Abstract
Patient: Male, 23
Final Diagnosis: Spontaneous coronary artery dissection
Symptoms: Chest discomfort • chest pain
Medication: —
Clinical Procedure: Coronary computed tomography angiography
Specialty: Radiology
Objective:
Rare disease
Background:
Multidetector computed tomography (MDCT) has gained wide acceptance in the evaluation of the cardiovascular system. Of particular clinical interest is its ability to non-invasively evaluate coronary arteries in patients presenting to the emergency room. In acute coronary syndromes, myocardial ischemia is most often caused by atherosclerosis. We present a case of a rare cause of acute coronary syndrome, spontaneous coronary artery dissection (SCAD), which was initially evaluated with MDCT and followed by intravascular ultrasound (IVUS) and invasive coronary angiography (ICA). We discuss the findings and role of each modality with particular attention to coronary computed tomographic angiography (CCTA) in the diagnosis and management of SCAD. As the use of CCTA in the emergency department continues to rise, radiologists must become familiar with CT appearance of SCAD.
Case Report:
We report the multidetector computed tomography (MDCT), intravascular ultrasound (IVUS), and invasive coronary angiography (ICA) findings in a case of spontaneous coronary artery dissection of the left anterior descending artery in a previously healthy 23-year-old man. The role of coronary computed tomographic angiography (CCTA) in diagnosis and management of this potentially life-threatening condition is discussed.
Conclusions:
In the clinical setting of acute coronary syndrome, SCAD must be a consideration, particularly in young patients without clear risk factors for coronary artery disease and in women in the peripartum period. CCTA is a very helpful diagnostic tool to diagnose the condition in a non-invasive manner and to follow up after treatment.
MeSH Keywords: Coronary Angiography; Coronary Vessel Anomalies; Dissection; Multidetector Computed Tomography; Ultrasonography, Interventional
Background
Multidetector computed tomography (MDCT) has gained wide acceptance in the evaluation of the cardiovascular system. Of particular clinical interest is its ability to non-invasively evaluate the coronary arteries in patients presenting to the emergency room. In acute coronary syndromes, myocardial ischemia is most often caused by atherosclerosis. We present a case of a rare cause of acute coronary syndrome, spontaneous coronary artery dissection (SCAD), which was initially evaluated with MDCT and followed by intravascular ultrasound (IVUS) and invasive coronary angiography (ICA). We discuss the findings and role of each modality, with particular attention to coronary computed tomographic angiography (CCTA) in the diagnosis and management of SCAD. As the use of CCTA in Emergency Departments continues to rise, radiologists must become familiar with CT appearance of SCAD.
Case Report
We report the case of a healthy 23-year-old Hispanic male without significant past medical history, who began to experience chest discomfort 2 days prior to presentation. When the pain suddenly intensified, he presented to the Emergency Department. The patient denied strenuous exercise, use of drugs, shortness of breath, palpitations, diaphoresis, or nausea. In the Emergency Room, nitrates relieved the pain. After being monitored overnight, it was felt that given his age and lack of risk factors there was a low probability that his chest discomfort had a cardiac etiology. Results of a urine drug screen were negative. Serial cardiac markers, however, did reveal mildly elevated troponin levels (troponin-I 0.2). EKG revealed sinus rhythm with diffuse 1-millimeter ST elevations.
A prospectively-triggered, electrocardiographically (ECG) synchronized CCTA using a 64-slice MDCT was performed. Post processing of the transaxial source images was carried out on a dedicated workstation. The study revealed no atherosclerosis or congenital coronary anomaly. Cardiac morphology was normal. Transaxial and post-processed images, however, clearly demonstrated a dissection of the mid-portion of the left anterior descending (LAD) artery. A false channel, intimal flap, and an intramural hematoma were noted to cause narrowing of the true lumen. Distal vascular opacification was seen (Figures 1–5).
Figure 1.
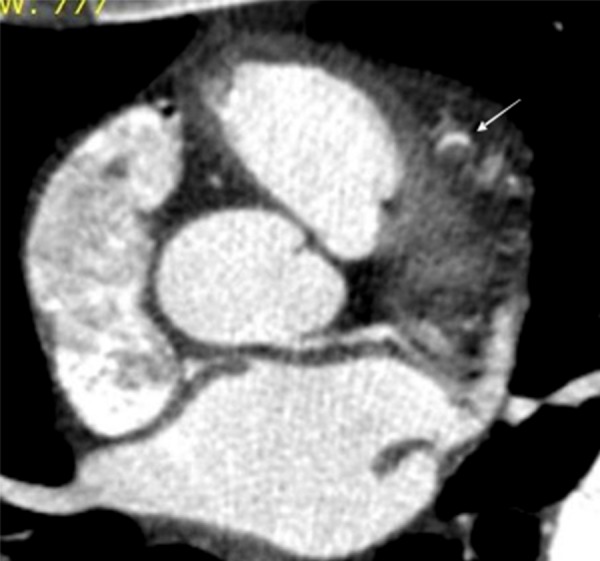
Transaxial source image of a CCTA shows an eccentric hypodense intramural hematoma severely narrowing the crescent-shaped lumen (white arrow) at the midportion of the LAD.
Figure 5.
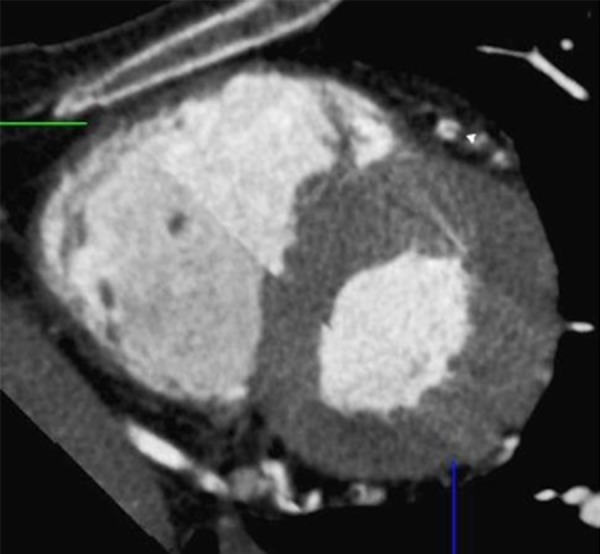
Using double oblique images of a multiplanar reconstruction, a cross-sectional orthogonal view of the LAD is obtained confirming the presence of a dissecting flap and a false lumen (white arrow).
Because of persistent signs of ischemia, the patient underwent invasive catheter-based intervention early in the morning. IVUS confirmed the presence of an intramural hematoma and showed the extent of the dissection (Figure 6). During ICA, cranial views better showed the luminal narrowing and differential vessel opacification caused by the dissection (Figure 7). To prevent propagation of the dissection and maintain patency of the true lumen, a stent was deployed. The false lumen was obliterated and the luminal narrowing resolved. Homogeneous opacification of the entire vessel was observed on repeat angiography (Figure 8). The patient tolerated the procedure well and his symptoms resolved. He was subsequently discharged home without complications.
Figure 6.
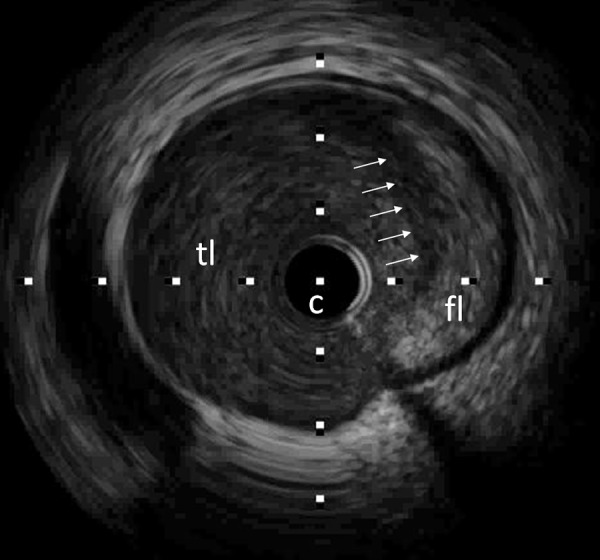
Intracoronary ultrasound (IVUS) of the corresponding LAD segment demonstrates a false lumen (fl) occupied by an echogenic mass (intramural hematoma). The true lumen (tl) is compressed and narrowed (white arrows: flap, c: catheter).
Figure 7.
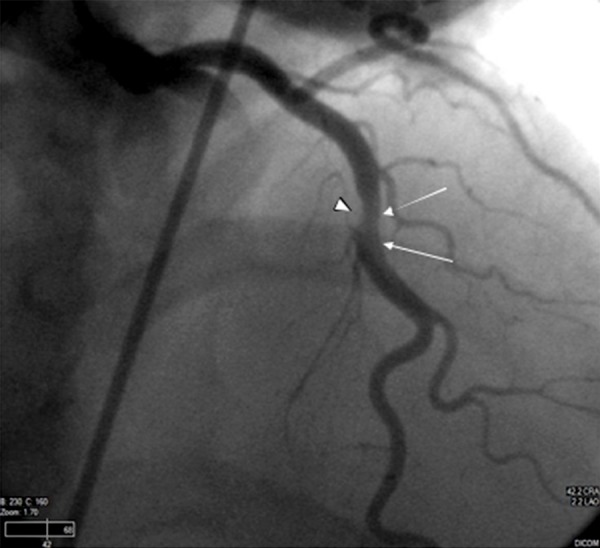
LAD cranial view demonstrates eccentric narrowing (arrowhead) with differential luminal opacification at its midportion (white arrows). While there is no direct visualization of the intramural hematoma or intimal flap, these findings correlate well with the known dissection per prior CCTA.
Figure 8.
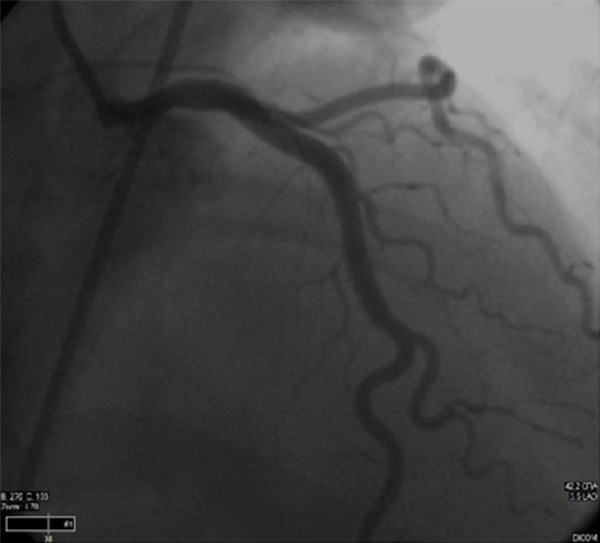
After stenting, the stenosis has resolved and the vessel opacifies uniformly throughout.
Discussion
SCAD is a rare cause of an acute coronary syndrome, with a reported incidence of 0.7% to 1.1% in published angiography series [1]. It may result in acute myocardial infarction and sudden death, or congestive heart failure if it results in chronic ischemia [2,3]. SCAD typically occurs in white, relatively young people (mean age of 42.6±10 years) without risk factors for coronary artery disease, a traditionally low-risk group in whom CCTA has been advocated in the Emergency Department [1,4]. Studies have shown most cases are women, especially those in a post-partum state. In most cases, the insult occurred a few weeks after delivery, with an average time-delay of about 1 month [5,6]. In addition, a large retrospective cohort study performed by Tweet et al. corroborated the previous claim and also revealed physical activity as a precipitating factor for SCAD in the male population. The same study reported a low proportion of diabetes mellitus and hypertension in SCAD patients, but indicated a higher rate of tobacco use and hyperlipidemia among males.
The etiology of SCAD is unclear, but certain vascular findings and morphologic abnormalities have been identified and associated with SCAD incidence and recurrence. Unlike aortic dissections, SCAD is not believed to be secondary to hyper-tension or atherosclerosis [2,5]. Spontaneous rupture of the vasa vasorum secondary to vascular shear stress, and abnormal connective tissue are postulated etiologies. A recent study that reviewed coronary angiograms in patients with SCAD proposed coronary tortuosity as marker for SCAD due to its high prevalence among the patient population, as well as the high rate of recurrence of dissection in tortuous segments [7]. The study also revealed a significant association between tortuous coronary arteries and extracoronal vasculopathy. A high prevalence of non-atherosclerotic SCAD in women with concomitant fibromuscular dysplasia (FMD) has been indicated, suggesting FMD as a predisposing factor for SCAD [8]. One series presented half of its patients with FMD in the external iliac arteries, but the condition has also been noted in the carotid and visceral arteries [9]. Other extracoronary vascular abnormalities such as aneurysms and dissections have also been observed in SCAD patients, suggesting underlying systemic vasculopathy as a causative factor [9]. For this reason Liang et al. propose that a CTA be performed from the neck to the pelvis in SCAD patients, in order to identify patients at risk of recurrence. Once the patient’s risk of recurrence has been established, serial follow-up examinations can be planned accordingly. Other implicated etiologies include sudden increases in intra-thoracic pressure (e.g., during retching, vigorous exercise, sexual intercourse), and progesterone-induced microstructural changes of the vessel wall [6,10–12]. Angiitis has also been considered, as several cases have shown eosinophilic infiltration of the affected coronary adventitia [13].
SCAD is characterized by the development of a hematoma in the outer third of the vessel media, forcing the intimal-medial layer towards the true lumen, with resultant stenosis. Using angiography, the severity of dissection can be categorized from A to E according to the National Heart, Lung, and Blood Institute classification system. Types A and B contain a wide dissection lumen, C and D have a thin dissection lumen, and type E is distinguished by a flap that protrudes into the true lumen of the artery [14]. If present, an intimal tear may decompress the hematoma in the false lumen and allow communication with the true lumen. This explains the variable findings during ICA: if an intimal tear is present, the typical appearance of an intimal flap with opacification and delayed washout of the false lumen can be noted. Conversely, if an intimal tear is absent, the appearance of the intramural hematoma tends to be less specific and may appear as a smooth narrow end vessel or simulate an intracoronary thrombus or atherosclerotic stenosis [3,6]. In these circumstances, the correct diagnosis of dissection using ICA alone may be elusive.
The addition of intracoronary ultrasound may be helpful in these cases. IVUS allows for the identification of coronary dissections, even when there are no angiographic features to suggest the diagnosis [15]. Although lumen-intimal interface cannot be sharply delineated, IVUS is a valuable complementary diagnostic tool that allows assessment of the diseased artery segment, and visualization and characterization of false lumen area and content [16]. It can also guide percutaneous coronary intervention by identifying the false lumen and ensuring that the stent is appropriately placed in the true lumen. In addition, IVUS can confirm sealing of the dissection and adequate stent expansion [5].
Advances in multidetector CT technology during the last decade have made noninvasive computed tomographic evaluation of the coronary arteries possible. With the faster gantry rotation times and increase in detector rows, the newest-generation scanners provide a near isotropic spatial resolution of 0.4 mm3 and a temporal resolution of 165 milliseconds with 64-slice MDCT and 83 milliseconds with dual source CT [17]. An added value of CCTA is the potential to generate additional image types using the source (transaxial) dataset, including multiplanar reconstructions (MPR), maximum intensity projections (MIP), curved formats, and volume rendered images. In this manner, each vessel can be tracked independently and the overlap of vessels, which frequently complicates ICA interpretation, is avoided. The vessel in question can be evaluated from a nearly infinite number of viewing angles, including cross-section, optimizing the diagnostic yield of the study. As in our case, the pathology is identified and studied in different views, increasing confidence in the diagnosis.
The 2010 Expert Consensus Document on Coronary Computed Tomographic Angiography underlined the utility of CCTA, which, by virtue of its high negative predictive value, can rule-out significant obstructive coronary disease with a very high degree of confidence and in a non-invasive manner. Another consideration in our case was the possibility of the patient having a congenital coronary anomaly with an inter-arterial course, which could account for his symptoms and clinical findings. Of note, for this clinical scenario, CCTA received the highest Appropriate Use Score (9) in the 2010 Appropriate Use Criteria for Cardiac Computed Tomography [18,19].
There are no uniform recommendations for the management of SCAD. Treatment is tailored to each individual case, taking into consideration the site and extent of the dissection within the coronary system, distal coronary flow, signs of ongoing ischemia, and the hemodynamic status of the patient. In hemodynamically stable patients with limited dissections and adequate antegrade coronary flow, a trial of medical therapy has resulted in favorable long-term outcomes, even when multiple vessels have been involved [10]. Beta-blockade and antiplatelet agents are recommended to reduce vascular shear force and thrombus formation, respectively. Thrombolytic agents, on the other hand, may worsen the clinical scenario by enlarging the intramural hematoma and propagating the dissection [5,6]. Like thrombolytic agents, stent placement can cause loss of coronary blood flow by propagating the dissection or displacing the intramural hematoma, as well as other complications related to passing the wire through the false lumen of the dissected coronary vessel during the percutaneous coronary intervention. For these reasons, stenting should be reserved for patients with limited single-vessel dissection and ongoing signs of ischemia or infarction [1]. Coronary artery bypass grafting (CABG) is best reserved for dissection involving the left main coronary artery, multivessel involvement, failed stenting, or ongoing ischemia despite medical therapy [3]. Whether medical therapy is instituted or stenting or CABG is performed, MDCT can be useful in following patients with SCAD. Coronary occlusion, in-stent re-stenosis, and by-pass graft patency can all be assessed with CCTA [20,21].
Conclusions
In the clinical setting of acute coronary syndrome, SCAD must be a consideration, particularly in young patients without clear risk factors for coronary artery disease and in women in the peripartum period. CCTA is a very helpful diagnostic tool to use in diagnosing SCAD in a non-invasive manner, as well as during follow-up after treatment.
Figure 2.
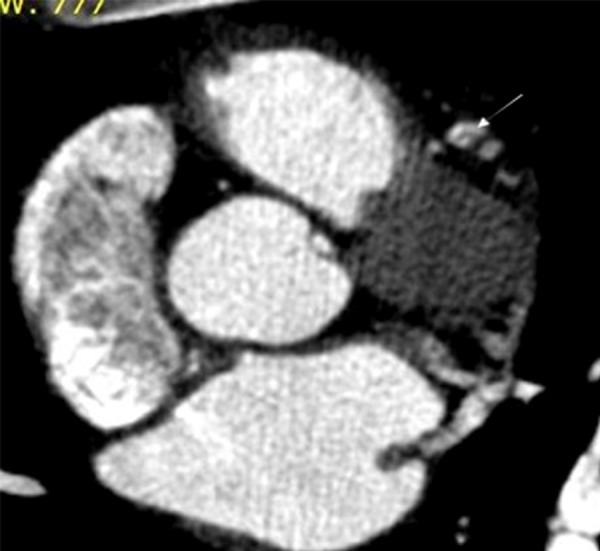
At a slightly more distal level, a curvilinear intraluminal density representing an intimal flap is noted (white arrow). There is opacification of a smaller false lumen.
Figure 3.
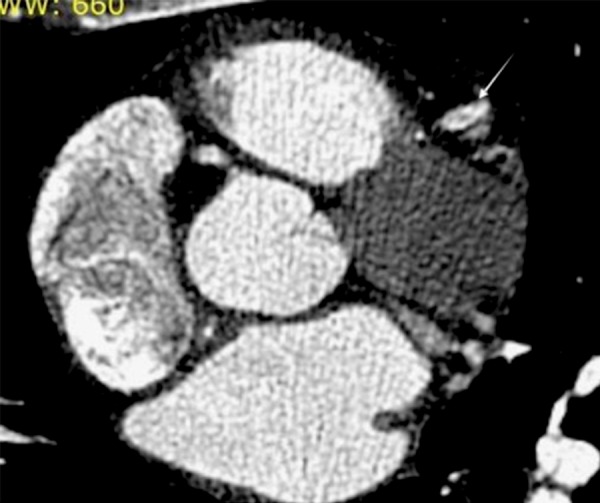
The intimal flap terminates a few millimeters more distally. Both true and false channels are demonstrated (white arrow). There is adequate opacification of the vessel distally (not shown).
Figure 4.
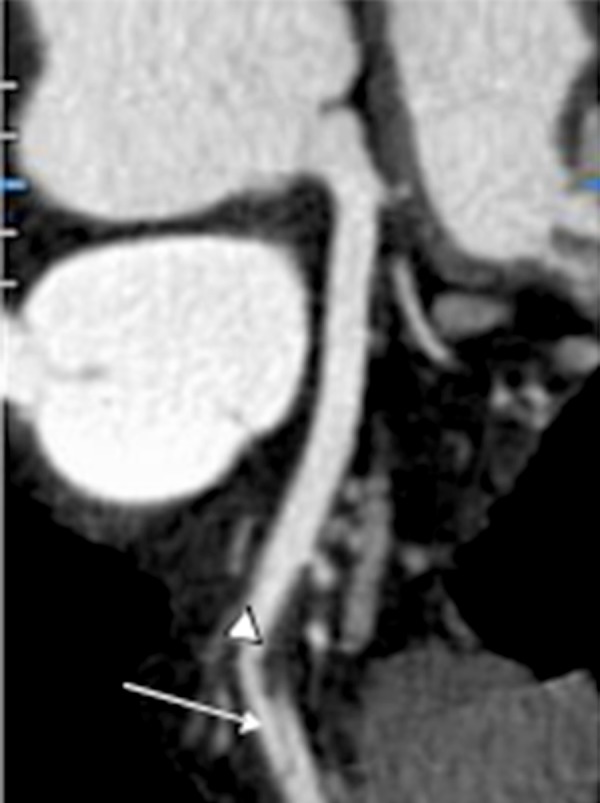
Curved multiplanar reformation nicely shows the intramural hematoma (arrowhead) and dissection flap, as seen in type E coronary artery dissections (white arrow) [10]. The vessel is free of atherosclerosis.
References:
- 1.Tweet M, Hayes S, Pitta S, et al. Clinical Features, Management, and Prognosis of Spontaneous Coronary Artery Dissection. Circulation. 2012;126:579–88. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, O’Rourke RA, Walsh R. Hurst’s The Heart. 12th ed. New York: McGraw-Hill; 2008. pp. 1287–88. [Google Scholar]
- 3.Dwyer N, Galligan L, Harle R. Spontaneous coronary artery dissection and associated CT coronary angiographic findings: a case report and review. Heart Lung Circ. 2007;16(2):127–30. doi: 10.1016/j.hlc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Hollander JE, Litt HI, Chase M, et al. Computed tomography coronary angiography for rapid disposition of low-risk emergency department patients with chest pain syndromes. Acad Emerg Med. 2007;14(2):112–16. doi: 10.1197/j.aem.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 5.Gowda R, Sacchi T, Khan I. Clinical perspectives of the primary spontaneous coronary artery dissection. Int J Cardiol. 2005;105(3):334–36. doi: 10.1016/j.ijcard.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Arnold J, West N, Van Gaal W, et al. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound. 2008;6:24. doi: 10.1186/1476-7120-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eleid M, Guddeti R, Tweet M, et al. Coronary Artery Tortuosity in Spontaneous Coronary Artery Dissection Angiographic Characteristics and Clinical Implications. Circ Cardiovasc Interv. 2014;7:656–62. doi: 10.1161/CIRCINTERVENTIONS.114.001676. [DOI] [PubMed] [Google Scholar]
- 8.Saw J, Ricci D, Starovoytov A, et al. Spontaneous Coronary Artery Dissection Prevalence of Predisposing Conditions Including Fibromuscular Dysplasia in a Tertiary Center Cohort. JAAC Cardiovasc Interv. 2013;6(1):44–52. doi: 10.1016/j.jcin.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Liang J, Prasad M, Tweet M, et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Computed Tomogr. 2014;8:189–97. doi: 10.1016/j.jcct.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Davidson C. Spontaneous multivessel coronary artery dissection in a long-distance runner successfully treated with oral antiplatelet therapy. J Invasive Cardiol. 2002;14(11):675–78. [PubMed] [Google Scholar]
- 11.Velusamy M, Fisherkeller M, Keenan M, et al. Spontaneous coronary artery dissection in a young woman precipitated by retching. J Invasive Cardiol. 2002;14(4):198–201. [PubMed] [Google Scholar]
- 12.Schifferdecker B, Pacifico L, Ramsaran E, et al. Spontaneous coronary artery dissection associated with sexual intercourse. Am J Cardiol. 2004;93(10):1323–24. doi: 10.1016/j.amjcard.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Shigeyama J, Ito S, Kondo H, et al. Angiographic Classification of Coronary Artery Dissections after Plain Balloon Angioplasty for Prediction of Regression at Follow-up. Jpn Heart J. 2001;42(4):393–408. doi: 10.1536/jhj.42.393. [DOI] [PubMed] [Google Scholar]
- 14.Kearney P, Singh H, Hutter J, et al. Spontaneous coronary artery dissection: a report of three cases and review of the literature. Postrgrad Med J. 1993;69(818):940–45. doi: 10.1136/pgmj.69.818.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maehara A, Mintz G, Castagna M, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002;89(4):466–88. doi: 10.1016/s0002-9149(01)02272-x. [DOI] [PubMed] [Google Scholar]
- 16.Paulo M, Sandoval J, Lennie V, et al. Combined Use of OCT and IVUS in Spontaneous Coronary Artery Dissection. JACC Cardiovasc Imaging. 2013;6(7):830–32. doi: 10.1016/j.jcmg.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Patel S, Lieberman S. Computed Tomography of Coronary Artery Disease. Semin Roentgenol. 2008;43(2):122–35. doi: 10.1053/j.ro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Taylor A, Cerqueira M, Hodgson J, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/ NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Mark D, Berman D, Budoff M, et al. 2010 Expert Consensus Document on Coronary Computed Tomographic Angiography. Circulation. 2010;121:2509–43. doi: 10.1161/CIR.0b013e3181d4b618. [DOI] [PubMed] [Google Scholar]
- 20.Jones C, Chin K, Yang G, et al. Coronary Artery Bypass Graft Imaging with 64-Slice Multislice Computed Tomography: Literature Review. Semin Ultrasound CT MRI. 2008;29(3):204–13. doi: 10.1053/j.sult.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Jones C, Chin K, Hamady M, et al. Coronary Stent Assessment with 64-Slice Multi-slice Computed Tomography: A Literature Review. Semin Ultrasound CT MRI. 2008;29(3):214–22. doi: 10.1053/j.sult.2008.02.007. [DOI] [PubMed] [Google Scholar]


