Abstract
Background
Animal models in pain research have suggested that inclusion of both evoked and nonevoked behavioral measures are needed to better reflect the human pain experience. Individuals with chronic pain are known to experience spontaneous pain, in addition to pain, following exposure to an external stimulus. Recently, the Dynamic Weight Bearing (DWB) apparatus was developed to assess for nonevoked hyperalgesia by capturing weight bearing and surface distribution in the paws of mice following acute inflammation.
Objectives
The aim of this study was to evaluate the DWB test as a measure of nonevoked hyperalgesia.
Method
The experimental group received an intraplantar injection to the left hind paw of the inflammatory agent—Complete Freund's Adjuvant (CFA)—while the vehicle control group received a saline injection, and the naïve control group had no treatment. Caliper and plethysmometer were used to verify inflammation, and the hot-plate test was used as a measure for stimulus evoked hyperalgesia. Data were collected at baseline, three hours, one, three, and seven days after injection.
Results
Mice injected with CFA showed a statistically significant higher mean paw thickness and volume displacement compared to vehicle and naïve control groups. In the hot-plate testing, CFA-treated mice showed lower response temperature at seven days compared to other groups. On the DWB test, CFA-treated mice showed a reduction in the ipsilateral paw load and surface area compared to the contralateral paw load at Day 1, Day 3 and Day 7.
Discussion
Mice with inflammation demonstrated alterations in weight bearing, as well as increased thermal hyperalgesia in comparison to controls groups. These findings support the use of the DWB test as a tool for measuring nonevoked inflammatory hyperalgesia in a mouse model.
Keywords: animal models, hyperalgesia, inflammatory pain, weight bearing
Pain is a critically important public health concern affecting more than 100 million adults in the United States, and costing $635 billion annually in the treatment cost, lost productivity, and disability cost (Institute of Medicine [IOM], 2011). The IOM has deemed chronic pain as a national challenge requiring the participation of government agencies, healthcare providers, healthcare professional associations, educators, and public and private funders of health care to improve the prevention, assessment, and management of the various types of pain. Patients with chronic pain conditions report disruptions in mental health, performance of daily activities, and sexual function, as well as an overall reduction in quality of life (Fine, 2011; IOM, 2011; Kalaydjian & Merikangas, 2008). Despite the debilitating physical and substantial economic burden associated with chronic pain, the biological underpinnings of many pain conditions have yet to be elucidated and effective treatment remains challenge. Animal-behavioral pain models in preclinical research have played an integral role in the advancement of knowledge regarding mechanistic insight into pain and the development and identification of novel therapeutic targets for chronic pain conditions (Berge, 2011; Gregory et al., 2013; Kissin, 2010). Therefore, it is crucial to establish appropriate measurement techniques to facilitate preclinical evaluation of putative medicines and their potential effects (Tétreault, Dansereau, Doré-Savard, Beaudet, & Sarret, 2011).
Background
Due to the challenges in studying the underlying mechanisms of pain in human participants, behavioral methods of assessing pain utilizing laboratory animal models of pain are often employed. Many of these methods involve observations of animals for behavioral responses that infer nociception and hyperalgesia. Nociceptive responses are behavioral reactions indicative of the pain triggered by stimulation of peripheral nerve fibers (e.g., nociceptors) that respond only to stimuli approaching or exceeding harmful intensity (Loeser & Treede, 2008). Hyperalgesia is an increase in pain sensitivity and is inferred from a decrease and/or an increase in the threshold response. Examples of conventional measurements for thermal and mechanical pain include the hot/cold plate and von Frey filament tests (see Table, Supplemental Digital Content 1). These tests investigate evoked, reflexive nociceptive responses (i.e., paw withdrawal, paw licking) to experimenter-introduced external stimuli (i.e., temperature, nylon filament). While these tests may adequately assess pain following a noxious stimulus, they do not address the spontaneous or continuous pain that occurs in pain conditions. Recent reviews of animal pain models have suggested that inclusion of both evoked and nonevoked behavioral measures are needed to improve animal models of pain and better reflect the human pain experience (Berge, 2011; Gregory et al, 2013; Mogil, 2009; Whittaker & Howarth, 2014).
Weight Bearing in Clinical and Preclinical Models of Pain
Weight-bearing evaluation has been used to monitor disease progress and treatment effectiveness in clinical inflammatory pain conditions, including osteoarthritis and rheumatoid arthritis in humans (Neugebauer, Han, Adwanikar, Fu, & Ji, 2007; van der Leeden et al., 2008; Wickman, Pinzur, Kadanoff, & Juknelis, 2004). With the aim of making rodent pain model experimental findings more translatable to the human pain conditions, instruments assessing weight bearing have been increasingly utilized (Doré-Savard et al., 2010; Ferland, Laverty, Beaudry, & Vachon, 2011; Möller, Berge, & Hamers, 2008; Robinson, Sargent, & Hatcher, 2012; Tétreault et al., 2011). The dynamic weight bearing (DWB) device is based on pressure sensors and an open recording area for mice to move without restraint, and is capable of measuring multiple weight-bearing parameters. The DWB test has shown alterations in weight bearing in inflammatory pain models in both mice and rats (Doré-Savard et al., 2010; Lolignier et al., 2011; Robinson et al., 2012; Tétreault et al., 2011). As stated earlier, studies comprising evoked and nonevoked measures may increase their clinical relevance. None of these prior studies have incorporated a well-established assay for thermal hyperalgesia in addition to weight bearing—all within the same group of mice. Therefore, the aim of this study is to evaluate the DWB test as a measure of nonevoked pain, while also incorporating a test for evoked thermal pain sensitivity in the Complete Freund’s Adjuvant (CFA) mouse model of inflammatory pain.
Materials and Method
Animals
This study followed the International Association for the Study of Pain (IASP) guidelines for investigations of pain in animals (Zimmerman, 1986). The study received approval from the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine (Protocol #0613006). Forty-five adult male C57BL/6J mice at nine weeks (21–30g; Jackson Laboratory, Bar Harbor, ME) were used in this study. Mice were housed on a 12:12 hour light: dark cycle with food and water available ad libitum.
Research Design
This randomized case control study used the CFA-induced persistent inflammatory pain model (Möller et al., 2008; Parvathy & Masocha, 2013; Robinson et al., 2012). The treatment group received an injection of CFA in the left hind paw, the vehicle control group received an injection of saline in the left hind paw, and the naïve control group received no injection. The right hind paw was used as an internal control for the DWB test. Forty-five mice were randomized into the experimental, vehicle, and naïve control groups, resulting in 15 mice per group. A total of four measurement tools were employed in this study:
the caliper;
the plethysmometer were used to verify that CFA injection produced hind paw inflammation;
the hot plate test confirmed the development of hyperalgesia; and
the DWB test assessed weight-bearing distribution.
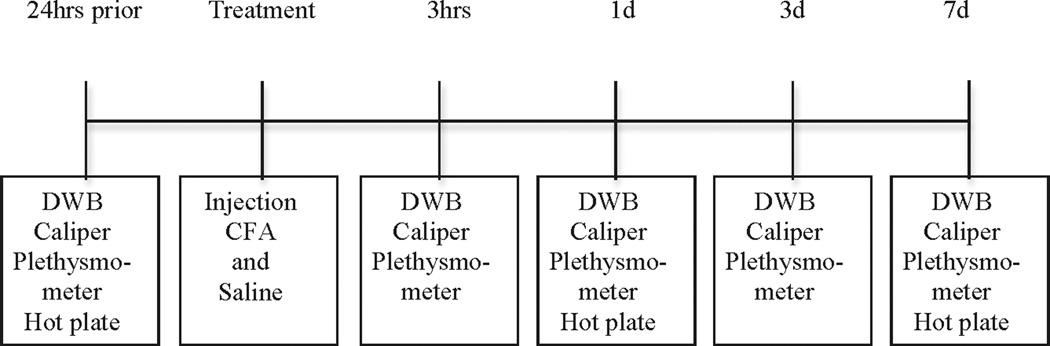
Baseline assessments were performed on all measurement 24 hours prior to treatment. Post-treatment measurements were taken at three hours, one, three, and seven days (Figure 1). Measurements were always conducted in the following order: DWB, caliper, and plethysmometer. To minimize stress in the mice, the hot-plate test was completed at baseline, one day and seven days following the plethysmometer. All mice were tested on all measures individually and sequentially (i.e., mouse one from the naïve control group followed by mouse one from the vehicle control group, followed by mouse one from the experimental group, followed by mouse two from the naïve control group, etc.). This experimental order was maintained throughout the study for all measurement tools.
FIGURE 1.
Experimental timeline.
Inflammatory Pain Model
Induction of inflammation
To induce peripheral inflammation in the experimental group, mice received a subcutaneous injection of 20 µl of CFA into the plantar surface of the left hind paw. CFA is an inflammatory agent consisting of 1 mg Mycobacterium tuberculosis (heat killed and dried), 0.85 mL of paraffin oil, and 0.15 mL mannide monooleate. CFA causes an augmented immune response producing localized erythema and edema (Sigma-Aldrich, 2013). CFA has been shown to exhibit an inflammatory effect three hours post injection and may persist for up to four weeks (Renn & Dorsey, 2011). The vehicle control group received an injection of 20 µl saline in the left hind paw; the naïve control group received no injection.
Verification of inflammation
To verify peripheral inflammation, each mouse hind paw was measured with a caliper and a plethysmometer. The caliper was used to measure paw thickness in mm of the dorsal-plantar axis at the metatarsal level (Morris, 2003). Each paw was measured three times and an average was calculated. The plethysmometer was used to measure paw volume by calculating fluid volume displacement in a measurement chamber (Morris, 2003). The plethysmometer consists of two interconnected, clear acrylic tubes filled with a conductive solution, and it is connected to a decoder that digitally displays the volume displaced (Harvard Apparatus). One at a time, each hind paw was lowered up to the lateral malleolus into the smaller acrylic tube—the larger tube reflected the water volume displaced and the decoder displayed the result in mL. Each hind paw was measured twice and an average was calculated.
Behavioral Testing
Hot-plate test
Using an incremental hot/cold plate (IITC, Woodland Hills, CA), the thermal nocifensive response was measured by calculating the temperature at which a mouse displays a positive stimulus-response behavior, which is defined for this study as licking either hind paw. Each mouse was placed individually on a 30°C (non-noxious temperature) hot plate inside a clear acrylic cylinder. The temperature was increased at a ramp rate of 10°C per minute. An observer ended the test once the mouse licked either hind paw or the hot plate reached the machine designated safety cutoff temperature of 50°C. The temperature was measured to the nearest 0.1 degree. Each mouse was measured twice and an average was calculated. The presence of thermal hyperalgesia was defined as a decrease in the temperature that elicits paw licking from the baseline, pre-inflammation measurement.
Dynamic weight bearing
The DWB test (Bioseb, Pinellas Park, FL) calculates multiple parameters in freely moving mice, including weight bearing for each paw (gram and percent of total animal weight), weight for grouped front and rear paws (gram and percent total animal weight), left/right and front/rear weight ratio, surface for each paw, surface for grouped front and rear paws, and variability for each parameter. Parameters are given for the duration of different postures (i.e., four paws and rearing) over the whole experiment and total time spent on each paw over the whole experiment. The parameters utilized in this study were weight on each paw in grams, surface of each paw in mm2, and front to rear weight ratio. Each mouse was individually placed in an 11cm wide×11cm long×22 cm high Plexiglas enclosure with a floor sensor containing 1,936 pressure transducers. The mouse was allowed to move freely for five minutes while a camera recorded each movement. The video recordings and pressure data relayed from the floor sensors were transmitted to a computer and stored for analysis. Following the recordings, an observer validated the data by comparing the live recordings with a scaled map of activated sensors. The observer verified the accuracy in placement of rear and front right paw; rear and front left paw, and other areas such as the tail. The DWB software then determined surface and pressure parameters automatically. Each mouse was measured once at each time point.
Statistical Analysis
Statistical analyses were conducted with SPSS 21.0, and graphs were created with GraphPad Prism version 6.0d. Values were expressed as mean ± Standard Error of the Mean (SEM). Comparison of mouse weight in grams was analyzed with a one-way analysis of variance (ANOVA). Comparisons between groups and across time points were analyzed by two-way repeated measures ANOVA. Greenhouse-Geisser estimates were utilized when there was a violation of sphericity. To determine which groups and time points differed, Bonferonni post-hoc tests were conducted for a statistically significant ANOVA with nominal p values of 0.05 for caliper, plethysmometer, and hot plate test. To assess within group differences in DWB ipsilateral and contralateral weight bearing and surface distribution in the hind paws, dependent samples t-tests were conducted for each group separately. Means for all comparisons are available in Supplemental Digital Content 2.
Results
Inflammatory Response
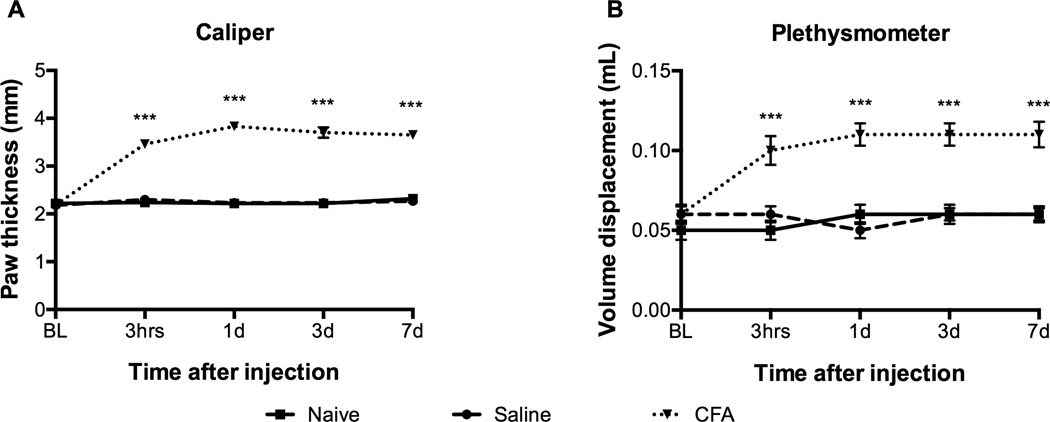
A two-way repeated measures ANOVA showed there was a significant main effect for time (F (3, 42) = 94.09, p < .001), treatment group (F (2, 42) = 261.10, p < .001), and interaction between time and treatment (F (6, 42) = 77.77, p < .001) for the caliper measurements (i.e., paw thickness). There was a similar significant main effect for time (F (3, 42) = 32.88, p < .001), treatment group (F (2, 42) = 17.96, p < .001), and interaction between time and treatment (F (6, 42) = 26.55, p < .001) for the plethysmometer measurements (i.e., paw volume). There was no significant difference between the groups at baseline for either paw thickness or paw volume. CFA-treated mice showed a higher mean paw thickness and volume displacement compared to vehicle (p < .001) and naïve (p < .001) control groups in the ipsilateral paw, beginning at three hours post CFA injection and continuing for the experimental duration (p < .001) (Figure 2). Analysis of simple effects for time showed that paw thickness and paw volume were significantly greater than baseline in CFA-treated mice at three hours, one day, three days, and seven days (p < .01). Vehicle and naïve groups showed no difference in mean paw thickness and volume displacement compared to each other through the duration of the experiment. When percent change was calculated for the caliper measurement of CFA-treated mice, ipsilateral paw thickness had increased by 58% at three hours and 75% on Day 1 from baseline. The plethysmometer recorded an average of .04 to .05 mL increase in volume displacement in the ipsilateral paw of CFA-treated mice compared to vehicle and naïve control groups from baseline. The results indicated that the CFA was successful in inducing inflammation as soon as three hours and up to seven days following injection. After paw inflammation, the mice continued to eat, drink, groom, and explore their environment normally.
FIGURE 2.
CFA significantly increased paw thickness as measured by (a) caliper and (b) plethysmometer at three hours, one, three, and seven days. Mean ± SEM; *** p < .001.
Nocifensive Behavior
Hot-plate test
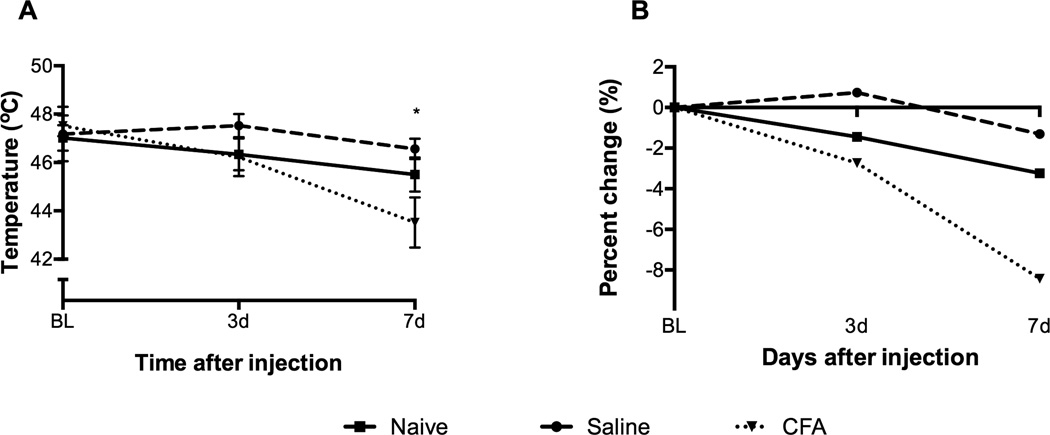
There was a significant main effect for time and time by treatment group interaction for the hot-plate test. There was no significant difference between the groups at baseline. Thermal hyperalgesia as measured by the hot-plate test was apparent in the CFA-treated mice by Day 3. There was a statistical significance in thermal hyperalgesia as compared to baseline by Day 7 in CFA treated mice (p = .001) (Figure 3). The CFA-treated mice posted an 8.2% decrease in response temperature by Day 7 compared to the temperature recorded at baseline; the percent decrease in the naïve group was 3.2% and in the saline group 1.3% (Figure 3B). By Day 7, CFA-treated mice recorded a positive response at 43.5 degrees; the naïve group was two degrees higher (45.5°C); and the saline-treated mice recorded the highest temperature response (46.6°C) (Figure 3A). CFA-treated mice responded at a significantly lower temperature than the saline group at Day 7 (p < .05). Naïve and saline groups did not show any significant difference between each other or across time. Overall, these results showed that CFA-treated mice licked their hind paw at lower temperatures compared to saline-treated mice indicating that CFA was successful in producing thermal hyperalgesia by Day 7.
FIGURE 3.
(A) CFA-treated mice (n = 15) withdrew their rear paw at a significantly shorter time compared to naïve and saline treated mice by Day 7. (B) CFA-treated mice posted an 8.2% decrease in response time on Day 7 compared to baseline time. Mean ± SEM; * p < .05.
Dynamic weight bearing
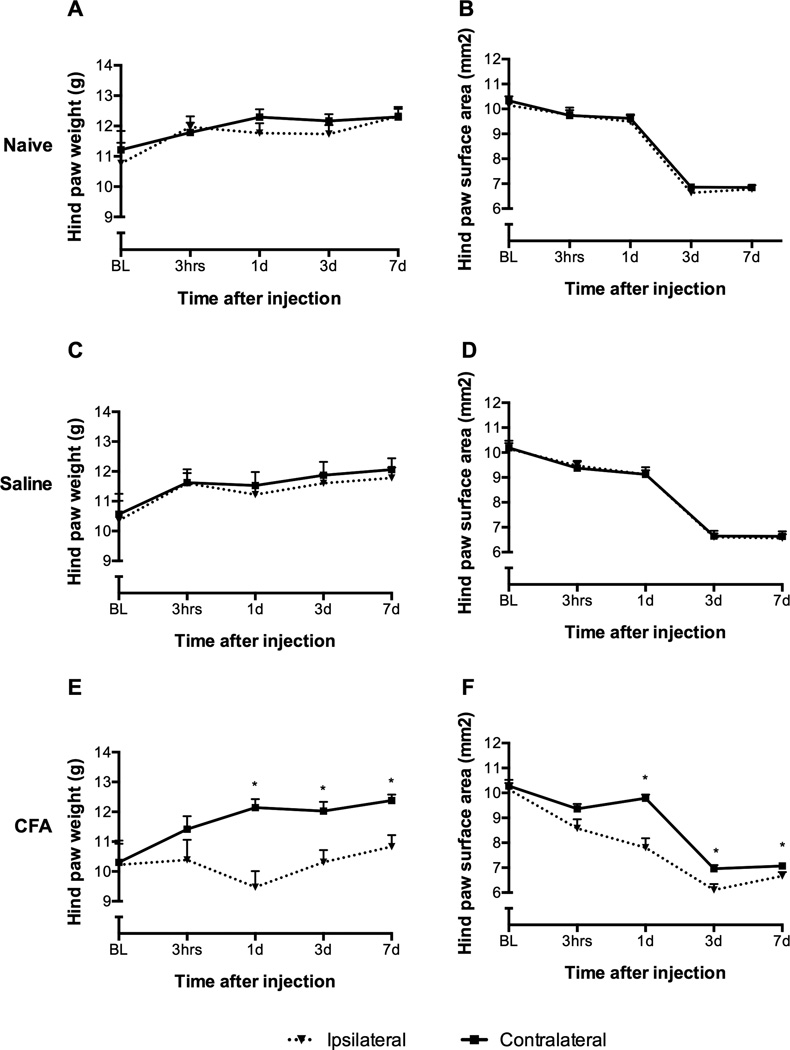
Weight bearing as measured by DWB was noted as a reduction in the ipsilateral paw load compared to the contralateral paw load at Day 1 (p < .001), Day 3 (p = .006), and Day 7 (p = .002) for CFA-treated mice, but no difference in paw load was found in the saline-treated or naïve mice (Figure 4). On Day 1, CFA-treated mice recorded a 7.3% drop in ipsilateral paw load, while the contralateral paw load increased by 17.8% from baseline. Differences were also found in the ipsilateral paw surface area compared to the contralateral paw surface area at Day 1 (p < .001), Day 3 (p = .004), and Day 7 (p = .009) for CFA treated mice, but not for saline-treated or naïve mice (Figure 4). While the CFA-treated mice showed a higher proportion of front–to-rear ratio in weight bearing compared to the saline treated and naïve mice, this was not statistically significant. The results of the paw thickness measurements and the behavioral response to thermal stimulation confirmed the presence of inflammation with hyperalgesia; this indicates that the differences between the CFA group and the vehicle and naïve groups in the DWB test are likely due to the presence of inflammatory hyperalgesia or allodynia. Taken together, the findings of this study suggest that the DWB instrument is a valid instrument to identify nocifensive behaviors that are not stimulus-evoked.
FIGURE 4.
CFA-treated mice showed a significant reduction in ipsilateral hind paw weight bearing and surface area compared to contralateral hind paw on days one, three, and seven as measured with DWB (E, F). Saline-treated (C, D) and naïve (A, B) mice showed no difference in weight bearing or surface area. Mean ± SEM; *p < .05.
Discussion
In order to increase the clinical translation of this study, mice were evaluated in both evoked (i.e., hot plate) and nonevoked (i.e., DWB) pain conditions. The CFA model of inflammatory pain has been used in previous research to model inflammatory conditions such as rheumatoid arthritis, tendonitis, and general inflammatory pain (Cobos et al., 2012; Gregory et al., 2013; Tétreault et al., 2011). CFA-treated mice evidenced greater thermal hyperalgesia in comparison to vehicle-control and naïve mice when exposed to a heat stimulus. CFA-treated mice also demonstrated alterations in weight bearing, including reduced paw load and surface distribution for the affected paw. These findings support the use of DWB test as a tool for measuring nonevoked inflammatory hyperalgesia in the mouse model. This approach is highly translatable as it measures continuous and/or spontaneous pain often associated with clinical inflammatory pain conditions.
Overall, the results are consistent with other studies that have utilized the DWB test to assess alterations in weight bearing following induction of inflammation (Cobos et al., 2012; Doré-Savard et al., 2010; Lolignier et al., 2011; Robinson et al., 2012; Tétreault et al., 2011). This study contributes to this literature as none of these prior studies employed a measure of evoked hyperalgesia (i.e., thermal hyperalgesia) in addition to weight bearing. To further strengthen the validity of the findings, all mice were subjected to all experimental procedures in a standardized order, and repeated measurements were taken for methods involving experimental subjectivity. Inflammation was verified at each data collection point by both bilateral paw thickness and paw volume displacement. Weight bearing was always assessed prior to inflammation verification and the hot-plate test to minimize the possible influence of animal handling required for these assessments and exposure to a noxious thermal stimulus on weight bearing behavior.
Other tests used to assess weight-bearing and nociceptive behavior in preclinical pain models include the CatWalk gait analysis system and the Incapacitance test (Möller et al., 2008; Parvathy & Masocha, 2013). The CatWalk gait analysis system (Noldus Information Technology, The Netherlands) consists of an enclosed walkway with a glass plate floor in which the mouse is to walk from one end to the other. Light is illuminated where the mouse paws make contact with the glass plate resulting in an image of the paw print to estimate weight bearing. The Incapacitance test involves positioning the animal in a chamber with their hind paws on two separate weight-bearing sensors. Both the CatWalk and Incapacitance tests involve confinement of the animal and do not allow calculation of weight bearing parameters that occur during typical rodent actions, such as rearing and exploring their environment. Assessment of weight bearing in freely moving mice permitted by the DWB test may be more clinically relevant as it analyzes continuous weight-bearing behavior as a method to measure spontaneous, nonevoked pain.
There is minimal literature reporting the use of the DWB apparatus as a measure of nonevoked inflammatory hyperalgesia in mice (Cobos et al., 2012; Robinison et al., 2012). This study further reinforces its relevance to study in mice. The use of the DWB test in mice offers possibilities for future research evaluating novel analgesics in inflammatory models, as well as other mouse models of pain, including surgical, neuropathic, and cancer. Furthermore, with the increase of transgenic mouse models, valid behavioral methods for use in mice are required.
Limitations
There are a few limitations in this study. Experimenter blinding was not employed in this study. While blinding would have been ideal, it was not possible as the CFA-injected paws become markedly red and edematous within three hours of injection. Although several minutes longer than static weight-bearing measurement, the observation period in DWB is still quite brief at five minutes. And, even though the DWB test enclosure allows freedom of movement, there is likely to be some confinement and displacement associated stress. For the hot-plate test, paw licking was the solitary indication of thermal pain. Even though this increased consistency, other studies have utilized any sign that could be indicative of thermal pain (paw licking, flinching, jump response; Abu-Ghefreh & Masocha, 2010). Finally, there was no statistical control for the multiple number of post-hoc comparisons conducted, thus increasing the risk of a type I error.
Conclusions
Individuals with inflammatory pain conditions (e.g., rheumatoid and osteoarthritis) experience spontaneous pain, as well as pain in response to an external stimulus; therefore, it is imperative that preclinical animal models and associated behavioral assessment methods strive to closely reflect the clinical pain experience. The current findings suggest the DWB test may be a valuable instrument for future research investigating pain conditions using a mouse model.
Supplementary Material
Footnotes
The authors have no conflicts of interest to report.
Supplemental Digital Content 1. Table that illustrates examples of conventional measurements for thermal and mechanical pain include the hot/cold plate and von Frey filament tests. Doc
Supplemental Digital Content 2. Table that includes means for all comparisons. Pdf
Contributor Information
Mari A. Griffioen, University of Maryland School of Nursing.
Valerie H. Dernetz, University of Maryland School of Nursing.
Gee Su Yang, University of Maryland School of Nursing.
Kathleen A. Griffith, University of Maryland School of Nursing.
Susan G. Dorsey, University of Maryland School of Nursing.
Cynthia L. Renn, University of Maryland School of Nursing.
References
- Abu-Ghefreh AA, Masocha W. Enhancement of antinociception by coadministration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naïve mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskeletal Disorders. 2010;11:276. doi: 10.1186/1471-2474-11-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge O-G. Predictive validity of behavioural animal models for chronic pain. British Journal of Pharmacology. 2011;164:1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré-Savard L, Otis V, Belleville K, Lemire M, Archambault M, Tremblay L, Sarret P. Behavioral, medical imaging and histopathological features of a new rat model of bone cancer pain. PloS One. 2010;5:e13774. doi: 10.1371/journal.pone.0013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacology Biochemistry and Behavior. 2011;97:603–610. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Fine PG. Long-term consequences of chronic pain: Mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Medicine. 2011;12:996–1004. doi: 10.1111/j.1526-4637.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: Disease models and outcome measures. Journal of Pain. 2013;14:1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosomatic Medicine. 2008;70:773–780. doi: 10.1097/PSY.0b013e31817f9e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I. The development of new analgesics over the past 50 years: A lack of real breakthrough drugs. Anesthesia & Analgesia. 2010;110:780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Treede R-D. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Lolignier S, Amsalem M, Maingret F, Padilla F, Gabriac M, Chapuy E, Busserolles J. Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PloS One. 2011;6:e23083. doi: 10.1371/journal.pone.0023083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: Progress and challenges. Nature Reviews Neuroscience. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Möller KÄ, Berge O-G, Hamers FPT. Using the Cat Walk method to assess weight-bearing and pain behaviour in walking rats with ankle joint monoarthritis induced by carrageenan: Effects of morphine and rofecoxib. Journal of Neuroscience Methods. 2008;174:1–9. doi: 10.1016/j.jneumeth.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Morris CJ. Carrageenan-induced paw edema in the rat and mouse. In: Winyard PG, Willoughby DA, editors. Methods in molecular biology: Inflammation protocols. Totowa, NJ: Humana Press; 2003. pp. 117–118. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Molecular Pain. 2007;3:1–13. doi: 10.1186/1744-8069-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund’s adjuvant-induced arthritis using the CatWalk system. BMC Musculoskeletal Disorders. 2013;14:14. doi: 10.1186/1471-2474-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn CL, Dorsey SG. From mouse to man: The efficacy of animal models of human disease in genetic and genomic research. Annual Review of Nursing Research. 2011;29:99–112. doi: 10.1891/0739-6686.29.99. [DOI] [PubMed] [Google Scholar]
- Robinson I, Sargent B, Hatcher JP. Use of dynamic weight bearing as a novel end-point for the assessment of Freund’s Complete Adjuvant induced hypersensitivity in mice. Neuroscience Letters. 2012;524:107–110. doi: 10.1016/j.neulet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Sigma-Aldrich Freund’s adjuvant, complete. 2013 Retrieved from http://www.sigmaaldrich.com/catalog/product/sigma/f5881?lang=en®ion=US. [Google Scholar]
- Tétreault P, Dansereau M-A, Doré-Savard L, Beaudet N, Sarret P. Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiology & Behavior. 2011;104:495–502. doi: 10.1016/j.physbeh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- van der Leeden M, Steultjens MP, Terwee CB, Rosenbaum D, Turner D, Woodburn J, Dekker J. A systematic review of instruments measuring foot function, foot pain, and foot-related disability in patients with rheumatoid arthritis. Arthritis Care & Research. 2008;59:1257–1269. doi: 10.1002/art.24016. [DOI] [PubMed] [Google Scholar]
- Whittaker AL, Howarth GS. Use of spontaneous behavior measures to assess pain in laboratory rats and mice: How are we progressing? Applied Animal Behaviour Science. 2014;151:1–12. [Google Scholar]
- Wickman AM, Pinzur MS, Kadanoff R, Juknelis D. Health-related quality of life for patients with rheumatoid arthritis foot involvement. Foot & Ankle International. 2004;25:19–26. doi: 10.1177/107110070402500105. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Ethical considerations in relation to pain in animal experimentation. Acta Physiologica Scandinavica: Supplementum. 1986;554:221–233. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.