful improvements in pain and function (P < 0.0001) compared with baseline [80]. Response rates were higher for patients with grade 3 hip OA treated with hylan G-F 20 compared with MPA-treated patients, while similar relief was found for patients with grade 2 hip OA [80]. In addition, hylan G-F 20 also demonstrated effectiveness in one observational [81] and one retrospective study [82], relieving symptoms and improving hip function in patients eligible for total hip replacement.
In contrast, one controlled study that evaluated the efficacy of Hyalgan found no significant benefits associated with treatment when compared with saline [83]. In this first known randomized, placebo-controlled trial of HA injections in the hip joint, Hyalgan was not significantly effective for any study endpoint, including the primary outcome (pain on walking) during the 3 months of intervention [83]. While a significant improvement could not be shown which could be a result of the small sample size, the effect size did indicate a small clinical improvement with Hyalgan treatment [83]. Taken together, available data from these off-label investigations of HA injections indicate that hylan G-F 20 (Synvisc) was the only HA to potentially demonstrate improvements in pain and function in the hip [81].
Ankle
Several preliminary studies have evaluated the effects of HAs in the treatment of ankle OA. Hylan G-F 20 demonstrated effectiveness in two open-label studies, resulting in significant improvements in pain with or without ankle arthroscopy and yielded positive results in a single-blinded trial [84, 85]. In four small studies, Hyalgan and Orthovisc were shown to improve pain and function [86–89]. Larger, randomized controlled studies are needed to support the use of HAs in treating ankle OA.
Thumb Base
Several HAs are associated with treatment benefits among individuals with OA involving the base of the thumb. For example, hylan G-F 20 was shown to improve pain and function in patients with thumb base (CMC) OA over 6 months in placebo-controlled trial [90] and open-label pilot study [91]. In addition, a randomized, clinical trial of 60 patients found that treatment with Synvisc resulted in improved grip strength and pain in patients with CMC OA [92].
In CMC OA, Orthovisc was effective in reducing pain and more effective in improving some aspects of hand function compared with steroids [93]. A small pilot study showed that Hyalgan was also effective in CMC OA [94].
Temporomandibular Joint
Research is still emerging in this area as very few studies have provided evidence of efficacy in treating OA of the temporomandibular joint. Currently, only hylan G-F 20 and Hyalgan have shown any positive effects. When given 14 days apart, two IA injections of Synvisc significantly reduced pain intensity versus two injections of corticosteroids [95]. Hyalgan has been found to markedly improve several outcomes (e.g., maximum mouth opening and movements, mastication efficiency, pain at mastication, pain at phonation, pain at rest, functional limitation, and subjective efficacy) over a 6-month period in a small open-label study of 76 patients when combined with arthrocentesis [96].
Shoulder
A recent meta-analysis, which included randomized, controlled trials that compared the efficacy of HA injections with placebo, demonstrated that HAs are effective treatments for chronic shoulder pain [97]. Articles were identified through database searches where study endpoints included pain, shoulder range of motion, total functional score, and comparison between HA and steroid injections [97]. Based on 19 randomized trials that were pooled for analysis, HA injections were effective for alleviating chronic shoulder pain [97]. The primary outcome of global pain intensity was evaluated in 10 trials (835 patients) having a favorable pooled standardized mean difference of 0.40 (95% CI 0.22, 0.59; P = 0.00002) for HA treatment compared with placebo [97]. Moreover, several studies have shown that hylan G-F 20 improves pain and function in patients with glenohumeral OA for up to 6 months [98–100]. In another study, Hyalgan improved persistent shoulder pain that was refractory to standard treatments, but no improvement was observed in the primary outcome of improvement in shoulder pain on movement at 13 weeks after the initiation of treatment based on use of a 100-mm visual analog scale (VAS) and the treatment effect through 26 weeks [101].
THE ROLE OF VS IN THE MULTIMODAL TREATMENT OF KNEE OA
The treatment of OA symptoms requires an integrated approach [38] and the combined use of both non-pharmacologic and pharmacologic treatments provide the greatest opportunity for pain relief and preservation of joint function. Multimodal therapy has been shown to demonstrate superior efficacy when one OA treatment modality is combined with another [102].A multimodal approach to the treatment of knee OA can provide excellent pain relief and a return to function [102]. By reducing pain through its impact on peripheral pain receptors and synovial tissue, as well as its role in enhancing the viscoelastic properties of synovial fluid, VS has become an integral part of the multimodal treatment of knee OA [3]. Numerous studies have shown that combined use of HAs plus conventional care including exercise is more effective than conventional care alone [103– 107]. In addition, combining HAs with other pharmacotherapies can also be considered, as this approach has been shown to be effective [108, 109]. For example, de Campos and colleagues recently published a study showing concomitant treatment of hylan G-F 20 with a corticosteroid improved pain scores for the first week compared with those injected with hylan G-F 20 alone, although the benefit of combined therapy did not persist after the first week [109].
Evidence for the use of VS Earlier in the OA Treatment Paradigm
Current treatment algorithms, such as those developed by the American College of Rheumatology and Osteoarthritis Research Society International, position HA as second-line treatment following non-pharmacologic therapies for persons who are unresponsive to acetaminophen or NSAIDs, or cannot take these medications [11, 12]. However, there is evidence to support the use of HAs earlier in the OA treatment paradigm [3, 110–114]. Patients with lower grades of OA reported a significantly (P < 0.05) better or much better overall response to hylan G-F 20 than patients with higher grades of OA [111], and patients with grade 3 OA found significantly (P < 0.05) more reduced VAS pain scores than patients with Grade 4 OA in another study [112]. Patients diagnosed with OA within a year were more likely to benefit from hylan G-F 20 than those patients who had been diagnosed longer than a year [114]. Evanich and colleagues found that patients treated with hylan G-F 20 with less severe radiographic OA were more likely to benefit from treatment than patients with more severe radiographic disease [113]. Compared with patients with more severe OA, patients with less severe disease who received an HA product required fewer analgesics or use of assistive devices [110]. Collectively these findings suggest that using HA soon after OA has been diagnosed could benefit patients.
In addition, HAs are at least as effective as NSAIDs if not more effective [3], and have been shown to be safer than NSAIDs and COX-2 inhibitors [3, 16, 78, 115, 116]. Hyaluronans are not associated with the potentially serious GI problems of NSAIDs or the CV safety issues of the newer COX-2 inhibitors. Given their demonstrated efficacy and safety and the potential drawbacks of these more conservative therapies, HAs can subsequently potentially decrease the need for concomitant NSAIDs and corticosteroids [103, 104, 114, 117, 118], although hylans and HAs are not currently indicated for concomitant use with any other therapeutics.
While this review was not systematic, it reflects a comprehensive overview of published studies using IA injections of VS as a treatment option to ameliorate OA-related pain. Current guidelines recommend simple analgesics (acetaminophen, NSAIDs) as the first option for treating OA pain, and using opioids, IA corticosteroids, and IA hylans/HAs after the simple analgesics fail to successfully alleviate the pain or are not tolerated [11, 12]. Existing data suggest that the sustained pain relief with VS treatments outweighs the low incidence of treatment-related side effects (that are transient and for the most part restricted to the site of injection) among patients with OA knee pain [59, 78, 110, 112]. When treating OA patients, the primary care physician should recommend treatment plans tailored to each individual.
CONCLUSION
In conclusion, VS is a valuable OA knee treatment option for primary care physicians to understand. Primary care physicians who encounter patients with knee OA pain can learn how to inject HAs in their offices, or refer those patients to orthopedists or rheumatologists to inject VS to alleviate OA pain.
ACKNOWLEDGEMENTS
Dr. Reid is the guarantor for this article and takes responsibility for the integrity of the work as a whole. Sanofi (formerly Genzyme Biosurgery) provided support for medical writing services to Nicole Cooper and Susan Bijur, PhD, of Precise Publications, LLC. No other funding or sponsorship was received for this study or publication of this article.
Abbreviation
- HA
Hylan/hyaluronic acid
- PBS
Phosphate buffered saline
Footnotes
Conflict of interest. Dr. Reid has served as a consultant for Sanofi, Pfizer, and Endo Pharmaceuticals.
Compliance with ethics guidelines. This article does not contain any studies with human or animal subjects performed by the author.
REFERENCES
- 1.A National Public Health Agenda for Osteoarthritis. [Accessed Aug 24, 2012];Centers for Disease Control and Prevention. 2010 www.cdc.gov/arthritis/docs/OAagenda.pdf.
- 2.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 3.Brzusek D, Petron D. Treating knee osteoarthritis with intra-articular hyaluronans. Curr Med Res Opin. 2008;24:3307–3322. doi: 10.1185/03007990802490124. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthr Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hootman JM, Murphy LB, Helmick CG. Arthritis as a potential barrier to physical activity among adults with obesity—United States, 2007–2009. Morb Mortal Wkly Rep. 2011;60:614–615. [PubMed] [Google Scholar]
- 7.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanism of action. Arthr Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagga H, Burkhardt D, Sambrook P, March L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946–950. [PubMed] [Google Scholar]
- 9.Balazs EA. Some aspects of the aging and radiation sensitivity of the intercellular matrix with special regard to hyaluronic acid in synovial fluid and vitreous. Aging Connect Skeletal Tissue. 1969:107–122. [Google Scholar]
- 10.Felson DT, Gross KD, Nevitt MC, et al. The effects of impaired joint position sense on the development and progression of pain and structural damage in knee osteoarthritis. Arthr Rheum. 2009;61:1070–1076. doi: 10.1002/art.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthr Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Altman R, Barkin RL. Topical therapy for osteoarthritis: clinical and pharmacologic perspectives. Postgrad Med. 2009;121:139–147. doi: 10.3810/pgm.2009.03.1986. [DOI] [PubMed] [Google Scholar]
- 14.Altman RD, Fowler PJ. Pharmacologic treatment of knee osteoarthritis in athletic women. Phys Sportsmed. 2011;39:39–44. doi: 10.3810/psm.2011.09.1919. [DOI] [PubMed] [Google Scholar]
- 15.Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med. 2010;77:19–27. doi: 10.3949/ccjm.77a.09084. [DOI] [PubMed] [Google Scholar]
- 16.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 17.Chou R, Helfand M, Peterson K, Dana T, Roberts C. Drug class review on cyclo-oxygenase (COX)-2 inhibitors and non-steroidal anti-inflammatory drugs (NSAIDs) final report update. Oregon Health and Science University. 2006 [PubMed] [Google Scholar]
- 18.Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2012;32:1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA acetaminophen announcement. [Accessed Oct 30, 2013];Nevada Department of Health and Human Services. 2012 www.medicaid.nv.gov/Downloads/provider/web_announcement_468_20120425.pdf.
- 20.FDA. [Accessed Mar 5, 2013];Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) 2005 www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm 150314.htm.
- 21.Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthr Cartil. 2007;15:957–965. doi: 10.1016/j.joca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD005522.pub2. CD005522. [DOI] [PubMed] [Google Scholar]
- 23.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 24.FDA issues draft guidance on abuse-deterrent opioids. [Accessed Mar 5, 2013];US Food and Drug Administration. 2013 www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm334785.htm.
- 25.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;2 doi: 10.1002/14651858.CD005328. CD005328. [DOI] [PubMed] [Google Scholar]
- 26.Daly E, Gray A, Barlow D, Mcpherson K, Roche M, Vessey M. Measuring the impact of menopausal symptoms on quality of life. BMJ. 1993;307:836–840. doi: 10.1136/bmj.307.6908.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balch HW, Gibson JM, El-Ghobarey AF, Bain LS, Lynch MP. Repeated corticosteroid injections into knee joints. Rheumatol Rehabil. 1977;16:137–140. doi: 10.1093/rheumatology/16.3.137. [DOI] [PubMed] [Google Scholar]
- 28.Tehranzadeh J, Booya F, Root J. Cartilage metabolism in osteoarthritis and the influence of viscosupplementation and steroid: a review. Acta Radiol. 2005;46:288–296. doi: 10.1080/02841850510016027. [DOI] [PubMed] [Google Scholar]
- 29.Synvisc-One® (hylan G-F 20) Prescribing Information. [Accessed Mar 5, 2013];Genzyme Biosurgery. 2013 http://synviscone.com/*/media/SynviscOneUS/Files/Synvisc-OnePI-70240104.pdf.
- 30.Synvisc® (hylan G-F 20) Prescribing Information. [Accessed Mar 5, 2013];Genzyme Biosurgery. 2013 http://synviscone.com/*/media/SynviscOneUS/Files/synvisc_PI.pdf.
- 31.Euflexxa® (1% sodium hyaluronate) Prescribing Information. [Accessed Mar 5, 2013];Ferring Pharmaceuticals. 2013 www.euflexxa.com/assets/euflexxa_physician-f87c5c8e9b6f9317c6cfd0b7df1b48c6.pdf.
- 32.Hyalgan® (sodium hyaluronate) Prescribing Information. [Accessed Mar 5, 2013];Sanofi-aventis. 2013 www.hyalgan.com/download/hyalgan_pi.pdf.
- 33.Supartz® (sodium hyaluronate) Prescribing Information. Seikagaku Corporation. 2013 http://supartzprofessional.com/docs/PackageInsert.pdf.
- 34.Orthovisc® (sodium hyaluronate) Prescribing Information. [Accessed Mar 5, 2013];Anika Pharmaceuticals. 2013 www.orthoviscline.com/sites/default/files/file/Orthovisc_Package_Insert.pdf.
- 35.Gel-One® (cross-linked hyaluronate) Prescribing Information. [Accessed Mar 5, 2013];Seikagaku Corporation. 2013 www.zimmer.com/content/pdf/en-US/Gel-One_Pkg_Insert_Final.pdf.
- 36.Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 37.Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—meta-analysis. Osteoarthr Cartil. 2011;19:611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg VM, Goldberg L. Intra-articular hyaluronans: the treatment of knee pain in osteoarthritis. J Pain Res. 2010;3:51–56. doi: 10.2147/jpr.s4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grecomoro G, La Sala F, Francavilla G. Rheologic changes in the synovial fluid of patients with gonarthritis induced by intraarticular infiltration of hyaluronic acid. Int J Tissue React. 2001;23:67–71. [PubMed] [Google Scholar]
- 40.Wang Y, Hall S, Hanna F, et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195. doi: 10.1186/1471-2474-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creamer P, Sharif M, George E, et al. Intra-articular hyaluronic acid in osteoarthritis of the knee: an investigation into mechanisms of action. Osteoarthr Cartil. 1994;2:133–140. doi: 10.1016/s1063-4584(05)80063-9. [DOI] [PubMed] [Google Scholar]
- 42.Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther Res. 1994;55:220–232. [Google Scholar]
- 43.Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20:410–423. doi: 10.1016/s0149-2918(98)80052-0. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology. 2002;41:1240–1248. doi: 10.1093/rheumatology/41.11.1240. [DOI] [PubMed] [Google Scholar]
- 45.Grecomoro G, Martorana U, Di Marco C. Intraarticular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmatherapeutica. 1987;5:137–141. [PubMed] [Google Scholar]
- 46.Dixon AS, Jacoby RK, Berry H, Hamilton EBD. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin. 1988;11:205–213. doi: 10.1185/03007998809114237. [DOI] [PubMed] [Google Scholar]
- 47.Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthr Cartil. 1993;1:97–103. doi: 10.1016/s1063-4584(05)80024-x. [DOI] [PubMed] [Google Scholar]
- 48.Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25:2203–2212. [PubMed] [Google Scholar]
- 49.Puhl W, Bernau A, Greiling H, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthr Cartil. 1993;1:233–241. doi: 10.1016/s1063-4584(05)80329-2. [DOI] [PubMed] [Google Scholar]
- 50.Lohmander LS, Dalen N, Englund G, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Ann Rheum Dis. 1996;55:424–431. doi: 10.1136/ard.55.7.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day R, Brooks P, Conaghan PG, Petersen M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol. 2004;31:775–782. [PubMed] [Google Scholar]
- 52.Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin Orthop Relat Res. 2001;385:130–143. doi: 10.1097/00003086-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 53.Kotevoglu N, Iybozkurt PC, Hz O, Toktas H, Kuran B. A prospective randomized controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26:325–330. doi: 10.1007/s00296-005-0611-0. [DOI] [PubMed] [Google Scholar]
- 54.Neustadt D, Caldwell J, Bell M, Wade J, Gimbel J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: a randomized, controlled, multicenter trial. J Rheumatol. 2005;32:1928–1936. [PubMed] [Google Scholar]
- 55.Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial) Semin Arthritis Rheum. 2009;39:1–9. doi: 10.1016/j.semarthrit.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Tamir E, Robinson D, Koren R, Agar G, Halperin N. Intra-articular hyaluronan injections for the treatment of osteoarthritis of the knee: a randomized, double blind, placebo controlled study. Clin Exp Rheumatol. 2001;19:265–270. [PubMed] [Google Scholar]
- 57.Altman RD, Rosen JE, Bloch DA, Hatoum HT. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthr Cartil. 2011;19:1169–1175. doi: 10.1016/j.joca.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113–119. doi: 10.1136/ard.2008.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2012;20:350–356. doi: 10.1016/j.joca.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Strand V, Barafat HSB, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3:297–304. doi: 10.1177/1947603512451024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee (review) Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD005321.pub2. CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86-A:538–545. doi: 10.2106/00004623-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–3121. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 64.Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. Biodrugs. 2012;26:257–268. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 65.Arrich J, Piribauer F, Mad P, Schmid D, Klaushofer K, Mullner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039–1043. doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell J, Bellamy N, Gee T. Differences between systematic reviews/meta-analyses of hyaluronic acid/hyaluronan/hylan in osteoarthritis of the knee. Osteoarthr Cartil. 2007;15:1424–1436. doi: 10.1016/j.joca.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of Hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15:318–324. doi: 10.1016/j.knee.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 68.Karatosun V, Unver B, Gocen Z, Sen A. Comparison of two hyaluronan drugs in patients with advanced osteoarthritis of the knee. A prospective, randomized, double-blind study with long term follow-up. Clin Exp Rheumatol. 2005;23:213–218. [PubMed] [Google Scholar]
- 69.Juni P, Reichenbach S, Trelle S, et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthr Rheum. 2007;56:3610–3619. doi: 10.1002/art.23026. [DOI] [PubMed] [Google Scholar]
- 70.Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2006;14:154–162. doi: 10.1016/j.joca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Maheu E, Zaim M, Appelboom T, et al. Comparative efficacy and safety of two different molecular weight (MW) hyaluronans F60027 and Hylan G-F20 in symptomatic osteoarthritis of the knee (KOA). Results of a non inferiority, prospective, randomized, controlled trial. Clin Exp Rheumatol. 2011;29:527–535. [PubMed] [Google Scholar]
- 72.Briggs KK, Matheny LM, Steadman JR. Can hylan G-F 20 with corticosteroid meet the expectations of osteoarthritis patients? Am J Orthop (Belle Mead NJ) 2012;41:311–315. [PubMed] [Google Scholar]
- 73.Whitman C, Allen D, Comadoll JL, Thomason HC, Oweida SJ. A retrospective study of SUPARTZ® and repeat treatment for osteoarthritis pain in the knee. J Manag Care Med. 2010;13:43–47. [Google Scholar]
- 74.Kotz R, Kolarz G. Intra-articular hyaluronic acid: duration of effect and results of repeated treatment cycles. Am J Orthop. 1999;28(Suppl 11):5–7. [PubMed] [Google Scholar]
- 75.Raynauld JP, Goldsmith CH, Bellamy N, et al. Effectiveness and safety of repeat courses of hylan G-F 20 in patients with knee osteoarthritis. Osteoarthr Cartil. 2005;13:111–119. doi: 10.1016/j.joca.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 76.Scali JJ. Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: a long-term study. Eur J Rheumatol Inflamm. 1995;15:57–62. [Google Scholar]
- 77.Waddell DD, Cefalu CA, Bricker DC. A second course of hylan G-F 20 for the treatment of osteoarthritic knee pain: 12-month patient follow-up. J Knee Surg. 2005;18:7–15. doi: 10.1055/s-0030-1248152. [DOI] [PubMed] [Google Scholar]
- 78.Waddell DD. The tolerability of viscosupplementation: low incidence and clinical management of local adverse events. Curr Med Res Opin. 2003;19:575–580. doi: 10.1185/030079903125002243. [DOI] [PubMed] [Google Scholar]
- 79.Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31:333–343. [PubMed] [Google Scholar]
- 80.Spitzer AI, Bockow BI, Brander VA, et al. Hylan G-F 20 improves hip osteoarthritis: a prospective, randomized study. Phys Sportsmed. 2010;38:35–47. doi: 10.3810/psm.2010.06.1781. [DOI] [PubMed] [Google Scholar]
- 81.Migliore A, Tormenta S, Massafra U, et al. Intra-articular administration of hylan G-F 20 in patients with symptomatic hip osteoarthritis: tolerability and effectiveness in a large cohort study in clinical practice. Curr Med Res Opin. 2008;24:1309–1316. doi: 10.1185/030079908x291930. [DOI] [PubMed] [Google Scholar]
- 82.Migliore A, Bizzi E, Massafra U, Bella A. The impact of treatment with hylan G-F 20 on progression to total hip arthroplasty in patients with symptomatic hip OA: a retrospective study. Curr Med Res Opin. 2012;28:755–760. doi: 10.1185/03007995.2011.645563. [DOI] [PubMed] [Google Scholar]
- 83.Qvistgaard E, Christensen R, Torp-Pedersen S, Bliddal H. Intra-articular treatment of hip osteoarthritis: a randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthr Cartil. 2006;14:163–170. doi: 10.1016/j.joca.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Carpenter B, Motley T. The role of viscosupplementation in the ankle using hylan G-F 20. J Foot Ankle Surg. 2008;47:377–384. doi: 10.1053/j.jfas.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 85.Witteveen AG, Giannini S, Guido G, et al. A prospective multi-centre, open study of the safety and efficacy of hylan G-F 20 (Synvisc) in patients with symptomatic ankle (talo-crural) osteoarthritis. Foot Ankle Surg. 2008;14:145–152. doi: 10.1016/j.fas.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Salk RS, Chang TJ, D’Costa WF, Soomekh DJ, Grogan KA. Sodium hyaluronate in the treatment of osteoarthritis of the ankle: a controlled, randomized, double-blind pilot study. J Bone Joint Surg Am. 2006;88:295–302. doi: 10.2106/JBJS.E.00193. [DOI] [PubMed] [Google Scholar]
- 87.Cohen MM, Altman RD, Hollstrom R, Hollstrom C, Sun C, Gipson B. Safety and efficacy of intra-articular sodium hyaluronate (Hyalgan) in a randomized, double-blind study for osteoarthritis of the ankle. Foot Ankle Int. 2008;29:657–663. doi: 10.3113/FAI.2008.0657. [DOI] [PubMed] [Google Scholar]
- 88.Sun SF, Hsu CW, Sun HP, Chou YJ, Li HJ, Wang JL. The effect of three weekly intra-articular injections of hyaluronate on pain, function, and balance in patients with unilateral ankle arthritis. J Bone Joint Surg Am. 2011;93:1720–1726. doi: 10.2106/JBJS.J.00315. [DOI] [PubMed] [Google Scholar]
- 89.Witteveen AG, Sierevelt IN, Blankevoort L, Kerkhoffs GM, van Dijk CN. Intra-articular sodium hyaluronate injections in the osteoarthritic ankle joint: effects, safety and dose dependency. Foot Ankle Surg. 2010;16:159–163. doi: 10.1016/j.fas.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Figen Ayhan F, U¨stun N. The evaluation of efficacy and tolerability of hylan G-F 20 in bilateral thumb base osteoarthritis: 6 months follow-up. Clin Rheumatol. 2009;28:535–541. doi: 10.1007/s10067-008-1080-0. [DOI] [PubMed] [Google Scholar]
- 91.Mandl LA, Hotchkiss RN, Adler RS, et al. Injectable hyaluronan for the treatment of carpometacarpal osteoarthritis: open label pilot trial. Curr Med Res Opin. 2009;25:2103–2108. doi: 10.1185/03007990903084016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heyworth BE, Lee JH, Kim PD, Lipton CB, Strauch RJ, Rosenwasser MP. Hylan versus corticosteroid versus placebo for treatment of Basal joint arthritis: a prospective, randomized, double-blinded clinical trial. J Hang Surg Am. 2008;33:40–48. doi: 10.1016/j.jhsa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Stahl S, Karsh-Zafrir I, Ratzon N, Rosenberg N. Comparison of intraarticular injection of depot corticosteroid and hyaluronic acid for treatment of degenerative trapeziometacarpal joints. J Clin Rheumatol. 2005;11:299–302. doi: 10.1097/01.rhu.0000191194.39926.c9. [DOI] [PubMed] [Google Scholar]
- 94.Schumacher HR, Meador R, Sieck M, Mohammed Y. Pilot investigation of hyaluronate injections for first metacarpal-carpal (MC-C) osteoarthritis. J Clin Rheumatol. 2004;10:59–62. doi: 10.1097/01.rhu.0000120894.49180.99. [DOI] [PubMed] [Google Scholar]
- 95.Bjornland T, Gjaerum AA, Moystad A. Osteoarthritis of the temporomandibular joint: an evaluation of the effects and complications of corticosteroid injection compared with injection with sodium hyaluronate. J Oral Rehabil. 2007;34:583–589. doi: 10.1111/j.1365-2842.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 96.Manfredini D, Bonnini S, Arboretti R, Guarda-Nardini L. Temporomandibular joint osteoarthritis: an open label trial of 76 patients treated with arthrocentesis plus hyaluronic acid injections. Int J Oral Maxillofac Surg. 2009;38:827–834. doi: 10.1016/j.ijom.2009.03.715. [DOI] [PubMed] [Google Scholar]
- 97.Saito S, Furuya T, Kotake S. Therapeutic effects of hyaluronate injections in patients with chronic painful shoulder: a meta-analysis of randomized controlled trials. Arthr Care Res (Hoboken) 2010;62:1009–1018. doi: 10.1002/acr.20174. [DOI] [PubMed] [Google Scholar]
- 98.Brander VA, Gomberawalla A, Chambers M, Bowen M, Nuber G. Efficacy and safety of hylan G-F 20 for symptomatic glenohumeral osteoarthritis: a prospective, pilot study. PM R. 2010;2:259–267. doi: 10.1016/j.pmrj.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 99.Merolla G, Sperling JW, Paladini P, Porcellini G. Efficacy of Hylan G-F 20 versus 6-methylprednisolone acetate in painful shoulder osteoarthritis: a retrospective controlled trial. Musculoskelet Surg. 2011;95:215–224. doi: 10.1007/s12306-011-0138-3. [DOI] [PubMed] [Google Scholar]
- 100.Silverstein E, Leger R, Shea KP. The use of intra-articular hylan G-F 20 in the treatment of symptomatic osteoarthritis of the shoulder: a preliminary study. Am J Sports Med. 2007;35:979–985. doi: 10.1177/0363546507300256. [DOI] [PubMed] [Google Scholar]
- 101.Blaine T, Moskowitz R, Udell J, et al. Treatment of persistent shoulder pain with sodium hyaluronate: a randomized, controlled trial. A multicenter study. J Bone Joint Surg Am. 2008;90:970–979. doi: 10.2106/JBJS.F.01116. [DOI] [PubMed] [Google Scholar]
- 102.Langworthy MJ, Saad A, Langworthy NM. Conservative treatment modalities and outcomes for osteoarthritis: the concomitant pyramid of treatment. Phys Sportsmed. 2010;38:133–145. doi: 10.3810/psm.2010.06.1792. [DOI] [PubMed] [Google Scholar]
- 103.Raynauld JP, Torrance G, Band P, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): clinical results. Osteoarthr Cartil. 2002;10:506–517. doi: 10.1053/joca.2002.0798. [DOI] [PubMed] [Google Scholar]
- 104.Kahan A, Lleu PL, Salin L. Prospective randomized study comparing the medicoeconomic benefits of hylan G-F 20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine. 2003;70:276–281. doi: 10.1016/s1297-319x(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 105.Brander VA, Stadler TS. Functional improvement with hylan G-F 20 in patients with knee osteoarthritis. Phys Sportsmed. 2009;37:38–48. doi: 10.3810/psm.2009.10.1728. [DOI] [PubMed] [Google Scholar]
- 106.Karatosun V, Unver B, Gocen Z, Sen A, Gunal I. Intra-articular hyaluronic acid is compared with progressive knee exercises in osteoarthritis of the knee: a prospective randomized trial with long-term follow-up. Rheumatol Int. 2006;26:277–284. doi: 10.1007/s00296-005-0592-z. [DOI] [PubMed] [Google Scholar]
- 107.Atamaz F, Kirazli Y, Akkoc Y. A comparison of two different intra-articular hyaluronan drugs and physical therapy in the management of knee osteoarthritis. Rheumatol Int. 2006;26:873–878. doi: 10.1007/s00296-005-0096-x. [DOI] [PubMed] [Google Scholar]
- 108.Lee SC, Rha DW, Chang WH. Rapid analgesic onset of intra-articular hyaluronic acid with ketorolac in osteoarthritis of the knee. J Back Musculoskelet Rehabil. 2011;24:31–38. doi: 10.3233/BMR-2011-0272. [DOI] [PubMed] [Google Scholar]
- 109.de Campos GC, Rezende MU, Pailo AF, Frucchi R, Camargo OP. Adding triamcinolone improves viscosupplementation: a randomized clinical trial. Clin Orthop Relat Res. 2013;471:613–620. doi: 10.1007/s11999-012-2659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neustadt DH. Long-term efficacy and safety of intra-articular sodium hyaluronate (Hyalgan) in patients with osteoarthritis of the knee. Clin Exp Rheumatol. 2003;21:307–311. [PubMed] [Google Scholar]
- 111.Lussier A, Cividino AA, McFarlane CA, Olszynski WP, Potashner WJ, De Medicis R. Viscosupplementation with hylan for the treatment of osteoarthritis: findings from clinical practice in Canada. J Rheumatol. 1996;23:1579–1585. [PubMed] [Google Scholar]
- 112.Waddell DD, Bricker DC. Hylan G-F 20 tolerability with repeat treatment in a large orthopedic practice: a retrospective review. J Surg Orthop Adv. 2006;15:53–59. [PubMed] [Google Scholar]
- 113.Evanich JD, Evanich CJ, Wright MB, Rydlewicz JA. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res. 2001;390:173–181. doi: 10.1097/00003086-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 114.Kemper F, Gebhardt U, Meng T, Murray C. Tolerability and short-term effectiveness of hylan G-F 20 in 4253 patients with osteoarthritis of the knee in clinical practice. Curr Med Res Opin. 2005;21:1261–1269. doi: 10.1185/030079905X56501. [DOI] [PubMed] [Google Scholar]
- 115.Dickson DJ, Hosie G, English JR. A double-blind, placebo-controlled comparison of hylan G-F 20 against diclofenac in knee osteoarthritis. J Clin Res. 2001;4:41–52. [Google Scholar]
- 116.Kean WF, Rainsford KD, Kean IR. Management of chronic musculoskeletal pain in the elderly: opinions on oral medication use. Inflammopharmacology. 2008;16:53–75. doi: 10.1007/s10787-008-1623-7. [DOI] [PubMed] [Google Scholar]
- 117.Lee S, Park D, Chmell SJ. Viscosupplementation with hylan G-F 20 (Synvisc®): pain and mobility observations from 74 consecutive patients. J Knee Surg. 2004;17:73–77. doi: 10.1055/s-0030-1248202. [DOI] [PubMed] [Google Scholar]
- 118.Waddell DD, Bricker D. Clinical experience with the effectiveness and tolerability of hylan G-F 20 in 1047 patients with osteoarthritis of the knee. J Knee Surg. 2006;19:19–27. doi: 10.1055/s-0030-1248072. [DOI] [PubMed] [Google Scholar]
- 119.Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthr Rheum. 2009;61:1704–1711. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]