Table 5.
[CoII(Por)]-catalysed trans-selective β-lactam synthesis using different N-tosylhydrazone sodium salts and imines.[a]
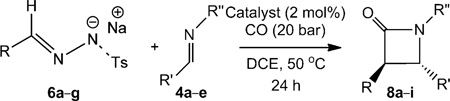 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | R | R′ | R″ | Yield (%)[b] (trans:cis) |
| 1 | [CoII(P1)] | Ph | Ph | Me | 64 (8a) (>95:5) |
| 2 | [CoII(P2)] | Ph | Ph | Me | 65 (8a) (>95:5) |
| 3 | [CoII(P3)] | Ph | Ph | Me | 65 (8a) (>95:5) |
| 4 | [CoII(P1)] | Ph | Ph | PhCH2 | 62 (8b) (>90:10) |
| 5 | [CoII(P1)] | Ph | pClC6H4 | Me | 63 (8c) (>95:5) |
| 6 | [CoII(P1)] | Ph | pOMeC6H4 | Me | 73 (8d)(> 90:10) |
| 7 | [CoII(P1)] | pMeC6H4 | Ph | Me | 77 (8e) (>95:5) |
| 8 | [CoII(P1)] | pClC6H4 | Ph | Me | 62 (8f) (>95:5) |
| 9 | [CoII(P1)] | pMeOC6H4 | Ph | Me | 73 (8g) (>85:15) |
| 10 | [CoII(P1)] | 2-napthyl | Ph | Me | 67 (8h) (>95:5) |
| 11 | [CoII(P1)] | PhCH = CH | Ph | Me | 52 (8i) (>95:5) |
Stoichiometry: N-tosylhydrazone:imine=1:2.
Isolated yields after column chromatography.
