Abstract
Cross-sectional research suggests that individuals at risk for internalizing disorders show differential activation levels and/or dynamics of stress-sensitive physiological systems, possibly reflecting a process of stress sensitization. However, there is little longitudinal research to clarify how the development of these systems over time relates to activation during acute stress, and how aspects of such activation map onto internalizing symptoms. We investigated children’s (n=107) diurnal hypothalamic-pituitary-adrenal activity via salivary cortisol (morning and evening levels) across 29 assessments spanning 6+ years, and related longitudinal patterns to acute stress responses at the end of this period (age 9–10). Associations with child psychiatric symptoms at age 10 were also examined to determine internalizing risk profiles. Increasing morning cortisol levels across assessments predicted less of a cortisol decline following interpersonal stress at age 9, and higher cortisol levels during performance stress at age 10. These same profiles of high and/or sustained cortisol elevation during psychosocial stress were associated with child anxiety symptoms. Results suggest developmental sensitization to stress—reflected in rising morning cortisol and eventual hyperactivation during acute stress exposure—may distinguish children at risk for internalizing disorders.
Keywords: Cortisol, Stress, Development, Internalizing Risk
Early life experiences are fundamental in shaping both the development of the stress response system and how an individual responds to stress across the lifespan. Exposure to a wide range of adversity (low socioeconomic status, child maltreatment, parental depression, etc.) has been associated with the development of internalizing syndromes (i.e., depression, anxiety) later in life (Dougherty, Klein, Rose, & Laptook, 2011; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008), and dysregulation of stress-sensitive systems—in particular, the hypothalamic-pituitary-adrenal (HPA) axis—has been proposed as a mechanism in this path (e.g., Gunnar & Quevedo, 2007). Although there is wide support for the link between early adversity and HPA axis dysregulation, a clear and specific characterization of ‘dysregulation’ is currently lacking in the literature. For example, HPA axis dysregulation has been alternatively characterized as higher or lower levels of HPA activity during certain times of day, higher or lower levels of peak HPA activation during acute stress, and exaggerated acute stress reactivity and/or an impaired recovery slope. Both mixed findings from cross-sectional investigations and limitations in the modeling techniques used to analyze HPA activation have left the field without a precise conceptualization of what stress dysregulation is and how it arises.
Early adversity appears to differentially affect components of HPA axis functioning across development. Work with preschoolers suggests that HPA hyporeactivity (as commonly indicated by an attenuated cortisol response to a laboratory stressor), as well as low diurnal cortisol levels, may serve as risk factors for later internalizing difficulties in this younger population, particularly in the context of early adversity (Hankin, Badanes, Abela, & Watamura, 2010; Badanes, Watamura, & Hankin, 2011). On the other hand, there is a relatively clear relationship in adults and adolescents between HPA axis hyperreactivity (indicated by heightened and/or extended cortisol response to a laboratory stressor), early stress exposure, and increased risk for internalizing psychopathology, such as depression or anxiety (Southwick, Vythilingham, & Charney, 2005; Ehlert, Gaab, & Heinrichs, 2001; Young & Korszun, 1998; Guerry & Hastings, 2011). One approach to understanding these risk processes is to use a narrow lens, deconstructing diurnal and/or acute response cortisol measures into more specific components such as the cortisol awakening response, diurnal slopes, and peak stress reactivity vs. recovery slopes. This approach has led to important refinements in knowledge about the nature of stress dysregulation (see Lopez-Duran, Mayer, & Abelson, 2014; Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012; Vargas & Lopez-Duran,). Another approach is to use a wide lens, connecting patterns of diurnal activation and acute response over time.
Although differences in HPA function appear to exist between high-risk young children and adolescents, no longitudinal work to our knowledge has investigated the developmental trajectory of stress system activation from early to late childhood as a predictor of later stress responsivity and internalizing problems in an attempt to explain these differences. The field needs a more integrative account of HPA axis dysregulation that considers how particular profiles of diurnal activation across childhood map onto acute responses to psychosocial stressors, and which aspects of these stress responses underlie which types of symptoms. We propose that a better understanding of which children within an adverse environmental context go on to develop internalizing psychopathology will come from shining a light on the dynamic HPA mechanisms underlying risk. In particular, discerning which developmental trajectories of children’s diurnal cortisol predict which aspects of responsiveness to acute stress will allow for a more complete characterization of stress sensitivity. To our knowledge, few longitudinal studies have examined children’s diurnal cortisol levels across multiple years (for exceptions see Shirtcliff et al., 2012; Trickett, Noll, Susman, Shenk, & Putnam, 2010) or taken into consideration both diurnal cortisol and acute stress responsiveness (Badanes et al., 2011). The present study investigates 1) how developmental changes in HPA axis functioning (i.e., diurnal cortisol levels) in a population of children exposed to moderate to high adversity relate to acute HPA axis responses (i.e., patterns of reactivity/recovery) in late childhood, and 2) how these acute HPA axis responses relate to anxiety-related psychopathology in late childhood.
Stress Sensitization Hypothesis
A potentially useful explanatory frame for understanding how changes in stress responsivity may give rise to internalizing problems is provided by the stress sensitization (or “kindling”) hypothesis. This model proposes that adverse experiences, including both dysphoric episodes and the life events that precede them, sensitize an individual’s stress response system, heightening reactivity to stress (Post, 1992; Heim & Nemeroff, 2001). As a result, individuals become more susceptible to physical and mental health problems following lower levels of subsequent stress (Post, 1992; Heim et al., 2008). Although the stress sensitization hypothesis was originally presented as a theory to explain the lifelong vulnerability to and recurrence of depression, it has since been extended to explain anxiety as well (e.g., McLaughlin, Conron, Koenen, & Gilman, 2010). Overall, this theory contributes to the understanding of how early experience with stress increases one’s vulnerability to internalizing problems.
Research across the developmental spectrum has provided support for the stress sensitization hypothesis. In particular, childhood adversity predicts a higher likelihood of clinically significant depression and/or anxiety upon exposure to subsequent stressors in children (Rudolph & Flynn, 2007), adolescents (Espejo et al., 2007; Harkness, Bruce, & Lumley, 2006), and adults (Hammen, Henry, & Daley, 2000; McLaughlin et al., 2010). However, despite the accumulating support based on retrospective reports for the stress sensitization hypothesis, much remains unknown about how stress sensitization unfolds over the course of development. In particular, the biological substrates of stress sensitization require further longitudinal study to determine how sensitization occurs.
The HPA Axis as a Marker of Stress Sensitivity
The HPA axis is the neuroendocrine system responsible for coordinating the body’s response to stressors via cortisol output from the adrenal gland (Sapolsky, Romero, & Munck, 2000). Beyond showing reactivity to acute stress, cortisol also demonstrates a diurnal pattern of release that contributes to the regulation of various other physiological systems, such as immune function (Kirschbaum et al., 1990; McEwen, 2006). Excessive stress exposure may cause dysregulation of normal HPA axis functioning, as manifested in altered levels and dynamics of cortisol responsiveness to acute stressors and/or disrupted diurnal patterns of cortisol release (McEwen, 2006). Because the HPA axis exhibits a protracted course of development, not reaching full maturity until adolescence (Gunnar & Donzella, 2002), experiences of early adversity can have lasting effects on this system. Here, we propose that the sensitization process outlined above based on behavioral research will be reflected in a particular developmental HPA profile; specifically, “stress sensitive” children are those who respond to adversity exposure with increasing basal (diurnal) HPA activation over time, resulting in heightened and/or sustained activation during acute stress situations, and ultimately the emergence of internalizing syndromes.
Diurnal cortisol
Considerable cross-sectional research links early adversity exposure with dysregulated diurnal HPA activity (e.g., Cicchetti & Rogosch, 2001; Bruce, Fisher, Pears, & Levine, 2009; Cutuli, Wiik, Herbers, Gunnar, & Masten, 2010; Shirtcliff & Essex, 2008). The directionality of this dysregulation has been varied across studies in children (for review, see Tarullo & Gunnar, 2006). Importantly, the extent to which dysregulated diurnal HPA activity manifests as hypercortisolism vs. hypocortisolism may depend on the age of assessment. In preschoolers, levels of early and concurrent stress have been shown to relate to diurnal cortisol such that high levels of early stress (i.e., in infancy) and concurrent stress are associated with high afternoon cortisol levels (Essex, Klein, Cho, & Kalin, 2002). High levels of early adversity in a sample of preschool-aged homeless children also have been associated with high morning cortisol levels (Cutuli et al., 2010). Alternatively, attenuated morning cortisol levels have been found in preschool-aged maltreated children in foster care (Bruce et al., 2009). These results suggest that elevated or attenuated diurnal cortisol may be associated with early adversity. A resolution to the question of which pattern signifies dysregulation may require broadening the lens from one point in time to examine changes across child development.
Key longitudinal investigations suggest that HPA axis dysregulation may manifest as stress sensitization over time following early adversity, as evidenced by increasing diurnal cortisol levels throughout childhood. Longitudinal work by Trickett and colleagues (2010) found in a population of children with a history of sexual abuse that cortisol hyperactivity (as evidenced by heightened morning cortisol levels) characterizes the developmental period from middle to late childhood. Another study of adopted children demonstrated that increasing adversity levels between ages 4.5 and 6 related to higher morning cortisol (Laurent et al., 2013). Finally, a longitudinal study of youth cortisol measured at ages 9, 11, 13, and 15 showed that for children exposed to multiple types of early life stress, increases in mental health symptoms were associated with increased morning cortisol across time (Essex et al., 2011). These results are consistent in suggesting that adversity relates to elevations in diurnal HPA activity during childhood. Yet, none of these studies incorporates response to an acute stressor, a critical marker of stress sensitivity as outlined above. To distinguish possible stress sensitization paths to internalizing disorders, prospective links between developmental changes in diurnal activation and acute responsiveness must be clarified.
Cortisol Response to Acute Stressors
Like diurnal cortisol, cortisol response to stressors has been examined extensively cross-sectionally. The most commonly used laboratory stressor tasks include the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum, Jobst, Wustmans, Kirschbaum, Rauh, & Hellhammer, 1997) and problem-solving tasks (see Granger, Weisz, & Kauneckis, 1994). These tasks elicit different types of stress, with the TSST-C serving as a performance stressor and problem-solving tasks—often completed with a caregiver—serving as interpersonal stressors. Both performance and interpersonal stressors have been shown to elicit HPA axis reactivity in children (Gunnar, Talge, & Herrera, 2009), though typical responses and developmental shifts may differ across stress types. In particular, whereas the magnitude of response and age-related increases appear stronger for performance stress, some individuals (i.e., females) may react more to interpersonal stress (Stroud et al., 2009; Stroud, Salovey, & Epel, 2002). Thus, examining cortisol reactivity and recovery across stressors promises the most complete view of HPA regulation and dysregulation.
Hyperreactivity, hyporeactivity, and/or nonrecovery following an acute stressor have each been associated with a variety of internalizing problems. Increased reactivity to a variety of psychosocial stressors (e.g., frustrating tasks, maternal separation) has been observed in depressed preschoolers compared to their nondepressed peers (Luby et al., 2003). In addition, anxious elementary and middle school children demonstrated increased cortisol reactivity to the TSST-C compared to their non-anxious peers (Mathewson et al., 2012), and increased cortisol reactivity to a parent-child conflict task has been shown in children referred to a clinic for internalizing, externalizing, and co-occuring problems (Granger et al., 1994). In contrast, Hankin and colleagues (2010) found in a longitudinal study from preschool through adolescence that high-risk, dysphoric children showed hyporeactivity to a stressor in preschool and 3rd grade, but hyperreactivity to a stressor in 9th grade, illustrating a developmental shift in cortisol response for at-risk youth. In addition to activation during the stressor itself, a failure to downregulate HPA activity following stress termination has been identified as a marker of internalizing problems in adults (Burke, Davis, Otte, & Mohr, 2005) and more recently in adolescents (Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013). Sustained cortisol elevations following the TSST appear to be particularly evident in adolescents with current internalizing problems (i.e., depression) and a history of early adversity as well as current chronic stress (Rao et al., 2008). These results are consistent with a stress sensitization characterization of HPA axis dysregulation underlying internalizing disorders; however, within-child research connecting increasing basal (diurnal) activation to heightened and/or prolonged acute stress responses would be needed to test this idea.
Diurnal Cortisol as a Predictor of Acute Cortisol Response
Limited work has examined diurnal cortisol and acute cortisol response within the same sample, let alone within children exposed to adversity. One study examined both diurnal and acute cortisol responses in depressed, at-risk, and a control group of adults and found that while diurnal cortisol levels (i.e., slope and cortisol awakening response [CAR]) did not differ between depressed and at-risk individuals, cortisol levels did differ between these groups during recovery following a laboratory stressor (Dienes, Hazel, & Hammen,, 2013). When assessed within the same study, diurnal cortisol and cortisol reactivity have typically shown differential relations to variables of interest (e.g., trait optimism in adults and ADHD in youth; Endrighi, Hamer, & Steptoe, 2011; Pesonen et al., 2011). Together, these studies suggest that valuable information can be gained by examining both diurnal and acute stress cortisol levels in the same population. However, none of these studies examined diurnal cortisol—whether at one time or across a developmental period—as a predictor of elevated cortisol in response to stress, a critical next step in understanding HPA axis dysregulation. To our knowledge, no research has prospectively examined how developmental shifts in child diurnal HPA activity over multiple years may predict HPA responses to acute stress in late childhood, and how these physiological profiles relate to early signs of internalizing disorders. Addressing this gap would significantly increase our understanding of the nature of HPA axis dysregulation and aid in early identification of stress-sensitive children exposed to adversity who will go on to develop internalizing disorders.
The Present Study
The present study seeks to more clearly define HPA axis dysregulation in terms of stress sensitization through an investigation of the links between diurnal HPA activation across childhood, acute HPA responses, and anxiety disorder symptoms in late childhood. This investigation was conducted in a sample of children from low-income families, some of whom were placed in foster care; thus, all children were exposed to at least moderate levels of early adversity, with some experiencing more extreme forms (i.e., maltreatment, parental separation). Anxiety disorders were selected as the outcome focus because, developmentally and ontologically, their onset typically precedes that of the depressive disorders with which they are often comorbid, and thus may best represent a behavioral phenotype at risk for varying internalizing disorders later in life (Breslau, Schultz, & Peterson, 1995; Cole, Peeke, Martin, Truglio, & Seroczynski, 1998). We examined children’s diurnal cortisol (morning and evening) across 6+ years, as well as their acute cortisol response trajectories during both performance and interpersonal stress tasks at the end of this period (age 9–10). Symptoms of anxiety-related psychopathology were assessed at age 10 to determine internalizing risk profiles.
In this study we aimed to test the overarching hypothesis that a process of stress sensitization characterizes internalizing-related HPA dysregulation by addressing the following unanswered questions: 1) How does the developmental trajectory of diurnal (morning and evening) cortisol from early to late childhood relate to cortisol trajectories in response to stress in late childhood? 2) How do levels and/or dynamics of acute stress cortisol responses relate to various forms of internalizing (anxiety) risk in late childhood? and 3) How can the knowledge from the previous two questions inform how we understand and define HPA axis dysregulation?
Consistent with previous cortisol literature and the stress sensitization model outlined above, we predicted that elevations in daily HPA functioning would lead to hyperactivation during acute stress and internalizing problems in later childhood. More specifically, we predicted that children who displayed increasing diurnal cortisol across assessments would show higher cortisol levels during stress and more extended cortisol elevations following stress in late childhood, which in turn would relate to symptoms of anxiety disorders. We approached specific associations between aspects of the cortisol response and anxiety syndrome presentations in an exploratory fashion.
Method
Participants
Preschool-aged (3–6-year old) children (n = 177) were recruited from a public child welfare agency and the community in a moderate-sized Pacific Northwest city. The sample comprised foster children who were randomly assigned to the Multidimensional Treatment Foster Care for Preschoolers (MTFC-P) intervention condition (n = 57) or to a regular foster care (RFC) comparison condition (n = 60), and a group of same-aged, low-income community children (CC) who had not been involved in the child welfare system (n = 60). Intervention effects were not a focus of the current study, but see Fisher and Chamberlain (2000) for further information about MTFC-P. There were no differences between the three groups on child age, gender, or ethnicity. Across groups, the sample was 89% European American, 1% African American, 5% Latino, and 5% Native American, representative of the community from which children were recruited.
Of the total sample, a subset (n = 107) with child diagnostic data available at the final assessment of the study was included in the current investigation. A comparison of cases included versus those not included showed that the former tended to have higher cortisol levels at the beginning of the study, t(175) = 3.17, p < .05, and more of a decline in daily cortisol over the course of the study period, t(175) = 2.67, p < .05. The sample included in analyses also contained a lower proportion of RFC children, χ2(1) = 15.88, p < .05, compared to the group of children missing CSI data. See Table 1 for further descriptive information about the sample.
Table 1.
Sample Descriptives
| Range | M, SD | |
|---|---|---|
| Age at first assessment | 2.99 – 6.78 | 4.40, .83 |
| Age at final assessment | 8.74 – 12.88 | 10.30, .80 |
| CSI Symptoms | ||
| Obsessive-Compulsive | 0 – 2 | .20, .44 |
| Post-Traumatic Stress | 0 – 1 | .15, .36 |
| Social Phobia | 0 – 3 | 1.05, .40 |
| Specific Phobia | 0 – 1 | .30, .46 |
| Generalized Anxiety | 0 – 2 | .21, .51 |
| Percent | ||
| Child Sex | ||
| Males | 53.7 | |
| Parent Education (Caregiver 1, 2) | ||
| ≤ High School | 32.6, 44.2 | |
| Some College | 59.9, 40.6 | |
| ≥ 4-Yr College | 7.4, 15.2 | |
| Gross Household Income | ||
| < $20,000 | 26.3 | |
| $20,000–$39,999 | 38.6 | |
| $40,000–$59,999 | 27.5 | |
| ≥ $60,000 | 7.6 | |
Procedure
All children completed daily cortisol assessments 29 times across 6+ years: the first 25 assessments were at 1-month intervals, followed by a gap in data collection (M = 32 months), and then 4 further assessments occurred at 6-month intervals. At each assessment, saliva samples were collected for cortisol assay in the morning and evening over two consecutive days.
At the final assessment waves, when children were approximately 9 and 10 years old, respectively, they participated in a 2-hour laboratory visit involving acute stress tasks shown in prior research to activate the HPA axis (Gunnar, Talge, & Herrera, 2009). All assessments occurred in the mid-late afternoon to control for diurnal variation in cortisol output. After an initial adaptation period, during which both the child and accompanying caregiver completed study measures, children were presented with the primary stress task (Problem-Solving at first lab assessment, TSST-C at second lab assessment). Following the stress task, children completed further (non-stressful) study measures focusing on well-being, developmental status, and other domains.
For the Problem Solving Task, both the parent and child were asked to select an issue to discuss from a list of potential problem topics (e.g., conflicts about the child’s school performance, helping out around the house, whom s/he spends time with, what the child eats or wears – see Robin & Foster, 1989). Parent and child were presented with the parent’s topic first and asked to “discuss the issue and try to come up with the best solution or idea for how to handle this issue” for 5 minutes alone. This process was then repeated for the child’s topic.
The Trier Social Stress Task for Children (TSST-C; Buske-Kirschbaum et al., 1997) is a standardized protocol in which children give a speech and perform mental arithmetic aloud in front of two unfamiliar adult judges. For the speech portion, children are asked to imagine they have been accused of stealing money from a friend and must prepare a speech explaining that they have not done it to their teacher and principal. After a 5-minute preparation period, children give the 5-minute speech in front of the judges, with scripted prompts reminding them of the remaining time during each phase. After the speech, children are asked to perform serial subtractions aloud for 5 minutes, with the judges correcting any errors. Judges maintain a somber demeanor throughout the task phases, but adopt a more friendly demeanor after the tasks are completed, reminding the child that this is only pretend and congratulating him/her on the performance.
Measures
Salivary cortisol collection
Daily saliva collections occurred 30 min after the child awoke and before eating or drinking (AM) and 30 min before bedtime (PM). Caregivers were trained by research staff to complete saliva collection at home following procedures described in Schwartz and colleagues (1998). The child was given a piece of Trident® Original sugarless gum to stimulate saliva flow. After 1 minute of chewing, the child spit the gum out, and the caregiver tipped a Salivette® absorbent roll from a protective plastic tube into the child’s mouth without touching the roll. The child was instructed to keep the roll in his/her mouth for 1 minute without touching it, after which the caregiver assisted the child in re-inserting the roll into the protective tube. The caregiver then wrote the date and time of collection on the tube label, and samples were kept in participants’ freezers until collection for assay by research staff.
During each of the laboratory assessments, six salivary cortisol samples were collected from the child. The first sample was taken soon after arrival at the lab, the second was taken immediately prior to the stress task (M = 23 min later, SD = 5 min), and the remaining four samples were taken at 15-min intervals from the start of the task. Children were asked to chew a piece of Trident® Original sugarless gum to stimulate salivation before expelling saliva through a straw into a pre-labeled vial. Samples were stored at −20° C before shipping for assay.
Salivary cortisol assay and missing data
Samples were assayed using the High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, State College, PA), with samples from each child assayed in the same batch to minimize within-subject variability. All samples were assayed in duplicate and averaged, with duplicates varying more than 15% reassayed. Intraassay and interassay coefficients of variance were 2.7% and 11.0%. To minimize extraneous sources of cortisol variability, children who routinely used steroid-based medications (e.g., asthma inhalers) were excluded from the study, and caregivers were instructed to avoid sampling when their child periodically used steroid-based medications or was ill. Caregiver responses to questionnaires regarding sampling times and child eating and sleeping behaviors on sampling days were also examined to ensure compliance with sampling guidelines.
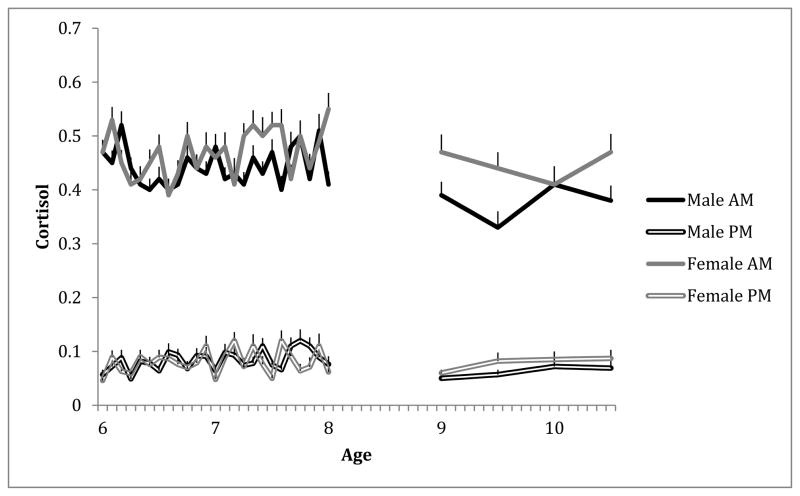
Of 20,532 possible daily samples, 1190 (5.8%) were missing because the caregiver failed to collect and/or return the sample, 30 (.1%) were missing because the cortisol value was out-of-range (> 2.0 ug/dl), 208 (1%) were missing because the sample time was incorrect (difference of > 30 min between sample tube and diary times or time did not correspond to the specified sampling window), and 4400 (21.4%) were missing for other reasons (i.e., family or caseworker refusal, lost sample, child unable to do the collection). Of 648 possible Problem-Solving session samples from the 108 children participating at that wave, 5 (.8%) were missing due to insufficient volume, 6 (1%) were missing due to incorrect collection time, and another 6 (1%) were missing due to labeling error. Of 642 possible TSST-C session samples from the 107 children participating at that wave, 2 (.3%) were missing due to insufficient volume, 6 (.9%) were missing due to incorrect collection time, and 4 (.6%) were missing due to labeling error, with another 9 (1.3%) missing for unknown reasons. Finally, of possible control variables considered—i.e., child age, sex, medication use, illness, sleep parameters (duration, night waking, wake time), collection time—only collection time related consistently to cortisol values, so this was included as a control in analyses1. Figure 1 displays observed morning and evening cortisol scores separately for male and female children across assessments.
Figure 1.
Mean observed morning and evening cortisol levels over time.
Child Symptom Inventory (CSI)
At the final assessment time, parents were asked about their child’s psychological symptoms using a standard diagnostic tool, the CSI (Gadow & Sprafkin, 1994). This interview (administered here in parent-report questionnaire form) includes DSM-based questions about youth diagnostic categories, including anxiety disorders. Total symptom counts for generalized anxiety disorder (GAD), obsessive-compulsive disorder (OCD), social phobia, specific phobia, and post-traumatic stress disorder (PTSD) were used to index child anxiety-related psychopathology (see Table 1 for description of the sample). Symptom scores, with the exception of specific phobia, were positively skewed and were thus log-transformed prior to analysis.
Data Analysis Plan
Hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002) was used to account for dependency in the data. This approach splits variability into within-person (Level 1) and between-person (Level 2) components; at Level 1, child cortisol trajectories across diurnal assessments or laboratory samples were modeled, which could in turn be explained by child psychopathology variables at Level 2. An advantage of HLM is that it allows missing data at Level 1 while using full information maximum likelihood (FIML) methods to arrive at parameter estimates; thus, children with missing cortisol data could still be included in analyses.
For illustration, the two-level equations addressing hypothesized links between (1) morning cortisol parameters and acute stress responses, and (2) anxiety symptoms and acute cortisol responses are shown:
Morning Cortisol Related to Acute Stress Responses
- Level 1 (within-child)
-
Level 2 (between-child)
(similar equations explain βT1-P2)
Child Anxiety Symptoms Related to Acute Stress Responses
- Level 1 (within-child)
-
Level 2 (between-child)
(similar equations explain βT1-P2)
Results
Baseline Models
Prior to testing explanatory models, baseline models with no predictors were fit to determine the best way of modeling (a) morning/evening cortisol scores from preschool through late childhood, and (b) acute stress responses in late childhood. In addition, parameter estimates from diurnal cortisol models were extracted to use as predictors in subsequent analyses.
First, children’s morning and evening cortisol levels across the 29 assessment occasions were modeled with intercept and linear slope terms, reflecting change in cortisol across months since study enrollment. All models controlled for sample collection time. On average, children showed a significant change in morning, but not evening, cortisol levels over time (starting level = .48, p < .001 and monthly slope = −.001, p < .001 for morning; term significantly improved model fit for morning cortisol, χ2(3) = 44.34, p < .001 (nonsignificant improvement for evening cortisol, χ2[3] = 5.99, ns). Significant between-child variability in both intercepts and slopes suggested that differences in children’s morning cortisol trajectories (i.e., some increasing, others decreasing over time) could be explained by individual difference predictors. Therefore, the linear model was retained for children’s morning cortisol, and an intercept-only model for evening cortisol. Each child’s estimated cortisol intercepts (morning starting level, evening mean level) and morning slope (monthly change) were retained to test as predictors of acute responsiveness.
Next, children’s cortisol levels during acute stress sessions were modeled with quadratic growth curves. Positive skew in cortisol scores was corrected by applying a natural log transformation. Models were centered at the modal peak stress sample (collected 15–20 minutes after task completion) so that intercepts represented cortisol levels during stress, linear terms indicated the child’s cortisol slope (increasing or decreasing) at that point in the session, and quadratic terms reflected the overall steepness of response curves. Separately modeling trajectories from the Problem-Solving and TSST-C sessions improved fit over a model that collapsed across sessions, χ2(18) = 388.48, p < .001.
On average, children’s (log-transformed) cortisol levels were declining at the peak stress sample (Problem-Solving linear = −.29, p < .001 from intercept = −2.87, p < .001; TSST-C linear = −.30, p < .001 from intercept = −2.78, p < .001). Although the average quadratic terms across children were not significant (Problem-Solving quadratic = .082, p = .10; TSST-C quadratic = −.13, p = .12), the fit improvement associated with adding quadratic parameters (χ2[13] = 48.25, p < .001) suggested that acute responses could not be adequately captured by linear models. Furthermore, significant between-child variability in all trajectory terms confirmed individual differences in response that could be predicted by children’s daily cortisol and/or mental health variables. This means that neither of these commonly used psychosocial stress tasks elicited significant reactivity in the sample as a whole, and average response trajectories could best be characterized as declining across the session. However, this decline did not follow a simple linear course, and children varied in the degree to which they displayed reactivity/recovery curves.
Daily Cortisol Over Time Related to Acute Responses (Table 2)
Table 2.
Children’s Morning Cortisol Over Time Related to Acute Stress Responses
| Predictor | TSST-C Session | Problem-Solving Session | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intercept β, p |
Linear β, p |
Quadratic β, p |
Intercept β, p |
Linear β, p |
Quadratic β, p |
|
| Mean Trajectory | −.018, .851 | −.44, < .001 | −.19, .116 | −.14, .082 | −.42, < .001 | .11, .098 |
| Starting Cortisol Level (first daily assessment) | −.019, .849 | −.045, .602 | −.13, .202 | −.030, .735 | −.11, .027 | −.12, .050 |
| Cortisol Monthly Slope (change across 29 assessments) | .24, .009 | .006, .947 | −.039, .689 | .046, .553 | .13, .019 | .12, .042 |
Note. Child cortisol scores were log-transformed to correct positive skew.
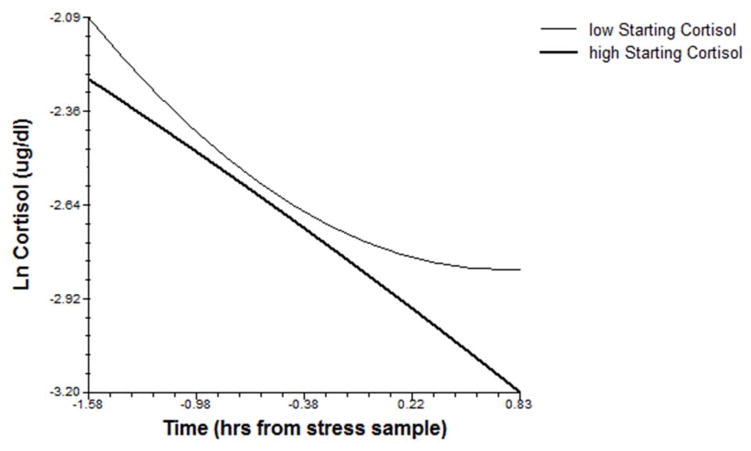
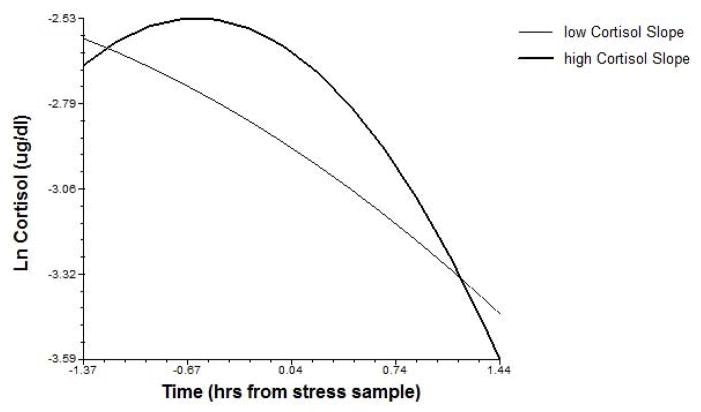
To determine whether children’s developmental patterns of diurnal cortisol related to acute stress response trajectories in late childhood, their starting morning cortisol and monthly slope, as well as mean evening cortisol, were tested as predictors of Problem-Solving and TSST-C stress trajectories. Significant effects of these terms were consistent with a sensitization model; children with lower starting morning cortisol levels showed sustained cortisol elevation (more positive slope) following Problem-Solving stress, and those whose morning cortisol levels increased over time additionally showed higher cortisol levels during TSST-C stress (see Figures 2–3). Evening cortisol levels did not relate to acute stress responses.
Figure 2.
Morning cortisol levels in preschool predict cortisol slopes during Problem-Solving stress in late childhood (shown at upper and lower quartile values of starting cortisol levels).
Figure 3.
Change in morning cortisol levels from preschool through late childhood predict cortisol levels during TSST-C stress in late childhood (shown at upper and lower quartile values of cortisol slopes).
Acute Cortisol Responses Related to Anxiety Symptoms (Table 3)
Table 3.
Children’s Acute Cortisol Responses Related to CSI Anxiety Symptoms
| Predictor | TSST-C Session | Problem-Solving Session | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intercept β, SE |
Linear β, SE |
Quadratic β, SE |
Intercept β, SE |
Linear β, SE |
Quadratic β, SE |
|
| Mean Trajectory | −.14, .075 | −.44, < .001 | −.19, .106 | −.018, .855 | −.42, < .001 | .12, .094 |
| Obsessive-Compulsive | .090, .257 | −.058, .587 | .12, .379 | .018, .821 | .16, .026 | .049, .496 |
| Post-Traumatic Stress | .17, .046 | .031, .687 | −.014, .911 | .030, .660 | .16, .013 | .060, .294 |
| Specific Phobia | .28, .001 | .12, .109 | −.050, .658 | .077, .354 | .031, .688 | .009, .908 |
| Generalized Anxiety | .17, .009 | −.0007, .993 | .20, .026 | .26, < .001 | −.005, .937 | −.13, .123 |
Note. Child cortisol and symptom scores (except specific phobia) were log-transformed to correct positive skew.
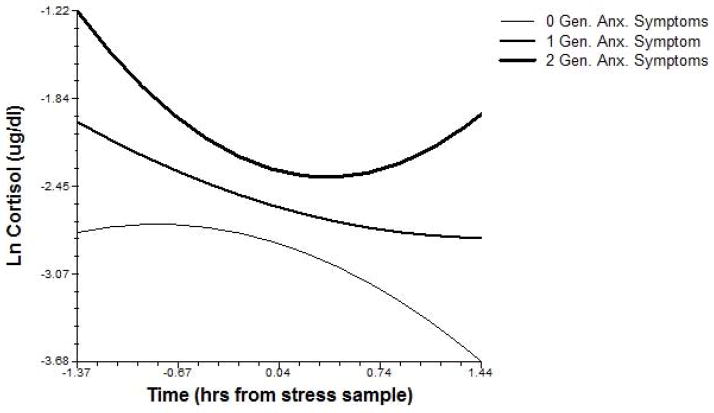
Anxiety disorder symptoms were also tested in relation to acute cortisol response trajectories. Obsessive-compulsive symptoms related to sustained cortisol elevation following Problem-Solving stress, as indexed by a more positive linear term. Specific phobia symptoms related to higher TSST-C stress cortisol levels, and PTSD symptoms related to both higher TSST-C cortisol and sustained elevation following Problem-Solving stress. Finally, generalized anxiety symptoms related to higher cortisol levels during both TSST-C and Problem-Solving stress, and a flatter TSST-C stress response curve (more positive quadratic; see Figure 4).
Figure 4.
Children’s generalized anxiety symptoms relate to cortisol levels and response dynamics during TSST-C stress in late childhood (shown at observed symptom values for the sample).
Alternative Model Testing
While continuous growth curve models are useful for describing the shape and dynamics of overall response trajectories, they cannot give specific information about effects involving pre- vs. post-task components of the stress response. Further, the modal peak (at the post-task sample) does not necessarily apply to every child, and registering models such that the intercept represents each child’s peak may better represent individual responses (see Lopez-Duran, Mayer, & Abelson, 2014). To better interpret effects identified above, piecewise growth models with individual peak registration were fit to separately estimate children’s (a) pre-peak slopes, (b) peak levels, and (c) post-peak slopes. The baseline model showed that children’s cortisol declined, on average, during both portions of the Problem-Solving session (pre-peak slope = −.31, p < .001; log-transformed peak level = −2.84, p < .001; post-peak slope = −.31, p < .001) and during the second portion of the TSST-C session (pre-peak slope = −.08, ns; log-transformed peak level = −2.71, p < .001; post-peak slope = −.48, p < .001). Consistent with the quadratic model results above, this means that neither task elicited acute reactivity in the sample as a whole; however, significant between-child variability in all terms again suggested that some children did react, whereas others showed no change or continuously declined across the session.
Results of explanatory peak-registered models generally echoed effects reported above on child stress session cortisol intercepts (which mapped onto peak levels) and slopes (post-peak slopes). Specifically, morning cortisol slopes (β = .27, p = .006) and specific phobia symptoms (β = .23, p = .01) related positively to TSST-C peak cortisol levels, generalized anxiety symptoms related positively to peak cortisol levels during both sessions (β = .29, p = .003 for Problem Solving; β = .14, p = .02 for TSST-C), and PTSD symptoms related positively to post-peak Problem-Solving slopes (β = .25, p = .009). These models also shed further light on the source of quadratic effects; whereas the generalized anxiety effect on TSST-C curves appeared to be driven by the pre-peak slope (β = −.19, p = .06), morning cortisol effects on Problem-Solving curves were driven by the post-peak slope (starting level β = −.20, p = .05; monthly slope β = .21, p = .03).
Another question is whether effects on cortisol levels were specific to stress responses, or represented more general differences in HPA activation. Models centered at the first sample in the Problem-Solving or TSST-C session were examined, providing partial support for task-specificity. In particular, these models revealed nonsignificant associations between pre-task TSST-C cortisol intercepts and both PTSD (β = .12, p = .19) and specific phobia symptoms (β = .12, p = .22), and between pre-task Problem-Solving cortisol intercepts and generalized anxiety symptoms (β = .12, p = .07). Pre-task TSST-C intercepts were significantly related to morning cortisol slopes (β = .21, p = .02) and generalized anxiety symptoms (β = .32, p < .001); thus, these two effects reflected more generally elevated HPA activation, and not task-specific elevation.
In summary, the above models show that children who began with low morning HPA activation during preschool but who increased across childhood were more likely to show elevated and/or extended activation during psychosocial stress. These patterns were, in turn, associated with different anxiety symptom presentations in late childhood.
Discussion
Prior research has sought to characterize the nature of HPA dysregulation by focusing on specific components of diurnal or acute stress function. In this study we extend this work with an integrative account of stress sensitivity mechanisms that give rise to internalizing disorders by examining both diurnal levels of HPA activity across childhood and acute stress response trajectories in a sample of children exposed to a range of early adversity. Longitudinal assessments of morning and evening cortisol highlighted rising morning cortisol as a predictor of heightened cortisol during stress and/or sustained elevation following stress, each of which mapped onto particular sets of anxiety symptoms. Evening cortisol, on the other hand, did not relate to acute stress responsiveness. Based on these results, it appears that an increasing morning HPA profile rendered children especially sensitive to the effects of relatively low-level psychosocial stressors (as represented by Problem-Solving and TSST-C tasks), with high and/or extended stress activation fueling different internalizing disorder presentations. By contrast, children who started with higher levels of morning HPA activity that modulated downward across childhood appeared protected. The current findings help to refine our understanding of how different aspects of HPA function (i.e., diurnal and acute stress activity) relate to one another in a high-risk developmental context. This study further confirms with a prospective longitudinal design what had long been suspected based on retrospective reports of adults—i.e., that a process of stress sensitization, characterized by increasing diurnal activation culminating in heightened activity during/following stress, underlies internalizing syndromes. This is an important step, given the possibility of differing neurobiological profiles for anxiety in adults vs. children (Garcia de Miguel, Nutt, Hood, & Davies, 2012).
As predicted, children whose diurnal cortisol levels started low but increased over time showed elevated cortisol and declined more slowly in the context of acute performance and/or interpersonal stress. Although the idea that basal cortisol levels and acute responsiveness represent related aspects of an underlying HPA profile has been discussed (Boyce & Ellis, 2005), there is very little empirical research linking the two, particularly in childhood. Evidence from studies such as this is needed to determine how these HPA indices relate at different points in development. The current findings support positive links between morning cortisol slopes from early-late childhood and cortisol levels both preceding and during acute stress in late childhood. It may be that children who adapt to the stresses of daily life by calibrating their morning HPA activation upward bring this threat readiness to acute stress contexts, fueling high/extended responses to anticipated and actual stress that feed back into higher cortisol as they confront the day. Some explanations for this pattern include a lower threshold for HPA activation and/or a deficit in the HPA negative feedback mechanism, possibly reflecting (epi)genetic influences on the hypothalamus and upstream inputs (i.e., enhanced amygdala, suppressed hippocampal function; see Simmons, Howard, Simpson, Akil, & Clinton, 2012). Consistent with the body of evidence linking HPA hyperactivation to anxiety-related behaviors (e.g., Deussing & Wurst, 2005), this physiological profile in turn may feed (and be fed by) anxiety symptoms.
However, this coupling of diurnal cortisol and acute responsiveness is not necessarily universal; even within this study, differences were found across morning and evening cortisol, and between stress tasks. Although definitive statements about the importance specifically of morning cortisol must await further replication, it may be that the stress readiness associated with HPA activation at the start of the day best captures the sensitization process underlying anxiety-related psychopathology, whereas difficulties downregulating stress at the end of the day map onto different symptom profiles. In addition, we found effects in the same direction but of differing strengths (and not always significant) across Problem-Solving and TSST-C stress sessions. Given that child age (9 or 10) and stress type (interpersonal or performance) were confounded in this design, we cannot definitively disentangle the source of differences. The fact that morning cortisol levels at the beginning of the study related to Problem-Solving trajectories only, whereas change in cortisol levels over time additionally related to TSST-C trajectories, is consistent with a timing effect—i.e., it may be harder to detect effects of earlier HPA function after a longer time lag. Similarly, development may help to understand internalizing symptom effects in that anxiety-related difficulties downregulating cortisol following stress at age 9 may give rise to higher absolute cortisol levels at age 10. At the same time, it is possible that ongoing elevation following interpersonal stress and heightened activation during performance stress are particularly relevant for early signs of anxiety disorders. Further investigation of both child and stressor factors that drive the strength of diurnal-acute stress HPA associations is warranted.
Although the same basic stress sensitivity profile—i.e., low starting morning cortisol that increased over time, higher acute stress cortisol levels and/or sustained elevation—was implicated in various forms of anxiety disorder, certain forms better represented each aspect of this pattern. Given the paucity of research clearly differentiating HPA features of different childhood anxiety disorders (see Faravelli et al., 2012), any interpretation of these differences remains speculative. However, we hope that by considering links between HPA function and behavioral signatures of each disorder, we offer a base on which further research can build.
Whereas children with OCD symptoms failed to show the typical post-stress cortisol decline (but not higher cortisol levels), those with symptoms of specific phobia showed the opposite—high cortisol levels during stress, but no difference in stress dynamics. The idea that child OCD is characterized not by higher arousal, but by lengthier arousal, fits with previous research indicating a blunted cortisol response to stress in children and adolescents with OCD (Gustafsson, Gustafsson, Ivarsson, & Nelson, 2008). It also maps onto the typical presentation of the disorder, which involves less anxious arousal and more perseveration than most of the other anxiety disorders. On the other side, phobias more clearly involve subjective (and, according to the current results, neuroendocrine) hyperarousal. Though preliminary, these results suggest a possible divergence between anxiety problems involving heightened vs. extended arousal, which could have meaningful implications for screening and intervention. Based on the current study design, it is unknown whether divergent phenotypes could be detected in early childhood, or represent an emergent property of the developing child; future research should examine HPA reactivity/recovery parameters at multiple times from infancy through late childhood to discern this.
PTSD and GAD symptoms were characterized by both higher cortisol during stress (as well as, for GAD, higher cortisol preceding performance stress) and less dynamic response trajectories. Following the argument outlined above, these disorders involve both a prominent hyperarousal component and a maintenance of anxiety even in the absence of immediate threat. This pattern dovetails with PTSD symptoms of hypervigilance and reactivation of trauma-related responses, perhaps due to incomplete stress recovery. Although PTSD is often characterized by hypocortisolism in adults, previous studies in youth trauma survivors have demonstrated elevated cortisol in response to both performance (TSST-C) and parent-child problem-solving stress (Dietz et al., 2013; Saxbe, Margolin, Spies Shapiro, & Baucom, 2012). Further, initially elevated child cortisol following trauma has been shown to give way to lower cortisol levels over time (Pervanidou & Chrousos, 2012; Trickett, et al., 2010); thus, the current findings may represent an early phase of this HPA hyper- to hypoactivation process.
In contrast to the positive effects on post-task cortisol slopes outlined above (detected using both quadratic growth curves and piecewise models), GAD symptoms were additionally associated with more negative pre-task slopes (detectable only with the latter approach). This illustrates the utility of examining stress responsiveness in multiple ways and is consistent with a chronically heightened state of alert—i.e., elevated HPA activation even before stress exposure that only partially diminishes once the stressor has passed. GAD is indeed distinguished by chronic physical, cognitive, and affective manifestations of anxiety, with a trait-like rather than episodic presentation. Given this stability, as well as high comorbidity with depression, child GAD may represent the prototype for emergent internalizing risk. Surprisingly, relatively few studies have addressed HPA function in children with GAD, though available findings are consistent with a deficit in HPA negative feedback that would explain rising cortisol levels (Pfeffer, Altemus, Heo, & Jiang, 2007). Further work tracing HPA function and both GAD and depressive symptoms from childhood through adolescence (the typical onset of depression) may help to clarify which characteristics, if any, are specific to anxiety, and which underlie a broader vulnerability to internalizing disorder.
While the current study sheds light on stress dysregulation processes by identifying prospective links between children’s diurnal and acute stress HPA activity and anxiety symptoms, it is not without limitations. An important limitation is that we did not directly examine children’s adversity exposure in relation to HPA function. All of the children involved in this study can be assumed to have faced some degree of adversity due to low socioeconomic status, which may be enough to push vulnerable children toward HPA sensitization. The proxy for differential adversity exposure in this study—i.e., membership in regular foster care vs. treatment foster care vs. community group—did not appear to play a large role in these findings, though moderated effects that were found supported heightened HPA sensitization in high-risk (regular foster care) children. In line with this argument for contextual stress sensitization, another recent study in this sample showed that even though regular foster care children typically showed lower cortisol levels than their lower-risk counterparts, higher cortisol in this group only was associated with anxiety symptoms (Authors, in press). Another limitation lies in the relatively low levels and range of anxiety psychopathology in this sample, which would be expected to attenuate effects. Future research should investigate both short-term and chronic adversity effects on longitudinal HPA regulation and mental health in samples at high risk for internalizing disorders due to family history and/or early symptom presentation.
Interpretation of the current results must be tempered by the fact that neither the Problem-Solving nor the TSST-C stressor elicited significant cortisol reactivity in the sample as a whole, and average trajectories were characterized by declining slopes across the sessions. It may be that children in this sample typically experienced greater anticipatory stress coming to the lab than task-related reactivity—this would be consistent with greater pre-task declines in the first (Problem-Solving) lab session—and we cannot assume that post-task slope effects reflect acute stress recovery in the narrow sense. This does not negate the fact that individual differences in response dynamics related to child adjustment were detected, but it does underline the message that care should be taken in discussing the origins of stress session effects (i.e., which are specific to stress-induced cortisol levels vs. prior activation, the extent to which cortisol declines represent task-related recovery vs. recovery from more generalized stress). These distinctions will need to be more thoroughly teased apart with research designs that capture cortisol measures both at home and at repeated intervals during different laboratory stress situations.
While the current study design offered a more complete assessment of the stress response than is typical by including two separate stress paradigms, it did not allow a separation of developmental from stressor type effects. Further studies involving both performance and interpersonal stressors at different ages will be needed to fully interpret these effects. It would also be useful to follow children into adolescence to obtain a better picture of which HPA signatures predict early-onset vs. later-onset symptoms of both anxiety and depression. This study yields a preliminary framework for understanding how diurnal cortisol across childhood relates to acute stress parameters and symptoms in late childhood; future efforts that include multiple longitudinal measures of each of these constructs will advance an integrated developmental model that better pinpoints when and how stress dysregulation arises. Finally, denser cortisol measurement would allow further consideration of specific constructs such as the cortisol awakening response and diurnal slopes, in addition to peak reactivity/recovery and change over time. Research designs that enable the use of both a narrow and wide lens on stress system functioning promise to elucidate more fully the nature of HPA dysregulation.
For now, this study adds a critical piece to the puzzle of where internalizing disorders start by delineating childhood paths from rising morning cortisol to acute stress hypersensitivity to anxiety symptoms. With a clearer understanding of who is at greatest risk and why, we will be in a better position to offer timely support to the children and families who need it most.
Acknowledgments
The support for this research was provided by the grants MH59780 and MH65046 from the National Institute of Mental Health.
Footnotes
Child sex was found to relate to morning cortisol slopes, but primary predictor effects were unaffected by inclusion of this variable. Wake time related to morning cortisol levels, but this effect was not as strong as that of collection time (with which it was confounded). Thus, reported models included only the collection time control.
References
- Authors. HPA stability for children in foster care: Mental health implications and moderation by early intervention. Developmental Psychobiology. doi: 10.1002/dev.21226. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Research. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol Levels in preschool aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cole DA, Peeke LG, Martin JM, Truglio R, Seroczynski AD. A longitudinal look at the relation between depression and anxiety in children and adolescents. Journal of Consulting and Clinical Psychology. 1998;66:451–460. doi: 10.1037//0022-006x.66.3.451. [DOI] [PubMed] [Google Scholar]
- Cutuli JJ, Wiik KL, Herbers JE, Gunnar MR, Masten AS. Cortisol function among early school-aged homeless children. Psychoneuroendocrinology. 2010;35:833–845. doi: 10.1016/j.psyneuen.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, Wurst W. Dissecting the genetic effect of the CRH system on anxiety and stress-related behavior. Comptes Rendus Biologies. 2005;328:199–212. doi: 10.1016/j.crvi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Dienes KA, Hazel NA, Hammen C. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38:927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz LJ, Stoyak S, Melhem N, Porta G, Matthews KA, Walker Payne M, Brent DA. Cortisol response to social stress in parentally bereaved youth. Biological Psychiatry. 2013;73:379–387. doi: 10.1016/j.biopsych.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-Pituitary Adrenal Axis Reactivity in the Preschool-Age Offspring of Depressed Parents Moderation by Early Parenting. Psychological Science. 2011;22:650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Endrighi R, Hamer M, Steptoe A. Associations of trait optimism with diurnal neuroendocrine activity, cortisol responses to mental stress, and subjective stress measures in healthy men and women. Psychosomatic Medicine. 2011;73:672–678. doi: 10.1097/PSY.0b013e31822f9cd7. [DOI] [PubMed] [Google Scholar]
- Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman JM, Bor W. Stress sensitization and adolescent depressive severity as a function of childhood adversity: a link to anxiety disorders. Journal of Abnormal Child Psychology. 2007;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM. Influence of early life stress on later hypothalamic pituitary adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Lo Sauro C, Godini L, Lelli L, Benni L, Pietrini F, Lazzeretti L, Talamba GA, Fioravanti G, Ricca V. Childhood stressful events, HPA axis and anxiety disorders. World Journal of Psychiatry. 2012;2:13–25. doi: 10.5498/wjp.v2.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventories Manual. Stony Brook, NY: Checkmate Plus; 1994. [Google Scholar]
- Garcia de Miguel B, Nutt DJ, Hood SD, Davies SJC. Elucidation of neurobiology of anxiety disorders in children through pharmacological challenge tests and cortisol measurements: A systematic review. Journal of Psychopharmacology. 2012;26:431–442. doi: 10.1177/0269881110372818. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review. 2011;14:135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Gustafsson PA, Ivarsson T, Nelson N. Diurnal cortisol levels and cortisol response in youths with obsessive-compulsive disorder. Neuropsychobiology. 2008;57:14–21. doi: 10.1159/000123117. [DOI] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. Journal of Consulting and Clinical Psychology. 2000;68:782–787. [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Bruce AE, Lumley MN. The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. Journal of Abnormal Psychology. 2006;115:730–741. doi: 10.1037/0021-843X.115.4.730. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, Hellhammer DH. Cortisol and behavior: 2. Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology. 1990;15:297–307. doi: 10.1016/0306-4530(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, Leve LD. Stress system development from age 4.5 to 6: Family environment predictors and adjustment implications of HPA activity stability versus change. Developmental Psychobiology. 2013 doi: 10.1002/dev.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Mayer SE, Abelson JL. Modeling neuroendocrine reactivity in salivary cortisol: Adjusting for peak latency variability. Stress. 2014 doi: 10.3109/10253890.2014.915517. epub May 8. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Miskovic V, Cunningham CE, McHolm AE, Boyle MH, Schmidt LA. Salivary cortisol, socioemotional functioning, and academic performance in anxious and non-anxious children of elementary and middle school age. Early Education & Development. 2012;23:74–95. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 2006;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61:611–619. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Kajantie E, Jones A, Pyhala R, Lahti J, Heinonen K, Eriksson JG, Strandberg TE, Raikkonen K. Symptoms of attention deficit hyperactivity disorder in children are associated with cortisol responses to psychosocial stress but not with daily cortisol levels. Journal of Psychiatric Research. 2011;45:1471–1476. doi: 10.1016/j.jpsychires.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the September 11, 2001 terror attacks. Biological Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of Psychosocial Stress Into the Neurobiology. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Sage; 2002. [Google Scholar]
- Robin AL, Foster SL. Negotiating parent-adolescent conflict: A behavioral-family systems approach. Guilford Press; 2002. [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology. 2007;19:497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Margolin G, Spies Shapiro LA, Baucom BR. Does dampened physiological reactivity protect youth in aggressive family environments? Child Development. 2012;83:821–830. doi: 10.1111/j.1467-8624.2012.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RK, Howard JL, Simpson DN, Akil H, Clinton SM. DNA methylation in the developing hippocampus and amygdala of anxiety-prone versus risk-taking rats. Developmental Neuroscience. 2012;34:58–67. doi: 10.1159/000336641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment*. Annual Review of Clinical Psychology. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology. 2013;41:1–12. doi: 10.1007/s10802-013-9740-1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle C, Shirtcliff EA, Lemergy-Chalfant K, Goldsmith HH. Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Hormones and Behavior. 2012;62:36–42. doi: 10.1016/j.yhbeh.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas I, Lopez-Duran N. Dissecting the impact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology. 2014;40:10–16. doi: 10.1016/j.psyneuen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A. Psychoneuroendocrinology of depression: hypothalamic-pituitary-gonadal axis. Psychiatric Clinics of North America. 1998;21:309–323. doi: 10.1016/s0193-953x(05)70007-1. [DOI] [PubMed] [Google Scholar]