Abstract
Animal studies suggest that neuroactive steroids, in particular progesterone and its metabolites, have stress-dampening effects. However, few studies have explored these effects in humans. In this study, we investigated the effects of acute progesterone administration upon responses to the Trier Social Stress Test (TSST).
Healthy men participated in the TSST 3.5h after intramuscular injection of 0, 50 or 100mg progesterone (N=16, 14 and 14). We measured cardiovascular (heart rate, blood pressure), hormonal (plasma adrenocorticotrophic hormone, cortisol and noradrenaline) and subjective (e.g. anxiety, arousal) responses to stress in the three groups.
Before the TSST, progesterone injections increased plasma levels without altering physiological or subjective states. Stress produced its expected physiological and subjective effects among placebo-treated individuals. Progesterone 50mg attenuated peak increases in plasma cortisol and reduced changes in negative mood and alertness after stress, yet it increased plasma noradrenaline and systolic blood pressure. Progesterone 100mg also attenuated stress-induced increases in alertness and arousal yet it potentiated stress-induced increases in diastolic pressure. Thus, progesterone dampened some of the psychological effects of stress but produced inconsistent effects upon physiological stress responses.
Keywords: progesterone, allopregnanolone, stress, TSST
An acute physiological or psychological threat initiates a cascade of events called the stress response, which serves to counteract the threat. The stress response is crucial to survival and must be readily activated, but must also be limited in duration to avoid harmful effects (McEwen, 2008). One mechanism thought to regulate the activity of stress systems is the release of neuroactive steroids (Girdler & Klatzkin, 2007). In particular, progesterone and its metabolites allopregnanolone and pregnanolone, which bind potently to the GABAA receptor (Majewska, Harrison, Schwartz, Barker, & Paul, 1986; Paul & Purdy, 1992) have been implicated in restoring normal GABAergic tone and hypothalamic-pituitary-adrenal (HPA) axis activity after a stressor (Barbaccia et al, 1996b). Thus, it might be expected that high levels of progesterone and its neuroactive metabolites might dampen stress responses in humans.
Several lines of evidence suggest that neuroactive steroids may play a role in returning homeostasis after a stressful event. First, neuroactive steroids are released in response to acute stress; animal and human studies show that progesterone and allopregnanolone are elevated after stress (Barbaccia et al., 1996a, b, c; Barbaccia, Serra, Purdy, & Biggio, 2001; Childs & de Wit, 2009; Droogleever Fortuyn et al., 2004; Purdy, Morrow, Moore, & Paul, 1991) and that stress increases brain levels of enzymes that convert progesterone to allopregnanolone (Sanchez, Torres, Gavete, & Ortega, 2008). Second, the physiological effects of these agents oppose hormonal stress responses; animal studies show that allopregnanolone attenuates stress-induced increases in adrenocorticotrophic hormone and corticosterone (Patchev, Hassan, Holsboer, & Almeida, 1996). Finally, the behavioural effects of these agents are also consistent with a role in combating the subjective effects of stress; progesterone, via its metabolite allopregnanolone, produces sedative effects in humans (de Wit, Schmitt, Purdy, & Hauger, 2001; Freeman, Purdy, Coutifaris, Rickels, & Paul, 1993; Soderpalm, Lindsey, Purdy, Hauger, & de Wit, 2004) and decreases anxiety-like behaviours in animals (Bitran, Shiekh, & McLeod, 1995). Thus, the available evidence suggests that stress-induced increases in progesterone and allopregnanolone may be an endogenous mechanism that counteracts increases in anxiety and limits hypothalamic pituitary adrenal axis activation.
In this exploratory study we investigated the anti-stress effects of progesterone in humans. Using a randomised double-blind between-subjects design, healthy male volunteers (N=44) received intramuscular injections of 0, 50, or 100mg progesterone three hours before participating in a standardised psychosocial stress task, the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993). We assessed the effects of the treatments and TSST upon cardiovascular (heart rate, blood pressure), hormonal (plasma adrenocorticotrophic hormone - ACTH, cortisol and noradrenaline) and subjective measures. We hypothesised that progesterone would attenuate stress-induced increases in cortisol and subjective distress.
Methods
Participants
Healthy non–smoking men (N=44) aged 18–35 years participated in the study. The exclusion criteria included serious medical conditions, current or prior diagnosis of Axis I disorder, body mass index outside of 19–26 kg/m2, abnormal blood pressure or electrocardiogram, and night shift work. Participants were told that the aim of study was to investigate the effects of a drug upon responses to a verbal task; they were not told the specific nature of the drug or task.
Procedure
The University of Chicago Institutional Review Committee approved the protocol. Subjects participated in a single, 7h session. On arrival at the General Clinical Research Center (GCRC) at 8am, participants submitted breath and urine samples to test for recent use of drugs or alcohol; no one tested positive. Participants ate a standard breakfast and then a snack at 11:15am prepared by the GCRC staff. At 8:30am, an intravenous catheter was placed in the participant’s non-dominant arm for collection of blood samples. At 9am, baseline samples of blood and measures of blood pressure and mood were obtained. Heart rate was measured continuously (Mini-Logger® system). At 9:15am, participants received an intramuscular (IM) injection of 0, 50 or 100mg progesterone. These doses have been previously administered to healthy individuals and were well tolerated (de Wit et al., 2001). Higher doses (e.g., 200mg) are likely to produce sedative effects (Soderpalm et al., 2004) which would complicate the interpretation of this study. Individuals were randomly assigned to treatment groups. Progesterone was administered intramuscularly because there is a higher relative bioavailability in comparison to orally administered progesterone (Simon et al., 1993) and also because oral administration produces highly variable plasma levels of progesterone (Freeman, Weinstock, Rickels, Sondheimer, & Coutifaris, 1992) due to individual differences in first pass metabolism. Allopregnanolone was not accessible in a bio-available form to administer directly. Physiological, subjective and hormonal measures were collected hourly until 12:15pm. At 12:45pm (3.5h after progesterone injections) individuals were taken to an adjacent room to perform the TSST, a mock job interview that consisted of 5min public speaking and 5min mental arithmetic (Childs & de Wit, 2009). Plasma progesterone peaks approximately 3.5–5h after an intramuscular injection at the doses used here (de Wit et al., 2001), and in our previous acute stress study, progesterone peaked 20min after stressor onset (Childs & de Wit, 2009). Thus, peak plasma progesterone from the injections in the present study were expected to coincide with the peak post-stress levels (at approximately 4h). At the end of the task the participant was escorted back to the patient room. Physiological, subjective and hormonal measures were obtained immediately and then at 10, 20, 30, and 60min after the task. Participants were fully debriefed and received payment at a debriefing session.
Dependent Measures
The GCRC Core Laboratory determined levels of cortisol in plasma (Immulite®1000 Cortisol, sensitivity=0.2ug/dl). The University of Chicago Medical Endocrinology Laboratory determined levels of ACTH and progesterone in plasma (Immulite®2000 Progesterone, sensitivity=0.1ng/ml). Mayo Medical Laboratories determined levels of plasma noradrenaline (Test code 8532, NA sensitivity=70pg/ml). Participants reported subjective mood using an experimental version of the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971) and Visual Analogue Scales (VAS; Folstein & Luria, 1973).
Statistical Analysis
Sample size calculations were based upon responses to the TSST in untreated healthy non-smoking males (Childs & de Wit, 2009). Demographic characteristics were compared between groups using Independent Samples t-Tests and Pearson’s Chi-squared tests. Among placebo-treated individuals, the effects of the TSST upon dependent measures were assessed by comparing pre-stress values with those obtained immediately after the stress task using Student’s paired t-tests. Treatment effects before and after stress were analysed using two-factor analysis of variance (ANOVA) for repeated measures, with Time as within-subjects factors and Treatment Group as between-subjects factor. Significant interactions were further investigated by one-factor ANOVA and Student’s paired t-tests with Bonferroni correction for multiple comparisons. Peak change scores i.e., the maximum positive or negative change from the pre-task baseline at any time point after the TSST, were analysed by Student’s unpaired t-tests. The effects of progesterone were analysed separately for each dose as previous studies report paradoxical and bimodal effects of progesterone and its metabolites (Andreen, Sundstrom-Poromaa, Bixo, Nyberg, & Backstrom, 2006; Fish, Faccidomo, DeBold, & Miczek, 2001 et al., 2009).
All analyses were conducted using SPSS version 16.0 for Windows. Repeated measures ANOVAs were performed with Greenhouse Geisser correction where violations of sphericity were observed. Differences were considered to be significant if p<0.05. Effect sizes are reported using partial eta squared (ηp2) for analyses of variance; 0.01, 0.06 and 0.14 are considered respectively small, medium and large effect sizes.
Results
Demographic characteristics
Most participants were European American (68%) full-time students (70%) in their early twenties (21.9 ± 3.1 years). The groups did not differ in demographic characteristics (Table 1).
Table 1.
Demographic characteristics of participants in each treatment group
| 0mg (N=16) | 50mg (N=14) | 100mg (N=14) | |
|---|---|---|---|
| Agea | 21.8±0.9 | 21.2±0.7 | 22.6±0.8 |
|
| |||
| Body Mass Indexa (kg/m2) | 22.0±0.6 | 23.6±0.6 | 23.6±0.4 |
|
| |||
| Full time Student (#) | 11 | 12 | 8 |
|
| |||
| Education (%) | |||
| High school | 13 | 7 | 14 |
| Partial college | 56 | 93 | 57 |
| College degree | 31 | 0 | 29 |
|
| |||
| Caffeine consumptiona (cups/week) | 3.9±1.0 | 5.0±1.6 | 6.0±2.2 |
|
| |||
| Alcohol consumptiona (drinks/week) | 4.7±1.0 | 5.3±0.9 | 4.9±1.7 |
|
| |||
| Race (%) | |||
| White | 63 | 79 | 64 |
| Black | 12 | 0 | 14 |
| Asian | 6 | 14 | 8 |
| Other | 19 | 7 | 14 |
|
| |||
| Drug Use (% ever used) | |||
| Cannabis | 69 | 50 | 64 |
| Stimulants | 25 | 0 | 7 |
| Opiates | 31 | 7 | 21 |
| Tranquilizers | 0 | 0 | 0 |
| Hallucinogens | 19 | 7 | 14 |
Data is presented as mean±SEM.
Effects of Progesterone before Stress
At baseline (before injections) the mean concentration of progesterone in plasma was 0.89 ± 0.11 ng/ml among all participants and did not differ between the three treatment groups. There were also no differences between the groups in other physiological and subjective measures before injections. Progesterone injections significantly increased plasma progesterone in comparison to placebo injections [50mg Treatment*Time F(3,75)=32.8 p<0.001 ηp2=0.57; 100mg Treatment*Time F(3,75)=54.7 p<0.001 ηp2=0.69]. Before stress, mean plasma progesterone was respectively 0.57±0.04, 30.58±4.32 and 38.97±3.98 ng/ml among individuals in the 0, 50 and 100mg progesterone groups. Progesterone injections did not significantly influence cardiovascular and hormonal measures or self-reported subjective state before the tasks, although individuals in the placebo group reported feeling more Drowsy overall i.e., both before and after administration of progesterone, in comparison to the 100mg treatment group before participating in the stress tasks [Treatment F(1,26)=5.1 p<0.05 ηp2=0.16].
Effects of the TSST
As expected, the TSST significantly increased heart rate, blood pressure, and levels of cortisol, ACTH, noradrenaline and progesterone in plasma among individuals in the placebo treatment group (Table 2). Stress also produced an increase in feelings of anxiety and negative mood (POMS Anger, Anxiety, Confusion, and Depression).
Table 2.
Effects of the TSST among placebo-treated individuals. Data represent mean±SEM at baseline (pre-stress) and immediately after the TSST (post-stress).
| Measure | Pre-stress | Post-stress | t(df) |
|---|---|---|---|
| Systolic Blood Pressure (mm Hg) | 115.8 ± 3.1 | 130.3 ± 2.9 | −8.0(15)*** |
| Diastolic Blood Pressure (mm Hg) | 64.1 ± 1.6 | 70.2 ± 2.2 | −2.8(15)* |
| Plasma Cortisol (ug/dl) | 7.9 ± 0.8 | 14.7 ± 1.1 | −5.3(15)*** |
| Plasma ACTH (pg/ml) | 16.4 ± 1.4 | 28.6 ± 5.4 | −2.4(13)* |
| Noradrenaline (ng/ml) | 173.0 ± 15.5 | 347.6 ± 43.4 | −5.4(14)*** |
| Progesterone (ng/ml) | 0.6 ± .04 | 0.7 ± .06 | −2.5(12)* |
| STAI | 35.0 ± 2.4 | 46.1 ± 2.6 | −4.4(15)*** |
| POMS Anger | 0.2 ±.09 | 0.5 ± 0.1 | −3.4(15)** |
| POMS Arousal | 0.6 ± 0.3 | 1.3 ± 0.4 | −2.1(15)* |
| POMS Confusion | 0.6 ± 0.2 | 1.2 ± 0.2 | −2.6(15)* |
| POMS Depression | 0.4 ±0.2 | 0.8 ± 0.3 | −2.6(15)* |
| Heart Rate Baseline | 71.5 ± 2.4 | 89.9 ± 3.8 | −7.2(14)*** |
indicates a significant difference between pre- and post-stress measurements (Student’s paired t-test, *p<0.05, **p<0.01, ***p<0.001).
Effects of progesterone upon responses to the TSST: Cardiovascular Measures
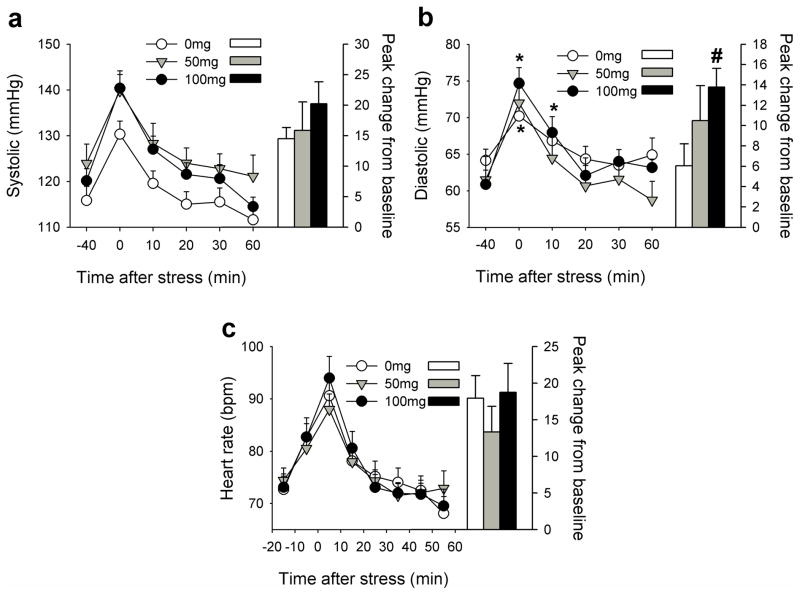
In comparison to placebo, 50mg progesterone increased systolic blood pressure overall [Treatment F(1,28)=4.8 p<0.05 ηp2=0.15] but did not significantly alter TSST-induced increases. Progesterone 100mg potentiated and prolonged TSST-induced increases in diastolic blood pressure [Treatment*Time F(5,140)=2.5 p<0.05 ηp2=0.08]; blood pressure remained significantly elevated above baseline for 10mins after the TSST among individuals treated with 100mg progesterone and the peak change from pre-task baseline was significantly greater after 100mg compared to placebo [t(28)=−2.7 p<0.05, Figure 1). Progesterone treatments did not significantly alter TSST-induced changes in heart rate.
Figure 1. Effects of the TSST upon systolic blood pressure (a), diastolic blood pressure (b) and heart rate (c) among individuals treated with 0, 50 and 100mg progesterone.
Data points indicate mean±SEM values at repeated times before and after the TSST (left axis) and bars indicate the peak change from −40min (right axis). Asterisks indicate a significant difference from −40min (Student’s paired t-test with Bonferroni correction, p<0.001). # indicates a significant difference from 0mg (Student’s unpaired t-test, p<0.05).
Hormonal Measures
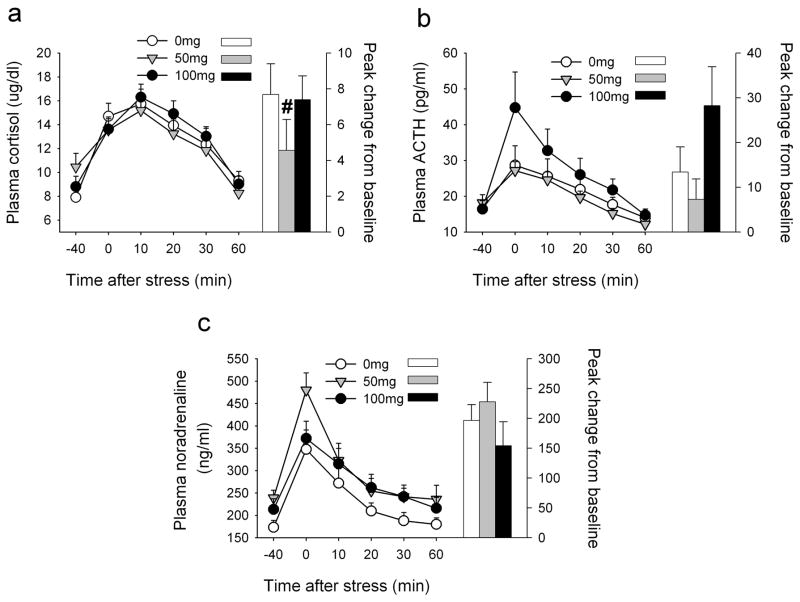
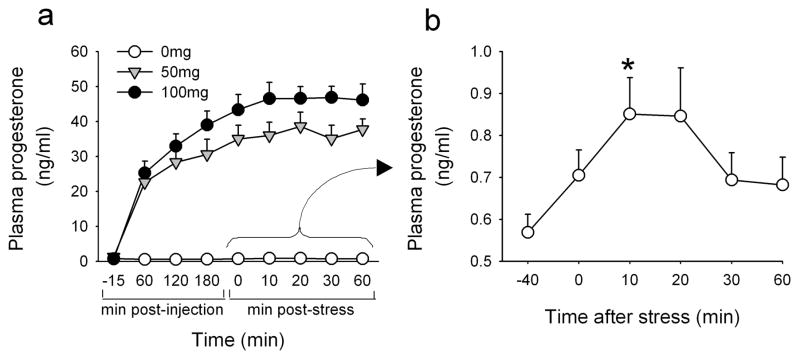
In comparison to placebo, progesterone 50mg significantly attenuated peak increases in plasma cortisol after the TSST [t(28)=2.1 p<0.05] and there was a trend to a reduced response over time [Treatment*Time F(5,140)=2.2 p=0.06 ηp2=0.10, Figure 2]. ACTH responses to the task did not differ among the groups although there was a trend to potentiated responses by 100mg progesterone [Treatment*Time F(5,95)=2.0 p=0.08 ηp2=0.10, Figure 2]. In comparison to placebo, progesterone 50mg significantly elevated plasma noradrenaline overall [Treatment F(1,24)=4.1 p=0.05 ηp2=0.15, Figure 2]. The TSST significantly increased plasma progesterone among individuals who received placebo [Treatment*Time F(5,60)=5.5 p<0.001 ηp2=0.32, Figure 3b]. Among progesterone-treated individuals, plasma progesterone continued to rise after the TSST; levels peaked and reached a plateau at 10–20 min post-stress, a time coinciding with peak increases in progesterone in the placebo group (Figure 3a, b).
Figure 2. Effects of the TSST upon plasma cortisol (a), ACTH (b) and noradrenaline (c) among individuals treated with 0, 50 and 100mg progesterone.
Data points indicate mean±SEM values at repeated times before and after the TSST (left axis) and bars indicate the peak change from −40min (right axis). # indicates a significant difference from 0mg (Student’s unpaired t-test, p<0.05).
Figure 3. Plasma progesterone levels as a function of time among individuals treated with 0, 50 and 100mg progesterone (a) and changes in plasma progesterone after the TSST among individuals treated with 0mg progesterone (b).
Data points indicate mean±SEM values at repeated times before and after the TSST. Asterisks indicate a significant difference from −40min (Student’s paired t-test with Bonferroni correction, p<0.001).
Subjective Measures
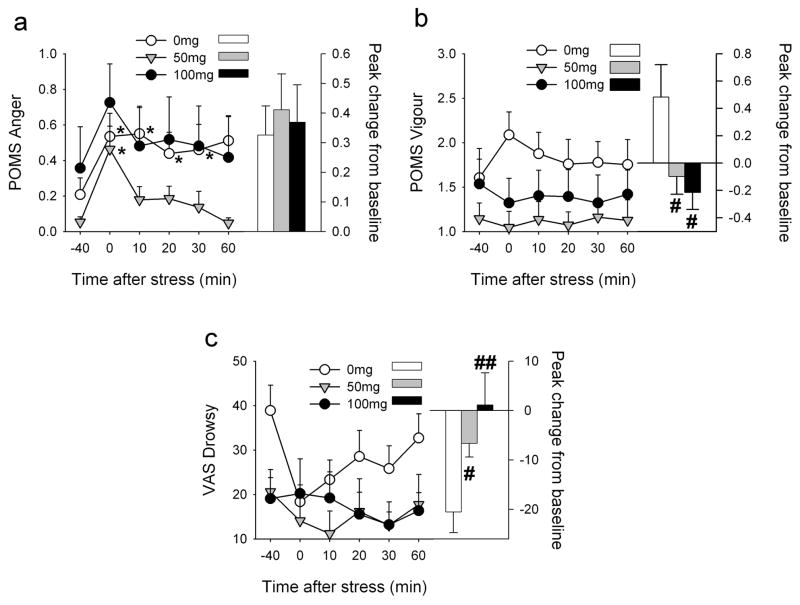
In comparison to the placebo group, individuals treated with progesterone 50mg exhibited less negative mood after the TSST; peak increases in POMS Anger did not differ between the groups but mood returned to baseline much more quickly among those treated with 50mg [Treatment*Time F(5,140)=2.6 p<0.05 ηp2=0.09]. Post hoc tests showed that scores for POMS Anger were significantly elevated above baseline immediately after the TSST among progesterone-treated individuals yet remained significantly elevated for 30min after stress among placebo-treated individuals (Figure 4). Progesterone-treatment attenuated TSST-induced increases in POMS Vigour [50mg Treatment F(1,28)=4.9 p<0.05 ηp2=0.15; 100mg Treatment*Time F(5,140)=2.5 p<0.05 ηp2=0.08] and decreases in VAS Drowsy [100mg Treatment*Time F(5,130)=3.0 p<0.05 ηp2=0.10]. Similarly, progesterone attenuated peak changes in POMS Vigour [50mg t(24)=−2.7 p<0.05; 100mg t(26)=−2.1 p<0.01] and VAS Drowsy [50mg t(28)=2.1 p<0.05; 100mg t(28)=2.5 p<0.05] after the TSST. Since baseline values of VAS Drowsy differed significantly between the placebo and 100mg treatment groups before stress, we also analysed change from baseline values at each time point. These analyses confirmed that 100mg progesterone attenuated stress-induced decreases in VAS Drowsy, even after taking into account elevated ratings of Drowsy among placebo-treated individuals before the stress procedure [Treatment*Time F(4,104)=2.9 p<0.05 ηp2=0.19].
Figure 4. Effects of the TSST upon POMS Anger (a), Vigour (b) and VAS Drowsy (c) among individuals treated with 0, 50 and 100mg progesterone.
Data points indicate mean±SEM values at repeated times before and after the TSST (left axis) and bars indicate the peak change from −40min (right axis). Asterisks indicate a significant difference from −40min (Student’s paired t-test with Bonferroni correction, p<0.001). # indicates a significant difference from 0mg (Student’s unpaired t-test, #p<0.05 ##p<0.01).
Discussion
The results of this study are consistent with evidence from recent non-human and human studies which suggest that progesterone, perhaps via conversion to allopregnanolone and pregnanolone, modulates responses to stress. In laboratory animals, swim stress elevates central levels of enzymes that convert progesterone to allopregnanolone (Sanchez et al., 2008), and brain and plasma levels of progesterone and allopregnanolone are elevated after acute stressors including swim stress, foot-shock, and CO2 inhalation (Barbaccia et al., 1996a,1996b, 1996c; Barbaccia et al., 2001; Purdy et al., 1991). Further, allopregnanolone attenuates stress-induced increases in adrenocorticotrophic hormone and corticosterone (Patchev et al., 1996). In humans, stress induces increases in progesterone and allopregnanolone (Childs & de Wit, 2009; Droogleever Fortuyn et al., 2004). In the present study, we found that progesterone (50, 100mg) attenuated stress-induced increases in alertness, arousal and negative mood. However, unexpectedly progesterone did not consistently attenuate physiological responses to the stress; 50mg progesterone attenuated stress-induced increases in plasma cortisol but increased plasma noradrenaline and systolic blood pressure overall, whereas 100mg progesterone potentiated stress-induced increases in diastolic blood pressure. These results suggest that progesterone may preferentially play a role in psychological or subjective responses to stress in humans.
Progesterone significantly attenuated stress-induced increases in POMS Vigor i.e., mental activation, and decreases in VAS Drowsy, which is consistent with a sedative profile of subjective effects. Although previous studies have reported clear sedative effects of progesterone itself in humans (de Wit et al., 2001; Freeman et al., 1992; Soderpalm et al., 2004) it is notable that progesterone in our study did not increase self-reports of sedation before the stress task began. The plasma levels of progesterone after the 50 and 100 mg doses were comparable to plasma concentrations in the earlier studies i.e., up to three-fold higher than those observed during the mid-luteal phase in women (Redei & Freeman, 1995). It is not clear why the participants did not report subjective sedation. However, the subjective effects of progesterone became apparent during the stress challenge. Progesterone attenuated stress-induced increases in anger and frustration and promoted recovery from negative mood changes after stress. These findings are consistent with the idea that progesterone metabolites such as allopregnanolone and pregnanolone play a role in recovery from stress. Although we did not measure plasma levels of the progesterone metabolites in this exploratory study, we and others (de Wit et al., 2001; Freeman et al., 1993; Soderpalm et al., 2004) found high correlations between plasma levels of progesterone, allopregnanolone and pregnanolone, and that the time course of plasma allopregnanolone closely follows that of plasma progesterone after oral and intramuscular progesterone (Andreen, Spigset, Andersson, Nyberg, Backstrom, 2006; de Wit et al., 2001; Soderpalm et al., 2004). Moreover, Freeman et al. (1993) found that individual differences in levels of progesterone and its metabolites were correlated with subjective measures and performance in cognitive tasks. Thus, our findings provide support for the theory that progesterone, most likely via conversion to neuroactive metabolites, promotes psychological recovery after stress.
Contrary to our hypothesis, progesterone did not consistently attenuate physiological responses to stress. Although 50mg progesterone attenuated the increase in cortisol after stress, the 100mg dose tended to potentiate ACTH responses. This is in contrast to animal data showing that progesterone attenuated stress-induced increases in ACTH (Patchev et al., 1996). Indeed, there is accumulating evidence to show that the effects of progesterone, allopregnanolone and other GABAA modulators are bimodal and paradoxical (Andreen et al., 2009). For example, there are biphasic effects of GABAA steroids upon mood in humans (Andreen, Bixo, Nyberg, Sundstrom-Poromaa, & Backstrom, 2003; Andreen et al., 2005, Andreen, Sundstrom-Poromaa, Bixo, Nyberg, & Backstrom, 2006; Bjorn et al., 2002) and behavioural effects in animals (Beauchamp, Ormerod, Jhamandas, Boegman, & Beninger, 2000; Fish, Faccidomo, DeBold, & Miczek, 2001; Gourley, Debold, Yin, Cook, & Miczek, 2005; Miczek, DeBold, van Erp, & Tornatzky, 1997; Yoshimura and Ogawa, 1989) i.e., low doses increase negative mood, aggression and anxiety-like behaviours while high doses are calming and reduce aggressive and anxiety-like behaviours. Thus, it follows that there may also be bimodal effects of GABAA steroids upon HPA axis activation. The precise mechanism of the biphasic action is unknown but may involve disinhibition and specific effects at certain GABAA receptor subtypes (see Andreen et al., 2009). It is also interesting that the 100mg dose increased plasma progesterone only slightly above that seen in the 50mg group which may indicate that progesterone was converted to other mineralocorticoids e.g., aldosterone, in this group.
Progesterone 50mg increased noradrenaline and systolic blood pressure overall although it did not alter response to stress in these measures. These findings are in line with previous findings in animal studies, which have also shown that progesterone increases noradrenaline (Sandoval, Gong, & Davis, 2007) and systolic blood pressure (Sharkey et al., 2005). Yet, they are in contrast to previous human studies which reported that progesterone reduced plasma noradrenaline (Tollan, Oian, Kjeldsen, Eide, & Maltau, 1993) and cardiovascular measures (Evans and Foltin, 2006; Rylance et al., 1985; Sofuoglu, Mitchell and Kosten, 2004). Thus, our results suggest that the beneficial effects of progesterone administration upon subjective responses to stress are counterbalanced by its less advantageous hypertensive action.
A limitation of this study is that the sample size is relatively small. There is rather large inter-individual variation in the effects of stress (Kudielka, Hellhammer, & Wust, 2009), and there was also modest variability in the plasma levels of progesterone. Thus, the sample may not have been large enough to demonstrate small-medium size drug effects. Further, we used a similar pretreatment period for each dose of progesterone. Since there is variation in the time to peak plasma levels between different progesterone doses after intramuscular injection (de Wit et al., 2001; Soderpalm et al., 2004), the effects of progesterone may have been assessed at different phases of absorption i.e., approaching peak plasma levels with 50mg vs. following peak plasma levels with 100mg. Although it appeared that peak levels of progesterone were achieved at approximately the same time in the 50 and 100 mg groups, subtle differences in the time to peak plasma concentrations between the groups may be related to the differential effects of progesterone doses upon hormonal measures. In this exploratory study we chose to study the effects of progesterone upon stress responses in men only. First, we previously reported clear differences in levels of progesterone after stress in men (Childs and de Wit, 2009), differences in women differed with menstrual cycle (Childs and de Wit, In Press). Also, there are sex differences in stress responses between men and women and in women between different phases of the menstrual cycle, which may influence progesterone-stress interactions. In addition, animal studies show that the effects of progesterone and other GABAA modulators differ depending upon the length of exposure (Biggio et al., 2006; Brot, Akwa, Purdy, Koob, & Britton, 1997; Gullinello and Smith, 2003), and therefore differences in baseline levels of progesterone between men and women are also likely to influence progesterone-stress interactions. Thus, the findings of this experiment cannot be generalized to the effects of progesterone upon stress responses in women, which remain to be studied.
In conclusion, administration of progesterone dampened subjective responses to acute stress without altering mood alone. The effects of progesterone upon other physiological and hormonal responses to stress were not dose dependent and provide mixed support for the role of progesterone and its metabolites in restoring homeostasis after a stressful event. These results should be extended to other populations i.e., women and anxiety-sensitive individuals.
Acknowledgments
These experiments complied with current US laws. The authors declare that they have no conflicts of interest. This research was supported by NIDA (DA02812) and the University of Chicago Hospital’s GCRC (USPHS MO1RR000555); the NIDA and University of Chicago Hospital’s GCRC had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. We thank Leslie Sidney and Joshua Shulruff for their technical assistance.
References
- Andreen L, Bixo M, Nyberg S, Sundstrom-Poromaa I, Backstrom T. Progesterone effects during sequential hormone replacement therapy. European Journal of Endocrinology. 2003;148:571–577. doi: 10.1530/eje.0.1480571. [DOI] [PubMed] [Google Scholar]
- Andreen L, Nyberg S, Turkmen S, van Wingen G, Fernandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34:1121–1132. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Andreen L, Spigset O, Andersson A, Nyberg S, Backstrom T. Pharmacokinetics of progesterone and its metabolites allopregnanolone and pregnanolone after oral administration of low-dose progesterone. Maturitas. 2006;54:238–244. doi: 10.1016/j.maturitas.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Andersson A, Nyberg S, Backstrom T. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology. 2005;30:212–224. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom T. Allopregnanolone concentration and mood--a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology (Berl) 2006;187:209–221. doi: 10.1007/s00213-006-0417-0. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, et al. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacology Biochemistry and Behavior. 1996a;54:205–210. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996b;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Perra C, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996c;35:1299–1305. doi: 10.1016/s0028-3908(96)00067-6. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. International Review of Neurobiology. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Ormerod BK, Jhamandas K, Boegman RJ, Beninger RJ. Neurosteroids and reward: allopregnanolone produces a conditioned place aversion in rats. Pharmacology, Biochemistry, and Behavior. 2000;67:29–35. doi: 10.1016/s0091-3057(00)00299-9. [DOI] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Mostallino MC, Sanna E, et al. Plastic neuronal changes in GABA(A) receptor gene expression induced by progesterone metabolites: in vitro molecular and functional studies. Pharmacology, Biochemistry, and Behavior. 2006;84:545–554. doi: 10.1016/j.pbb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. Journal of Neuroendocrinology. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bjorn I, Bixo M, Nojd KS, Collberg P, Nyberg S, Sundstrom-Poromaa I, et al. The impact of different doses of medroxyprogesterone acetate on mood symptoms in sequential hormonal therapy. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2002;16:1–8. [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. European journal of pharmacology. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl) 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Schmitt L, Purdy R, Hauger R. Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroendocrinology. 2001;26:697–710. doi: 10.1016/s0306-4530(01)00024-5. [DOI] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptor density. Psychoneuroendocrinology. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, DeBold JF, Miczek KA. Alcohol, allopregnanolone and aggression in mice. Psychopharmacology (Berl) 2001;153:473–483. doi: 10.1007/s002130000587. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychological Medicine. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Purdy RH, Coutifaris C, Rickels K, Paul SM. Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendocrinology. 1993;58:478–484. doi: 10.1159/000126579. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo-controlled study of effects of oral progesterone on performance and mood. British Journal of Clinical Pharmacology. 1992;33:293–298. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacology and Therapeutics. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Debold JF, Yin W, Cook J, Miczek KA. Benzodiazepines and heightened aggressive behavior in rats: reduction by GABA(A)/alpha(1) receptor antagonists. Psychopharmacology (Berl) 2005;178:232–240. doi: 10.1007/s00213-004-1987-3. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. The Journal of pharmacology and experimental therapeutics. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L, editors. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Miczek KA, DeBold JF, van Erp AM, Tornatzky W. Alcohol, GABAA-benzodiazepine receptor complex, and aggression. Recent developments in alcoholism. 1997;13:139–171. doi: 10.1007/0-306-47141-8_9. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. Faseb J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E, Freeman EW. Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology. 1995;20:259–267. doi: 10.1016/0306-4530(94)00057-h. [DOI] [PubMed] [Google Scholar]
- Rylance PB, Brincat M, Lafferty K, De Trafford JC, Brincat S, Parsons V, et al. Natural progesterone and antihypertensive action. British medical journal (Clinical research ed) 1985;290:13–14. doi: 10.1136/bmj.290.6461.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P, Torres JM, Gavete P, Ortega E. Effects of swim stress on mRNA and protein levels of steroid 5alpha-reductase isozymes in prefrontal cortex of adult male rats. Neurochemistry International. 2008;52:426–431. doi: 10.1016/j.neuint.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Gong B, Davis SN. Antecedent short-term central nervous system administration of estrogen and progesterone alters counterregulatory responses to hypoglycemia in conscious male rats. American Journal of Physiology Endocrinology and Metabolism. 2007;293:E1511–1516. doi: 10.1152/ajpendo.00340.2007. [DOI] [PubMed] [Google Scholar]
- Sharkey LC, Kirchain S, McCune SA, Simpson GI, Archambault EZ, Boatright NK, et al. Progesterone increases blood pressure in spontaneous gestational hypertension in rats. American Journal of Hypertension. 2005;18:36–43. doi: 10.1016/j.amjhyper.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Simon JA, Robinson DE, Andrews MC, Hildebrand JR, 3rd, Rocci ML, Jr, Blake RE, et al. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertility and Sterility. 1993;60:26–33. [PubMed] [Google Scholar]
- Soderpalm AH, Lindsey S, Purdy RH, Hauger R, Wit de H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacology, Biochemistry, and Behavior. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Tollan A, Oian P, Kjeldsen SE, Eide I, Maltau JM. Progesterone reduces sympathetic tone without changing blood pressure or fluid balance in men. Gynecologic and obstetric investigation. 1993;36:234–238. doi: 10.1159/000292636. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Ogawa N. Acute and chronic effects of psychotropic drugs on maternal aggression in mice. Psychopharmacology (Berl) 1989;97:339–342. doi: 10.1007/BF00439447. [DOI] [PubMed] [Google Scholar]