Abstract
The immune system in the female reproductive tract (FRT) does not mount an attack against human immunodeficiency virus (HIV) or other sexually transmitted infections (STI) with a single endogenously produced microbicide or with a single arm of the immune system. Instead, the body deploys dozens of innate antimicrobials to the secretions of the FRT. Working together, these antimicrobials along with mucosal antibodies attack viral, bacterial, and fungal targets. Within the FRT, the unique challenges of protection against sexually transmitted pathogens coupled with the need to sustain the development of an allogeneic fetus, has evolved in such a way that sex hormones precisely regulate immune function to accomplish both tasks. The studies presented in this review demonstrate that estradiol (E2) and progesterone secreted during the menstrual cycle act both directly and indirectly on epithelial cells, fibroblasts and immune cells in the reproductive tract to modify immune function in a way that is unique to specific sites throughout the FRT. As presented in this review, studies from our laboratory and others demonstrate that the innate and adaptive immune systems are under hormonal control, that protection varies with the stage of the menstrual cycle and as such, is dampened during the secretory stage of the cycle to optimize conditions for fertilization and pregnancy. In doing so, a window of STI vulnerability is created during which potential pathogens including HIV enter the reproductive tract to infect host targets.
Keywords: Epithelial cells, female reproductive tract, fibroblasts, immune cells, mucosal immunity, sex hormones
Introduction
Sexually transmitted infections (STI) have reached epidemic proportions throughout the world with more than 20 pathogens transmitted through sexual intercourse. The World Health Organization estimates that more than 300 million new infections of Trichonomas vaginalis, Chlamydia trachomatis, or Neisseria gonorrhea occur annually (http://www.who.int/mediacentre/factsheets/fs110/en/index.html). Some STI can be transmitted vertically to the fetus, resulting in preterm deliveries and/or life-threatening systemic illness in newborn infants. Generally, adolescents and young adults are the demographic age groups most frequently affected with STI, and women are more likely than men to suffer the consequences of these serious infections (http://www.who.int/hiv/data/2009_global_summary.png UGso-tAe2009). 1
Human Immunodeficiency Virus (HIV) is unique in its rapid spread and the depth of its impact. With 25 million deaths worldwide and an additional 33.2 million (of which 50% are women) infected, HIV is one of the world’s worst pandemics.2 Since the 1980s, in parts of the world HIV has shifted from a disease spread predominantly through contaminated needles, blood products and male-male sexual contact, to a sexually transmitted disease in which women worldwide are more likely to be infected than men (http://www.who.int/mediacentre/factsheets/fs360/en/index.html: HIV/AIDS2012). Presently, women and girls make up almost 57% of all people infected with HIV in Sub-Saharan Africa, where a striking 76% of young people (aged 15–24 years) living with HIV are female.2
Within the female reproductive tract (FRT), the mucosal immune system functions as the first line of defense.3–5 In response to the unique requirements of balancing immune protection with procreation, the immune system in the FRT, which consists of both innate and adaptive immune components, is responsive to and precisely regulated by the sex hormones estradiol (E2) and progesterone, both of which are produced in a cyclic fashion by the ovary over the course of the menstrual cycle. In preparing the reproductive tract for fertilization and implantation, E2 and progesterone simultaneously regulate the immune system in the Fallopian tubes, uterus, cervix, and vagina to complement the reproductive process (see ref. 6 for review).
Key constituents of the mucosal immune system in the FRT are a dynamic population of immune cells which migrate into the uterus, cervix and vagina as well as resident epithelial cells and supportive stromal cells.6 Sex hormones influence the migration of macrophages and dendritic cells (DC) as well as T and B cells by affecting the expression of adhesion molecules and chemotactic factors.6–9 Epithelial cells, in addition to providing barrier protection, transport immunoglobulins (IgA and IgG) into FRT secretions, and produce antimicrobials that are both bactericidal and viricidal.7,10 Through the production of cytokines and chemokines, these cells signal the recruitment and activation of other cells of the innate and adaptive immune systems. In this dynamic balance, epithelial cells, fibroblasts, and immune cells throughout the FRT respond directly to E2 and progesterone, as well as indirectly to the cytokines and growth factors. What is clear is that this responsiveness is part of the bidirectional communication that occurs in which epithelial cells direct both reproductive as well as immune function to maintain an effective level of protection which distinguishes between pathogens, commensals, allogeneic sperm, and the developing fetus. The pleiotropic capacity of epithelial cells has led to their recognition as sentinels, the functions of which are only now being recognized.6,11,12
This review focuses on our current knowledge regarding the role of epithelial cells, fibroblasts, and immune cells in the human FRT, with special emphasis on protection against STI. Our goal is to highlight some of the unique responses of these cells to E2 and progesterone and to point out that, in addition to direct hormonal effects on particular cells, there are the equally important indirect actions of E2 and progesterone mediated through growth factors, cytokines, and chemokines.
Menstrual cycle effects on epithelial cells and immune cells in the FRT
The immune system in the FRT has evolved to be responsive to and precisely regulated by E2 and progesterone, which are produced in a cyclic fashion by the ovary during the menstrual cycle. In preparing the reproductive tract for fertilization and implantation, E2 and progesterone simultaneously regulate the immune system in the Fallopian tubes, uterus, cervix, and vagina to complement the reproductive process (see ref. 5 for review). Over the course of the menstrual cycle, immune cells are present in substantial numbers and non-uniformly distributed in both the stromal layer and the epithelium of the FRT.13–16 Flow cytometry analyses of enzymatically and mechanically dispersed tissue fragments demonstrated that leukocytes are 6–20% of the total number of cells within the FRT.17 When natural killer (NK) cells were included in this analysis, the population of immune cells doubled.18 These studies indicated that T-lymphocytes (CD4+ and/or CD8+) are a major constituent of reproductive tract leukocytes from all tissues. The Fallopian tube contained granulocytes as a second major constituent, but were significantly less numerous in the other tissues. All tissues contained B-lymphocytes and monocytes as clearly detectable but minor components. Over the course of the menstrual cycle, subtle changes were observed in the migration of macrophages, B cells and neutrophils into the lower tract,17,19 and in DC entering the squamous epithelium.20
Immune Cell Migration
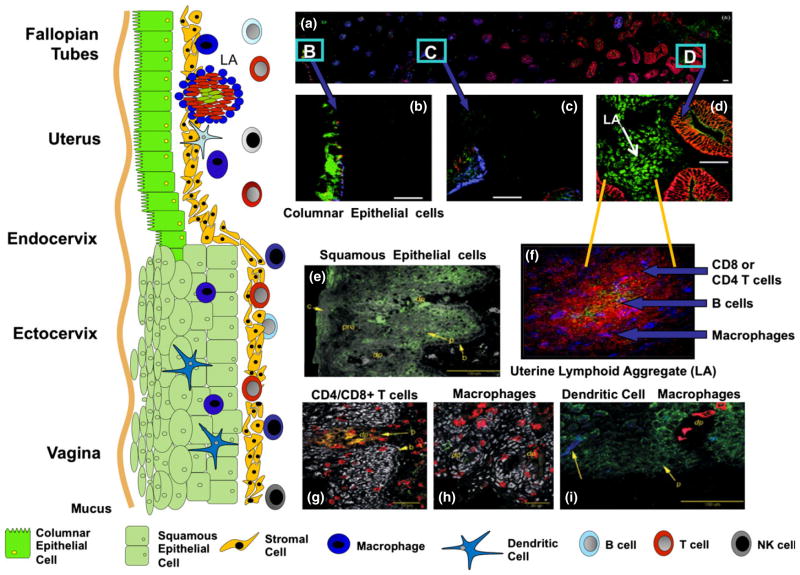
As seen in Fig. 1, the distribution of immune cells in the FRT varies with the site examined. Exclusive to the uterus are lymphoid aggregates, which consist of a B cell core surrounded by T cells and an outer halo of macrophages (Fig. 1a, f). The B cell core (CD19+) was most often seen in large aggregates present in the late proliferative and secretory stages of the cycle.21 Phenotypic analysis indicated that T cells are almost exclusively CD3+, CD8+, and CD4−. Aggregates containing only cells of the CD3+CD4+ phenotype were occasionally found as were individual CD4+ cells located outside the aggregates in the stroma. Monocytes/macrophages (CD14+ cells) were found as a mantle around the T cells. FRT aggregates are anatomically and functionally distinct from Peyer’s patches in the intestine.21 The size of lymphoid aggregates was found to vary with the stage of the menstrual cycle, and was significantly larger during the secretory (3000–4000 cells) than the proliferative stage (300–400 cells). The distribution and frequency of CD8+ T cells in aggregates using expression of Vb2 or Vb8 as markers of clonality and Ki-67 as a marker of dividing cells led to the conclusion that lymphoid aggregates form largely by the trafficking of cells to nucleation sites within the endometrium (EM), rather than by division of precursor cells.22 The lower reproductive tract, while lacking lymphoid aggregates, contains a full spectrum of immune cells located both within the submucosa and epithelial lining (Fig. 1g–i).
Fig. 1.
Schematic of the mucosal immune system throughout the human female reproductive tract (FRT). As seen in the drawing on the left side, the vagina and ectocervix are lined with squamous epithelial cells Columnar cells are present throughout the upper FRT including the endocervix, uterine endometrium (EM), and Fallopian tubes. Panel a–d: are confocal photomicrographs showing the distribution of immune cells throughout out the uterus. Frozen sections were directly stained with three fluorescently tagged monoclonal antibodies: an epithelial cell specific antibody (Cy3 labeled clone BerEP4, Red color panels a–d), anti-CCR5 (FITC labeled clone 2D7, Green color panels a–d) and anti-CXCR4 (Cy5 labeled 12G5, blue color panels a–d). Panel (a) Epithelial gland extending from the myometrial interface (far left) to the luminal epithelium (right-hand side). Panels (b) through d show higher magnification fields from the same region. The luminal epithelium, in contrast with the adjacent glands, retains a relatively intense BerEP4 expression (red color panels a and d). CCR5 expression is prominent on the lymphoid aggregates located in the stratum basalis immediately adjacent to the myometrium (‘LA’ in panel b). Epithelial expression of CCR5 is low in the stratum basalis and increases in the proximal third of the stratum functionalis. Panel f: This photo plate consists of a lymphoid aggregate in the uterine EM at the late proliferative stage of the menstrual cycle. The three fluorochromes are T cells (Cy3-anti CD3, red), B cells (FITC anti CD19, green) and macrophages (Cy5-anti CD14, blue). Panel e: Vaginal squamous cells expressing GalCer (green) is expressed on parabasal epithelial cells (p) and in the surface regions of the cornified layer. Panel g: CD8+ T cells present in both the submucosa and the squamous epithelium. Panel h: CD14 expression is found on both stroma and squamous epithelium macrophages. Panel i: CD1a positive dendritic cells (DC) are present in the squamous epithelium (blue). Panel a–d27; Panel f21; Panel e, g, h, i.28
Cytotoxic T Lymphocyte Activity
Coincident with aggregate formation in the uterus, White et al.23 found that CD8+ cytotoxic T lymphocyte (CTL) activity, measured in a redirected lysis assay, is suppressed in the uterus and Fallopian tubes during the secretory stage of the cycle. This suppression occurs without any drop in CD8+ T cell numbers. In the ectocervix and vagina, in contrast with the upper FRT, we found that CTL activity was measurable in tissues from women at the proliferative or secretory stages of the menstrual cycle.24
Chemokine Receptor Expression
Recognized as important molecules in implantation, 25,26 we measured the expression of chemokine receptors CXCR4 and CCR5 as well as CD4 on uterine epithelial cells and found that expression varies with the stage of the menstrual cycle (Fig. 1a–d).27 All three were low during the proliferative stage of the cycle, peaked at the time of ovulation and then either plateaued (CXCR4, CD4) or declined (CCR5) during the secretory stage of the cycle.27 Chemokine receptors on cultured endometrial epithelial cells showed an up-regulation and polarization of CXCR1, CXCR4, and CCR5 receptors when a human blastocyst was present.25 The distribution and regulation of these receptors in the endometrial epithelium and the human blastocyst suggest that each is essential in the apposition and adhesion phases of human implantation. In addition to expression in the upper FRT, leukocytes and epithelial cells in the lower tract (Fig. 1g–i) express CCR5 and GalCer on both.28 Yeaman et al.28 showed that basal and parabasal epithelial cells of the ectocervix express CD4, CCR5, and GalCer, unlike the midzone and superficial cells lining the lumen. Although changes in protein expression were not as pronounced as those seen in the uterus, histological evidence supported the conclusion that CD4 and CCR5 expression was greater during the proliferative stage than during the secretory stage of the cycle. An unintended consequence of chemokine expression in the upper and lower tract is that HIV-1, as a sexually transmitted pathogen, most likely uses these coreceptors to infect cells in the FRT.
Antibodies
The humoral immune system is hormonally controlled and varies with site analyzed and stage of the menstrual cycle (For review see ref. 10). In the uterus, levels of pIgR, the epithelial cell receptor responsible for transporting IgA from tissue to lumen, vary with the menstrual cycle.29 When expressed as the percentage of total protein, luminal uterine secretory component (SC) levels were highest during the secretory phase, significantly reduced during the proliferative phase and lowest during menstruation. Total SC was also greatest during the secretory phase, averaging approximately twofold higher than SC in proliferative and menstrual samples. In other studies, IgG levels in secretions from the uterine mucosa were highest during the periovulatory phase, whereas levels in the Fallopian tube were lowest at that time.30 This study reached the conclusion that each organ (Fallopian tubes, uterus, cervix, and vagina) and even different sites within each organ can respond independently from each other to changes in hormone levels, producing different types and amounts of secretory proteins. In the lower FRT, IgA, IgG, and lactoferrin levels in cervical mucus were depressed by 10–100-fold at midcycle relative to that seen early in the proliferative phase, only to rise toward the end of the menstrual cycle.31 When women were placed on oral contraceptives, immunoglobulins and lactoferrin levels were suppressed for the duration of hormone exposure. In other studies, in which cervical mucus was evaluated from 5 days before to 3 days after ovulation, IgA and IgG had a biphasic pattern with a peak before ovulation followed by a small increase after ovulation.32 Nardelli-Haefliger et al.33 demonstrated that titers of anti-human papillomavirus 16 virus-like particle (VLP) IgG in cervical secretions dropped approximately ninefold at midcycle during ovulatory cycles suggesting increased vulnerability to pathogens at midcycle.
Chemokines, Cytokines, and Antimicrobials
Chemokines and cytokines are central to the progressive tissue growth and remodeling that occur during each menstrual cycle while antimicrobials are secreted proteins that protect against pathogens.6 However, they are not mutually exclusive groupings, and many proteins are classified in both categories. For example, secretory leukocyte protease inhibitor (SLPI) is a potent inducer of neutrophil chemotaxis, alters Toll-like receptor (TLR) signaling, is bactericidal against Escherichia coli and Staphylococcus aureus amongst others, and possesses anti-HIV activity. While relatively little has been done in upper FRT during the menstrual cycle, several studies have demonstrated that ovarian stimulation markedly increases the profile of cytokines, chemokines, and growth factors in uterine secretions.34–36 Analysis of cervical-vaginal lavages (CVL) demonstrate that chemokines and cytokines (IL-6 and IL-8) as well as endogenously produced antimicrobials (SLPI, HBD2, HNP1–3, and lactoferrin) dropped significantly at midcycle (day 13) and remained depressed for 7–10 days, returning to proliferative stage levels just before menstruation.31,37 In contrast, total protein and TGFβ levels remained unchanged throughout the menstrual cycle. In other studies, human intestinal defensin-5 was highest in CVL during the secretory stage of the menstrual cycle.38 As discussed elsewhere,39 in contrast with CVL findings, studies using tampons for collection of vaginal fluid reported increased levels of HNP1–3, HBD2, and lysozyme while lactoferrin, HBD1, and SLPI decreased from proliferative to secretory stages of the menstrual cycle with no apparent mid-cycle decrease.39 When Dacron swabs were used, SLPI peaked at mid-cycle compared to proliferative and secretory stages.40 Further studies are needed to determine which recovery technique (CVL, tampon, or swab) most accurately reflects antimicrobial levels in the lower FRT.
Overall, these studies support the hypothesis that hormonal changes during the menstrual cycle regulate the immune system throughout the FRT in a way that is synchronized with reproductive function which optimizes FRT conditions for successful sperm migration, fertilization, implantation, and pregnancy. The extent to which changes in the immune system are directly or indirectly mediated by E2 is addressed in the following sections.
Sex hormone effects on epithelial cells
Epithelial Cell Barrier Function
Located throughout the FRT, epithelial cells provide a physical barrier to protect against microbial infection, as well as contribute to an environment for the transport and protection of sperm, ovum or conceptus.7,41 Stratified squamous epithelial cells line the vagina and ectocervix with tight junctions between cells in the basal layer. Tight junctions between the columnar epithelial cells maintain the integrity of the mucosal monolayer in the EM, endocervix, and Fallopian tube (Fig. 1). Disruption of the tight junctions in the upper FRT or damage to the squamous epithelial layer in the lower tract can lead to infection, resulting in infertility and potential life-threatening illness. The tight junction barrier permits epithelial cells to functionally polarize to respond to different stimuli and serve as a directional conduit for different factors. For example, the epithelial cell polymeric immunoglobulin receptor (pIgR) traverses the epithelial cell from the basolateral side to the apical side to release IgA into the lumen.42 Uterine epithelial cells secrete cytokines such as TGFβ preferentially at the basolateral surface and TNFα at the apical surface.43 In culture, columnar uterine epithelial cells generate an electrochemical gradient across an intact monolayer that reflects the presence of these tight junctions and the integrity of the physical barrier, thus barrier function can be measured as transepithelial resistance (TER).44–50
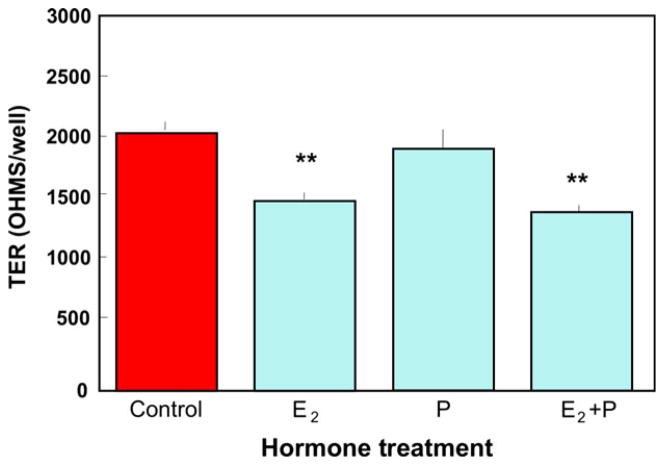
Physiological concentrations of E2 significantly reduce the TER of mouse epithelial cells7,51 and porcine vas deferens epithelia,52 as well as disrupt adherens junctions in endothelial cells.53 To determine the effect of E2 and progesterone on human uterine epithelial cell barrier integrity, we grew the highly differentiated cell line ECC-146 in cell inserts until they achieved a consistent maximum TER of about 2000 ohms/insert. As seen in Fig. 2, the addition of 5 × 10−8 M E2 for 48 hr reduced TER by more than 25%. Progesterone at 1 × 10−7 M had no effect on TER, and also failed to inhibit the reduction in TER caused by E2. A lower TER would result in an increase in fluid and molecule transport from the uterine tissue into the lumen, which can contribute to transport of sperm and conceptus. In other studies, Gorodeski examined the effects of hormones on TER in human normal epithelial vaginal-ectocervical cells from premenopausal and postmenopausal women and found that E2 induced an early transient decrease in TER through a remodeling of occludin. 54,55
Fig. 2.
Estradiol inhibits transepithelial resistance (TER) in the human uterine epithelial cell line ECC-1. ECC-1 cells were grown in media supplemented with stripped FBS until maximal TER of about 2000 ohms/well in cell inserts was obtained. Estradiol (5 × 10−8 M) and/or progesterone (1 × 10−7 M) were added to the basolateral compartment of some inserts and TER was measured after 48 hr. Four wells per group; **Significantly different (P < 0.01) from Control. From reference (145).
Human uterine epithelial cells express TLR 1 through 9 and are therefore prepared to respond to a range of pathogens that include viruses, bacteria, and fungi.56,57 To determine the effect of a TLR3 agonist on TER in the presence of E2, the dsRNA viral mimic poly (I:C) was added to primary polarized human uterine epithelial cells with and without a pre-treatment of E2 for 24 hr, and TER was measured. Estradiol treatment, similar to the ECC-1 cells, reduced TER in primary uterine cells. Unexpectedly, we found that in contrast with E2, poly (I:C) alone significantly increased TER. This finding suggested that epithelial cells increase barrier protection in response to viral challenge possibly to limit viral infection. Interestingly, when ECC-1 cells were pretreated with E2, TER stimulation by poly (I:C) is muted and remains unchanged relative to control values. These findings suggest that in the context of viral challenge, which involves TLR3 ligation, E2 lowers TER without affecting barrier protection. What remains to be determined is whether E2 reduced TER translates into lower barrier protection in the presence of a viral pathogen. In other studies, treatment of polarized ECC-1 cells with live but not killed Chlamydial trachomatis lowered TER and inhibited the expression of tight junction genes (ZO-1 and Claudin 2; L. Mukura, D. Hickey, J.V. Fahey, C.R. Wira, Submitted). Recognizing that chlamydia is a known risk factor for HIV infection in the FRT,58 these findings suggest a mechanism whereby STI co-infection can lead to viral access to HIV-target cells in the underlying stroma. Interestingly, Kaushic et al.59 demonstrated that exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. Disruption in barrier functions was associated with viral and bacterial translocation across the epithelial monolayers that was in part mediated through the paracrine effects of TNFα.
We have previously suggested that the secretory phase of the menstrual cycle is a time of risk for infection by HIV due to decreases in a number of innate and adaptive immune mechanisms.5 It is important for reproductive success that the FRT immune system be regulated to prevent attack on sperm and conceptus, and E2 is likely a major player in modulating specific FRT immune responses. Unfortunately, it is likely that the reduction in uterine epithelial cell TER due to E2 may also contribute to the potential for infection by microbial pathogens in the upper tract.60
To further understand the modulation of TER in uterine epithelial cells by E2, we seeded ECC-1 cells in cell inserts and treated the cells 24 hr later with E2, tamoxifen, or raloxifene. The latter two molecules are Selective Estrogen Receptor Modulators (SERMs) and are clinically relevant for the treatment of breast cancer (tamoxifen) and bone resorption (raloxifene). As shown in Table I, TER was affected by all treatments. Consistent with data shown above, E2 inhibited TER by more than 50%. This result infers that E2, increasing in concentration for the start of ovulation, may reduce barrier integrity and allow certain small pathogens such as HIV to enter underlying tissue and infect target cells. Both Tamoxifen and Raloxifene significantly enhanced TER over control values suggesting that the use of SERMs, perhaps in a cervical ring that is commercially available for contraceptive purposes, may be beneficial in the protection of infection by microorganisms that cause STI such as HIV-1. Thus, treatment with SERMs might be valuable in lowering HIV infection in the FRT.
Table I.
Effects of Estradiol, Tamoxifen, and Raloxifene on Transepithelial Resistance (TER) of ECC-1 Cells. ECC-1 Cells were Seeded in Cell Inserts and Treatments with Estradiol or the Selective Estrogen Receptor Modulators (SERMs) Began 24 hr After Seeding (Day 1, When TER was Just Developing and Averaged 350 ohm/well) and Cells were Re-Treated at 48 hr Intervals After TER Measurements were Assessed. TER was Affected by all Treatments; Estradiol Significantly Inhibited TER by More Than 50% Compared to Control. SERMs Enhanced TER
| Transepithelial resistance
| |||
|---|---|---|---|
| Days in Culture | 3 | 5 | 7 |
| Control | 2193 | 3203 | 3343 |
| 221 | 276 | 123 | |
| Estradiol | 1030# | 1297# | 1430# |
| 175 | 48 | 21 | |
| Tamoxifin | 3067** | 4520** | 5727** |
| 227 | 316 | 334 | |
| Raloxifene | 2960** | 6200** | 9620** |
| 698 | 408 | 811 | |
The data represent the mean and S.E.M. of four wells per group.
Significantly (P < 0.01) greater than controls.
Significantly (P < 0.01) lower than controls.
Chemokines, Cytokines, and Antimicrobials
Uterus
In addition to a physical barrier, uterine epithelial cells provide protection from pathogens through the secretions of antimicrobials, chemokines, and cytokines that recruit and activate immune cells of both the innate and adaptive immune systems.6 How E2 regulates the secretion of these soluble factors can determine whether infection will occur. The functions of antimicrobial factors in host defense are multi-faceted and range from direct killing of invading microbes (bacteria, fungi, and viruses) to linking innate and acquired immunity.7 Epithelial cells in the FRT secrete factors that exhibit potent antimicrobial activity including human α-defensin-5 (HD5), β-defensins 1–4 (HBD1–4), Elafin, and SLPI.12,38,61–64 These antimicrobial peptides, some of which are induced in response to TLR ligand exposure, are effective at inhibiting Gram-positive and Gram-negative bacteria, fungi, Neisseria gonorrhoeae, Chlamydia trachomatis, Candida albicans, Herpes Simplex Virus-2, and HIV-1.65–71 Other uterine epithelial cell factors with antiviral activity, notably against HIV-1, are the chemokines MIP-1α, MIP-1β, RANTES, and SDF-1α, which inhibit the ability of HIV-1 to bind coreceptors CCR5 and CXCR4 found on host target cells.72,73 More recently, we demonstrated that human uterine and Fallopian tube epithelial cells produce MIP3α and Trappin-2/Elafin, and that these recombinant peptides inhibit HIV infection.74,75 More recently, in addition to inhibition of extracellular attachment and transcytosis of HIV-1, exogenously added N-terminus-unmodified Elafin was able to enter the nucleus and reduce IL-8 secretion, attenuate NF-κB activation, and lower expression of innate viral sensors.76
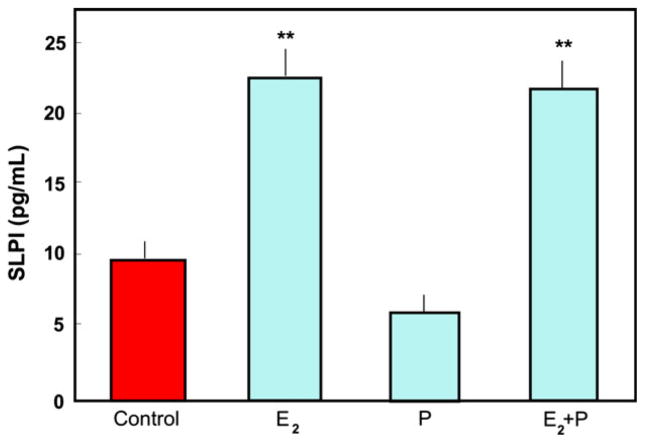
Sex hormones can alter the secretion of cytokines, chemokines and antimicrobials, and thus immune protection. As shown in Fig. 3, E2 significantly increased the secretion of SLPI by ECC-1 cells, a cell line characteristic of luminal epithelium; progesterone neither enhanced SLPI secretion nor negated the E2-induced effect. These data indicate that uterine epithelial cells constitutively secrete SLPI and that E2 enhances that secretion. Further studies demonstrated that apical secretions from polarized human uterine epithelial cells from pre-menopausal women, but not from post-menopausal women, had anti-bacterial activity against both gram+ and gram− bacteria.77 The anti-bacterial activity was partially neutralized with antibody to SLPI. These data support our working hypothesis that endocrine balance during the menstrual cycle modulates the sentinel function of epithelial cells and offers an explanation as why susceptibility to sexually transmitted pathogens varies with stage of the menstrual cycle.78
Fig. 3.
Estradiol stimulates secretion of secretory leukocyte protease inhibitor (SLPI) by ECC-1 cells. ECC-1 cells were grown to confluence and high transepithelial resistance in cell inserts. The media was changed and some inserts were cultured with 5 × 10−8 M E2 and/or progesterone (1 × 10−7 M) for 48 hr. Apical conditioned media were recovered, centrifuged to remove cell debris and SLPI was measured by ELISA. Four wells per group; **Significantly different (P < 0.01) from control or progesterone. From reference (12).
Estradiol regulates innate immunity by suppressing the secretion and/or expression of pro-inflammatory mediators by epithelial cells. For example, in human keratinocytes, Kande and Watanabe demonstrated the suppression of MCP-1, RANTES, and IP-10 with E2 treatment.79–81 Previous data in the rodent from our laboratory has also shown that E2 inhibits the constitutive and TLR-agonist-induced secretion of TNFα and MIP3α by uterine epithelial cells.82 As shown in Fig. 4, lipopolysaccharide (LPS) induced an approximate fourfold increase in IL-6 expression in ECC-1 cells; this increase was almost completely abrogated by pre-treatment with E2. These results are consistent with the anti-inflammatory actions of E2, which in the FRT may reduce HIV infection by inhibiting target cell migration and eliminating the ‘immune activated’ environment often associated with HIV infection. In similar experiments with polarized primary uterine epithelial cells, we found that E2 enhanced the secretion and mRNA expression of the antimicrobials SLPI and human HBD2.12 Concomitant with increased antimicrobial secretion in the presence of E2, there was a significant reduction in the LPS- and poly (I:C)-induced secretion of the proinflammatory cytokines MIF, IL-6, and IL-8, as well as a 70% inhibition of mRNA expression of NFkB in primary uterine epithelial cells. Since SLPI is known to inhibit NFkB expression,83–88 these findings suggest that E2 inhibition of pro-inflammatory cytokines may be mediated through SLPI regulation of NFkB. Overall, these findings indicate that the production of cytokines, chemokines, and antimicrobials by FRT epithelial cells are differentially regulated by E2. Furthermore, it suggests that, with E2 regulation, epithelial cells that line the uterine cavity have evolved immunologically to be sensitive to viral and bacterial infections as well as the constraints of procreation.
Fig. 4.
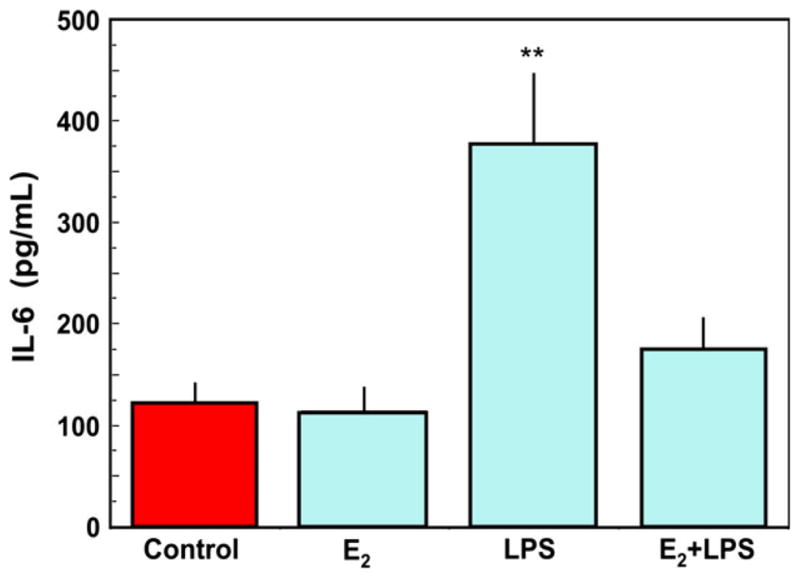
Estradiol inhibits lipopolysaccharide (LPS)-induced IL-6 secretion by uterine epithelial cells. Monolayer cultures of uterine epithelial cells at high transepithelial resistance were treated with 5 × 10−8 M E2 for 48 hr in the basolateral compartment of cell inserts with some wells receiving an apical treatment of ultra pure LPS (100 mg/mL) after 24 hr. Apical conditioned media were recovered, centrifuged to remove cell debris and IL-6 was measured by ELISA. LPS stimulated a significant increase in IL-6 secretion, which was mostly abrogated by pre-treatment with E2. Four wells per group; **Significantly different (P < 0.01) from Control, E2 and E2 + LPS. Adapted from reference (12).
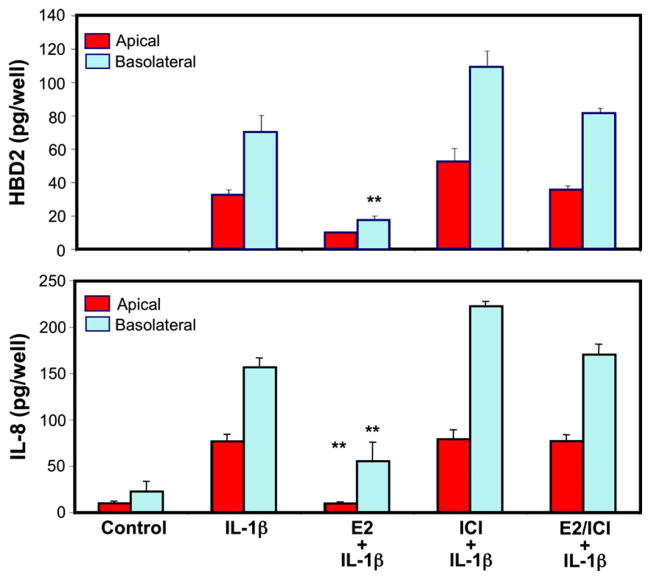
The human uterus is uniquely evolved in its ability to mount immune responses toward pathogens and, at the same time, tolerate an immunologically distinct fetus. Several studies have shown that uterine epithelial cells, when stimulated with pathogens or TLR agonists that mimic pathogenic antigens, can mount an immune response.56,63,89 In vivo, a massive proinflammatory response, although useful against pathogens, might be detrimental to the host and/or fetus. Therefore, immunological regulations are in place that can mitigate severe inflammatory reactions. One of the key molecules that can regulate inflammatory responses in the upper FRT is E2. Studies from our laboratory and others have demonstrated the down-regulation of immunologically relevant genes by E2. 12,90 For example, Schaefer et al.57 have shown that when ECC-1 cells were stimulated by IL-1β, the secretion of both HBD2 as well as IL-8 was enhanced. IL-1β is a cytokine involved in the generation of rapid and potent immune responses by upregulating genes involved in the induction of innate immunity (e.g. HBD2 and IL-8). Since the prolonged presence of IL-1β is detrimental in the FRT leading to conditions such as preeclampsia and endometriosis,91,92 immuno-modulators such as E2 can dampen this response effectively. As shown in Fig. 5, IL-1β enhanced the secretion of HBD2 and IL-8 by ECC-1 cells. Treatment with E2 significantly decreased the secretion of both proteins. Treatment with estrogen receptor antagonist ICI182780 abolished the E2 mediated down modulation of both genes indicating that the observed effect was mediated through the estrogen receptor. The inhibitory effect of E2 on IL-1β mediated inflammatory responses generated by uterine epithelial cells demonstrates a link between the endocrine and immune systems and may be crucial for dampening proinflammatory responses during the time of ovulation or pregnancy.
Fig. 5.
Estradiol inhibits IL-1β mediated secretion of HBD2 and IL-8 and the inhibition occurs though involvement of the estrogen receptor. ECC-1 cells were preincubated with E2, the pure ER antagonist ICI182780, or a combination of steroid and antagonist for 72 hr before stimulation with IL-1β (5 ng/mL). Apical and basolateral conditioned medium were collected following 24 hr stimulation and analyzed for HBD2 and IL-8 protein secretion by ELISA. The results are shown as the mean ± S.E.M. **Significantly different (P < 0.01) from control. Red bars, apical conditioned medium; Blue bars, basolateral conditioned medium. Adapted from reference (57).
Vagina
Vaginal surface area ranges from 65 to 108 cm93,94 and is lined by multiple layers of squamous non-keratinized epithelial cells95 which provide the physical barrier against pathogen entry in the lower reproductive tract. The vagina also sustains a commensal bacterial population that supports its major functions and creates a unique mucosal environment. Similar to uterine epithelial cells, vaginal epithelial cells express pattern recognition receptors such as TLRs, 2, 3, 5, and 6.96 TLR stimulation elicits proinflammatory cytokine production.96 Primary vaginal epithelial cells are sensitive to poly (I:C), a TLR3, RIG-I, and MDA5 viral agonist, which induces strong upregulation of IL-1β, IL6, IL8, and CCL2.96 TNFα is also secreted in response to stimulation by the microbial compounds LPS (TLR4) and peptidoglycan (TLR2).97 Overall, the vagina and its role in innate immune defense remains relatively understudied with the majority of experiments being conducted using cell lines rather than primary isolates.
In contrast with uterine epithelial cells, recent experiments using98 vaginal epithelial cells treated with E2 demonstrated significantly decreased production of antimicrobials HBD2 and Elafin in vitro over 48 hr (Fig. 6). Recognizing that in secretory phase of the menstrual cycle both E2 and progesterone are present at high concentrations, vaginal epithelial cells were incubated with progesterone or a combination of E2 and progesterone. Unlike E2 alone, progesterone alone had no effect on the secretion of HBD2 or Elafin. Interestingly, E2 and progesterone co-treated cells had reduced secretion of HBD2 beyond that seen with E2 alone. We also found that primary vaginal cell secretion of MIP3α was undetectable in secretions from control and E2-treated cells. A lack of MIP3α production by vaginal epithelial cells suggests an important role for leukocytes 99 and upper reproductive tract epithelial cells in its production, as its presence is detectable in vaginal fluids.100,101 The E2 suppressed HBD2 and Elafin secretion by squamous epithelial cells suggests decreased protection by vaginal epithelial cells in vivo during the ovulatory and secretory phase of the menstrual cycle when E2 levels are relatively high.5 These changes in protection could be secondary to physiological changes permitting the survival of sperm deposited in the tract. The opposing responses to E2 stimulation between the upper and lower tracts may be due to mechanistic changes arising from their differing embryonic origins.
Fig. 6.
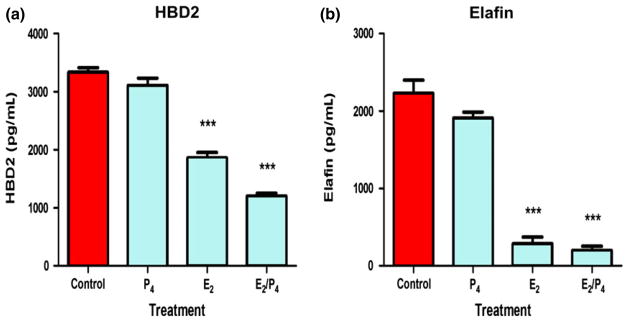
Estradiol decreases the production of HBD2 and elafin in primary vaginal squamous epithelial cells. Vaginal secretions and cells were recovered from volunteers using an Instead™ Menstrual Cup following insertion for 1 hr. Freshly isolated vaginal epithelial cells were incubated overnight in triplicate wells prior to treatment with P4, E2 or a combination of both for 48 hr. Conditioned media were recovered and analyzed by ELISA for the presence of HBD2 (a) and elafin (b). Data shown are from one volunteer that is representative of two experiments from two different volunteers. ***P < 0.001 with respect to control. From reference (98).
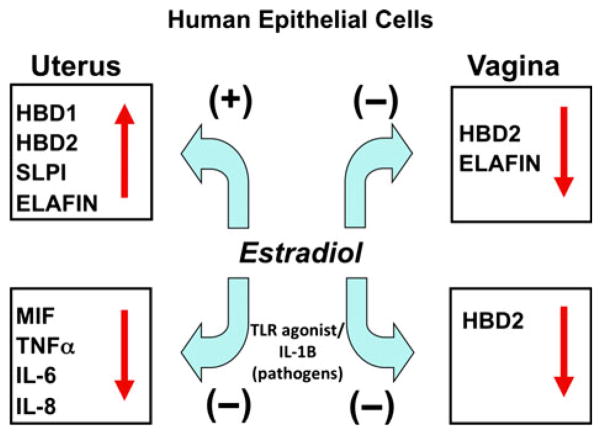
Overall, as seen in Fig. 7, E2 exerts both stimulatory and inhibitory effects on cytokines, chemokines and antimicrobials secreted by uterine and vaginal epithelial cells. As shown in this Figure, E2 increases antimicrobial secretion by uterine epithelial cells while inhibiting their secretion by vaginal squamous cells in culture. In contrast, E2 dampens uterine and vaginal epithelial cytokine/chemokine responses to TLR agonists and IL-1β. Given the midcycle suppression of some of these cytokines, chemokines and antimicrobial during the menstrual cycle,5,37 our findings suggest that these changes are due, in part, to the direct effects of E2 on squamous cells rather than a dilution by cervical mucus. Whether comparable changes occur in response to E2 in secretions from the uterine lumen remain to be determined.
Fig. 7.
Schematic of estradiol regulation of innate immune function by human epithelial cells in the upper and lower female reproductive tract. In the uterus, E2 enhances the secretion of antimicrobial factors and reduces the secretion of induced pro-inflammatory mediators. In contrast with the uterus, E2 inhibits both the constitutive and induced secretion of antimicrobials in the vagina.
Direct and indirect effects of sex hormones on fibroblasts and immune cells in the FRT
Fibroblasts
Fibroblasts are key structural components of reproductive tissues. However, recent findings have shown they are not passive bystanders, but active players in the immune response. Epithelial cell interactions with underlying stromal fibroblasts are essential in facilitating steroid hormone-induced growth and development in the EM. Cooke et al.102 used FRT tissues from estrogen receptor knockout mice to demonstrate that underlying estrogen receptor positive stromal cells regulate the differentiation of adjacent epithelial cells. These studies indicated that E2 acts on stromal cells to release one or more paracrine factors that then modulate E2 effects on FRT epithelial cell growth and differentiation. Hepatocyte Growth Factor (HGF), a pleiotrophic agent initially shown to stimulate proliferation of hepatocytes in vivo,103 is produced primarily by stromal fibroblasts.104,105 HGF has been well characterized in terms of its normal physiological roles of increasing epithelial cell motility and proliferation, wound healing,106 angiogenesis,107,108 epithelial cell scattering, and embryogenesis109 (see review ref. 110). Using primary human uterine epithelial cells, Sugawara et al.111 found that HGF stimulates proliferation, migration, and morphological changes in human uterine epithelial cells and concluded that HGF may modulate the cyclic regeneration and development of the endometrial lining of the uterus.
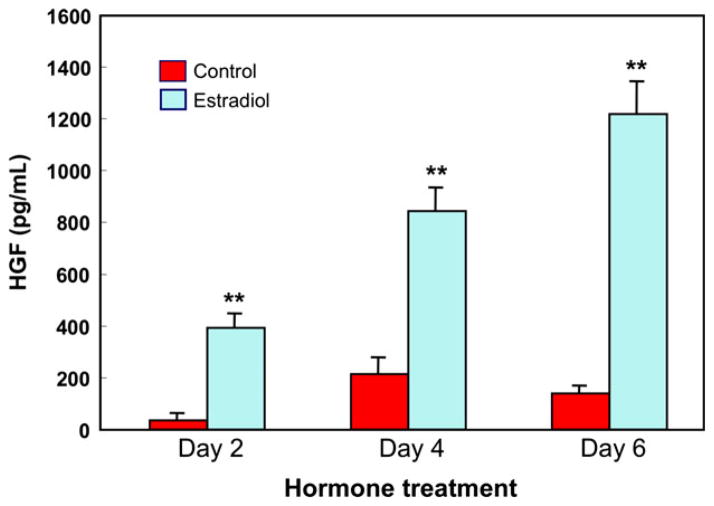
As shown in Fig. 8, treating uterine stromal fibroblasts with estradiol significantly increased HGF secretion; E2-induced secretion of HGF increased with the duration of exposure to E2.112 Cells treated with E2 for 6 days secreted three times more HGF than cells exposed for 2 days. The long-lasting effect of E2 on HGF secretion by uterine stromal fibroblasts could have implications for cancer or endometriosis where continued HGF secretion might lead to further proliferation of uterine epithelial cells.
Fig. 8.
Estradiol stimulates secretion of hepatocyte growth factor (HGF) by human uterine stromal fibroblasts. Uterine fibroblasts were cultured to confluence in 24 well plates and 1 × 10−8 M E2 or control media was added on Day 0 and every 2 days thereafter with media change. The conditioned media was recovered every 2 days, centrifuged and supernatants analyzed for HGF by ELISA. HGF secretion increased with E2 treatment and with on-going culture. Four wells per group; **significantly different from Control on the day shown (P < 0.01).112
In related studies using a co-culture system, mouse uterine stromal fibroblasts cultured below uterine polarized epithelial cells in cell inserts produced HGF that significantly increased TER.113 When epithelial cells and/or stromal cells were incubated with anti-HGF or anti-HGF receptor (HGFR) antibody prior to the addition of HGF, the effect of HGF was blocked. Addition of recombinant HGF to the basolateral compartment of polarized epithelial cells increased TER in a dose-dependent manner. These findings indicate that epithelial cells express the HGFR at their basolateral surfaces and that HGFR mediates the effects of HGF on TER.114 In contrast, when cells were incubated with TGFβ, TER was markedly but reversibly suppressed. Based on these findings, we conclude that TGFβ and HGF may play regulatory roles in modulating epithelial cell tight junctions. Further studies are needed to determine the relative contributions of hormone balance (Fig. 2 and Table I), pathogen exposure, cytokine, and growth factor secretion (Fig. 8), to innate and adaptive immune protection against pathogens in the FRT.
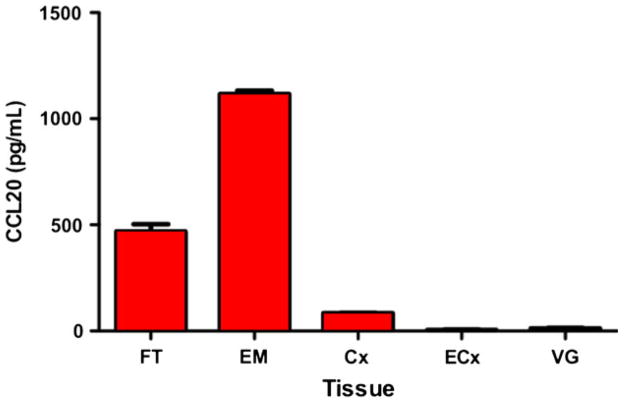
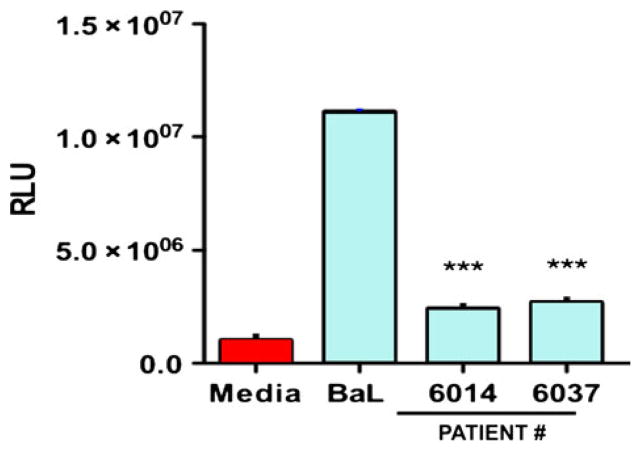
The role of fibroblasts in mediating the immune response against viral pathogens including HIV in the FRT is not well-understood and few studies have addressed this. We have shown that confluent layers of purified fibroblasts from the distinct anatomical regions of the FRT secrete a panel of anti-HIV factors including CCL20/MIP3α (Fig. 9), IL-8, RANTES, and SDF-1α (not shown). However, fibroblasts isolated from the EM and Fallopian tubes secreted substantially higher levels of CCL20 than those from the lower FRT. In preliminary studies, diluted conditioned media (CM) (1:4) from EM fibroblasts of two women showed anti-HIV activity, and reduced R5 (BaL) infection of TZM-bl cells by approximately 75% (Fig. 10). As a part of these studies, we found that FRT fibroblasts demonstrated site-specific differences when treated with E2. Estradiol stimulated SDF-1 production in EM fibroblasts but had no effect on fibroblasts isolated from the cervix or Fallopian tubes (M. Patel, M. Rodriguez-Garcia, J.V. Fahey, C.R. Wira, Submitted).
Fig. 9.
Fibroblasts were isolated from hysterectomy tissues of the Fallopian tubes (FT), endometrium (EM), cervix (Cx), ectocervix (ECx), and vagina (VG), and grown in culture for 48 hr. Conditioned medium from four patients per tissue was assayed for CCL20/MIP3α by ELISA. Fibroblasts from the upper female reproductive tract (FRT; FT, EM, Cx), but not the lower tract (ECx, VG), secrete CCL20/MIP3α.
Fig. 10.
Conditioned medium (CM) from EM fibroblasts in culture for 48 hr from two patients were diluted 1:4 in culture media and tested for anti-human immunodeficiency virus (HIV) activity in a TZM-bl assay. Fibroblast CM from each patient had potent anti-HIV activity against an R5 (BaL) reference virus. The mean ± S.E.M. relative light units (RLU) for media, virus alone (BaL) and the conditioned media from each patient are shown from quadruplicate treatments. ***P < 0.001.
Monocytes
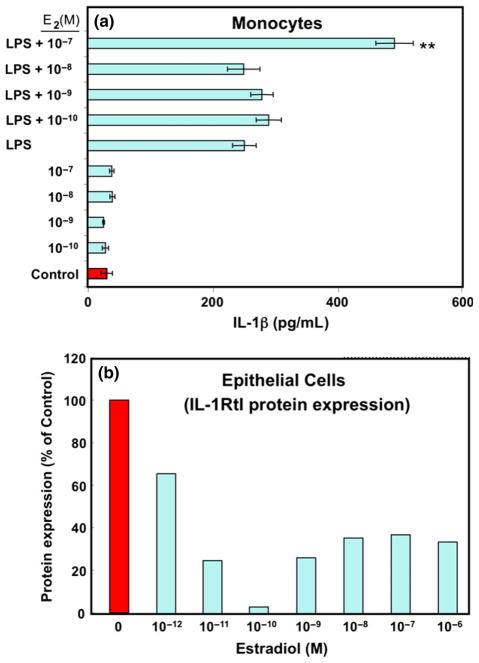
Monocytes circulate through the periphery, mediating immune recognition and pathogen clearance by phagocytosis and indirect stimulation of the immune system through production of key cytokines and chemokines. As shown in Fig. 11 (top), the TLR4 agonist LPS, the key antigenic component of many pathogenic bacteria, stimulates peripheral blood monocyte secretion of IL-1β, thereby causing a proinflammatory response. In the presence of E2, this response is further enhanced in a dose dependent manner (Fig. 11, bottom)115 and may be detrimental to the host especially in the FRT.91,92 In other studies, we found that increasing E2 levels in the FRT can down-modulate immune associated HBD2 and IL-8 responses to IL-1β (see Fig. 5). We have further shown that this response is mediated through a down-regulation of interleukin-1 receptor type I (IL-1RtI) protein expression (Fig. 11). Overall, E2 has the ability to enhance pathogen induced IL-1β expression by monocytes but at the same time decrease IL-1β receptor expression by epithelial cells. These studies demonstrate a close link between the endocrine and immune systems in the FRT that may be crucial for dampening proinflammatory responses during the time of ovulation or pregnancy.
Fig. 11.
Estradiol augments lipopolysaccharide (LPS)-induced IL-1β levels in monocytes (a). Peripheral blood monocytes were incubated with indicated concentrations of E2 for 24 hr and then stimulated with 10 ng/mL LPS for an additional 12 hr. Supernatants were collected from these cultures. IL-1β production was measured by ELISA. (b) Estradiol inhibits interleukin-1 receptor type I (IL-1RtI) protein expression. Whole cell lysates were generated from ECC-1 cells incubated with various concentrations of E2 for 72 hr. Proteins were resolved by 10% SDS-PAGE and detected with an anti-IL-1RtI antibody. Individual bands were scanned, and the intensity was quantified by computer analysis. The amount of IL-1RtI was normalized to GAPDH levels and plotted as a percentage of the control, **significantly different from Control (P < 0.01) (representative of two experiments).57,115
CD4+ T Cells
Even though sex hormones have been described to influence HIV infection in epidemiological studies and regulate different immune responses that may affect HIV infection, the direct role that female sex hormones play in altering the susceptibility of target cells to HIV-infection is largely unknown. To examine the direct effect of E2 on HIV-infection in an in vitro infection assay, purified CD4+ T cells (>98% purity) were activated in vitro in the presence or absence of E2 for 3 days and infected with R5 (HIV-1BaL) viral strain.116 Secreted and intracellular p24 were measured 7 days after infection as an indication of viral replication. As shown in Fig. 12a, when CD4+ T cells were treated with E2 prior to infection (pre) for 3 days, released p24 was significantly reduced 7 days after infection with HIV-1BaL (56% reduction; P = 0.024). However, when E2 was added for the entire length of incubation (pre/post) or 2 hr after infection (post) no differences were found compared to the control condition. This observation was confirmed by a significant reduction in the expression of intracellular p24 in CD4+ T cells pre-treated with E2 before infection (not shown).116 As a control to demonstrate that the shifts into the p24 positive population represent de novo infection and not residual virus, CD4+ T cells infected in the presence of azidothymidine (AZT) showed no infection (not shown). We investigated whether the reduction in HlV-1 susceptibility induced by E2 could be due to differences in activation. CD4+ T cells treated or not with E2 showed similar expression of CD25 and HLA-DR before HIV challenge (data not shown).
Fig. 12.
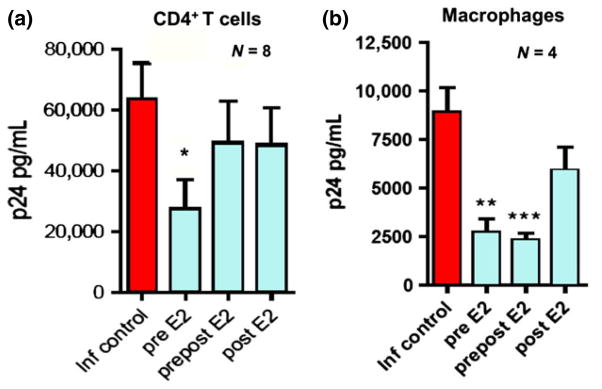
Effect of estradiol on Susceptibility of Blood CD4+ T cells and Macrophages to human immunodeficiency virus (HIV) Infection (BaL). (a) Released p24 levels in the culture media after 7 days of infection when CD4+ T cells where pre-treated with E2 (pre E2), treated with E2 before and after infection (prepost E2) or only after infection (post E2). (b) p24 levels released in to culture media after 7 days of infection when macrophages where pre-treated with E2 (pre E2), treated with E2 before and after infection (prepost E2) or only after infection (post E2). Bars represent mean ± S.E.M. from 8 (a) and 4 (b) independent experiments with different donors. *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from reference (116).
Since these experiments were conducted with cells from both female and male donors, we analyzed if any differences could be found between them. Infection levels in the infected controls from female donors were lower than male donors, with median p24 values of 38,085 versus 71,541 respectively (P = 0.03). Treatment with E2 prior to viral challenge significantly reduced HIV-infection in women (54.9% reduction; P = 0.03), but the reduction (18.3%) was not significant in men.
In other studies, recognizing that ethinyl estradiol (EE) is the main estrogenic component in most oral contraceptives and that contraceptives may be a risk factor for HIV infection,117,118 we investigated if EE would have the same effect as E2 in preventing HIV-infection of target cells. Following the same experimental design described above, CD4+ T cells were activated in the presence of EE and infected with an R5 viral strain. Irrespective of the time (pre) and length (pre/post/post) of hormone treatment, and unlike E2 that inhibited HIV infection, EE had no effect on CD4+ T cell infection with HIV-1BaL (not shown). Similar to the lack of inhibition with E2, we observed a pattern of reduced HIV-1IIIb viral replication when EE was added 2 hr after infection, but no statistical significance.
Macrophages
Recognizing that CD4+ T cells and macrophages are the most likely targets for HIV infection, we next focused on the effect of E2 on macrophage infection. Monocyte-derived macrophages were differentiated in vitro in the presence of E2 for 4 days and infected with HIV-1BaL. As shown in Fig. 12b, similar to our results with CD4+ T cells, viral replication was significantly reduced in macrophages differentiated in the presence of E2 (P < 0.0003). This reduction in susceptibility to HIV-infection was induced by differentiating the macrophages in the presence of E2 before infection (pre; 69% reduction) and maintained when E2 was added back to the culture 2 hr after infection (pre/post; 73% reduction). In contrast, E2 had no effect when added immediately after infection (post) (Fig. 12a, b). Intracellular p24 analysis confirmed a significant reduction in the percent of p24 positive cells when macrophages were differentiated in the presence of E2 (not shown). Intracellular p24 values correspond to de novo infection, since macrophages infected in the presence of AZT as a control show intracellular p24 values equal to uninfected controls. Comparison between macrophages derived from female monocytes or male monocytes showed no statistical differences, and in both cases E2 pre-treatment was able to significantly reduce HIV-infection (not shown). When Fig. 12a, b are compared, E2 appears to be more effective in reducing susceptibility to HIV-infection in macrophages than CD4+ T cells. Furthermore, the suppressive effect was maintained when E2 was present after infection. This maintenance of suppression represents a difference with respect to the effect observed in CD4+ T cells in that the inhibitory effect induced by pre-treatment with E2 is lost when E2 is present after infection (Fig. 12a).
In contrast with T cells, EE was able to decrease macrophage susceptibility to HIV-infection, When macrophages were differentiated in the presence of EE, released p24 was significantly reduced 7 days after infection (P < 0.005). When CD4+ T cells and macrophages from the same donors were analyzed in parallel to compare the effects of E2 and EE, intracellular p24 staining confirmed the lack of effect on CD4+ T cells but decreased the% of infected macrophages for both E2 and EE pretreatments. Pre-treatment with E2, however, was consistently 10–20% more effective than EE in suppressing viral replication (not shown).
In other studies, E2 suppresses HIV-infection in a dose-dependent manner with both T cells and macrophages. To investigate the possible mechanisms involved in E2 suppression of viral replication, we first examined the expression of CCR5, the main coreceptor for R5 viral strains. CD4+ T cells were activated in the presence of E2 and CCR5 expression was assayed by RNA and flow cytometry immediately prior to HIV-infection. No differences were observed in CD4+ T cell CCR5 gene expression after E2 treatment, relative to controls. Expression levels of CD4 were also measured, but no differences were found between controls and E2 treated cells (not shown). Macrophages differentiated in the presence of E2 or EE were also analyzed for the expression of CCR5 at the time of infection and neither E2 nor EE had any effect on CCR5 gene expression relative to controls. To better define the role of E2 in affecting viral entry, we used a single cycle, VSV-G pseudotyped virus, which enters the cells by endocytocis, keeping the need for receptor/coreceptor attachment and fusion.119 No differences in infection levels were found between the control conditions and cells pretreated with E2, strongly suggesting that E2 inhibits infection at the steps of viral entry or fusion.
Dendritic Cells
Expressed by DC, DC-SIGN is a calcium-dependent carbohydrate-binding protein.120,121 As an adhesion receptor DC-SIGN interacts with ICAM-2 on endothelial cells to both induce migration of DCs from blood to tissue122 and mediate clustering of DCs with naive T cells through binding of ICAM-3.120 Of equal importance in the FRT, DC-SIGN functions as a pattern recognition receptor that induces specific immune responses upon interaction with a number of pathogens.123 This carbohydrate recognition pattern is the basis of its broad specificity for different pathogens and might also be responsible for its distinct signaling properties. While initial target cells for mucosal transmission of HIV-1 in the reproductive tract have not been clearly determined, studies conducted in non-human primate models indicate the potential role of DC as initial targets for mucosal infection and systemic dissemination of HIV-1.124–126 DC can capture HIV-1 using surface expressed C-type Lectin Receptors (CLRs),127–129 among which DC-SIGN is the best characterized.122 HIV-1 captured by DC can be transferred to other target cells including CD4+ T lymphocytes via virological synapses, in a process referred to as trans infection.20,130 Understanding the mechanisms by which DC-SIGN and other HIV-1 receptors are regulated on mucosal target cells remains critical to designing strategies to prevent sexual transmission.
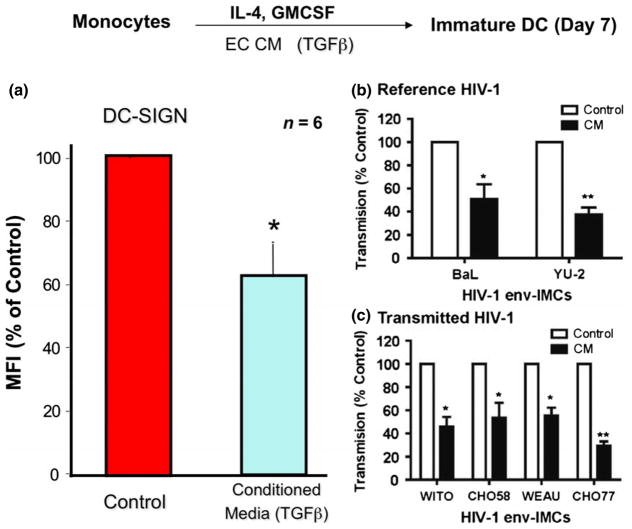
In previous studies aimed at elucidating the role of cell-cell interactions in the EM on the expression of HIV-1 receptors, we examined the effects of soluble factors produced by uterine epithelial cells on the expression of DC-SIGN by DC.131 In these experiments, immature DC were differentiated from human monocytes in the presence of IL-4 and GM-CSF. To assess the CM effects on DC-SIGN expression, DC were differentiated in the presence or absence of CM consisting of basolateral secretions from polarized epithelial cells for 7 days prior to flow cytometry. As shown in Fig. 13a, we found that uterine epithelial cell secretions (i.e. CM) decreased DC-SIGN expression on immature DC via a TGFβ mechanism. These results suggested that DC generated in the presence of CM (i.e. CM-DC) might have reduced capacity for trans infection of HIV-1 relative to control DC. To directly test this hypothesis, HIV-1 trans infection assays were undertaken (Fig. 13b). When immature DC were pulsed with HIV-1, prior to virus infection of TZM-bl cells, CM inhibited DC-mediated trans infection of HIV-1.131 These results provided direct evidence for uterine epithelial cell regulation of DC transmission of infection with reference and transmitted/founder HIV-1 variants. Interestingly, whereas recombinant TGFβ1 inhibited trans infection of prototypic reference HIV-1 by DC, TGFβ1 had a minimal effect on trans infection of transmitted/founder variants irrespective of the reporter system used to measure trans infection. These findings have immediate implications for designing strategies to prevent sexual transmission of HIV-1.
Fig. 13.
Inhibition of dendritic cells (DC)-SIGN on iDC by Uterine Epithelial Cell conditioned media (CM) Decreases Trans-infection of human immunodeficiency virus (HIV) to TZM-bl cells. Panel a. Averaged mean fluorescence intensity (MFI) values for DC-SIGN expression by DC from individual donors (n = 5) are presented as% MFI of Control DC ± standard error of mean (S.E.M.). *P = 0.035 (S.E.M.). Panel b and c. Reduced trans infection of HIV-1 by DC cultured with primary UEC CM. Shown is the effect of primary UEC CM on trans-infection by DC of HIV-1 reference HIV-1 (BaL or YU-2) (a) or transmitted/founder variants (b) (n = 5 blood donors). HIV-1 trans-infection assay was performed using TZM-bl reporter cells as targets as described in materials and methods. Unshaded histograms are Control DC and the black histograms are CM DC. The data are presented as% transmission 6 S.E.M. (Calculated from 4 or 5 separate experiments: see Table I) of HIV-1 by CM DC relative to Control DC. *P = 0.05, **P = 0.001. From reference (131).
We recently proposed a model for the existence of a window of vulnerability for HIV-1 infection during a woman’s menstrual cycle.5 During this critical period, 7–10 days of the normal menstrual cycle, critical components of innate and adaptive immune responses are suppressed by E2 and/or progesterone to facilitate reproductive processes. HIV-1 presumably exploits this time frame, during which antiviral factors are suppressed, to establish and propagate infection in the female genital tract mucosal.5 The reported findings showing that E2 increases DC-SIGN expression reveals yet another potential dimension by which sex steroid hormone may modulate susceptibility to HIV-1 infection in women. It is likely that in addition to suppressing antiviral effector mechanisms, the profile of sex steroid hormones present during the window of vulnerability in women may increase HIV-1 acquisition through enhanced expression of HIV-1 capture receptors by DC. More studies are needed to directly test the effects of sex steroid hormones on infection of DC with HIV-1.
NK Cells
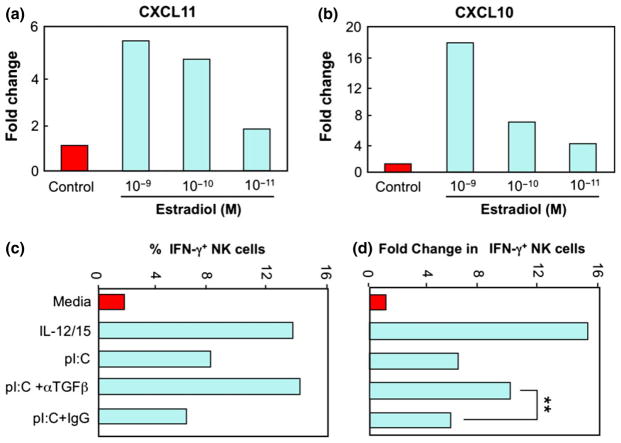
Natural killer (NK) cells are important in innate immunity, not only for their ability to kill certain tumour cells and viral-infected cells without prior immunization or MHC restriction, but also for their secretion of immunoregulatory cytokines that contribute to early host responses against viruses, bacteria and fungi.132 NK cells account for a large percentage of leukocytes in the human EM and, since their numbers increase as the menstrual cycle progresses,16–18,133 Sentman et al.134 hypothesized that recruitment and/or expansion of uterine NK cells are regulated by sex hormones. As shown in Fig. 14a, b, they tested the effect of various doses of E2 on human endometrial slices and found that 10−9 M E2 significantly enhanced mRNA expression of the chemokines CXCL10 and CXCL11. Since the estrogen receptor inhibitor ICI182780 blocked the E2-induced increase of these two chemokines, the effect was dependent upon E2 receptor activity (not shown). Progesterone at 10−8 M also increased the mRNA expression of CXCL10 (8.6-fold) and CXCL11 (12.2-fold) in the endometrial organ culture system.134 Sentman et al.134 have suggested that the sex hormones do not act directly on NK cells, but rather act on stromal and epithelial cells to produce chemokines for NK cell recruitment. Since E2 and progesterone stimulate production of these cytokines in the EM, it is likely that these sex hormones contribute to the migration of NK cells needed for immune surveillance, pathogen response, pregnancy, and normal menstrual cycle function.
Fig. 14.
Effect of estradiol and TGFβ on natural killer (NK) cell immune function. To examine the effect of E2 on chemokine expression, endometrial tissue sections were incubated with different concentrations of E2 as indicated for 48 hr, then snap frozen and stored at −80°C. Chemokines CXCL11 (a) and CXCL10 (b) were measured following the isolation of total RNA using TRIzol. Quantitative Real-Time PCR was used to determine the relative fold expression of each gene compared with medium only. In other studies, endogenous TGFβ suppression of poly (I:C)-induced interferon-γ (IFN-γ) production by uterine NK cells was measured (c and d). Uterine cells were isolated from a hysterectomy patient and cultured with media, IL-12 and IL-15, or poly (I:C) as indicated for 18 hr in the presence of blocking anti-TGFβ monoclonal antibodies (αTGFβ), control IgG (IgG) or media only (media). Uterine NK cells were then analyzed for intracellular IFN-γ production by flow cytometry (c). IL-12 and IL-15 in combination and poly (I:C) increase the percent of IFN-γ+ producing NK cells and antibody to TGFβ enhances the number of uterine NK cells induced by poly (I:C) that produce IFN-γ. In (d), data are expressed in terms of fold increase in IFN-γ+ NK cells. Adapted from134 (bottom) Adapted from reference (139). *P < 0.05.
One innate mechanism that regulates NK activity is TGFβ, which is regulated by sex hormones. TGFβ is typically produced in a pro-form that requires activation 135; the precise mechanisms that lead to activation of TGFβ in tissues are not well understood. It has previously been shown that uterine NK cell responses to monokines and blood cell NK cell responses to TLR agonists were inhibited by endogenous TGFβ.136,137 Uterine NK cells express TLR and can respond to TLR agonists by producing cytokines. 138 Eriksson et al.139 explored how endogenous TGFβ modulates the production of interferon-γ (IFN-γ) by human uterine NK cells. As shown in Fig. 14c, d, IL-12 and IL-15 in combination and poly (I:C) increase the percent of IFN-γ producing NK cells in the uterus, and antibody to TGFβ enhances the number and fold change of uterine NK cells induced by poly (I:C) that produce IFN-γ. Thus, endogenous TGFβ suppresses poly (I:C)-induced IFN-γ production by uterine NK cells. Since one way that E2 regulates FRT immunity is by modulating the production and/or activation of TGFβ, it is likely that E2 regulates NK activity via endogenous TGFβ.
Neutrophils
At mucosal surfaces, neutrophils are responsible for rapid elimination of potential pathogens through phagocytosis of microbes, release of antimicrobial compounds, and production of toxic oxygen and nitrogen species.140 In studies to determine whether sex hormones modulate neutrophil phenotype, Smith et al.19,141 examined blood neutrophils from women at days 7, 14, 21, and 28 of the menstrual cycle for expression of surface receptors, granule proteins, and intracellular cytokines. Blood neutrophil phenotype varied during the menstrual cycle with decreased expression of CD89 (IgA Fc receptor) and TNFα during the periovulatory period. In other studies, cytokines were analyzed for their ability to enhance the innate immune potential of neutrophils by altering receptor expression and cell function.142 These studies indicated that GM-CSF, known to be produced by FRT epithelial cells, acted synergistically with the chemoattractant IL-8 to promote neutrophil chemotaxis. Use of antibody neutralization and CM from primary confluent cultures of epithelial cells led to the conclusion that FRT epithelial cells are a potent source of neutrophil chemoattractant activity.
Sex hormone regulation of antimicrobial activity
We have previously shown that polarized human uterine epithelial cells secrete antimicrobial molecules that kill or inhibit pathogenic bacteria, fungi, and viruses.63,77,143 Apical secretion of these molecules is probably one reason why the uterine lumen has a relatively low number of microorganisms compared to the lower FRT despite flow of fluids throughout the entire tract.39 Secretion of antimicrobials is both constitutive and induced by TLR agonists and microorganisms, and is affected by many factors such as age, menstrual status, health, and the presence of chemokines, cytokines and steroid hormones.
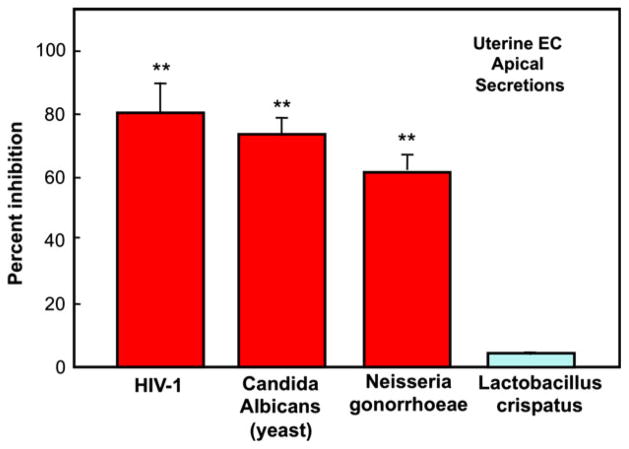
As seen in Fig. 15, uterine epithelial cell secretions inhibit Neisseria gonorrhoeae and Candida albicans (yeast and hyphal form), as well as reduce HIV-1 (R5) infection of target cells. Similar inhibition of pathogens is seen with secretions from Fallopian tube, cervical, and ectocervical epithelial cells (not shown). In contrast, none had an inhibitory effect on Lactobacillus crispatus, a common commensal in the vagina. Analysis of cytokines and chemokines in uterine secretions revealed several molecules that could account for pathogen inhibition.144 These findings provide definitive evidence for the critical role of epithelial cells in protecting the FRT from infections, without comprising the beneficial presence of L. crispatus, which is part of the normal vaginal microflora of humans.
Fig. 15.
Comparison of antimicrobial activity in secretions from uterine epithelial cells against human immunodeficiency virus (HIV)-1, Neisseria gonorrhoeae, Candida albicans, and Lactobacillus crispatus. Results shown are the percent inhibition obtained with uterine apical conditioned media (CM) derived from four cell inserts of one patient. For each pathogen, the colony-forming unit (CFU) obtained with CM incubation was compared with four controls of the microorganism incubated with media. **P < 0.01 compared with control.144 For HIV-1 analysis, CM (48 hr) from polarized uterine epithelial cells was diluted 1:10 with fresh media and incubated with BaL HIV-1 for 1 hr before addition to TZM-bl cells to assess infection. Results are compared with infection data obtained with each virus incubated with media alone. Data presented for HIV-1 are for 4 inserts per patient). **P < 0.01 compared with control. From reference (144).
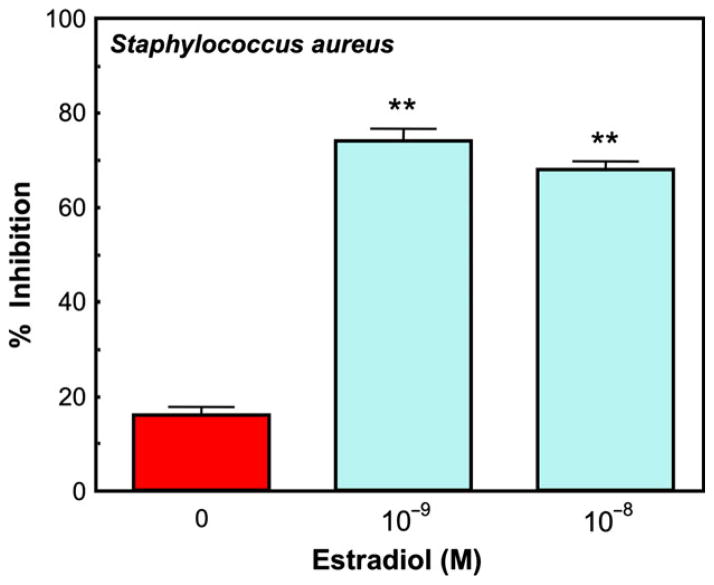
The presence of sex hormones can dramatically alter anti-bacterial activity by epithelial cells. Secretions from human uterine epithelial cells treated with multiple concentrations of E2 for at least 48 hr show greater inhibition of Staphylococcus aureus growth than those secretions from untreated cells (Fig. 16), suggesting that E2 induces the secretion of antimicrobial(s) in addition to that seen under control conditions. This reinforces the premise that E2 can have a significant effect on antimicrobial secretion by human uterine polarized epithelial cells, and suggests that antibacterial, antifungal and antiviral activity in cell secretions changes during the menstrual cycle.
Fig. 16.
Effect of estradiol on anti-bacterial activity by ECC-1 epithelial cells in culture. Monolayer cultures were treated with or without two concentrations of E2 for 48 hr. The apical conditioned media was recovered after 2 days, centrifuged and an aliquot of supernatants was then incubated with Staphylococcus aureus for 1 hr and the bacteria were cultured overnight. Control colony forming units (CFU) refers to the colonies grown in media in the absence of uterine epithelial cells. The inhibition seen with no E2 represents the bacteria that grew out in the presence of antimicrobials constitutively produced by the cells. Estradiol by itself had no effect on bacterial CFU (not shown). Four wells per group; **Significantly different (P < 0.01) from Control. From reference (12).
Conclusions
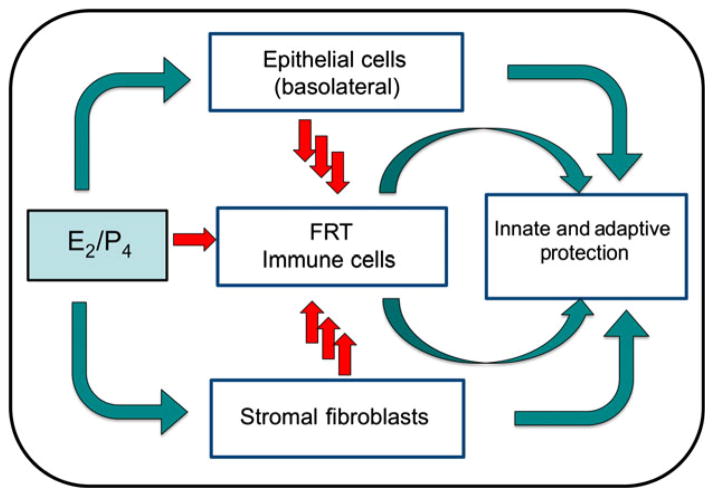
In conclusion, analysis of the FRT indicates that the innate and adaptive immune systems are present and functional throughout the menstrual cycle. Not widely appreciated is a growing body of data indicating that the immune system in the Fallopian tubes, uterus, cervix, and vagina function independently from each other to changes in hormone levels during the menstrual cycle. The net result is coordinated immune protection that complements the reproductive demands of each organ. As the first line of this protection, epithelial cells, macrophages, DC, NK cells, and neutrophils in the FRT function to meet the challenges of STI, while at the same time supporting an immunologically distinct fetal placental unit. The studies presented demonstrate overwhelmingly that E2 secreted by the ovary during the menstrual cycle acts both directly and indirectly on epithelial cells and other immune cells in the FRT to regulate a spectrum of immune functions specific to each site in the FRT. At the center of immune protection, epithelial cells that line the FRT provide a level of protection not previously recognized. Acting as a physical barrier, these cells are sentinels that function as an integral part of the innate and adaptive immune systems by recognizing and protecting against bacterial and viral challenges as well as signaling the recruitment and activation of underlying immune protection when pathogenic challenge exceeds their protective capacity. Along with CD4+ T cell, macrophage, DC, epithelial cell, and fibroblast responses are uniquely programmed to respond to hormone changes so that immune coverage occurs in a way that confers continuous protection. As shown in Fig. 17, this review indicates that epithelial, fibroblast and immune cell functions are complicated by their mutual interactions and the recognition that sex hormones alter cell-cell communication in the FRT to regulate innate and adaptive immune protection. As discussed previously,5 aspects of the innate, humoral, and cell-mediated immune systems are suppressed by sex hormones to optimize conditions for procreation. Suppression occurs in the upper (Fallopian tubes, uterus, endocervix) and lower (ectocervix and vagina) FRT, and coincides with the recruitment of potentially infectable cells and upregulation of coreceptors involved in pathogen uptake. The studies presented suggest that by understanding the ways in which sex hormones regulate epithelial, fibroblast, and immune cell function in the FRT, new avenues may be identified both to protect against potential pathogens and to enhance the quality of women’s reproductive health.
Fig. 17.
Schematic indicating the role of sex hormones in epithelial cell, fibroblast, and immune cell protection in the female reproductive tract (FRT) tissues from the upper and lower FRT. Estradiol and progesterone act on epithelial cells, fibroblasts, and immune cells both directly and indirectly through cytokines, chemokines, and antimicrobials to modulate innate and adaptive immune protection. Immune regulation varies with the site in the reproductive tract and may be either enhanced or suppressed by sex hormones to meet the combined challenges of procreation and pathogenic challenge.
Acknowledgments
This work was funded by a National Institutes of Health Grants AI51877, AI102838 (CRW), and by a grant from the Bill & Melinda Gates Foundation through the Grand Challenges Exploration Initiative (JVF/CRW).
References
- 1.UNAIDS. AIDS Epidemic Update, Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) Geneva, Switzerland: 2008. UNAIDS 2008. [Google Scholar]
- 2.UNAIDS. AIDS Epidemic Update JUNPoHAUaWHOW. Geneva, Switzerland: 2007. [Google Scholar]
- 3.Ogra P, Yamanaka T, Losonsky GA. Reproductive Immunology. In: Fleicher N, editor. Local immunologic defenses in the genital tract. New York: Alan R. Liss, Inc; 1981. pp. 381–394. [PubMed] [Google Scholar]
- 4.Wira CR, Fahey JV, White HD, Yeaman GR, Given AL, Howell AL. The mucosal immune system in the human female reproductive tract: influence of stage of the menstrual cycle and menopause on mucosal immunity in the uterus. In: Glasser S, Aplin J, Guidice L, Tabibzadeh S, editors. The Endometrium. New York: Taylor and Francis; 2002. pp. 371–404. [Google Scholar]
- 5.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 7.Wira CR, Grant-Tschudy KS, Crane-Godreau M. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:1–12. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 8.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 9.Fahey JV, Schaefer TM, Wira CR. Sex hormone modulation of human uterine epithelial cell immune responses. Integr Comp Biol. 2006;46:1082–1087. doi: 10.1093/icb/icl036. [DOI] [PubMed] [Google Scholar]
- 10.Kaushic C, Wira CR. IgA and reproductive tract immunity. In: Kaetzel AC, editor. Mucosal Immune Defense: Immunoglobulin. New York: Kluwer Academic/Plenum Publisher; 2008. pp. 291–320. [Google Scholar]
- 11.Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlenbach-Hellweg G. Histopathology of the Endometrium. 2. New York: Springer-Verlag; 1975. [Google Scholar]
- 14.Morris H, Edwards J, Tiltman A, Emms M. Endometrial lymphoid tissue: an immunohistological study. J Clin Pathol. 1985;38:644–652. doi: 10.1136/jcp.38.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulmer JN, Earl U. The expression of class II MHC gene products by fallopian tube epithelium in pregnancy and throughout the menstrual cycle. Immunology. 1987;61:207–213. [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt JS. Immunologically relevant cells in the uterus. Biol Reprod. 1994;50:461–466. doi: 10.1095/biolreprod50.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 18.Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, Sentman CL. Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol. 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Smith JM, Wira CR, Fanger MW, Shen L. Human fallopian tube neutrophils: a distinct phenotype from blood neutrophils. Am J Reprod Immunol. 2006;56:218–229. doi: 10.1111/j.1600-0897.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 20.Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman GR, Fazleabas AT, Wira CR. Endometrial lymphoid aggregates. Mucosal Immunol Update. 2004;12:6–8. [Google Scholar]
- 22.Yeaman GR, Collins JE, Fanger MW, Wira CR, Lydyard PL. T cell receptor Vb usage in human uterine endometrial lymphoid aggregates shows no clonal restriction: evidence that uterine lymphoid aggregates arise by cell trafficking. Immunology. 2001;102:434–440. doi: 10.1046/j.1365-2567.2001.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 24.White HD, Crassi K, Wira CR. Cytolytic functional activities of NK cells and cytotoxic T lymphocytes (CTL) are coordinately regulated in the human female reproductive tract. In: Husband AJ, Beagley KW, Clancey RL, Collins AM, Cripps AW, Emery DL, editors. Mucosal Solutions: Advances in Mucosal Immunology. Sydney, Australia: The University of Sydney; 1997. pp. 385–391. [Google Scholar]
- 25.Dominguez F, Galan A, Martin JJL, Remohi J, Pellicer A, Simón C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 26.Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–6167. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 27.Yeaman GR, Howell AL, Weldon S, Damien DJ, Collins JE, O’Connell DM, Asin SN, Wira CR, Fanger MW. HIV receptor and coreceptor expression on human uterine epithelial cells during the menstrual cycle. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Wira CR, Fanger MW, Howell AL. Chemokine receptor expression in the human cervix: implications for infection by the human immunodeficiency virus-type I (HIV-1) Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan DA, Richardson GS, MacLaughlin DT, Wira CR. Variations in the levels of secretory component in human uterine fluid during the menstrual cycle. J Steroid Biochem. 1984;20:509–513. doi: 10.1016/0022-4731(84)90263-2. [DOI] [PubMed] [Google Scholar]
- 30.Tauber PF, Wettich W, Nohlen M, Zaneveld LJ. Diffusible proteins of the mucosa of the human cervix, uterus, and fallopian tubes: distribution and variations during the menstrual cycle. Am J Obstet Gynecol. 1985;15:1115–1125. doi: 10.1016/0002-9378(85)90394-1. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher GFB. Soluble proteins in cervical mucus. In: Blandau RJ, Moghissi K, editors. The Biology of the Cervix. Chicago: The University of Chicago Press; 1973. pp. 201–233. [Google Scholar]
- 32.Kutteh KW, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses. 1998;14:S51–S55. [PubMed] [Google Scholar]
- 33.Nardelli-Haefliger DJ, Wirthner D, Schiller J, Lowy D, Hildesheim A, Ponci F, Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human Papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 34.Boomsma CM, Kavelaars A, Eijkemans MJ, Lentjes EG, Fauser BC, Heijnen CJ, Macklon NS. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod. 2009;24:1427–1435. doi: 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 35.Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, Garcia-Velasco JA, Matorras R, Prieto B, Gonzalez S, Nagore D, Simon L, Elortza F. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res. 2009;8:4622–4632. doi: 10.1021/pr9004426. [DOI] [PubMed] [Google Scholar]
- 36.Boomsma CM, Kavelaars A, Eijkemans MJ, Fauser BC, Heijnen CJ, Macklon NS. Ovarian stimulation for in vitro fertilization alters the intrauterine cytokine, chemokine, and growth factor milieu encountered by the embryo. Fertil Steril. 2010;94:1764–1768. doi: 10.1016/j.fertnstert.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 37.Keller M, Guzman E, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein S, Cole A, Cole A, Profy A, Wira C, Hogarty K, Herold B. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 38.Quayle AJ, Martin Porter E, Nussbaum AA, Wang YM, Brabec C, Yip K-P, Mok SC. Gene expression, immunolocalization and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 39.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriyama A, Shimoya K, Ogata I, Kimura T, Nakamura T, Wada H, Ohashi K, Azuma C, Saji F, Murata Y. Secretory leukocyte protease inhibitor (SLPI) concentrations in cervical mucus of women with normal menstrual cycle. Mol Hum Reprod. 1999;5:656–661. doi: 10.1093/molehr/5.7.656. [DOI] [PubMed] [Google Scholar]
- 41.Fahey JV, Kaushic C, Wira CR. Human uterine epithelial cells: influence of culture conditions and stromal cells on epithelial cell transepithelial cell resistance. In: Gupta SK, editor. Reproductive Immunology. New Delhi: Narosa Publishing House; 1999. pp. 366–378. [Google Scholar]
- 42.Wira CR, Stern J. Endocrine regulation of the mucosal immune system in the female reproductive tract: control of IgA, IgG, and secretory component during the reproductive cycle, at implantation and throughout pregnancy. In: Pasqualini JR, Scholler R, editors. Hormones and Fetal Pathophysiology. New York: Marcel Dekker Inc; 1992. pp. 343–368. [Google Scholar]
- 43.Grant KS, Wira CR. Effect of mouse uterine stromal cells on epithelial cell transepithelial resistance (TER) and TNFalpha and TGFbeta release in culture. Biol Reprod. 2003;69:1091–1098. doi: 10.1095/biolreprod.103.015495. [DOI] [PubMed] [Google Scholar]
- 44.Godfrey RWA. Human airway epithelial tight junctions. Microsc Res Tech. 1997;38:488–499. doi: 10.1002/(SICI)1097-0029(19970901)38:5<488::AID-JEMT5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Mo B, Vendrov AE, Palomino WA, DuPont BR, Apparao KB, Lessey BA. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75:387–394. doi: 10.1095/biolreprod.106.051870. [DOI] [PubMed] [Google Scholar]
- 47.Planchon S, Fiocchi C, Takafuji V, Roche JK. Transforming growth factor-beta1 preserves epithelial barrier function: identification of receptors, biochemical intermediates and cytokine antagonists. J Cell Physiol. 1999;181:55–66. doi: 10.1002/(SICI)1097-4652(199910)181:1<55::AID-JCP6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 48.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 49.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 50.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 51.Grant-Tschudy KS, Wira CR. Effect of estradiol on mouse uterine epithelial cell transepithelial resistance (TER) Am J Reprod Immunol. 2004;52:252–262. doi: 10.1111/j.1600-0897.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 52.Phillips ML, Schultz BD. Steroids modulate transepithelial resistance and Na+ absortion across cultured porcine vas deferens epithelia. Biol Reprod. 2002;66:1016–1023. doi: 10.1095/biolreprod66.4.1016. [DOI] [PubMed] [Google Scholar]
- 53.Groten T, Pierce AA, Huen AC, Schnaper HW. 17 beta-estradiol transiently disrupts adherens junctions in endothelial cells. FASEB J. 2005;19:1368–1370. doi: 10.1096/fj.04-2558fje. [DOI] [PubMed] [Google Scholar]
- 54.Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology. 2007;148:218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorodeski GI. Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women. Menopause. 2007;14:1012–1019. doi: 10.1097/gme.0b013e3180587eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer TM, Wright JA, Pioli PA, Wira CR. IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type 1 in uterine epithelial cells. J Immunol. 2005;175:6509–6516. doi: 10.4049/jimmunol.175.10.6509. [DOI] [PubMed] [Google Scholar]
- 58.Schust DJ, Ibana JA, Buckner LR, Ficarra M, Sugimoto J, Amedee AM, Quayle AJ. Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection. Curr HIV Res. 2012;10:218–227. doi: 10.2174/157016212800618093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr Womens Health Rev. 2008;4:102–117. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King AE, Critchley HO, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6:191–196. doi: 10.1093/molehr/6.2.191. [DOI] [PubMed] [Google Scholar]
- 62.King AE, Fleming DC, Critchley HO, Kelly RW. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59:1–16. doi: 10.1016/s0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 63.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 64.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human b-defensin-1, an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38:59–64. doi: 10.1016/S0928-8244(03)00106-8. [DOI] [PubMed] [Google Scholar]
- 66.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 67.Hocini H, Becquart P, Bouhlal H, Adle-Biassette H, Kazatchkine MD, Belec L. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin Diagn Lab Immunol. 2000;7:515–518. doi: 10.1128/cdli.7.3.515-518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porter E, Yang H, Yavagal S, Preza GC, Murillo O, Lima H, Greene S, Mahoozi L, Klein-Patel M, Diamond G, Gulati S, Ganz T, Rice PA, Quayle AJ. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun. 2005;73:4823–4833. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 70.Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–747. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- 71.Wahl S, McNeely T, Janoff E, Shugars D, Worley P, Tucker C, Orenstein J. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-1. Oral Dis. 1997;3(Suppl 1):S64–S69. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 72.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 73.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 74.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2010;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3 alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drannik AG, Nag K, Yao XD, Henrick BM, Ball TB, Plummer FA, Wachihi C, Kimani J, Rosenthal KL. Anti-HIV-1 activity of elafin depends on its nuclear localization and altered innate immune activation in female genital epithelial cells. PLoS One. 2012;7:e52738. doi: 10.1371/journal.pone.0052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 78.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 79.Kanda N, Watanabe S. 17Beta-estradiol inhibits MCP-1 production in human keratinocytes. J Invest Dermatol. 2003;120:1058–1066. doi: 10.1046/j.1523-1747.2003.12255.x. [DOI] [PubMed] [Google Scholar]
- 80.Kanda N, Watanabe S. 17beta-estradiol inhibits the production of RANTES in human keratinocytes. J Invest Dermatol. 2003;120:420–427. doi: 10.1046/j.1523-1747.2003.12067.x. [DOI] [PubMed] [Google Scholar]
- 81.Kanda N, Watanabe S. 17beta-estradiol inhibits the production of interferon-induced protein of 10 kDa by human keratinocytes. J Invest Dermatol. 2003;120:411–419. doi: 10.1046/j.1523-1747.2003.12066.x. [DOI] [PubMed] [Google Scholar]
- 82.Grant-Tschudy KS, Wira CR. Effect of oestradiol on mouse uterine epithelial cell tumour necrosis factor-alpha release is mediated through uterine stromal cells. Immunology. 2005;115:99–107. doi: 10.1111/j.1365-2567.2005.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greene CM, McElvaney NG, O’Neill SJ, Taggart CC. Secretory leucoprotease inhibitor impairs Toll-like receptor 2- and 4-mediated responses in monocytic cells. Infect Immun. 2004;72:3684–3687. doi: 10.1128/IAI.72.6.3684-3687.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lentsch AB, Jordan JA, Czermak BJ, Diehl KM, Younkin EM, Sarma V, Ward PA. Inhibition of NF-kappaB activation and augmentation of IkappaBbeta by secretory leukocyte protease inhibitor during lung inflammation. Am J Pathol. 1999;154:239–247. doi: 10.1016/s0002-9440(10)65270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lentsch AB, Yoshidome H, Warner RL, Ward PA, Edwards MJ. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology. 1999;117:953–961. doi: 10.1016/s0016-5085(99)70355-0. [DOI] [PubMed] [Google Scholar]
- 86.Nakamura A, Mori Y, Hagiwara K, Suzuki T, Sakakibara T, Kikuchi T, Igarashi T, Ebina M, Abe T, Miyazaki J, Takai T, Nukiwa T. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J Exp Med. 2003;197:669–674. doi: 10.1084/jem.20021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taggart CC, Cryan SA, Weldon S, Gibbons A, Greene CM, Kelly E, Low TB, O’Neill SJ, McElvaney NG. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med. 2005;202:1659–1668. doi: 10.1084/jem.20050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward PA, Lentsch AB. Endogenous regulation of the acute inflammatory response. Mol Cell Biochem. 2002;234:225–228. [PubMed] [Google Scholar]
- 89.Jorgenson RL, Young SL, Lesmeister MJ, Lyddon TD, Misfeldt ML. Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3-dependent responses to dsRNA. Hum Immunol. 2005;66:469–482. doi: 10.1016/j.humimm.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lesmeister MJ, Jorgenson RL, Young SL, Misfeldt ML. 17Beta-estradiol suppresses TLR3-induced cytokine and chemokine production in endometrial epithelial cells. Reprod Biol Endocrinol. 2005;3:74. doi: 10.1186/1477-7827-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- 92.Grosskinsky CM, Halme J. Endometriosis: the host response. Baillieres Clin Obstet Gynaecol. 1993;7:701–713. doi: 10.1016/s0950-3552(05)80459-6. [DOI] [PubMed] [Google Scholar]
- 93.Pendergrass PB, Belovicz MW, Reeves CA. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol Obstet Invest. 2003;55:110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
- 94.Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. Baseline dimensions of the human vagina. Hum Reprod. 2006;21:1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 95.Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006;273:195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 96.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, Chesson R, Spagnuolo RA, Pyles RB. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 97.Steele C, Fidel PL. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun. 2002;70:577–583. doi: 10.1128/IAI.70.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel MV, Fahey JV, Rossoll RM, Wira CR. Innate immunity in the vagina (part I): estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. Am J Reprod Immunol. 2013;69:463–474. doi: 10.1111/aji.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scapini P, Laudanna C, Pinardi C, Allavena P, Mantovani A, Sozzani S, Cassatella M. Neutrophils produce biologically active macrophage inflammatory protein-3a (MIP-3a)/CCL20 and MIP-3b/CCL19. Eur J Immunol. 2001;31:1981–1988. doi: 10.1002/1521-4141(200107)31:7<1981::aid-immu1981>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 100.Lahey T, Ghosh M, Fahey JV, Shen Z, Mukura LR, Song Y, Cu-Uvin S, Mayer KH, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Selective impact of HIV disease progression on the innate immune system in the human female reproductive tract. PLoS One. 2012;7:e38100. doi: 10.1371/journal.pone.0038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lail-Trecker M, Gulati R, Peluso JJ. A role for hepatocyte growth factor/scatter factor in regulating normal and neoplastic cells of reproductive tissues. J Soc Gynecol Investig. 1998;5:114–121. doi: 10.1016/s1071-5576(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 104.Kankuri E, Cholujova D, Comajova M, Vaheri A, Bizik J. Induction of hepatocyte growth factor/scatter factor by fibroblast clustering directly promotes tumor cell invasiveness. Cancer Res. 2005;65:9914–9922. doi: 10.1158/0008-5472.CAN-05-1559. [DOI] [PubMed] [Google Scholar]
- 105.Skibinski G, Elborn JS, Ennis M. Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L69–L76. doi: 10.1152/ajplung.00299.2006. [DOI] [PubMed] [Google Scholar]
- 106.Conway K, Price P, Harding KG, Jiang WG. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006;14:2–10. doi: 10.1111/j.1743-6109.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 107.Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morishita R, Aoki M, Hashiya N, Yamasaki K, Kurinami H, Shimizu S, Makino H, Takesya Y, Azuma J, Ogihara T. Therapeutic angiogenesis using hepatocyte growth factor (HGF) Curr Gene Ther. 2004;4:199–206. doi: 10.2174/1566523043346453. [DOI] [PubMed] [Google Scholar]
- 109.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 110.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- 111.Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A. Hepatocyte growth factor stimulated proliferation, migration, and lumen formation of human endometrial epithelial cells in vitro. Biol Reprod. 1997;57:936–942. doi: 10.1095/biolreprod57.4.936. [DOI] [PubMed] [Google Scholar]
- 112.Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril. 2009;92:1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grant-Tschudy KS, Wira CR. Hepatocyte growth factor regulation of uterine epithelial cell transepithelial resistance and tumor necrosis factor alpha release in culture. Biol Reprod. 2004;72:814–821. doi: 10.1095/biolreprod.104.035618. [DOI] [PubMed] [Google Scholar]
- 114.Grant-Tschudy KS, Wira CR. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J Reprod Immunol. 2005;67:1–12. doi: 10.1016/j.jri.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 115.Pioli PA, Weaver LK, Schaefer TM, Wright JA, Wira CR, Guyre PM. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. J Immunol. 2006;176:6647–6655. doi: 10.4049/jimmunol.176.11.6647. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS One. 2013;8:e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reichelderfer PS, Coombs RW, Wright DJ, Cohn J, Burns DN, Cu-Uvin S, Baron PA, Coheng MH, Landay AL, Beckner SK, Lewis SR, Kovacs AA. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS. 2000;14:2101–2107. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- 118.Benki S, Mostad SB, Richardson BA, Mandaliya K, Kreiss JK, Overbaugh J. Increased levels of HIV-1-infected cells in endocervical secretions after the luteinizing hormone surge. J Acquir Immune Defic Syndr. 2008;47:529–534. doi: 10.1097/QAI.0b013e318165b952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 121.Granelli-Piperno A, Pritsker A, Pack M, Shimeliovich I, Arrighi JF, Park CG, Trumpfheller C, Piguet V, Moran TM, Steinman RM. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 123.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 124.Miller CJ, McChesney M, Moore PF. Langerhans cells, macrophages and lymphocyte subsets in the cervix and vagina of rhesus macaques. Lab Invest. 1992;67:628–634. [PubMed] [Google Scholar]
- 125.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 126.Miller CJ. Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques. J Reprod Immunol. 1998;41:331–339. doi: 10.1016/s0165-0378(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 127.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 129.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 130.Wu L, Kewalramani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ochiel DO, Ochsenbauer C, Kappes JC, Ghosh M, Fahey JV, Wira CR. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS One. 2010;5:e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol. 1989;24:195–205. doi: 10.1016/0198-8859(89)90060-8. [DOI] [PubMed] [Google Scholar]
- 134.Sentman CL, Meadows SK, Wira CR, Eriksson M. Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol. 2004;173:6760–6766. doi: 10.4049/jimmunol.173.11.6760. [DOI] [PubMed] [Google Scholar]
- 135.Khalil N. Post translational activation of latent transforming growth factor beta (L-TGF-beta): clinical implications. Histol Histopathol. 2001;16:541–551. doi: 10.14670/HH-16.541. [DOI] [PubMed] [Google Scholar]
- 136.Eriksson M, Meadows SK, Wira CR, Sentman CL. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol. 2004;76:667–675. doi: 10.1189/jlb.0204090. [DOI] [PubMed] [Google Scholar]
- 137.Meadows SK, Eriksson M, Barber A, Sentman CL. Human NK cell IFN-gamma production is regulated by endogenous TGF-beta. Int Immunopharmacol. 2006;6:1020–1028. doi: 10.1016/j.intimp.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 138.Eriksson M, Meadows SK, Basu S, Mselle TF, Wira CR, Sentman CL. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J Immunol. 2006;176:6219–6224. doi: 10.4049/jimmunol.176.10.6219. [DOI] [PubMed] [Google Scholar]
- 139.Eriksson M, Meadows SK, Wira CR, Sentman CL. Endogenous transforming growth factor-beta inhibits toll-like receptor mediated activation of human uterine natural killer cells. Am J Reprod Immunol. 2006;56:321–328. doi: 10.1111/j.1600-0897.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 140.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 141.Smith JM, Shen Z, Wira CR, Fanger MW, Shen L. Effects of menstrual cycle status and gender on human neutrophil phenotype. Am J Reprod Immunol. 2007;58:111–119. doi: 10.1111/j.1600-0897.2007.00494.x. [DOI] [PubMed] [Google Scholar]
- 142.Shen L, Fahey JV, Hussey SB, Asin SN, Wira CR, Fanger MW. Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol. 2004;230:23–32. doi: 10.1016/j.cellimm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 143.Schaefer TM, Fahey JV, Wright JA, Wira CR. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly (I:C) Am J Reprod Immunol. 2005;54:193–202. doi: 10.1111/j.1600-0897.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 144.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4:335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]