Abstract
Activating NRAS mutations are found in 15-20% of melanomas. Immune therapies have become a mainstay in advanced melanoma treatment. We sought to evaluate whether tumor genotype (e.g. NRAS mutations) correlate with benefit from immune therapy in melanoma. We identified 229 melanoma patients treated with immune therapies (interleukin-2, ipilimumab, or anti-programmed cell-death-1/ligand-1 (PD-1/PD-L1)) at three centers, and compared clinical outcomes following immune therapy for patients with or without NRAS mutations.
Of the 229 melanoma patients, 60 had NRAS mutation, 53 had BRAF mutation, and 116 had NRAS/BRAF WT. The NRAS-mutant cohort had superior or a trend to superior outcomes compared to the other cohorts in terms of response to first-line immune therapy (28% vs. 16%, p=0.04), response to any line of immune therapy (32% vs. 20%, p=0.07), clinical benefit (response + stable disease lasting ≥24 weeks; 50% vs. 31%, p<0.01), and progression-free survival (median 4.1 vs. 2.9 months, p=0.09). Benefit from anti-PD-1/PD-L1 was particularly marked in the NRAS cohort (clinical benefit rate 73% vs. 35%). In an independent group of patient samples, NRAS-mutant melanoma had higher PD-L1 expression (although not statistically significant) compared to other genotypes (8/12 vs. 9/20 samples with ≥1% expression; 6/12 vs 6/20 samples with ≥5% expression), suggesting a potential mechanism for the clinical results. This retrospective study suggests that NRAS mutations in advanced melanoma correlate with increased benefit from immune-based therapies compared to other genetic subtypes. If confirmed by prospective studies, this may be explained in part by high rates of PD-L1 expression.
Keywords: Melanoma, NRAS, ipilimumab, anti-PD-1, IL-2, immunotherapy, checkpoint inhibitors, personalized cancer medicine
Introduction
The advent of molecular genetics has enabled classification of melanoma into clinically relevant subsets defined by the presence of specific ‘driver’ mutations, each with unique clinical and genetic features. These ‘driver’ mutations occur in multiple oncogenes, including BRAF, NRAS, and CKIT, and may serve as potential therapeutic targets. NRAS-mutant melanoma is a distinct cohort of this disease which comprises 15-20% of all melanomas and appears to confer a poor prognosis (1, 2). In contrast to BRAF-mutant melanoma, no effective small molecule inhibitors have been approved that specifically target NRAS, although MEK inhibitors have demonstrated modest clinical activity in a phase II trial (3). Additionally, melanomas without driver mutations in BRAF or NRAS (which comprise ~35% of all melanomas – hereafter referred to as “WT”) represent another challenging subgroup without genotype-directed treatments (4, 5). More effective therapeutic strategies both for NRAS-mutant and WT melanoma are urgently needed.
Immune therapies are playing an increasing role in the treatment of patients with metastatic melanoma, particularly when there is no specific targeted therapy available. Interleukin-2 (IL2) was a mainstay of melanoma therapy for many years resulting in durable remissions in 5-10% of patients despite severe acute toxicities (6). More recently, therapeutic approaches aimed at activating antitumor immunity through blockade of immune checkpoints have shown promise. Ipilimumab, a monoclonal antibody directed at cytotoxic T lymphocyte antigen-4 (CTLA-4), demonstrated a survival advantage in metastatic melanoma (7, 8). Newer checkpoint inhibitors targeting the programmed cell death-1/ligand (PD-1/PD-L1) axis (nivolumab, pembrolizumab [MK-3475], MPDL3280A etc.) have induced durable objective responses in 25-50% of patients in early trials (9-12). These novel immune-based therapies are better tolerated than IL2, although potentially severe autoimmune side effects still occur in some patients (13). Currently, no validated biomarkers have consistently predicted clinical responses to the immune therapies, although tumor expression of PD-L1 is likely associated with response to PD-1/PD-L1-directed therapies (9, 14). At present, it is unclear whether specific ‘driver’ mutations, such as NRASG12/G13/Q61 mutations, influence immune therapy outcomes. Pre-clinical studies have recently suggested that specific tumor driver mutations may affect the antitumor immune response through changes in expression of tumor antigens or checkpoint molecules, or production of immune-suppressive cytokines (15-18). In addition, several studies have suggested that while mutations in BRAF did not correlate consistently with response rates to immune therapy, NRAS mutations were associated with more frequent responses in patients treated with IL2 (19-21).
While we were assessing the clinical, pathologic, and therapeutic features affected by genotype in our database at Vanderbilt Ingram Cancer Center (VICC), we observed an association between NRAS mutations and response to immune therapy. Based on this finding, we hypothesized that NRAS mutations may impact the clinical outcome of melanoma patients treated with immune therapies. We further hypothesized that NRAS-mutant melanoma may be associated with increased expression of PD-L1, potentially contributing to its responsiveness to immune therapies. To investigate, we performed a retrospective study reviewing clinical information from melanoma patients treated at VICC, Memorial Sloan Kettering Cancer Center (MSKCC), and Massachusetts General Hospital (MGH) and assessed PD-L1 expression in an independent cohort of tumor samples. Our primary endpoints were response rate to immune therapy and clinical benefit rate (CBR; defined as response rate plus stable disease for ≥ 24 weeks); secondary endpoints were overall survival (OS), progression-free survival (PFS), and expression of PD-L1.
Patients and Methods
Study Population/Design
After institutional-review board approval was obtained, the electronic medical records were reviewed for patients with advanced melanoma seen at VICC, MSKCC, and MGH. Patients were included if they had biopsy-confirmed advanced melanoma, underwent molecular profiling for BRAF and NRAS mutations between July 1, 2010 and October 1, 2012, and were treated with immune therapies. Immune therapies included in this study were limited to high dose IL2, ipilimumab, anti-PD-1 (nivolumab [BMS-936558] or pembrolizumab [MK-3475]), and anti-PD-L1 (MPDL3280A). Only patients who received ≥1 week of high dose IL2 or >1 dose of ipilimumab or anti PD-1/PD-L1 were included. The study population included patients treated with immune therapies between January 1, 2005 and November 1, 2012. Results (OS and PFS) were updated through February 1, 2014. All patients underwent genotyping for “hotspot” mutations in BRAF and NRAS; most patients also underwent “hotspot” testing of other genes (i.e. CKIT, GNAQ, GNA11, MEK1 etc.) although this was not required. Melanomas with mutations identified in genes other than BRAFV600 or NRAS were included within the WT group. Our initial comparison was between NRAS-mutant and WT melanomas although we subsequently collected clinical data from BRAF-mutant melanoma patients from 2 centers (VICC and MGH).
Objective tumor responses were retrospectively investigator-assessed using the Response Evaluation in Solid Tumors (RECIST) 1.1 criteria as documented in radiographic study reports (PET or CT scans), provider notes, and/or tumor measurement forms (patients on experimental protocols had prospectively evaluated responses) (22). We evaluated whether patients experienced complete or partial response (CR/PR) to first-line immune therapy or to subsequent lines of immune therapy during their clinical course. Clinical benefit (CB) was also assessed, defined as CR, PR, or stable disease for ≥24 weeks. Any patient without a radiographically evaluable response was classified as a nonresponder. Responses to cytotoxic chemotherapy, molecularly targeted therapy, or additional experimental immune therapies were not assessed. All clinical data was obtained and maintained according to HIPAA standards.
Genetic analysis
Molecular profiling was performed by SNaPshot analysis (VICC and MGH) and Sequenom (MSKCC) on formalin-fixed paraffin-embedded tissue (FFPE). The SNaPshot process utilizes multiplex PCR, multiplex primer extension, and capillary electrophoresis, and has been extensively validated and described previously (4). The gene profiles performed for this study at VICC, MSKCC, and MGH are listed in Table S1, S2, and S3. Patients without identified mutations in NRAS or BRAF were classified as “WT.”
Immunohistochemistry analysis
Melanoma samples from patients with advanced melanoma naïve to immune checkpoint inhibitor therapy were selected based on genotype (NRAS-mutant, BRAFV600-mutant, WT). Expression of PD-L1 was measured by immunohistochemical (IHC) testing in FFPE tumor specimens with a rabbit monoclonal antihuman PD-L1 antibody and an assay developed by Dako®. The development of this assay has been previously described (12). Unstained slides were sent from VICC to the outside facility where laboratory personnel performed PD-L1 staining. A pathologist blinded to genotype and patient characteristics determined scores for clinical specimens. Samples were defined as positive at two thresholds: 1) if at least 1% or 2) if at least 5% of tumor cells exhibited membrane PD-L1 staining of any intensity in a section containing at least 100 cells that could be evaluated, as has been previously described (9, 12, 23).
Statistics
All statistical analyses were performed using SAS version 9.2. Comparisons were considered statistically significant for two-sided p-values <0.05. Categorical variables were summarized by frequencies in each study group; comparisons between the NRAS and non-NRAS groups (BRAF-mutant and WT) were performed using the Pearson chi-square test. The proportions of patients with best response of CR or PR, or stable disease, were compared between the NRAS and non-NRAS cohorts by the Pearson chi-square test. Differences in response rates and clinical benefit rates between groups for each individual therapy are displayed descriptively; we chose to avoid p-values due to small numbers per group and multiple comparisons. The proportion of patients with PD-L1 expression ≥1% or ≥5% within NRAS-mutant melanoma was compared to those with BRAF-mutant and WT melanoma were compared using the Fisher's exact test. PFS was defined as the time from first immune therapy to first progression or death. OS was calculated by date of first immune therapy to date of death for any reason. Patients alive at the last date of follow up were censored for OS; patients alive and progression-free were censored for PFS. PFS and OS distributions were estimated using the method of Kaplan and Meier and compared using the logrank test.
Results
Demographics
A total of 229 patients with advanced melanoma were included. Sixty (26%) melanomas harbored NRASG12/G13/Q61 mutations, 53 (23%) had BRAFV600 mutations, and 116 (51%) were WT for NRAS and BRAF by SNaPshot or Sequenom. Patient characteristics are shown in Table 1. The most common mutation identified was NRASQ61R in 28 cases (47%); 85% of NRAS mutations occurred in codon 61. Age, gender, elevated lactate dehydrogenase (LDH), and disease stage were not related to NRAS mutation status, although location of primary tumors differed significantly between NRAS and non-NRAS groups as previously described (5). Among the 53 patients with BRAFV600 mutations, 16 had received prior BRAF- and/or MEK-directed targeted therapies and 25 received these agents following their failure of first-line immune therapy.
Table 1.
Summary of clinical characteristics and treatment selection for NRAS-mutant, BRAF-mutant, and WT (NRAS/BRAF wild-type) cohorts.
| NRAS mutant (N = 60) | BRAF mutant (N=53) | WT (N=116) | p valuea | |
|---|---|---|---|---|
| Gender | p = 0.29 | |||
| Female | 21 (35) | 18 (34) | 29 (25) | |
| Male | 39 (65) | 35 (66) | 87 (75) | |
| Age | p = 0.11 | |||
| <60 years | 22 (37) | 35 (66) | 47 (41) | |
| ≥60 years | 38 (63) | 18 (34) | 69 (59) | |
| Stage | p = 0.78 | |||
| IIIc | 4 (7) | 1 (2) | 11 (10) | |
| M1a | 8 (13) | 8 (15) | 8 (6) | |
| M1b | 10 (17) | 5 (9) | 19 (16) | |
| M1c | 38 (63) | 39 (74) | 79 (68) | |
| Location of primary tumor | p = 0.02 | |||
| Head and Neck | 7 (11) | 8 (15) | 30 (26) | |
| Torso | 20 (33) | 22 (42) | 17 (15) | |
| Extremities | 20 (33) | 12 (23) | 19 (16) | |
| Uveal | 0 (0) | 0 (0) | 5 (4) | |
| Acral | 3 (5) | 0 (0) | 15 (13) | |
| Mucosal | 5 (8) | 0 (0) | 10 (9) | |
| Unknown | 5 (8) | 11 (21) | 20 (17) | |
| Lactate dehydrogenaseb | ||||
| <ULNc | 38 (76) | 20 (54) | 49 (65) | p = 0.07 |
| >ULN | 12 (24) | 17 (46) | 26 (35) | |
| Mutation detected | ||||
| NRASQ61R | 28 (47) | * | * | * |
| NRASQ61L | 5 (8) | * | * | |
| NRASQ61K | 15 (25) | * | * | |
| NRASQ61H | 3 (5) | * | * | |
| NRASG13R | 3 (5) | * | * | |
| NRASG13D | 1 (2) | * | * | |
| NRASG13C | 1 (2) | * | * | |
| NRASG12D | 2 (3) | * | * | |
| NRASG12C | 2 (3) | * | * | |
| BRAFV600E | * | 47 (89) | * | |
| BRAFV600K | * | 4 (8) | * | |
| BRAFV600R | * | 1 (2) | * | |
| BRAFV600D | * | 1 (2) | * | |
| Institution | ||||
| VICC | 26 (43) | 29 (55) | 58 (50) | |
| MSKCC | 24 (41) | 0 | 51 (46) | |
| MGH | 10 (17) | 24 (45) | 7 (6) | |
| Therapyd (1st Line) | p = 0.89 | |||
| IL-2 | 14 (23) | 27 (51) | 17 (15) | |
| Ipilimumab | 38 (63) | 19 (36) | 86 (74) | |
| Anti-PD-1/PD-L1 | 8 (13) | 7 (13) | 13 (11) | |
| Therapy (2nd Line) | p = 0.86 | |||
| IL-2 | 1 (10) | 2 (9) | 1 (4) | |
| Ipilimumab | 6 (70) | 13 (59) | 12 (52) | |
| Anti-PD-1/PD-L1 | 3 (20) | 7 (32) | 10 (43) | |
| Therapy (3rd Line) | * | |||
| IL-2 | 0 | 0 | 0 | |
| Ipilimumab | 0 | 0 | 2 | |
| Anti-PD-1/PD-L1 | 0 | 1 | 0 |
Pearson test between NRAS and non-NRAS genotypes.
Prior to initiation of immune based therapy, missing for some patients.
Upper limit of normal.
Only includes immune-based therapies
All 229 patients received ≥1 immune therapy regimen with 55 (24%) receiving a second line of immune therapy and 3 receiving two additional regimens (only including immune agents). First-line therapy consisted of high-dose IL2 in 25%, ipilimumab in 62%, and anti-PD-1/PD-L1 in 12% (Table 1). For those who received second-line immune therapy IL2 was administered in 7% of patients, ipilimumab in 56%, and anti-PD-1/PD-L1 in 36%. Regimen selection did not differ by NRAS mutation status for first (p=0.89) or second-line immunotherapy (p=0.86); the average number of different lines of immune therapy received per patient was 1.17 for the NRAS cohort, 1.22 for the WT group, and 1.44 in the BRAF group. Five patients (3 in the WT group and 2 in the NRAS group) received combination ipilimumab and nivolumab (BMS-936558) and were categorized in the anti-PD-1 group for the subgroup analysis. Nine patients (three in each group) also received ipilimumab in combination with other agents (temozolomide, dacarbazine, fotemustine, GM-CSF, bevacizumab, imiquimod) on experimental protocols; these were classified as having received ipilimumab.
Patient Outcomes
We assessed the association of NRAS mutation and response to therapy. We compared the proportion of patients in each group who experienced a complete or partial response to immune therapy at any time during their clinical course (Table 2). In the NRAS group, 19 of 60 patients (32%) had a CR or PR compared to 34 of 169 (20%) in the non-NRAS groups (p=0.07). We then compared the proportion of patients who achieved clinical benefit; this comparison more strongly favored the NRAS group (50% vs. 30%, p<0.01). Assessing first-line immune therapy only, we observed increased benefit for the NRAS-mutant cohort in terms of overall response rate (ORR; 28% vs. 16%, p=0.04) and clinical benefit rate (CBR; 45% vs. 26%, p<0.01). Of note, patients in the BRAF cohort treated with BRAF and/or MEK inhibitors prior to immune therapy had a seemingly lower, although not statistically significant ORR than those naïve to BRAF or MEK inhibitors (13% vs. 27%, p=0.25), consistent with previous studies (24).
Table 2.
Response rate and clinical benefit by NRAS status.
| NRAS mutant | BRAF mutant | WT | p valuea | |
|---|---|---|---|---|
| Best Response to any line of immune therapy | N = 60 | N = 53 | N = 116 | |
| CR/PR | 19 (32%) | 12 (23%) | 22 (19%) | p = 0.068 |
| SD/ PD | 41 (68%) | 41 (77%) | 94 (81%) | |
| CR/PR/SD | 30 (50%) | 16 (30%) | 34 (29%) | p = 0.004 |
| PD | 30 (50%) | 37 (70%) | 82 (71%) | |
| Response to first line immune therapy | N = 60 | N = 53 | N = 116 | |
| CR/PR | 17 (28%) | 8 (15%) | 19 (16%) | p = 0.037 |
| SD/PD | 43 (72%) | 45 (85%) | 97 (84%) | |
| CR/PR/SD | 27 (45%) | 13 (25%) | 31 (27%) | p = 0.006 |
| PD | 33 (55%) | 40 (75%) | 85 (73%) | |
Pearson-chi square test p value for NRAS-mutant vs. non-NRAS-mutant patients.
Abbreviations: CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease
We then examined whether NRAS mutation status affected the ORR and CBR for different types of immune-based therapy (Table 3). We observed that patients with NRAS-mutant melanoma who received anti-PD-1 or anti-PD-L1 agents had markedly increased benefit compared to WT and BRAF-mutant patients (ORR 64% vs. 30%, CBR 73% vs. 35%; n=48). Increased incidence of clinical benefit was also demonstrated for NRAS-mutant patients who received ipilimumab (ORR 19% vs. 11%, CBR 42% vs. 19%; n=169). In patients receiving IL2, the response rate and clinical benefit appeared similar between groups. Since many patients received multiple lines of therapy, we did not compare ORR between groups with formal statistical analysis.
Table 3.
Response rate and clinical benefit by immune therapy type.
| NRAS mutant | BRAF mutant | WT | |
|---|---|---|---|
| Anti PD-1/PD-L1 | N = 11 | N=14 | N = 23 |
| Objective Response | 7 (64%) | 3 (21%) | 8 (35%) |
| Clinical Benefit | 8 (73%) | 3 (21%) | 10 (43%) |
| Ipilimumab | N = 43 | N=31 | N = 95 |
| Objective Response | 8 (19%) | 4 (13%) | 10 (11%) |
| Clinical Benefit | 18 (42%) | 5 (16%) | 19 (20%) |
| IL-2 | N = 15 | N=29 | N = 19 |
| Objective Response | 5 (33%) | 6 (21%) | 5 (26%) |
| Clinical Benefit | 5 (33%) | 11 (34%) | 7 (37%) |
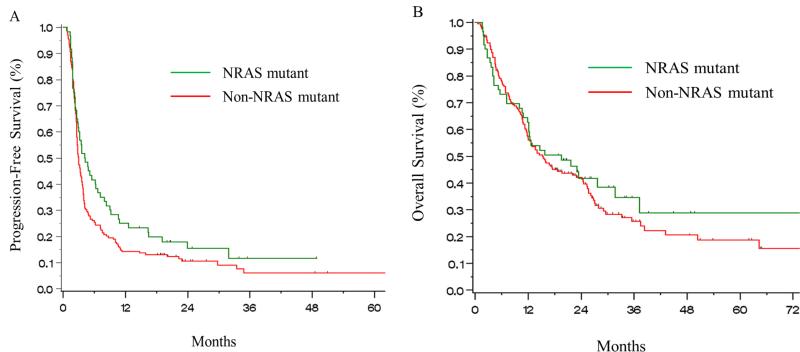
We evaluated PFS and OS for all patients with NRAS mutations compared to the non-NRAS cohorts from initiation of first-line immune therapy using the Kaplan-Meier analysis. We noted a trend toward improved PFS for patients with NRAS mutations (Figure 1a) and equivalent overall survival (Figure 1b). Median duration of PFS was 4.1 months for NRAS-mutant patients vs. 2.9 months for the non-NRAS cohort (log rank p=0.08); the median OS was 19.5 vs. 15.2 months (log rank p=0.51). When examining the non-NRAS cohort further, median PFS was 3.3 months for the WT group and 2.4 months for patients with BRAF mutations; median OS was 13.9 months in the WT cohort and 16 months in the BRAF group. In the BRAF cohort, PFS among those who previously received BRAF and/or MEK inhibitors was equivalent to those who had not received these therapies (2.0 vs. 2.7 months, log rank p=0.28) but OS strongly favored treatment-naïve patients (7.9 months vs. 25.7 months, log rank p=0.01).
Figure 1.
Kaplan-Meier curves of (A) Progression-Free Survival and (B) Overall Survival from first-line immune-based therapy for NRAS-mutant and non-NRAS-mutant (BRAF-mutant and WT) cohorts.
Since NRAS mutations have been previously associated with inferior OS (1, 2), we hypothesized that patients in the NRAS cohort who did not benefit from immune therapies would have rapid progression and death. In an exploratory, descriptive analysis, we assessed the outcome for patients who did not experience clinical benefit from immune-based therapies (poor responders) with evaluable follow up at 6 months following start of therapy. Among NRAS-mutant poor responders, only 46% (13 of 28) were alive at 6 months compared to 67% (78 of 117) of non-NRAS-mutant poor responders. This finding suggests that NRAS-mutant melanoma patients who do not benefit from immune therapy retain a poor prognosis.
PD-L1 Expression
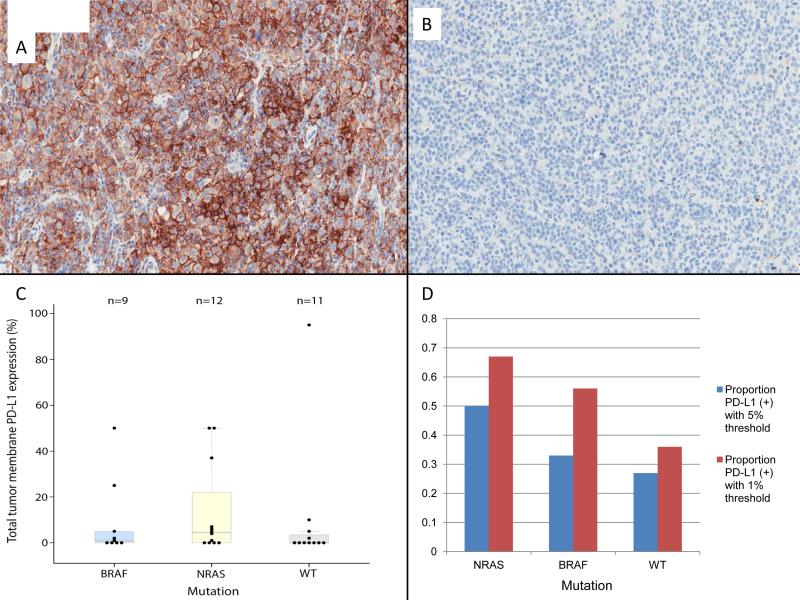
To investigate the potential mechanisms underlying our clinical observation, we selected an independent cohort of 39 archived samples from patients with advanced melanoma (NRAS-mutant=15, WT=14, BRAF-mutant=10). Patients were naïve to systemic therapy in most cases and had not received immune checkpoint inhibitor therapy (see Table S4 for prior therapies and clinical characteristics). Seven samples were not evaluable due to low tumor content or excessive pigmentation precluding assessment. Using a 1% cutoff, NRAS-mutant samples appeared to have a non-statistically significant trend toward higher expression of PD-L1 (8 of 12) compared to WT (4 of 11) and BRAF-mutant (5 of 9).Using a cutoff of ≥5% expression of PD-L1, a potentially higher proportion of NRAS-mutant samples were PD-L1-positive (6 of 12), compared to WT (3 of 11) and BRAF-mutant (3 of 9). (Figure 2 and table S4). Difference between PD-L1 expression between the NRAS-mutant cohort and non-NRAS groups were not statistically significant (p = 0.23, 1% cutoff; p = 0.26, 5% cutoff; fisher's exact test).
Figure 2.
Immunohistochemical analysis of tumor cell-surface expression of PD-L1 from representative samples (20x). Panel A shows an NRAS-mutant melanoma sample with strongly positive expression (~50% of cells); Panel B shows a WT melanoma with <1% of cells with PDL1 expression. Panel C shows the distribution of PD-L1 staining by genotype. Panel D shows number of samples evaluated by genotype and whether they were positive for PD-L1 expression (≥5%) or negative (<5%).
Discussion
We hypothesized that ‘driver mutation’ status may influence response to immune therapies, specifically examining the cohort of melanoma patients harboring activating NRAS mutations. Data from our multi-institutional retrospective analysis suggest that patients with NRAS-mutant melanoma experience higher rates of objective response or prolonged stable disease from immune therapy compared to those with BRAF-mutant and NRAS/BRAF WT melanoma. This benefit was particularly notable for the novel immune checkpoint inhibitors (ipilimumab and anti-PD-1/PD-L1). Although only small numbers were treated, the clinical benefit rate was unexpectedly high with anti-PD-1 or anti-PD-L1, occurring in 8 of 11 patients with NRAS-mutant melanoma compared to only 13 of 37 patients in the non-NRAS-mutant cohorts. This finding could have implications for molecular testing, treatment decision making, and provides early insights into the complex relationship between tumor genetics and the immune response.
In contrast to BRAF-mutant melanoma, no effective molecularly-targeted therapeutic strategies have yet been approved for NRAS-mutant or WT melanoma. Immune therapies, therefore, are the cornerstones of therapy for these subtypes. A recent study by Joseph and colleagues showed that IL2 treatment provided superior efficacy for patients with NRAS-mutant compared to patients with BRAF/NRAS WT melanoma (ORR of 47% vs. 15%), although this analysis included only 15 NRAS-mutant melanoma patients (19). Our study is much larger and extends to the immune checkpoint inhibitors. It should be noted that while several clinical endpoints strongly favored the NRAS population (ORR to first-line therapy, CBR to any and first-line therapy), only a trend was observed for several others (ORR to any therapy, PFS, OS).
We identified a potential partial explanation for this clinical observation. Since previous studies have shown that PD-L1 expression correlates with response to anti-PD-1, we hypothesized that PD-L1 may be differentially expressed in NRAS-mutant melanoma (9, 25). We attempted to answer this question using a relatively small, separate cohort of samples from patients that were naïve to treatment with immune checkpoint inhibitors. We observed that PDL1 expression appeared modestly higher in NRAS-mutant resected tumor samples compared to BRAF-mutant or WT melanoma. This result did not reach statistical significance and needs additional confirmation.
Of interest, a recent study demonstrated that PD-L1 expression did not differ between genotypes in melanoma cell lines (26), suggesting that mutant NRAS does not induce constitutive expression of PD-L1 but may be associated with enhanced immunogenicity in vivo. One potential hypothesis is that NRAS mutations may occur in melanomas with higher mutational burden, a factor linked with higher response rates to immune therapy (27, 28). Results from a large next-generation sequencing study showed that while not universally elevated, the total mutational burden appeared higher in NRAS-mutant melanoma than in melanomas of other subtypes (29). Profiling in larger populations of total somatic mutational burden, mutationally generated neo-epitopes, tumor-infiltrating lymphocytes, and melanoma lineage antigens are needed to provide additional insight. Understanding the link between NRAS mutations and other genetic alterations in melanoma with the antitumor immune response may also provide a better rationale for approaching combination strategies of molecularly-targeted and immune-based therapy. In addition, PD-L1 expression in melanoma and other cancers has been linked to poor prognosis, although this remains controversial (30-34). Based on our observation, it could be speculated that elevated PD-L1 expression may contribute both to the inferior prognosis of NRAS-mutant melanoma and improved response rates to anti-PD-1.
We observed only a trend toward improvements in overall and progression-free survival in our study. Since previous studies have indicated that unselected patients with NRAS mutations have an inferior overall prognosis, we hypothesized that rapid progression and death in non-responding patients may explain this finding (1, 2). This was supported by our observation that “poor responding” patients in the NRAS cohort (patients without clinical benefit from immune-based therapies) tended to have rapid disease progression and death. Presumably, the lack of approved molecularly targeted agents and aggressive natural history of NRAS-mutant melanoma has a negative impact on survival. This analysis was exploratory only; the influence of NRAS mutations on overall survival will need additional follow-up in larger cohorts treated with immune therapy.
Of note, this study included a relatively low percentage of patients harboring BRAF mutations (23%, n=53), likely due in part to physician preference for BRAF-targeted therapy during the study time period. Interestingly, response rates and PFS were not significantly inferior among patients previously treated with BRAF or MEK inhibitors. Overall survival, on the other hand, was vastly superior in the BRAF/MEK inhibitor naïve group. The population of BRAF/MEK inhibitor pre-treated patients could adversely influence clinical outcomes compared to the NRAS and WT cohorts. Conversely, the availability of these therapies upon immune therapy progression may actually skew OS in favor of the BRAF-mutant group, since no such therapies were available to the other subgroups. The low patient numbers and variable pre-treatment, therefore, limits the conclusions for the BRAF-mutant cohort and additional study is needed to define the activity of immune therapy for this genetic subtype.
Our study has several other limitations. Patients identified for this study had received several different immune therapies at three centers across the United States spanning a period of approximately eight years. In addition, genotyping for BRAF and especially for NRAS mutations was not widely available prior to 2010; therefore for patients treated before that time, analysis was largely limited to those surviving to obtain genotyping. Genotyping was also performed with three different assays that could also introduce heterogeneity, although each platform has been extensively validated. Also, somewhat distinct results were identified between immune therapies (no difference with IL2, more clinical benefit with ipilimumab, and higher responses with anti-PD-1/PD-L1); this may reflect divergent interactions of mutant NRAS with the immune response or may be a consequence of small sample size in each group. Finally, there was heterogeneity in the manner that responses to immune therapies were monitored, as some patients were enrolled in clinical trials and others were receiving treatment as standard-of-care. Therefore, prospective studies will be needed to confirm our observations.
In conclusion, we suggest that immune therapies, particularly immune checkpoint inhibitors, may be particularly effective treatment options for NRAS-mutant melanoma, a challenging cohort of patients with a poor prognosis. Mechanistic support for this assertion is suggested by high levels of PD-L1 expression in NRAS-mutant melanoma in a small cohort (although no statistically significant difference was noted). Prospective analyses and further mechanistic studies are needed to validate this finding. We are hopeful that these types of studies bring us a step closer to the goal of identifying predictive markers for immune therapy.
Supplementary Material
Acknowledgements
The authors thank Christine Horak and Jason Simon of Bristol Myers Squibb for their assistance in obtaining PD-L1 staining, Dr. William Pao for his advice throughout the study, Dr. Laetitia Borsu for assistance with Sequenom analyses, and Holly Crandall for her role in the VICC melanoma tissue repository.
Research Support: Douglas Johnson: K12 CA 0906525; Christine Lovly: K12 CA 0906525, Damon Runyon Clinical Investigator Award. Jeffrey Sosman: K24 Grant, American Cancer Society Professorship, Ingram Professorship. The project described was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Additionally, the authors thank the Joyce Family Foundation, the Martell Foundation, and the Bradford Family Foundation. The MSKCC Sequenom facility was supported by the Anbinder Fund.
Footnotes
Conflicts of Interest: CML: research grants from AstraZeneca and Novartis. AJI: ownership in ArcherDx and advisory board in BioReference Labs. The other authors have no conflicts to disclose.
Presented in part at the American Society of Clinical Oncology meeting in 2013, Chicago, IL; DB Johnson et al. NRAS mutation: A potential biomarker of clinical response to immune-based therapies in metastatic melanoma (MM).J Clin Oncol 31, 2013 (suppl; abstr 9019)
References
- 1.Jakob JA, Bassett RL, Jr., Ng CS, Curry JL, Joseph RW, Alvarado GC, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118:4014–23. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devitt B, Liu W, Salemi R, Wolfe R, Kelly J, Tzen CY, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24:666–72. doi: 10.1111/j.1755-148X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 3.Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249–56. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 4.Lovly CM, Dahlman KB, Fohn LE, Su Z, Dias-Santagata D, Hicks DJ, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7:e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31:9010. [Google Scholar]
- 12.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–30. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–8. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tompers Frederick D, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 18.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–94. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 19.Joseph RW, Sullivan RJ, Harrell R, Stemke-Hale K, Panka D, Manoukian G, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. 2012;35:66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahabi V, Whitney G, Hamid O, Schmidt H, Chasalow SD, Alaparthy S, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:733–7. doi: 10.1007/s00262-012-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sznol M, Kluger HM, Callahan MK, Postow MA, Gordon RA, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol. 2014;32:LBA 9003. [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackerman A, McDermott DF, Lawrence DP, et al. Outcomes of patients with malignant melanoma treated with immunotherapy prior to or after vemurafenib. J Clin Oncol. 2012;30:8569. [Google Scholar]
- 25.Weber JS, Kudchadkar RR, Gibney GT, De Conti RC, Yu B, et al. Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumab. J Clin Oncol. 2013;31:9011. [Google Scholar]
- 26.Atefi MS, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, et al. Effects of MAPK and PI3K Pathways on PD-L1 Expression in Melanoma. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014 doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria JC, et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). ECCO Annual Congress. 2013:3408. [Google Scholar]
- 29.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–14. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 31.Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117:2192–201. doi: 10.1002/cncr.25747. [DOI] [PubMed] [Google Scholar]
- 32.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, et al. Tumor Infiltrating PD1-Positive Lymphocytes and the Expression of PD-L1 Predict Poor Prognosis of Soft Tissue Sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 34.Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749–56. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.