Abstract
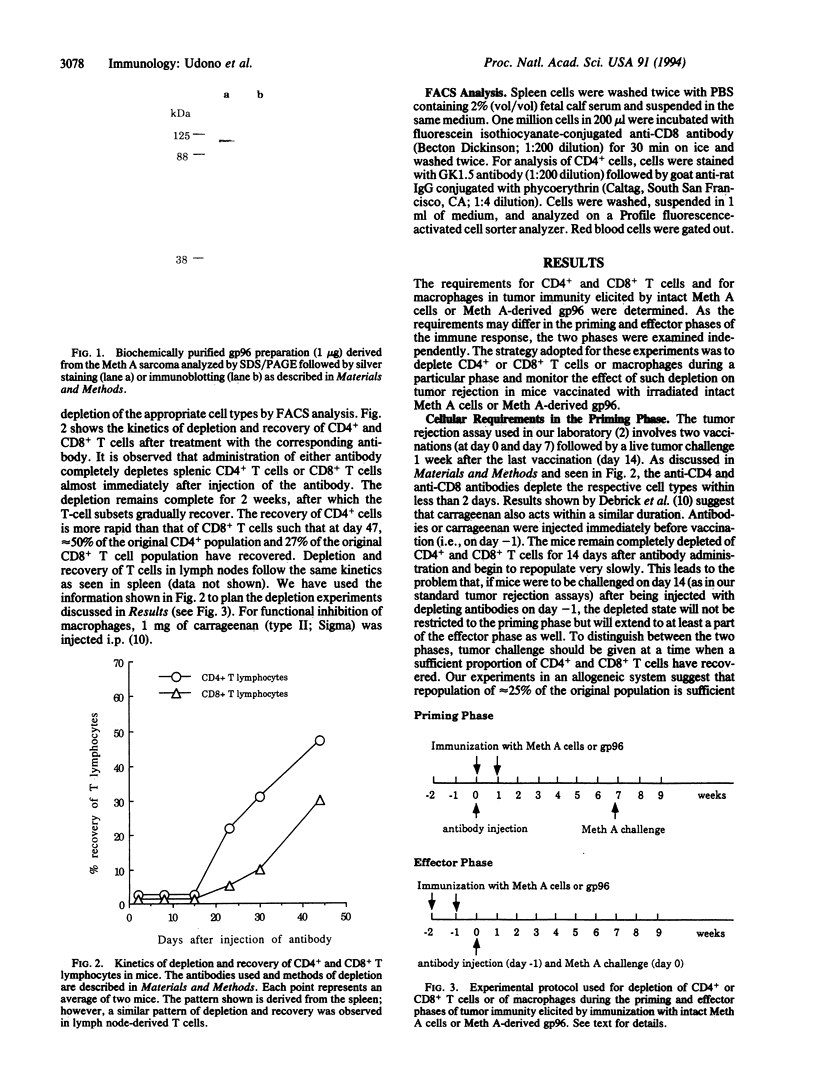
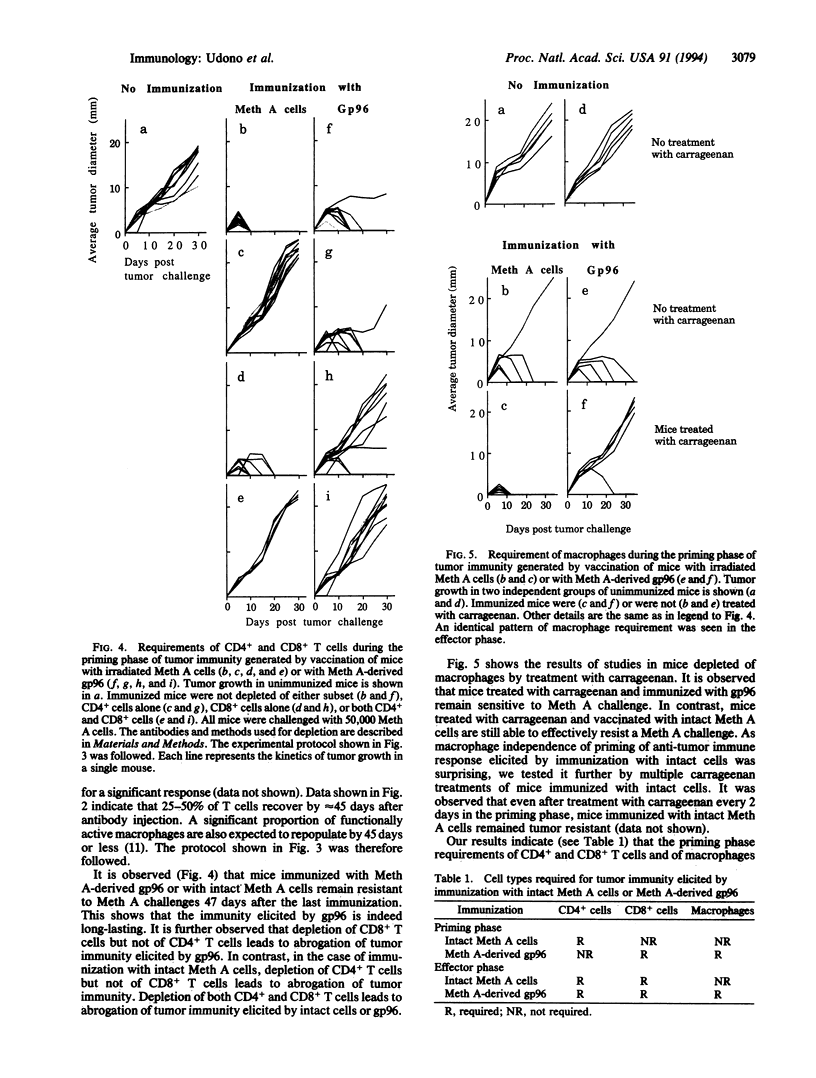
Purified preparations of 96-kDa heat shock proteins (gp96) have been previously shown to elicit tumor-specific immunity to the tumor from which gp96 is obtained but not to antigenically distinct chemically induced tumors. The cellular requirements of gp96-elicited immunity have been examined. It is observed that depletion of CD8+, but not CD4+, T cells in the priming phase abrogates the immunity elicited by gp96. The CD8+ T cells elicited by immunization with gp96 are active at least up to 5 weeks after immunization. Depletion of macrophages by treatment of mice with carrageenan during the priming phase also results in loss of gp96-elicited immunity. In the effector phase, all three compartments, CD4+ and CD8+ T cells and macrophages, are required. Immunity elicited by whole irradiated tumor cells shows a different profile of cellular requirements. In contrast to immunization with gp96, depletion of CD4+, but not CD8+, T cells during priming with whole tumor cells abrogates tumor immunity. Further, ablation of macrophage function during priming or effector phases has no effect on tumor immunity elicited by whole cells. Our results suggest the existence of a macrophage-dependent and a macrophage-independent pathway of tumor immunity. Our observations also show that in spite of exogenous administration, vaccination with gp96 preparations elicits a CD8+ T-cell response in vivo, and it is therefore a useful method of vaccination against cancer and infectious diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blachere N. E., Udono H., Janetzki S., Li Z., Heike M., Srivastava P. K. Heat shock protein vaccines against cancer. J Immunother Emphasis Tumor Immunol. 1993 Nov;14(4):352–356. doi: 10.1097/00002371-199311000-00016. [DOI] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Cudkowicz G., Yung Y. P. Abrogation of resistance to foreign bone marrow grafts by carrageenans. I. Studies with the anti-macrophage agent seakem carrageenan. J Immunol. 1977 Aug;119(2):483–487. [PubMed] [Google Scholar]
- De Leo A. B., Becker M., Lu L., Law L. W. Properties of a M(r) 110,000 tumor rejection antigen of the chemically induced BALB/c Meth A sarcoma. Cancer Res. 1993 Apr 1;53(7):1602–1607. [PubMed] [Google Scholar]
- Debrick J. E., Campbell P. A., Staerz U. D. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991 Nov 1;147(9):2846–2851. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., Allison J. P. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993 Jun 1;177(6):1791–1796. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Clark K., Benacerraf B., Rock K. L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Srivastava P. K. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993 Aug;12(8):3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama E., Uenaka A. Effect of in vivo administration of Lyt antibodies. Lyt phenotype of T cells in lymphoid tissues and blocking of tumor rejection. J Exp Med. 1985 Feb 1;161(2):345–355. doi: 10.1084/jem.161.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino M. A., Jr, Srivastava P. K., Oettgen H. F., DeLeo A. B. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. Cancer Res. 1987 Oct 1;47(19):5074–5079. [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Shu S., Chou T., Rosenberg S. A. In vitro differentiation of T-cells capable of mediating the regression of established syngeneic tumors in mice. Cancer Res. 1987 Mar 1;47(5):1354–1360. [PubMed] [Google Scholar]
- Srivastava P. K., DeLeo A. B., Old L. J. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986 May;83(10):3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., Maki R. G. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- Srivastava P. K., Old L. J. Individually distinct transplantation antigens of chemically induced mouse tumors. Immunol Today. 1988 Mar;9(3):78–83. doi: 10.1016/0167-5699(88)91269-8. [DOI] [PubMed] [Google Scholar]
- Srivastava P. K. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–177. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Srivastava P. K., Udono H., Blachere N. E., Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39(2):93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Udono H., Mieno M., Shiku H., Nakayama E. The roles of CD8+ and CD4+ cells in tumor rejection. Jpn J Cancer Res. 1989 Jul;80(7):649–654. doi: 10.1111/j.1349-7006.1989.tb01692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa H., Chang A. E., Shu S. Y. Cellular interactions in effector cell generation and tumor regression mediated by anti-CD3/interleukin 2-activated tumor-draining lymph node cells. Cancer Res. 1992 Mar 1;52(5):1129–1136. [PubMed] [Google Scholar]
- Yung Y. P., Cudkowicz G. Abrogation of resistance to foreign bone marrow grafts by carrageenans. II. Studies with the anti-macrophage agents iota, kappa, and lambda-carrageenans. J Immunol. 1977 Oct;119(4):1310–1315. [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]




