Abstract
Drug addiction is characterized by maladaptive decision-making and dysfunctional brain circuitry regulating motivated behaviors, resulting in loss of the behavioral flexibility needed to abstain from drug seeking. Hence, addicts face high risk of relapse even after prolonged periods of abstinence from drug use. This is thought to result from long-lasting drug-induced neuroadaptations of glutamate and dopaminergic transmission in the mesocorticolimbic and corticostriatal circuits where group II metabotropic glutamate receptors (mGlu2/3 receptors) are densely expressed. mGlu2/3 receptors presynaptically control glutamate as well as dopamine release throughout the mesocorticolimbic structures involved in reward processing and drug seeking, and their function is reduced after prolonged exposure to drugs of abuse. In pre-clinical models, mGlu2/3 receptors have been shown to regulate both reward processing and drug seeking, in part through the capacity to control release of dopamine and glutamate respectively. Specifically, mGlu2/3 receptor agonists administered systemically or locally into certain brain structures reduce the rewarding value of commonly abused drugs and inhibit the reinstatement of drug seeking. Given the ability of mGlu2/3 receptor agonists to compensate for and possibly reverse drug-induced neuroadaptations in mesocorticolimbic circuitry, this class of receptors emerges as a new therapeutic target for reducing relapse in drug addiction.
Keywords: addiction, glutamate, group II metabotropic glutamate receptors (mGlu2/3 receptors), LY379268, LY341495
1) Addiction as a cognitive disorder
Drug addiction is often described as a cognitive disorder that is characterized by maladaptive decision-making and dysfunctional motivational circuits (Koob and Le Moal 2001; Kalivas and Volkow 2005; Schoenbaum, Roesch et al. 2006). Addicts lose interest in obtaining natural reward and choose to seek drugs of abuse despite their insights into the adverse outcomes of their decision. Thus, addicts lack the necessary behavioral flexibility required to implement their stated desire to abstain from drug seeking. Instead, they engage in repeated drug seeking and exhibit increased vulnerability to relapse even after prolonged periods of withdrawal (Kalivas and O'Brien 2008). This is thought to result from long-lasting neuroadaptations in the brain circuitry regulating motivated behaviors caused by repeated exposure to drugs of abuse (Kalivas and Volkow 2005; Graybiel 2008). Emerging research studies over the past few years illustrate the role of glutamate neurotransmission in the neurobiology of addiction (Kalivas 2009). In particular, this review will describe the physiology and drug-induced pathologies in group II metabotropic glutamate receptors (mGlu2/3 receptors) supporting therapeutic interventions targeting this receptor class in treating drug addiction.
2) Neurocircuitry of motivated behavior and relapse
It is well established that increased vulnerability to relapse after chronic exposure to drugs of abuse is rooted in the long term neuroadaptations in the neural circuitry of normal goal oriented behavior (Kalivas and Volkow 2005). The prefrontal cortex (PFC) glutamatergic projection to the ventral striatum (nucleus accumbens; NAc) is a key component of the circuit involved in initiating and learning adaptive behaviors (Hyman, Malenka et al. 2006; Graybiel 2008), and this projection is in turn regulated by mesocorticolimbic dopaminergic projections from the ventral tegmental area (VTA), signaling the salience and facilitating learning of the relevant experience (Schultz and Dickinson 2000; Redgrave and Gurney 2006). Projections into PFC and NAc from other brain areas like hippocampus and basolateral amygdala are thought to provide previously learned, relevant contextual and emotional information associated with the experience at hand (Swanson and Petrovich 1998; Bast, Zhang et al. 2001; Phelps and LeDoux 2005; Rudy and Matus-Amat 2005). As well, other areas like the extended amygdala (bed Nucleus of stria terminalis, central amygdala, and NAc shell) sending projections into PFC/NAc convey signals about the organism's internal state that contribute to ongoing information processing (Swanson and Petrovich 1998; Kelly and Strick 2004; Reynolds and Zahm 2005). Importantly, in addition to regulating adaptive behavioral responses, the PFC-NAc glutamatergic pathway is involved in addiction related behaviors, and is necessary and sufficient for reinstating drug seeking behavior in some animal models of relapse (Figure 1) (Kalivas and Volkow 2005).
Figure 1.
The neurocircuitry of relapse. Drug, cue, and stress induced relapse after cocaine self-administration require the projections from prefrontal cortex (PFC) to nucleus accumbens (NAc) to ventral pallidum (VP) (red pathway), commonly referred to as the final common pathway. In addition, cue induced relapse depends on basolateral amygdala (BLA) projections to PFC, and stress induced relapse requires activation of the extended amygdala (Ext Amyg) – ventral tegmental area (VTA) pathway, which in turn feeds into PFC and NAc. PFC is not involved in context induced relapse after abstinence; instead, projections from hippocampus (Hipp) to dorsal striatum (DS) (Fuchs, Evans et al. 2005; Fuchs, Branham et al. 2006) and NAc shell are thought to be involved (Bossert, Gray et al. 2005).
Lesion or inactivation of the prelimbic PFC or NAc prevents reinstatement of drug seeking in extinguished animals, while stimulation of either promotes drug seeking in the absence of a drug- or cue-prime (Cornish, Duffy et al. 1999; Cornish and Kalivas 2000; Di Ciano and Everitt 2001; McFarland and Kalivas 2001; Capriles, Rodaros et al. 2003; McFarland, Lapish et al. 2003; McLaughlin and See 2003; McFarland, Davidge et al. 2004; Peters, LaLumiere et al. 2008). Moreover, behavioral electrophysiological data indicate an increase in firing rate of PFC pyramidal neurons (Sun and Rebec 2006), as well as subpopulations of NAc medium spiny neurons (MSN) (Carelli and Ijames 2000) during reinstatement of cocaine seeking. Human imaging studies have also identified correlations between craving and increased activity in the ventral orbital cortex, cingulate cortex, and ventral striatum upon drug or cue exposure (Breiter, Gollub et al. 1997; Breiter and Rosen 1999; Wilson, Sayette et al. 2004; Kufahl, Li et al. 2005; Risinger, Salmeron et al. 2005).
In addition, other brain regions are compulsory depending on the stimulus used to elicit drug seeking (Figure 1). For example, cue primed relapse requires basolateral amygdala while stress induced relapse requires the extended amygdala (Shaham, Erb et al. 2000; See 2002; McFarland, Davidge et al. 2004). Corresponding to the discovery of this circuitry, many laboratories have focused on identifying and reversing enduring neuroadaptations in the relapse circuitry encompassing protein biochemistry, gene expression, spine morphology, and electrophysiology (Hyman, Malenka et al. 2006; Kauer and Malenka 2007; Kalivas 2009). Different animal models are used to examine aspects of drug reward, including intracranial self stimulation, conditioned place preference, or self-administration of the relevant substance, while other models are thought to reflect enduring brain changes associated with increased reinforcing value and/or vulnerability to relapse, including locomotor sensitization and reinstatement of drug seeking (Epstein, Preston et al. 2006; Sanchis-Segura and Spanagel 2006). Using these animal models, mGlu2/3 receptors have emerged as an important substrate for drug-induced neuroadaptations and one that may have utility in regulating drug seeking and other addiction related behaviors.
3) mGlu2/3 receptors and regulation of neurotransmitter release
Group II metabotropic receptor (mGlu2/3 receptors) family includes 2 subtypes both coupled to Gi proteins; mGlu2 receptors are expressed outside the active zone on presynaptic axon terminals to negatively regulate neurotransmitter release, while mGlu3 receptors are localized pre- and post-synaptically as well as on glia with a less clear overall function, but including negative regulation of transmitter release (Ohishi, Shigemoto et al. 1993; Testa, Friberg et al. 1998; Schoepp 2001; Tamaru, Nomura et al. 2001; Richards, Messer et al. 2005). mGlu2/3 receptors can be homosynaptic, regulating glutamate release, or heterosynaptic regulating release of dopamine and γ-aminobutyric acid (GABA) (Hu, Duffy et al. 1999; Schoepp 2001; Karasawa, Yoshimizu et al. 2006; Xi, Kiyatkin et al. 2010). Gi coupling of mGlu2/3 receptors controls release through different mechanisms including activation of presynaptic K+ channels, inhibition of presynaptic Ca+ channels, or direct interference with vesicular release (Anwyl 1999).
In the PFC, mGlu2/3 receptors appear to be tonically activated by endogenous glutamate. Microdialysis studies reveal an increase in PFC glutamate levels upon infusion of a selective mGlu2/3 receptor antagonist (LY341495) (Melendez, Vuthiganon et al. 2005; Xie and Steketee 2008). However, perfusion of a selective agonist ((2R,4R)-4-aminopyrrolidine-2,4-dycarboxylate (APDC)) was without effect, suggesting the presence of ceiling-like glutamatergic tone on mGlu2/3 receptors (Melendez, Vuthiganon et al. 2005). In addition, infusion of the antagonist LY341495 in the prefrontal cortex increased glutamate levels in subcortical regions of the reward circuitry including the nucleus accumbens and ventral tegmental area (Xie and Steketee 2008). This is possibly due to reduced inhibition resulting in facilitated excitatory output from the PFC.
In the nucleus accumbens, data support the presence of endogenous glutamatergic tone on mGlu2/3 receptors regulating both glutamate and dopamine levels. Electrophysiological recordings from NAc slices reveal presynaptic autoregulation of glutamate release by mGlu2/3 receptors. Bath application of the selective agonists (S)-4-carboxy-3-hydroxyphenylglycine ((1S,3S)-ACPD) and (2S,1’S,2’S)-2-(2’-carboxycyclopropyl)glycine (L-CCG1) increased paired pulse ratios and reduced miniature excitatory post synaptic currents (mEPSC) frequency without affecting their amplitude, pointing to a presynaptic mode of action (Manzoni, Michel et al. 1997). In addition, in vivo microdialysis studies reveal glutamatergic tone on mGlu2/3 receptors as indicated by increased glutamate release upon selective antagonist LY143495 perfusion into NAc, while agonist (APDC) reduced extracellular glutamate levels (Xi, Baker et al. 2002). mGlu2/3 receptors regulate synaptic release in addition to glutamate efflux through Na+ independent cystine-glutamate antiporter through Ca2+ and protein kinase A (PKA) dependent cellular mechanisms (Xi, Baker et al. 2002).
Dopamine release in NAc is also controlled by mGlu2/3 receptors. Intra-accumbens infusion of direct (LY354740; (2S, 1’R,2’R,3’R)-2-(2,3-dicarboxycyclopropyl) glycine (DCG-4); LY379268) or indirect agonists (2-(phosphonomethyl)-pentanedioic acid [2-PMPA], inhibitor of N-acetylaspartylglutamate [NAAG] peptidase, thereby increasing NAAG levels, an endogenous mGlu3 receptor agonist) reduce, while antagonists (MGS0039; α-methyl-4-phosphonophenylglycine (MPPG)) increase basal dopamine levels measured with microdialysis probes (Hu, Duffy et al. 1999; Greenslade and Mitchell 2004; Karasawa, Yoshimizu et al. 2006; Xi, Kiyatkin et al. 2010). While this regulation depends on activation of voltage-dependent Ca2+ channels (Hu, Duffy et al. 1999) pointing to vesicular release, it is not clear if it is mediated directly via heterosynaptic mGlu2/3 receptors on dopaminergic terminals, especially since some studies failed to identify significant mGlu2/3 receptor mRNA levels in midbrain neurons projecting to the ventral striatum (Ohishi, Shigemoto et al. 1993). Another possibility is that mGlu2/3 receptors regulate glutamatergic terminals on accumbens medium spiny neurons which in turn project onto dopaminergic cells in the ventral tegmental area (VTA) (Kalivas, Churchill et al. 1993). Furthermore, mGlu2/3 receptors regulate glutamate release in VTA (Manzoni and Williams 1999), hippocampus (Marco 2004), bed nucleus of stria terminalis (BNST) (Grueter and Winder 2005) and other regions within the motivational circuit (Poisik, Raju et al. 2005).
4) Addiction causes neuroadaptations in mGlu2/3 receptors
Repeated exposure to drugs of abuse alters mGlu2/3 receptors function. mGlu2/3 receptors inhibitory effects on excitatory transmission in VTA and NAc are enhanced after early withdrawal from chronic morphine (Manzoni and Williams 1999; Martin, Przewlocki et al. 1999). Different conclusions are drawn from some nicotine studies. Compared to control subjects, a higher dose of mGlu2/3 receptor agonist is required to elevate the threshold of intracranial self-stimulation after acute withdrawal from nicotine self-administration, suggesting a functional down-regulation of these receptors in early withdrawal from nicotine (Harrison, Gasparini et al. 2002; Kenny, Gasparini et al. 2003; Kenny and Markou 2004). Furthermore, using [35S] GTPγ S binding assay, Liechti et al. recently found that acute withdrawal from nicotine self administration reduces mGlu2/3 receptor function by blunting coupling to G proteins throughout the corticolimbic system (PFC, NAc, VTA, amygdala, hippocampus, and hypothalamus) (Liechti, Lhuillier et al. 2007). Acute withdrawal from cocaine also resulted in reduced mGlu2/3 receptor function in central amygdala neurons as measured with electrophysiological markers (Neugebauer, Zinebi et al. 2000). Furthermore, PFC and NAc mGlu2/3 receptor function is reduced after prolonged withdrawal from chronic cocaine (Xi, Ramamoorthy et al. 2002; Bowers, McFarland et al. 2004; Xie and Steketee 2008; Ghasemzadeh, Mueller et al. 2009; Xie and Steketee 2009). In the PFC and NAc, activator of G-protein signaling 3 (AGS3) protein which uncouples Gi subunits from their receptors (De Vries, Fischer et al. 2000) is overexpressed after chronic cocaine (Bowers, McFarland et al. 2004). Accordingly, mGlu2/3 receptor coupling to G protein is reduced in NAc after chronic cocaine or ethanol (Xi, Ramamoorthy et al. 2002; Bowers, Hopf et al. 2008; Ghasemzadeh, Mueller et al. 2009).
In addition to deficits in receptor density and/or Gi signaling, mGlu2/3 receptor-dependent plasticity is impaired after exposure to drugs of abuse. For example, chronic cocaine impairs mGlu2/3 receptor-dependent long term depression (LTD) in PFC pyramidal cells (Huang, Yang et al. 2007), and chronic morphine impairs mGlu2/3 receptor induced LTD at excitatory synapses in NAc medium spiny neurons (Robbe, Bockaert et al. 2002). This form of plasticity is pharmacologically induced by the bath application of a selective mGlu2/3 receptor agonist onto acute slices (Robbe, Alonso et al. 2002; Robbe, Bockaert et al. 2002). Therefore, the impaired LTD could be explained by reduced receptor function as discussed previously. More recently it was shown that LTP induced in the NAc by high frequency stimulation of the PFC was abolished after withdrawal from self-administered cocaine, and this resulted from a reduction in mGlu2/3 receptor stimulation (Moussawi et al., 2009). Thus, in the absence of endogenous stimulation of mGlu2/3 autoreceptors, the PFC to NAc synapses were already potentiated, thereby masking the induction of further LTP. Given the importance of neuroplasticity in learning and updating behaviors following changes in environmental contingencies (Malenka and Bear 2004; Whitlock, Heynen et al. 2006; De Roo, Klauser et al. 2008), impaired plasticity after chronic drugs of abuse (Robbe, Bockaert et al. 2002; Martin, Chen et al. 2006; Huang, Lin et al. 2007; Moussawi, Pacchioni et al. 2009) could reflect the inability of addicts to modify or stop compulsive drug seeking despite the detrimental results of this maladaptive behavior. Thus, reduced function of mGlu2/3 receptors may be related to behavioral inflexibility observed in drug addicts.
It is possible that dysfunctional negative feedback of mGlu2/3 autoreceptors could underlie enhanced glutamate release in NAc after prolonged withdrawal from drugs of abuse in response to the drug itself or associated cues (Pierce, Bell et al. 1996; Bell, Duffy et al. 2000; Hotsenpiller, Giorgetti et al. 2001; Baker, McFarland et al. 2003; Madayag, Lobner et al. 2007; LaLumiere and Kalivas 2008). Along these lines, mGlu2/3 receptor agonists were shown to prevent glutamate and dopamine overflow in NAc of rats sensitized to amphetamine (Kim, Austin et al. 2005), and mGlu2/3 receptor antagonists facilitate drug induced glutamate release in NAc after chronic cocaine self-administration (Xi, Gilbert et al. 2006). Enhanced glutamate release in NAc after cue or drug presentation in cocaine or heroin trained animals is a hallmark and prerequisite of relapse to drug seeking in some animal models (Kalivas 2009). Accordingly, by facilitating glutamate release, chronic drug-induced defects in mGlu2/3 receptor signaling could contribute to drug seeking and relapse. A corollary of these findings is that stimulating mGlu2/3 receptors may be a potential therapeutic strategy for drug addiction.
5) mGlu2/3 receptors regulate reward processing and drug seeking
a) Regulation of reward function
Animal studies show that mGlu2/3 receptors regulate both reward processing and drug seeking. While mGlu2 receptor knock-out mice show no gross behavioral abnormalities, they express higher cocaine-induced locomotor sensitization and stronger conditioned placed preference, suggesting an increased rewarding value of cocaine in absence of mGlu2 receptor signaling (Morishima, Miyakawa et al. 2005). In addition, these mice reveal increased dopamine and glutamate release in NAc in response to a cocaine injection (Morishima, Miyakawa et al. 2005) that could possibly underlie the enhanced reinforcing value of cocaine. Consistent with these findings, direct or indirect systemic mGlu2/3 receptor agonists (LY379268; 2-PMPA) reduce cocaine-induced release of dopamine in rat NAc and attenuate rewarding effects of cocaine as measured by cocaine self-administration and intracranial self-stimulation in squirrel monkeys as well as rats (Baptista, Martin-Fardon et al. 2004; Adewale, Platt et al. 2006; Xi, Kiyatkin et al. 2010). Similar conclusions can be drawn from alcohol and nicotine studies. mGlu2/3 receptor agonists (LY314582; LY379268) reduce nicotine self-administration (Liechti, Lhuillier et al. 2007) and precipitate elevations in intracranial self-stimulation threshold (Harrison, Gasparini et al. 2002; Kenny, Gasparini et al. 2003), implying negative regulation of reward function by mGlu2/3 receptors (Harrison, Gasparini et al. 2002; Kenny, Gasparini et al. 2003; Kenny and Markou 2004; Liechti and Markou 2007). On the other hand, 1 and 3 mg/kg doses of systemic LY379268 show no effects on heroin self-administration (Bossert, Busch et al. 2005) and only a higher systemic dose of LY379268 (5mg/kg dose) reduces alcohol self-administration (Bäckström and Hyytiä 2005). However, these latter results are complicated by the evidence that LY379268 suppresses locomotor activity at higher doses (Bäckström and Hyytiä 2005). While the mGlu2/3 receptor agonist dose used in most studies (3 mg/kg) was reported to suppress basal locomotor activity (Cartmell, Monn et al. 2000), the same dose showed no effect on rotarod activity (Cartmell, Monn et al. 2000), suggesting a relatively mild effect on locomotion. In addition, this 3 mg/kg dose revealed no effect on the ability of animals to respond for natural reinforcers (Baptista, Martin-Fardon et al. 2004; Bossert, Poles et al. 2006; Liechti, Lhuillier et al. 2007) or some drugs of abuse (Bäckström and Hyytiä 2005; Bossert, Busch et al. 2005). Finally, consistent with the effects of mGlu2/3 receptors on drug reinforcement, mGlu2/3 receptors antagonist LY341495 increases behavioral sensitization in cocaine treated rats (Yoon, Jang et al. 2008), while mGlu2/3 receptor agonists reduce amphetamine-induced locomotor sensitization (Kim and Vezina 2002). However, it is important to note that the latter study lacks a necessary control group for the effects of the mGlu2/3 receptors agonist LY379268 on locomotor activity in saline treated rats.
b) Regulation of drug seeking
mGlu2/3 receptors regulate drug seeking across different species. Systemic injections of the mGlu2/3 receptor agonist LY379268 blocks drug primed relapse in squirrel monkeys (Adewale, Platt et al. 2006) and rats (Peters and Kalivas 2006) after cocaine self-administration. Baptista et al. also showed a dose dependent reduction of cue-induced reinstatement of cocaine seeking (Baptista, Martin-Fardon et al. 2004). In addition, systemic mGlu2/3 receptor agonists prevent cue and/or context induced relapse to nicotine (Liechti, Lhuillier et al. 2007), alcohol (Bäckström and Hyytiä 2005; Rodd, McKinzie et al. 2006; Zhao, Dayas et al. 2006), heroin (Bossert, Liu et al. 2004; Bossert, Busch et al. 2005), and cocaine (Lu, Uejima et al. 2007).
The ability of mGlu2/3 receptors to control drug seeking is not limited to one brain region. Microinjections of mGlu2/3 receptor agonist LY379268 into NAc core block drug primed reinstatement of cocaine seeking (Peters and Kalivas 2006), while microinjections of LY379268 into VTA or NAc shell produced a dose-dependent antagonism of context induced reinstatement of heroin seeking (Bossert, Liu et al. 2004; Bossert, Gray et al. 2005), with only high doses showing an effect in NAc core. Substantia nigra and dorsal striatum microinjections were without effect (Bossert, Liu et al. 2004; Bossert, Gray et al. 2005). In addition, Lu et al. showed that LY379268 mincroinjection into the central but not basolateral amygdala nucleus reduced the incubation of cocaine craving and blocked cue induced relapse (Lu, Uejima et al. 2007).
Taken together, these behavioral studies support a conclusion that mGlu2/3 receptor stimulation negatively regulates drug-seeking. The site of action in the brain varies between studies, perhaps in part as a function of the drug being examined or the stimulus used to reinstate drug-seeking. Thus, while activation of mGlu2/3 receptors in the VTA or NAc shell inhibits context-induced reinstatement in heroin-trained animals, reinstatement to cocaine in cocaine-trained animals was blocked by mGlu2/3 receptor agonists into the core of the NAc. The difference between the neural substrates underlying the effects of mGlu2/3 receptor agonists on drug seeking could be explained by the different circuitry underlying contextual vs. cue or drug primed reinstatement. NAc shell is required for context induced reinstatement (Vorel, Liu et al. 2001; Taepavarapruk and Phillips 2003; Ito, Robbins et al. 2008), corresponding to the hippocampus projecting substantially to NAc shell as compared to core (Groenewegen, Vermeulen-Van der Zee et al. 1987). Moreover, the role of the VTA in context-induced drug seeking is consistent with the known role of dopamine agonists in the shell, but not the core, to promote drug seeking (Schmidt, Anderson et al. 2006), and dopamine antagonists to prevent relapse (Ciccocioppo, Sanna et al. 2001; Crombag, Grimm et al. 2002). Given that mGlu2/3 receptor agonists reduce both glutamate and dopamine levels in NAc (Hu, Duffy et al. 1999; Greenslade and Mitchell 2004; Karasawa, Yoshimizu et al. 2006; Xi, Kiyatkin et al. 2010), it is possible that LY379268 blockade of context-induced drug-seeking is caused by reducing extracellular levels of both glutamate and dopamine in NAc shell.
It is important to note that several studies reveal an effect by mGlu2/3 receptors on natural reward vs. drug seeking. For example, systemic or locally microinjected LY379268 blocked reinstatement to conventional reinforcers (food, sweet milk, sucrose) (Baptista, Martin-Fardon et al. 2004; Bossert, Poles et al. 2006; Peters and Kalivas 2006; Uejima, Bossert et al. 2007), without affecting their primary rewarding value (Baptista, Martin-Fardon et al. 2004; Bossert, Poles et al. 2006; Liechti, Lhuillier et al. 2007). Hence, drugs of abuse and natural reinforcers possibly share a common glutamatergic neurocircuitry in regulating behavior. This conclusion is corroborated by human imaging studies showing that natural and drug reinforcers activate overlapping cortico-striatal circuits (Daglish, Weinstein et al. 2003). However, the overlap may in part result from relatively poor resolution of neuroimaging since behavioral electrophysiological recordings support the idea of separate subcircuits for drugs of abuse and natural rewards within the larger motivational circuit (Carelli, Ijames et al. 2000; Carelli 2002; Carelli and Wondolowski 2003; Donita and Regina 2008).
6) Solving the puzzle: mGlu2/3 receptor agonists functionally compensate for long term drug induced neuroadaptations
mGlu2/3 receptor agonists proved to be effective in preventing relapse to different drugs of abuse when administered systemically or locally into certain brain regions (see above). While it is well established that these agonists reduce glutamatergic transmission in the relevant areas of the reward circuitry, the bigger picture relating this effect to long-term drug induced neuroadaptations underlying relapse and compulsive drug seeking remains obscure. In the following section, we will discuss possible mechanisms relating mGlu2/3 receptors and glutamate transmission to long lasting drug induced neural changes, derived primarily from studies in the NAc after chronic cocaine treatment.
As discussed in the beginning of this article, glutamatergic transmission in the NAc is essential for addiction related behaviors. Glutamate homeostasis in the NAc, defined here as the balance between synaptic and nonsynaptic extracellular glutamate release and elimination (Figure 2 A), plays an intricate role in regulating synaptic transmission and plasticity by altering ionotropic and metabotropic glutamate receptor stimulation (Warr, Takahashi et al. 1999; Moran, McFarland et al. 2005; Jourdain, Bergersen et al. 2007; Mulholland, Carpenter-Hyland et al. 2008). This includes glutamate transporter regulation of extracellular glutamate levels arising from both synaptic and glial release (Danbolt 2001). In NAc, extracellular nonsynaptic glutamate is primarily released from glial Na+ independent cystine-glutamate antiporter (Xc-), which exchanges extracellular cystine for intracellular glutamate (Baker, Xi et al. 2002; McBean 2002).
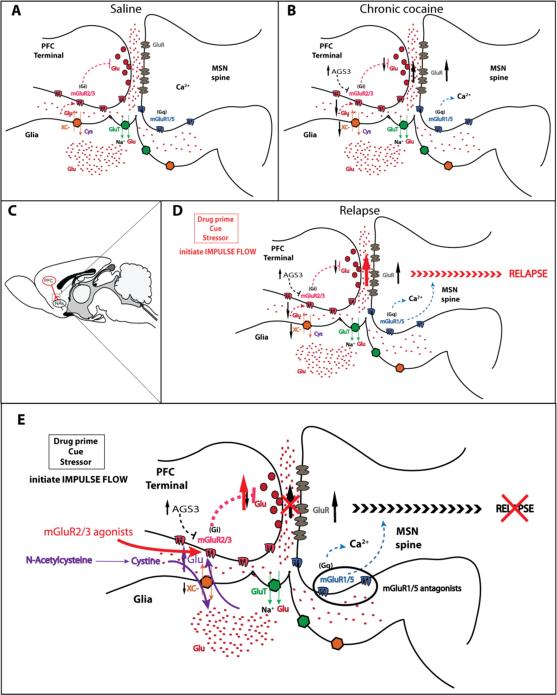
Figure 2.
(A) Glutamate homeostasis in a PFC-NAc excitatory synapse in a yoked saline rat. Extracellular glutamate regulates synaptic release from PFC terminals by stimulating mGlu2/3 receptors. (B) After chronic cocaine, basal extracellular glutamate is reduced and PFC-NAc synapses are potentiated pre- and post-synaptically as indicated by arrows (see text for details). (C) Cartoon of PFC-NAc pathway in a rat brain. (D) Stress, cues, or drug primes induce reinstatement of drug seeking by activating the potentiated PFC-NAc pathway. (E) Interfering with impulse flow pre- (NAcetylcysteine, mGlu2/3 receptor agonists) or post-synaptically (blocking ionotropic GluRs or mGlu5 receptors in NAc) prevents relapse.
Microdialysis measurements reveal reduced basal extracellular glutamate levels in NAc after withdrawal from chronic cocaine (Hotsenpiller, Giorgetti et al. 2001; Baker, McFarland et al. 2003; Madayag, Lobner et al. 2007; Berglind, Whitfield et al. 2009). This is associated with reduced Xc- system function (Baker, McFarland et al. 2003; Madayag, Lobner et al. 2007) and downregulation in the protein level of its catalytic subunit (Knackstedt, Melendez et al. 2010). In parallel with reduced basal levels of extracellular glutamate, microdialysis studies show that glutamate release from PFC afferents in the NAc is increased during reinstatement of drug seeking (McFarland, Lapish et al. 2003; Madayag, Lobner et al. 2007) (Figure 2 B-D). This latter change likely results in part from the marked down-regulation of glial glutamate transport after self-administered cocaine, and a corresponding escape of synaptically released glutamate from uptake into glia (Knackstedt, Melendez et al. 2010). Also, nonsynaptic extracellular glutamate derived from system Xc- activity normally modulates glutamate release from presynaptic prefrontal projections into NAc by providing tone on presynaptic inhibitory mGlu2/3 receptors (Xi, Baker et al. 2002; Losonczy, Somogyi et al. 2003; Moran, Melendez et al. 2003; Grueter and Winder 2005; Moran, McFarland et al. 2005), and hence regulates impulse flow from the PFC to NAc (Figure 2 D). Therefore, by reducing glutamatergic tone on mGlu2/3 receptors, withdrawal from chronic cocaine potentiates basal excitatory transmission at PFC-NAc synapses and increases glutamate release from PFC terminals during relapse to drug seeking (Pierce, Bell et al. 1996; Baker, McFarland et al. 2003; Kourrich, Rothwell et al. 2007; Madayag, Lobner et al. 2007; Conrad, Tseng et al. 2008; Berglind, Whitfield et al. 2009; Moussawi, Pacchioni et al. 2009).
Given the important role played by mGlu2/3 receptors in the regulation of synaptic glutamate release by nonsynaptic glutamate derived from system Xc- (figure 2), it follows that stimulation of mGlu2/3 receptors in NAc prevents reinstatement to cocaine seeking (Baptista, Martin-Fardon et al. 2004; Adewale, Platt et al. 2006; Peters and Kalivas 2006); likely by interfering with glutamatergic synaptic transmission and interrupting potentiated impulse flow from the PFC to NAc. This mechanism is supported by several studies using N-acetylcysteine (NAC) that acts as an indirect mGlu2/3 receptor agonist. NAC is a cystine prodrug currently approved for clinical use as an antioxidant (glutathione precursor) for acetaminophen toxicity and cystic fibrosis treatment. It has been shown to reduce compulsive gambling behavior (Grant, Kim et al. 2007), desire for cocaine use (LaRowe, Myrick et al. 2007), and number of cigarettes smoked (Knackstedt, LaRowe et al. 2009) in humans. In animal models of drug addiction, NAC prevents relapse to drug seeking (Baker, McFarland et al. 2003; Moran, McFarland et al. 2005; Madayag, Lobner et al. 2007; Kau, Madayag et al. 2008; Zhou and Kalivas 2008; Moussawi, Pacchioni et al. 2009). Acute administration of NAC reverses cocaine induced neuroadaptations related to glutamate homeostasis and synaptic transmission in NAc. By providing cystine and driving reduced Xc- transporter activity (Kau, Madayag et al. 2008), NAC normalizes extracellular glutamate levels in NAc after chronic cocaine (Baker, McFarland et al. 2003; Madayag, Lobner et al. 2007), reduces synaptic glutamate release and excitatory drive from the PFC after cue or drug exposure (Baker, McFarland et al. 2003; Madayag, Lobner et al. 2007; Zhou and Kalivas 2008), thereby preventing reinstatement of drug seeking. NAC was also shown to normalize PFC-NAc synaptic transmission by depotentiating these synapses, and restoring impaired plasticity (long term potentiation and long term depression, associated with learning and memory) in NAc after chronic cocaine (Moussawi, Pacchioni et al. 2009).
Akin to NAC, CB1 endocannabinoid receptor antagonist AM251 blocks relapse after cocaine self-administration and prevents increases in glutamate release in the NAc during reinstated cocaine seeking (Xi, Gilbert et al. 2006). AM251 inhibition of relapse was blocked by mGlu2/3 receptor antagonists suggesting a similar mechanism to NAC (Xi, Gilbert et al. 2006). Although the link between CB1 receptor and mGlu2/3 receptors remains to be clarified, it is interesting to note that NAC restores LTD by indirectly stimulating mGlu5 receptors, which is known to regulate LTD in the striatum via postsynaptic retrograde release of endocannabinoids (Robbe, Kopf et al. 2002; Sergeeva, Doreulee et al. 2007). Thus, the inhibition of drug seeking by NAC is associated with the simultaneous stimulation of mGlu2/3 and CB1 receptors.
7) Conclusions
mGlu2/3 receptors have been shown to regulate both reward function and drug seeking, in part through the capacity to control release of dopamine and glutamate respectively in mesocorticolimbic motivational circuitry. This interpretation is in agreement with the hypothesis that dopamine signaling controls drug reinforcement during early stages of drug addiction, while glutamate neurotransmission controls drug seeking at later stages (Kalivas and Volkow 2005). Given the effectiveness at reducing drug seeking after chronic exposure to different drugs of abuse, mGlu2/3 receptor agonists emerge as a promising potential treatment for relapse in drug addiction. The efficacy stems from an ability of mGlu2/3 receptor agonists to exert presynaptic inhibitory tone on glutamatergic terminals, which functionally compensates for drug-induced impaired glutamate homeostasis (Kalivas 2009), and normalizes excitatory synaptic transmission (Moussawi, Pacchioni et al. 2009). Furthermore, the modulatory nature of mGlu2/3 receptors allows them to selectively target extreme, pathological behaviors (compulsive drug seeking) without or minimally affecting normal responses to natural rewards. Taken together, these data indicate that mGlu2/3 receptor agonists may be therapeutically relevant ligands for reducing relapse in drug addiction.
References
- Adewale AS, Platt DM, et al. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318(2):922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29(1):83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. European Journal of Pharmacology. 2005;528(1-3):110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, et al. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22(20):9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, et al. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24(20):4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, et al. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139(1):39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, et al. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23(3):335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr., et al. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29(12):3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, et al. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16(9):1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, et al. Activation of Group II Metabotropic Glutamate Receptors in the Nucleus Accumbens Shell Attenuates Context-Induced Relapse to Heroin Seeking. Neuropsychopharmacology. 2005;31(10):2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, et al. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24(47):10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, et al. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behavioural Brain Research. 2006;173(1):148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, et al. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105(34):12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, et al. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42(2):269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, et al. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168(1-2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. [‘]natural’ reinforcement. Physiology & Behavior. 2002;76(3):379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Nucleus accumbens cell firing during maintenance, extinction, and reinstatement of cocaine self-administration behavior in rats. Brain Res. 2000;866(1-2):44–54. doi: 10.1016/s0006-8993(00)02217-4. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, et al. Evidence That Separate Neural Circuits in the Nucleus Accumbens Encode Cocaine Versus “Natural” (Water and Food) Reward. J. Neurosci. 2000;20(11):4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective Encoding of Cocaine versus Natural Rewards by Nucleus Accumbens Neurons Is Not Related to Chronic Drug Exposure. J. Neurosci. 2003;23(35):11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, et al. Tolerance to the motor impairment, but not to the reversal of PCP-induced motor activities by oral administration of the mGlu2/3 receptor agonist, LY379268. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(1):39–46. doi: 10.1007/s002109900151. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, et al. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98(4):1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, et al. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93(4):1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20(15):RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, et al. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27(6):1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Daglish MRC, Weinstein A, et al. Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage. 2003;20(4):1964–1970. doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, et al. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- De Vries L, Fischer T, et al. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc Natl Acad Sci U S A. 2000;97(26):14364–14369. doi: 10.1073/pnas.97.26.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25(3):341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Donita LR, Regina MC. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. European Journal of Neuroscience. 2008;28(9):1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, et al. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, et al. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, et al. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159(1):414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, et al. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Greenslade RG, Mitchell SN. Selective action of (-)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology. 2004;47(1):1–8. doi: 10.1016/j.neuropharm.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, et al. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23(1):103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30(7):1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Winder DG. Group II and III Metabotropic Glutamate Receptors Suppress Excitatory Synaptic Transmission in the Dorsolateral Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2005;30(7):1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, et al. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160(1):56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, et al. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14(11):1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hu G, Duffy P, et al. The Regulation of Dopamine Transmission by Metabotropic Glutamate Receptors. J Pharmacol Exp Ther. 1999;289(1):412–416. [PubMed] [Google Scholar]
- Huang CC, Lin HJ, et al. Repeated cocaine administration promotes long-term potentiation induction in rat medial prefrontal cortex. Cereb Cortex. 2007;17(8):1877–1888. doi: 10.1093/cercor/bhl096. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang PC, et al. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27(11):2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, et al. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, et al. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28(27):6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10(3):331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, et al. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57(4):1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, et al. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett. 2006;393(2-3):127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, et al. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155(2):530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, et al. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306(3):1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25(5):265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kim JH, Austin JD, et al. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21(1):295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P. The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav. 2002;73(2):333–337. doi: 10.1016/s0091-3057(02)00827-4. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, et al. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28(4):904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, et al. Is Cocaine Desire Reduced by N-Acetylcysteine? Am J Psychiatry. 2007;164(7):1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, et al. Metabotropic Glutamate 2/3 Receptors in the Ventral Tegmental Area and the Nucleus Accumbens Shell Are Involved in Behaviors Relating to Nicotine Dependence. J. Neurosci. 2007;27(34):9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochemical Pharmacology. 2007;74(8):1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Somogyi P, et al. Reduction of Excitatory Postsynaptic Responses by Persistently Active Metabotropic Glutamate Receptors in the Hippocampus. J Neurophysiol. 2003;89(4):1910–1919. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, et al. Systemic and Central Amygdala Injections of the mGluR2/3 Agonist LY379268 Attenuate the Expression of Incubation of Cocaine Craving. Biological Psychiatry. 2007;61(5):591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27(51):13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Michel JM, et al. Metabotropic glutamate receptors in the rat nucleus accumbens. Eur J Neurosci. 1997;9(7):1514–1523. doi: 10.1111/j.1460-9568.1997.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19(15):6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco C. Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway–CA1 EPSCs. European Journal of Neuroscience. 2004;19(10):2847–2858. doi: 10.1111/j.1460-9568.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- Martin G, Przewlocki R, et al. Chronic morphine treatment selectively augments metabotropic glutamate receptor-induced inhibition of N-methyl-D-aspartate receptor-mediated neurotransmission in nucleus accumbens. J Pharmacol Exp Ther. 1999;288(1):30–35. [PubMed] [Google Scholar]
- Martin M, Chen BT, et al. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9(7):868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23(7):299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, et al. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, et al. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1-2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, et al. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314(1):139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, et al. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25(27):6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, Melendez R, et al. Cystine/glutamate antiporter regulation of vesicular glutamate release. Ann N Y Acad Sci. 2003;1003:445–447. doi: 10.1196/annals.1300.048. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, et al. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005;102(11):4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, et al. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28(35):8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Zinebi F, et al. Cocaine and kindling alter the sensitivity of group II and III metabotropic glutamate receptors in the central amygdala. J Neurophysiol. 2000;84(2):759–770. doi: 10.1152/jn.2000.84.2.759. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, et al. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53(4):1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, et al. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335(2):252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186(2):143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, et al. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, et al. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J.Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisik O, Raju DV, et al. Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus. Neuropharmacology. 2005;49(Supplement 1):57–69. doi: 10.1016/j.neuropharm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7(12):967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25(50):11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G, Messer J, et al. Distribution and abundance of metabotropic glutamate receptor subtype 2 in rat brain revealed by [3H]LY354740 binding in vitro and quantitative radioautography: correlation with the sites of synthesis, expression, and agonist stimulation of [35S]GTPgammas binding. J Comp Neurol. 2005;487(1):15–27. doi: 10.1002/cne.20538. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, et al. Neural correlates of high and craving during cocaine self- administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, et al. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3- dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22(11):4346–4356. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Bockaert J, et al. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci. 2002;16(11):2231–2235. doi: 10.1046/j.1460-9568.2002.02273.x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, et al. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99(12):8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behavioural Brain Research. 2006;171(2):207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behav Neurosci. 2005;119(1):154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11(1):2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, et al. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23(1):219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, et al. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29(2):116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the Functions of Presynaptic Metabotropic Glutamate Receptors in the Central Nervous System. J Pharmacol Exp Ther. 2001;299(1):12–20. [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71(3):517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Doreulee N, et al. Long-term depression of cortico-striatal synaptic transmission by DHPG depends on endocannabinoid release and nitric oxide synthesis. Eur J Neurosci. 2007;26(7):1889–1894. doi: 10.1111/j.1460-9568.2007.05815.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, et al. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26(30):8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neurosciences. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Phillips AG. Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology (Berl) 2003;168(1-2):99–108. doi: 10.1007/s00213-002-1337-2. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, et al. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106(3):481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Testa CM, Friberg IK, et al. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390(1):5–19. [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, et al. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behavioural Brain Research. 2007;181(2):292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, et al. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292(5519):1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Warr O, Takahashi M, et al. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol. 1999;514(Pt 3):783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, et al. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, et al. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Baker DA, et al. Group II Metabotropic Glutamate Receptors Modulate Extracellular Glutamate in the Nucleus Accumbens. J Pharmacol Exp Ther. 2002;300(1):162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, et al. Cannabinoid CB1 Receptor Antagonist AM251 Inhibits Cocaine-Primed Relapse in Rats: Role of Glutamate in the Nucleus Accumbens. J. Neurosci. 2006;26(33):8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Kiyatkin M, et al. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2010;58(1):304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, et al. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303(2):608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Repeated exposure to cocaine alters the modulation of mesocorticolimbic glutamate transmission by medial prefrontal cortex Group II metabotropic glutamate receptors. J Neurochem. 2008;107(1):186–196. doi: 10.1111/j.1471-4159.2008.05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 2009;203(3):501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Jang JK, et al. Blockade of group II metabotropic glutamate receptors produces hyper-locomotion in cocaine pre-exposed rats by interactions with dopamine receptors. Neuropharmacology. 2008;55(4):555–559. doi: 10.1016/j.neuropharm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, et al. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26(39):9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-Acetylcysteine Reduces Extinction Responding and Induces Enduring Reductions in Cue- and Heroin-Induced Drug-Seeking. Biological Psychiatry. 2008;63(3):338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]