Virtually all animal species store energy for future needs in the form of fat. C. elegans store fat in intestinal epithelium, sharks store fat in the liver, but in most species, fat storage occurs in white adipose tissue (WAT)1. In normal weight humans, WAT represents 10-29% of body weight, making fat the largest organ in the body. If this were not enough, fat mass is increased in obesity, and we are in the midst of a worldwide epidemic of obesity. Indeed, two-thirds of the U.S. population and >1 billion people worldwide are either overweight or obese, and in some of these fat mass can exceed 100 kg. As a result, obesity-related pathologies, such as diabetes, dyslipidemias, hepatic steatosis and cardiovascular disease, have surpassed tobacco use as a cause of death. While we have a detailed understanding of the differentiation of preadipocytes to form adipocytes, very little is known about the origin of the preadipocyte.
Adipose tissue is generally regarded as having a mesodermal origin 1. It is believed that mesodermal/mesenchymal stem cells (MSCs) give rise to a common early precursor or adipoblast, which can develop into committed white and brown preadipocytes and ultimately mature adipocytes of different types (Figure 1). However, there are no unique markers on preadipocytes, and thus the exact nature of these precursors, the specific steps in commitment and differentiation, and the factors controlling these pathways are not clear. In this issue of Science, using lineage tracing with a knock-in reporter for PPARγ (including both the adipocyte-specific PPARγ2 isoform and the more widely expressed PPARγ1), Tang et al 2 have demonstrated the precursor of the white adipocyte to be a PPARγ+/sca1+/CD34+/CD31- mural cell or pericyte in the adipose vasculature. This cell population is also positive for smooth muscle actin (SMA), PDGFRβ and NG2, all markers of pericytes, but not perilipin, a marker of mature adipocytes. Prior to this study, the only known marker of preadipocytes was Pref-1 (also known as DLK-1), a cell surface protein in the EGF family 3. However, Pref-1 is not unique to the preadipocyte and has never proven suitable for isolation or tracking of these cells.
Figure 1.
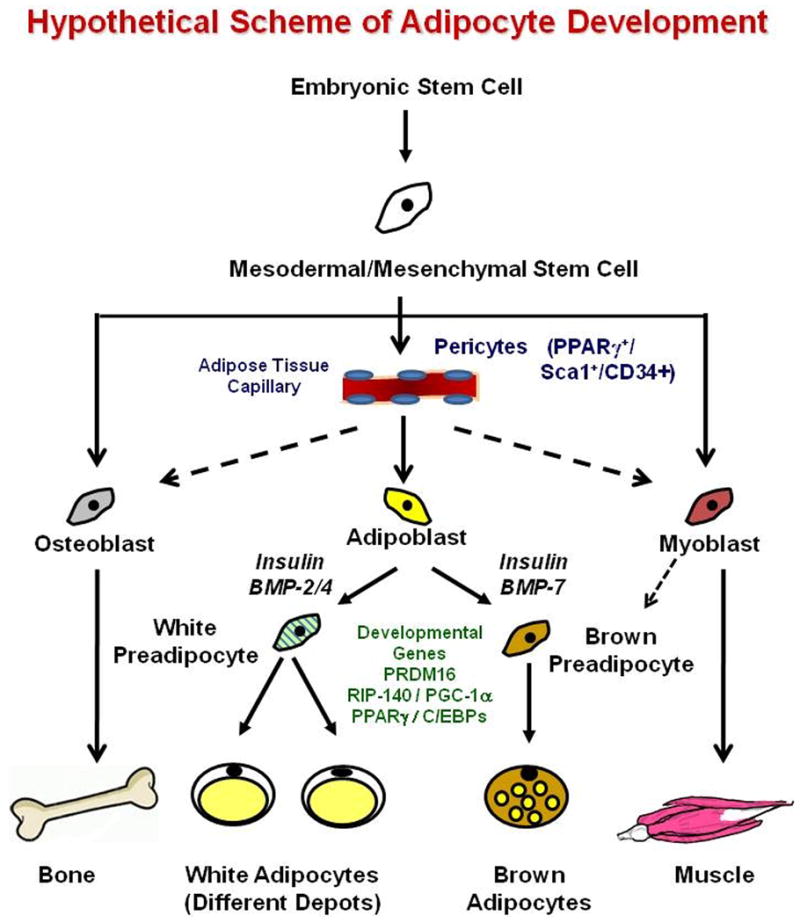
The current findings support previous work demonstrating that when the stromovascular fraction (SVF) of adipose is subjected to FACS, preadipocytes are enriched in the CD34+/CD31- fraction 4,5, and with studies suggesting that vascular supply to fat can be rate limiting in adipose accumulation 6. Although Tang, et al 2 find the PPARγ–marked cells only in vasculature of adipose tissue, perivascular cells from other locations have been shown to have adipogenic, myogenic, osteogenic and chondrogenic potential 7, with the switch between fates controlled in part by Wnt and growth factor signaling.
Multipotent stem cells can also be isolated from adipose SVF and may reside in a perivascular location and express CD34 and SMA 5. These adipose-derived stem cells can renew themselves for many passages in culture 8 and be induced to differentiate into adipose, muscle, bone, cartilage, endothelium, and even neuronal cells, similar to bone marrow-derived mesenchymal stem cells (BM-MSC) 9. Some studies have suggested that the adipocyte precursor is a circulating cell derived from bone marrow, although this is controversial. Both SVF-derived stem cells and BM-MSC share some surface markers, such as positivity for CD105 7, not present in the perivascular precursors identified by Tang, et al 2. Parenthetically, although most pericytes, adipocytes, myoblasts and osteoblasts have been suggested to be of mesodermal origin, in all four cases, similar cell types in the head and neck appear to derive from neural crest 10,11.
While this is a major step toward understanding the origins of fat, much remains to be learned. For example, recent studies indicate that the adipocyte population is more dynamic than previously believed. In humans, adipocyte number increases dramatically throughout the first two decades of life and continues to turnover at the rate of about 10% per year throughout adulthood 12. Individuals with early-onset obesity develop increased numbers of adipocytes and have increased adipocyte production rates as adults. How this relates to the origin of adipocytes in the vascular wall is unclear. In rodents, obesity can be limited by treatment with anti-angiogenic agents, however, this is thought to be an effect on endothelium and vascular supply rather than pericytes 6.
Adipose tissue is also heterogeneous, and how this relates to precursor lineage remains to be defined. WAT depots in different parts of the body have different developmental timing and different physiological effects. The most striking is the association of visceral adiposity, but not subcutaneous adiposity, with diabetes and other metabolic diseases 1. This may relate to intrinsic differences in WAT in different depots. Several laboratories have shown that adipocytes 13,14 and preadipocytes 15 from different WAT depots express different patterns of developmental genes (such as Hox, Shox, Engrailed, and Tbx), which are maintained through multiple generations in culture 16. The expression levels of some of these developmental genes exhibit strong correlations with fat mass and fat distribution 14.
In addition to white fat, mammals possess brown adipose tissue (BAT), which is important for basal and inducible energy expenditure in the form of thermogenesis through unique expression of mitochondrial uncoupling protein-1 (UCP-1) 17. Brown adipose tissue also appears to be of mesodermal origin, but the developmental patterns of BAT versus WAT are quite distinct. Thus, SVF isolated from BAT differentiates into UCP-1-expressing brown adipocytes, whereas SVF cells from WAT does not 18, and this has been supported by lineage tracing studies using the UCP-1 promoter 19. Indeed, brown preadipocytes possess a more “myogenic” gene expression signature 20, and recent lineage tracing studies with Myf5 have suggested a myogenic origin for BAT 21. Other factors known to be involved in brown versus white adipogenesis include different members of the BMP family 18, the transcriptional repressor RIP140 22, the transcriptional coactivator PGC-1α 23 and the zinc finger-containing protein PRDM16 21.
In summary, we are beginning to define the precursors of adipocytes and understand how adipose tissue can be so varied in its physiological and pathological roles. These new insights should provide new approaches to prevention and treatment of obesity and its associated morbidities.
References
- 1.Gesta S, Tseng YH, Kahn CR. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Tang W, et al. Science. 2008 [Google Scholar]
- 3.Smas CM, Sul HS. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 4.Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 5.Lin G, et al. Stem Cells Dev. 2008 doi: 10.1089/scd.2007.0186. [DOI] [PubMed] [Google Scholar]
- 6.Rupnick MA, et al. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisan M, et al. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, et al. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, et al. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 10.Billon N, et al. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller SM, et al. J Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 12.Spalding KL, et al. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 13.Cantile M, Procino A, D'Armiento M, Cindolo L, Cillo C. J Cell Physiol. 2003;194:225–236. doi: 10.1002/jcp.10210. [DOI] [PubMed] [Google Scholar]
- 14.Gesta S, et al. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchkonia T, et al. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 16.Tchkonia T, et al. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 17.Cannon B, Nedergaard J. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tseng YH, et al. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulin K, et al. Biochem J. 2001;356:659–664. doi: 10.1042/0264-6021:3560659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmons JA, et al. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seale P, et al. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiskinis E, et al. EMBO J. 2007;26:4831–4840. doi: 10.1038/sj.emboj.7601908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uldry M, et al. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]


