Abstract
Rationale
Alcohol use in adolescence is one of the most significant predictors of alcohol dependence in adulthood, yet the neurochemical mechanisms underlying this heightened vulnerability remain unknown. Whereas research has focused on characterizing adaptations in the mesolimbic dopamine (DA) system following ethanol exposure in adolescence, whether these changes persist into adulthood has yet to be determined.
Objectives
To investigate the effects of binge-intermittent ethanol administration in adolescence (P30-50) or early adulthood (P60-80) on DA in the nucleus accumbens (NAc) core after an ethanol challenge in adulthood following a period of abstinence.
Methods
Male Sprague Dawley rats (n=160) were administered intermittent ethanol injections, 1 g/kg or 3 g/kg, intraperitoneally (i.p.) every other day for 20 days starting on either P30 or 60. Following an ethanol-free period of either 7, 14 or 28 days, we measured DA efflux following an ethanol challenge (3 g/kg, i.p.) using electrochemical recording electrodes bilaterally implanted into the NAc core.
Results
Moderate dose ethanol administration (1 g/kg, i.p.) during adolescence significantly decreased ethanol-evoked DA release in adulthood at 7 and 14 days, but not 28 days, following pretreatment exposure compared to saline controls. Relative to rats pretreated with ethanol in adulthood, moderate dose ethanol in adolescence significantly reduced DA efflux at all time points measured. Additionally, adult rats pretreated with high dose ethanol administration (3 g/kg, i.p.) displayed significantly decreased DA compared to adolescents after 28 days of withdrawal.
Conclusions
Binge-intermittent ethanol administration during adolescence may induce age-dependent neuroadaptations in the mesolimbic DA system compared to ethanol-treated adults during protracted ethanol withdrawal.
Keywords: dopamine, ethanol, adolescence, binge, intermittent exposure
INTRODUCTION
Binge alcohol consumption during adolescence is a global problem approaching epidemic levels. In the United States, approximately 10 million respondents aged 12 to 20 report consuming alcohol in the previous month, with 15.8% classified as binge drinkers (SAMHSA 2012). Age of first alcohol use is a significant predictor of lifetime alcohol dependence (DeWit et al. 2000; Grant and Dawson 1997), suggesting adolescence may be a period of increased susceptibility to the harmful effects of alcohol. However, whether or not binge alcohol exposure in adolescence causes a neurobiological vulnerability to ethanol that persists into adulthood remains unclear.
Mesolimbic dopamine (DA) neurons mediate many of the rewarding properties of drugs and alcohol (Koob and Bloom 1988; Wise 1996). Acute exposure to ethanol increases DA neuron firing (Brodie et al. 1990; Gessa et al. 1985) and extracellular levels of DA in the nucleus accumbens (NAc) of animals and humans (Boileau et al. 2003; Imperato and Di Chiara 1986; Weiss et al. 1993). Age-dependent differences in DA neuron firing rates (McCutcheon et al. 2012; McCutcheon and Marinelli 2009) and basal DA concentrations (Badanich et al. 2006; Philpot et al. 2009) have been observed to peak in mid- to late adolescence and decline into adulthood. These developmental alterations in basal mesolimbic DA during adolescence are hypothesized to sensitize the dopaminergic system to the neurochemical effects of ethanol, which may contribute to further excessive alcohol intake (for review see Guerri and Pascual 2010; Spear and Varlinskaya 2010). In animal models, repeated binge ethanol administration in early adolescence increases basal NAc DA concentrations in adolescence (Pascual et al. 2009; Philpot et al. 2009), although it remains unclear whether basal DA is persistently altered into adulthood (Badanich et al. 2007; Sahr et al. 2004). Ethanol-induced DA efflux in the NAc after repeated ethanol administration is reduced in early stages of adolescence when compared to late adolescence and early adulthood (Pascual et al. 2009; Philpot et al. 2009). Furthermore, intermittent ethanol injections in early (P30-43), but not late (P45-58) adolescence increases daily consumption and motivation for ethanol in adulthood (Alaux-Cantin et al. 2013). These studies suggest there may be a temporal specificity for alcohol exposure in early adolescence and associated risk for future alcohol dependence that mirrors human epidemiological reports (DeWit et al. 2000; Grant and Dawson 1997).
Taken together, binge ethanol exposure in early adolescence may lead to greater vulnerability for future alcohol use through neuroadaptations in the mesolimbic DA system, including reduced DA efflux following ethanol exposure. Despite evidence implicating the mesolimbic DA system in adolescents’ differential vulnerability to alcohol compared to adults, it is unknown whether binge ethanol in adolescence results in changes in DA neurotransmission to an ethanol challenge in adulthood. Therefore, we investigated the effects of ethanol administration in adolescence (P30-50) or early adulthood (P60-80) on ethanol-induced DA efflux in the NAc core of adult rats following a period of abstinence. Our results suggest that binge-intermittent ethanol exposure in adolescence may induce different neuroadaptations in the mesolimbic DA system compared to adults following a period of abstinence.
METHODS
The following experiments were approved by the Institutional Care and Use Committee at the University of Memphis (Memphis, TN, USA) and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Efforts were made to reduce the number of animals used and to minimize animal pain and discomfort.
Animals and drug treatment
One hundred sixty male Sprague-Dawley rats (Harlan Inc., Indiana, USA) were used in the present study. Animals were delivered to the University of Memphis on either P28 or 58 and were housed three per cage in a temperature controlled environment (21 ± 1°C) with a 12 h light: 12 h dark cycle (lights on at 0600 h) and had ad libitum access to food and water. As previously described (Silvers et al. 2003; Silvers et al. 2006; Tokunaga et al. 2006), animals were administered 10% v/v ethanol, 1 g/kg or 3 g/kg, intraperitoneally (i.p.) every other day for 20 days in a binge-like fashion beginning on either P30 or 60. Control animals were administered an equal volume of saline. All ethanol exposures where conducted between 11:00 am and 2:00 pm. Experimental age groups were assigned based on P30 as an accepted estimate of the onset (as evidenced by no mature sperm) of adolescence in male rats while P60 considered early adulthood (as evidenced by mature sperm) (Odell 1990).
Surgery and chronoamperometric recordings
Following exposure to ethanol during adolescence or young adulthood, animals were ethanol free for either 7, 14 or 28 days. Upon completion of this ethanol-free period, subjects were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) with the incisor bar set at −3.3 mm (Paxinos and Watson 1997). Body temperature was maintained at 36 ± 0.5°C with a temperature regulated heating pad (TC-1000, CWE Inc., New York, NY, USA). In each rat, stearate-modified graphite electrochemical recording electrodes (o.d. 200 μm) were bilaterally implanted into the core of the NAc (coordinates: AP +1.2 mm from bregma, ML ±1.2 mm from midline and −6.5 mm from dura). These electrodes permit changes in DA oxidation current to be measured in vivo without interference from other oxidisable compounds in brain extracellular fluid (Blaha 1996; Blaha et al. 1996; Blaha and Phillips 1990; 1996). An Ag/AgCl reference and stainless steel auxiliary electrode combination was placed in surface contact with contralateral cortical tissue approximately 4 mm posterior to bregma.
Following implantation of all electrodes, repetitive chronoamperometric measurements were obtained using an electrometer (Echempro, GMA Technologies Inc., Vancouver, BC, Canada), and DA efflux was measured by applying a 1 sec potential pulse (from −.15 V to +.25 V vs Ag/AgCl) to each recording electrode at 30 sec intervals and monitoring changes in oxidation current at the end of each pulse. Once a stable 20 min baseline of chronoamperometric recordings was obtained, a challenge injection of 3 g/kg ethanol (10% v/v, i.p.) was administered and changes in DA oxidation current, corresponding to DA efflux, were recorded until signal returned to baseline values (approximately 240 minutes). To determine if injection stress during recording altered DA oxidation current levels, a separate set of control animals were administered saline injections during the pretreatment period, changes in DA oxidation current were monitored in response to a challenge dose of saline after a 7 day treatment-free period. No change in baseline current was found in animals pretreated with saline and tested with saline.
Histological analysis
Upon completion of data collection rats were sacrificed with a 0.5 ml intracardial injection of urethane (0.345 g/ml), and their brains removed, immersed overnight in 10% buffered formalin and then stored in 30% sucrose/10% formalin solution until sectioning. After fixation, 60 μm coronal sections were cut in a cryostat at −30°C, and recor ding electrode placements were determined under a light microscope and reproduced on representative coronal diagrams (Paxinos and Watson 1997).
Data collation and statistical analysis
Pre-drug baseline chronoamperometric currents were normalized to zero current values, and changes in response to ethanol or saline were presented as absolute changes (increases as positive and decreases as negative) in DA oxidation current, which served as the dependent variable. The recording electrode implanted in the left hemisphere in 4 rats became defective during testing and 8 rats had a defective electrode in the right hemisphere. Cell means were substituted for the missing data in these animals.
The general data analytic strategy was analysis of variance (ANOVA). The dependent variable was initially analyzed using Age (adolescent or adult), Pretreatment dose (saline, 1 g/kg or 3 g/kg EtOH) and Ethanol-free period (7, 14 or 28 days) as the between subjects factors, and Electrode side (left, right) and Time (0 to 240 minutes post-EtOH injection) as the within-subjects factors. As this initial ANOVA indicated that Electrode side had a non-significant influence on the results (Side; F=2.90, df=1,130, p=0.09) and failed to interact significantly with Age, Pretreatment dose or Ethanol-free period, it was dropped as a factor from all subsequent analyses. Thus, all analyses were conducted on DA oxidation current that was averaged between right and left electrodes, with 3 between- and 1 within-subjects factors. In all cases in which significant interactions occurred, simple main effects tests or Dunnett’s t-tests were used to identify the source of the interaction.
RESULTS
Histological analysis
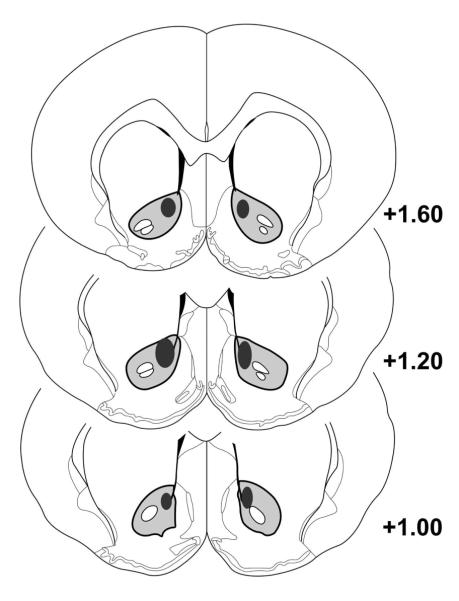
Figure 1 shows the distribution of recording electrodes. All DA recording electrodes (n=320) were localized within the NAc core, ranging from 1.6 to 1.0 mm anterior to bregma, 1.0 to 1.6 mm lateral to midline, and 6.0 to 6.8 mm ventral to dura).
Fig. 1.
Representative placements of electrodes within the NAc core. Black shaded area represents outer border of area encompassing all electrodes according to Paxinos and Watson (1997).
Dopamine oxidation current
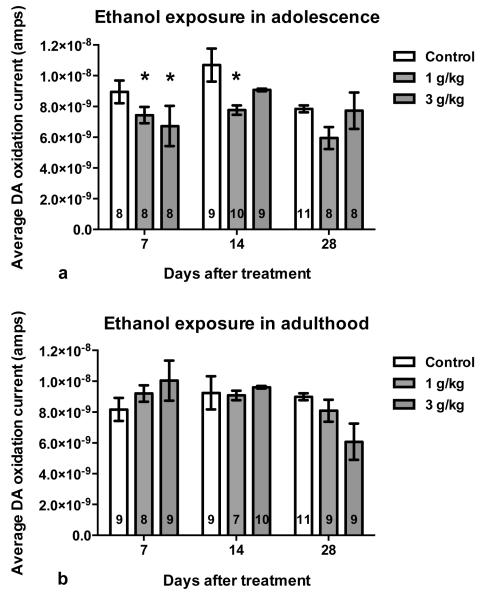
The initial ANOVA indicated that Age interacted with Ethanol-free period, Pretreatment dose and Time (F=1.52, df=96,1704, p<.001). In order to better understand this interaction, results were collapsed across Time within the recording sessions. Figure 2 shows average DA oxidation current after a 3 g/kg (i.p.) ethanol challenge at 7, 14 and 28 days following adolescent (top, n=79) or adult (bottom, n=81) intermittent pretreatment with saline, 1 or 3 g/kg ethanol. When averaged, an interaction between Age, Ethanol-free period and Pretreatment dose approached statistical significance (F=1.98, df=8,142, p=0.053; eta squared=0.09). In comparison to saline pretreated controls, rats pretreated with ethanol during adolescence displayed lower average DA oxidation currents although this effect tended to wane with time. DA oxidation currents in rats pretreated with ethanol during adulthood failed to differ from their respective saline controls regardless of when they received the ethanol challenge. In order to further explore the interaction among Age, Ethanol-free period and Pretreatment dose an additional ANOVA was conducted at each pretreatment dose which allowed us to determine (1) which pretreatment dose resulted in differences between adolescent and adult rats when challenged with 3 g/kg ethanol and (2) when, during the ethanol-free period, differences between adolescent and adult treated animals occurred. When these tests identified differences in the time course of the DA response, these differences were also investigated.
Fig. 2.
Average DA oxidation current (± SEM) after 3 g/kg (i.p.) ethanol challenge in adult rats pretreated with intermittent saline or ethanol (1 g/kg or 3 g/kg, i.p.) treatments in adolescence (a) or adulthood (b). Symbol (*p<.05) indicates DA current differs significantly from saline pretreatment controls by Dunnett’s post hoc. Group n included within each bar.
Group differences between adolescent and adult pretreatment
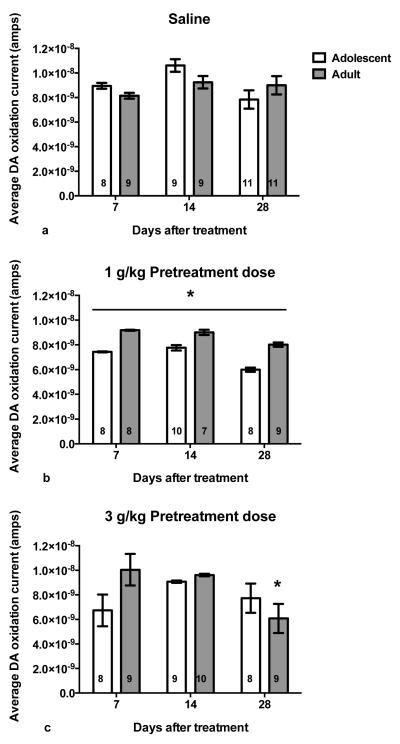
Figure 3 describes the age-related group differences following saline (top), 1 g/kg ethanol (middle) or 3 g/kg ethanol pretreatment (bottom). Regardless of injection-free period, rats pretreated with saline during adolescence (n=28) and adulthood (n=29) showed similar DA oxidation currents (Age × Ethanol-free period; F=1.43, df=2,51, p=0.248).
Fig. 3.
Average DA oxidation current (± SEM) after 3 g/kg ethanol challenge in adult rats pretreated with intermittent saline (a), 1 g/kg (i.p.) ethanol (b) or 3 g/kg ( i.p.) ethanol (c) treatments in adolescence or adulthood. Symbol (*p<.05) indicates DA current significantly differs between age groups at time point. Group n included within each bar.
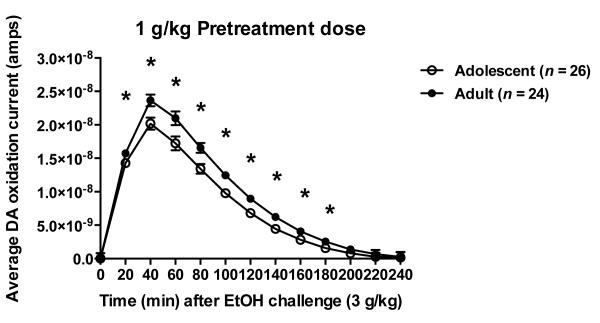
Following the 1 g/kg ethanol pretreatment (Fig. 3, middle), rats treated in adolescence (n=26) consistently showed a significant reduction in evoked DA response to the 3 g/kg ethanol challenge compared to adult (n=24) exposure (Age; F=5.07, df=1,44, p=0.029). In this case, age did not interact with ethanol-free period (F=0.09, df=2,44, p=0.911) indicating that this reduced DA response was maintained throughout the 28-day ethanol-free period. This ANOVA also identified a significant difference in the time course of the DA response that was maintained regardless of ethanol-free period (Age × Time; F=2.44, df=12,528, p=0.004). As shown in figure 4, although the peak response occurred in both groups at 40 minutes following the ethanol challenge (3 g/kg, i.p.), animals treated during adolescence showed a consistent and significant reduction in DA oxidation current between 20 and 180 minutes post-injection (Dunnett’s post hoc, p<0.05).
Fig. 4.
Average DA oxidation current response time course to a 3 g/kg ethanol challenge in the 1 g/kg ethanol pretreatment group averaged over challenge time (1, 2 and 4 weeks). Symbol (*p<.05) indicates currents significantly differ at time point by Dunnett’s post hoc.
As shown in figure 3 (bottom), ANOVA indicated that the effects of the 3 g/kg ethanol pretreatment varied between adolescent (n=25) and adult (n=28) treated animals as a function of ethanol-free period (Age × Ethanol-free period; F=4.26, df=2,47, p=0.020). In order to identify when group differences occurred, simple main effects tests were conducted at 7, 14 and 28 days after ethanol treatment. These tests indicated that adolescent and adult rats pretreated with 3 g/kg ethanol did not differ significantly following ethanol-free periods of 7 and 14 days, however rats pretreated in adolescence displayed a trend toward lower DA efflux at 7 days post-treatment (F=4.18, df=1,15, p=0.059; eta squared=0.22). Following 28 days of ethanol withdrawal, animals pretreated in adulthood had a significantly reduced DA response after receiving the 3 g/kg ethanol challenge in comparison to the adolescent pretreatment group (F=5.35, df=1,15, p=0.035).
DISCUSSION
In the present study, our results show that moderate binge-intermittent ethanol administration during adolescence results in reduced ethanol-evoked DA efflux in adulthood, suggesting a decline in responsivity of dopaminergic neurons to ethanol following adolescent exposure. As the period of abstinence increased, however, this reduction was less robust between rats treated with ethanol in adolescence and saline controls. Perhaps more importantly, when compared to rats treated with ethanol in adulthood, those treated in adolescence maintained reduced DA efflux regardless of abstinence period following ethanol exposure. These findings extend work on age differences in mesoaccumbens dopaminergic function following adolescent ethanol exposure and may contribute to determining the neurobiological basis of heightened vulnerability to alcohol use disorders observed in adults who begin drinking in adolescence.
Here we show that moderate dose (1 g/kg, i.p.) binge-intermittent ethanol administration in adolescence significantly decreased ethanol-stimulated DA release in the NAc core of adult rats. This result agrees with previous work showing that repeated binge-like ethanol administration in preadolescence and early adolescence, but not late adolescence and early adulthood, results in lower DA efflux in the NAc in response to a challenge dose of ethanol (Philpot et al. 2009). Our results extend these findings and suggest that reduced ethanol-evoked DA efflux in the NAc core may persist into adulthood. Moreover, we found that the overall reduction in DA efflux following adolescent ethanol compared to adult ethanol treatment was not due to a temporal shift in DA release, but a consistent reduction across time. A growing amount of evidence suggests that the DA response following ethanol administration in early adolescence mimics the developmental increase in DA that peaks later in adolescence, and results in alterations in synaptic regulation of DA release and sensitivity to ethanol (Pascual et al. 2009; Philpot et al. 2009). It is plausible that repeated ethanol administration increases dopaminergic activity at all stages of adolescence, but only ethanol exposure occurring in early adolescence results in a lasting decrease of ethanol-induced DA efflux into adulthood. This suggests there may be a specific developmental window for neuroadaptations to occur following repeated alcohol administration in adolescence. This hypothesis is further supported by a recent study showing that ethanol exposure in early, but not late, adolescence promotes increased consumption and motivation to drink alcohol, while also displaying lower c-Fos induction in the NAc shell in response to an ethanol challenge (Alaux-Cantin et al. 2013). While ethanol treatment in our study began in early adolescence (P30), future work is necessary to confirm that pretreatment beginning in late adolescence does not result in persistent decreases in DA efflux into adulthood, as suggested by adolescent findings by Philpot et al. (2009). Additionally, it should be noted that a similar blunting of the NAc DA response to cocaine has been observed in mice chronically treated with nicotine throughout adolescence, but tested as adults (Dickson et al. 2011). This raises the possibility that a common consequence to adolescent exposure to alcohol or drugs of abuse may be reduced dopaminergic response upon re-exposure in adulthood.
Although we observed a reduction in ethanol-evoked DA efflux in rats that were administered 1 g/kg intermittent ethanol in adolescence, we failed to observe statistical significance at 28 days following treatment when compared to saline controls. While a significant reduction in DA efflux was maintained regardless of ethanol-free period when compared to rats treated in adulthood, it is possible that adolescent ethanol exposure may not produce long-lasting modifications in the mesolimbic DA system later in life. Interestingly, adolescent alcohol use has been shown to be associated with altered dopamine signalling to risk, but not reward, at three months following ethanol drinking in adolescence (Nasrallah et al. 2011). Therefore, an alternate explanation could be that while ethanol-induced neurochemical adaptations occur within the mesolimbic system during adolescence, persistent effects within other brain regions may contribute greater to the risk of future alcohol problems.
Using a higher ethanol dose during pretreatment, rats administered ethanol in adulthood displayed a significantly reduced DA response after receiving the 3 g/kg ethanol challenge at 28 days withdrawal in comparison to rats administered ethanol in adolescence. These results indicate that dose- and time-dependent alterations in dopaminergic transmission in the NAc may exist following intermittent ethanol exposure. A similar study using this pretreatment dose (3 g/kg, i.p.) found no differences between adolescent and adult rats when examining ethanol-stimulated DA efflux in late adolescence following no abstinence period (Pascual et al. 2009). Despite variations in methodology between studies, it is possible ethanol pretreatment doses in adolescence vary in producing long-lasting dopaminergic alterations. Indeed, biphasic ethanol response curves have previously been reported, with high concentrations of ethanol decreasing evoked dopamine release (Budygin et al. 2001; Imperato and Di Chiara 1986; Yavich and Tiihonen 2000).
It is important to note that these results compare changes in the responsivity of mesoaccumbens dopaminergic neurons to a challenge dose of ethanol from a normalized baseline, and therefore cannot be represented as relative changes to a measured baseline. Additionally, changes in oxidation current, as reported, represent an indirect measure of tonic, not phasic, dopamine release. Chronoamperometric signals have previously been shown to correspond with changes in basal extracellular dopamine as measured by microdialysis (Blaha 1996; Blaha and Phillips 1990; 1996). However, future studies using microdialysis are warranted to establish a direct link between adolescent ethanol exposure and reduced ethanol-evoked dopamine efflux in adulthood.
Basal extracellular DA concentrations are suggested to peak in late adolescence, and decline into adulthood (Badanich et al. 2006; Philpot et al. 2009), although other studies have reported no age differences in basal extracellular DA in the NAc (Frantz et al. 2007; Natividad et al. 2010; Natividad et al. 2012). Adolescent exposure to ethanol may result in adaptive alterations in basal dopaminergic activity in addition to changes in ethanol-evoked activity as discussed in the present results. Future studies should aim to characterize potential age-related differences in basal extracellular dopamine following adolescent ethanol, and the effects of protracted withdrawal. Additionally, a limitation of this study is the use of a repeated, forced ethanol administration protocol and a single challenge dose of ethanol (3 g/kg). There is ample evidence stress significantly effects the dopaminergic system (for review see Ungless et al. 2010). Despite an abstinence period of at least 7 days in our study, it is possible repeated injection stress could influence our results. Therefore, further work is required to determine dose-response effects of ethanol in adulthood following adolescent pre-treatment using a model of ethanol self-administration.
In addition to age-dependent differences in dopamine release dynamics, dopamine uptake rates have previously been reported to vary across development, with adult rats showing greater uptake rates (Stamford 1989). Chronic ethanol administration has also been shown to increase dopamine uptake in the nucleus accumbens of adult rats (Budygin et al. 2007; Carroll et al. 2006). Therefore, we cannot rule out possible differential effects of dopamine uptake rates on the current results. However, quantitative microdialysis data has shown dopamine uptake unaffected by chronic, forced ethanol administration in adolescence and a recent study found no differences in dopamine uptake in dorsal striatum following voluntary ethanol exposure in adolescence (Badanich et al. 2007; Palm and Nylander 2014). Additionally, while urethane does not appear to change dopamine uptake in vivo (Sabeti et al. 2003), future studies using freely moving rats should be performed.
In summary, the results of this study suggest that binge-intermittent ethanol administration in adolescence induces neuroadaptations that may promote reduced dopamine release in response to future ethanol exposure in adulthood. These findings contribute toward understanding the neurochemical mechanisms underlying the addiction liability of individuals who begin drinking alcohol during adolescence and highlight the ongoing need for successful alcohol prevention programs for adolescents.
Acknowledgements
Funding supported by NIAAA, 1U01AA13509 to D.B.M. and 1U01AA13506 to G.M.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Administration SAaMHS . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings NSDUH Series H-44. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. European Journal of Pharmacology. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Chronic Ethanol Exposure During Adolescence Increases Basal Dopamine in the Nucleus Accumbens Septi During Adulthood. Alcoholism: Clinical and Experimental Research. 2007;31:895–900. doi: 10.1111/j.1530-0277.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Blaha CD. Evaluation of stearate-graphite paste electrodes for chronic measurement of extracellular dopamine concentrations in the mammalian brain. Pharmacol Biochem Behav. 1996;55:351–364. doi: 10.1016/s0091-3057(96)00104-9. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Liu D, Phillips AG. Improved electrochemical properties of stearate-graphite paste electrodes after albumin and phospholipid treatments. Biosens Bioelectron. 1996;11:63–79. doi: 10.1016/0956-5663(96)83714-6. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Phillips AG. Application of in vivo electrochemistry to the measurement of changes in dopamine release during intracranial self-stimulation. J Neurosci Methods. 1990;34:125–133. doi: 10.1016/0165-0270(90)90050-p. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Phillips AG. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviours. Behav Pharmacol. 1996;7:675–708. [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PEM, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of Acute Ethanol on Striatal Dopamine Neurotransmission in Ambulatory Rats. Journal of Pharmacology and Experimental Therapeutics. 2001;297:27–34. [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Dickson P, Rogers T, Lester D, Miller M, Matta S, Chesler E, Goldowitz D, Blaha C, Mittleman G. Genotype-dependent effects of adolescent nicotine exposure on dopamine functional dynamics in the nucleus accumbens shell in male and female mice: a potential mechanism underlying the gateway effect of nicotine. Psychopharmacology. 2011;215:631–642. doi: 10.1007/s00213-010-2159-2. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Varqui L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. Journal of Neurophysiology. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. European Journal of Neuroscience. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc Natl Acad Sci U S A. 2011;108:5466–71. doi: 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad L, Tejeda H, Torres O, O’Dell L. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Parsons LH, Torres OV, O’Dell LE. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. Journal of Neurochemistry. 2012;123:578–588. doi: 10.1111/j.1471-4159.2012.07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell WD. Sexual maturation in the rat. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the Onset of Puberty. Lippincott Williams & Williams; Baltimore, MD: 1990. pp. 183–210. [Google Scholar]
- Palm S, Nylander I. Dopamine Release Dynamics Change during Adolescence and after Voluntary Alcohol Intake. PLoS ONE. 2014;9:e96337. doi: 10.1371/journal.pone.0096337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. International Journal of Developmental Neuroscience. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Chloral hydrate and ethanol, but not urethane, alter the clearance of exogenous dopamine recorded by chronoamperometry in striatum of unrestrained rats. Neurosci Lett. 2003;343:9–12. doi: 10.1016/s0304-3940(03)00301-x. [DOI] [PubMed] [Google Scholar]
- Sahr AE, Thielen RJ, Lumeng L, Li TK, McBride WJ. Long-lasting alterations of the mesolimbic dopamine system after periadolescent ethanol drinking by alcohol-preferring rats. Alcoholism, clinical and experimental research. 2004;28:702–711. doi: 10.1097/01.alc.0000125344.79677.1c. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcoholism, clinical and experimental research. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O’Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J Neurochem. 1989;52:1582–9. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Silvers JM, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcoholism, clinical and experimental research. 2006;30:1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: Implications for addiction. Neuroscience and Biobehavioral Rev. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tiihonen J. Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. European Journal of Pharmacology. 2000;401:365–373. doi: 10.1016/s0014-2999(00)00456-8. [DOI] [PubMed] [Google Scholar]