Abstract
Purpose of review
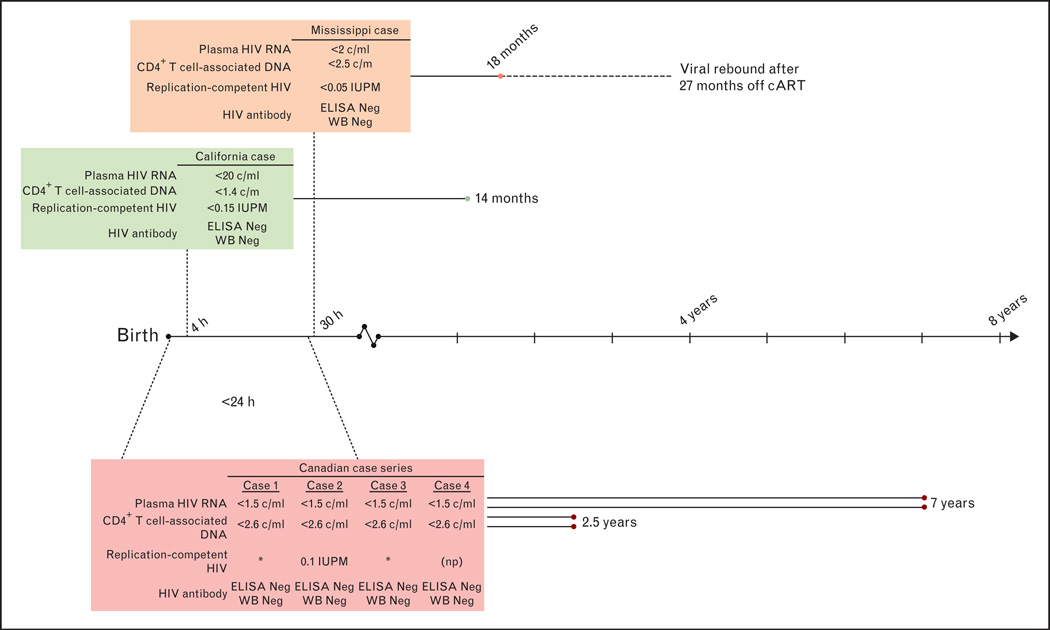
A single case of sustained HIV control in the absence of antiretroviral therapy or HIV-specific immune responses ensued following 18 months of combination antiretroviral therapy initiated at 30 h of age in a perinatally HIV-infected child (the Mississippi child). This case provides proof-of-concept that delay in HIV viremic rebound may ensue following very early treatment (VET) in perinatal infection, likely through marked reduction of latent replication-competent HIV reservoirs.
Recent findings
The latent HIV reservoir remains the critical barrier to remission. Several studies indicate that the earlier effective combination antiretroviral therapy is initiated, the smaller the size of the HIV reservoir. The unique ability of perinatally infected neonates to initiate VET at the time of birth maximizes the potential benefits of limiting latent reservoir size and permitting reservoir decay, likely lengthening the duration of remission and limiting the capacity for re-establishment of viremia.
Summary
This article covers the rationale and feasibility of VET to achieve sustained virologic remission in perinatal infection. Recent studies highlighting the effects of VET on biomarkers of HIV persistence in perinatal HIV infection are reviewed as well as implications and challenges for cure research in pediatric populations.
Keywords: HIV cure, HIV remission, latent reservoir, perinatal HIV
INTRODUCTION
The road to HIV cure has consisted of promising discoveries and disappointing developments. Despite significant progress in prevention of mother-to-child HIV transmission, about 2 40 000 incident HIV infections occur in infants each year [1]. Early combination antiretroviral therapy (cART) reduces mortality but lifelong therapy is needed because of early establishment of latent HIV reservoirs. Reduction in the size of latent HIV reservoirs is one step in the pathway toward HIV cure and characteristics of the infant immune system may contribute to this process. Optimism toward HIV remission and cure highlight the need for consideration of how we define cure, and how the timing and duration of cART affect the latent reservoir in perinatal HIV infection.
CASES OF VIROLOGIC REMISSION
Three adults and one child with HIV remission, defined as sustained virologic control without cART, have been reported (Table 1) [2■■,3,4,5■■]; however, only one adult is considered potentially cured with low levels of HIV RNA detected in a large volume of blood. After more than 7 years without cART, the Berlin patient lives without detectable replication-competent HIV and with diminishing levels of HIV-specific immune responses following allogeneic, myeloablative hematopoietic stem cell transplant (HSCT) from a delta32-CCR5 donor [5■■,6]. The Boston patients also underwent HSCT but with wild-type CCR5 donors [7] and remission after cART interruption was followed by virologic rebound 84 and 225 days later [4]. The Mississippi child, who received cART between 30 h of life and 18 months of age [2■■], remained free of replicating HIV without cART for 27 months before virologic rebound [8]. Although the virologic rebounds of the Boston patients and the Mississippi child were disappointing, they nonetheless demonstrate the capacity for long-term HIV remission. Moreover, these cases have facilitated the adoption of the term ‘remission’ (sustained virologic control without cART) rather than ‘cure’ (lifelong virologic control without cART) and an increasing recognition of a latent HIV reservoir capable of re-establishing viremia after long periods of undetectable HIV viremia without cART.
Table 1.
Biomarker profiles in the Mississippi child, Boston patients, and Berlin patient during HIV remission
| Mississippi child [2■■,3] | Boston patients [4] | Berlin patient [5■■] | ||
|---|---|---|---|---|
| Patient A | Patient B | |||
| Plasma HIV RNA | <2 copies/ml | <20 copies/ml | <0.4 copies/ml | Intermittent detectiona |
| Replication-competent HIV | <0.05 IU/106 cells | <0.007 IU/106 cells | <0.006 IU/106 cells | <1 IU/107–9 cells |
| Peripheral blood cells | ||||
| PBMC | 4.2 c/106 cells | <0.12 c/106 cells | <0.13 c/106 cells | Undetectable HIV RNA and DNA |
| Total CD4+ T cells | NA | NA | NA | Undetectable HIV RNA and DNA |
| Resting CD4+ T cells | <2.5 c/106 cells | NA | NA | NA |
| Activated CD4+ T cells | <2.6 c/106 cells | NA | NA | NA |
| Myeloid-derived cells | <11.5 c/106 cells | NA | NA | NA |
| HIV-specific T cell responses | Undetectable HIV-specific CD4+ and CD8+ T cell responses to gag and nef peptides | No PBMC activation to nef or gag peptides | No PBMC activation to nef or gag peptides | Low levels to gag peptides |
| HIV-specific antibodies | Undetectable | Decreased to low concentration | Decreased to low concentration | Low concentration and declining |
| Cerebrospinal fluid | NA | (<20 copies HIV RNA/ml) | NA | <0.1 copy HIV DNA/ml |
| Lymph node biopsy | NA | NA | NA | Undetectable HIV RNA and DNA |
| Rectal biopsy | NA | NA | <2.4 c/106 cells | Intermittent detection with <1 c/106 cells overall |
| Ileal biopsy | NA | NA | NA | <1 c/106 cells |
| Duration of remission | 27 months | 84 days | 225 days | Undefined |
Values represent most recent published data in the potential cure case of the Berlin patient and prior to virologic rebound for the Mississippi child and Boston patients.
c/106, copies HIV DNA per million cells; c/ml, copies HIV DNA per milliliter of blood; IU/106, infectious units per million cells; NA, sample not collected.
The intermittent detection of low level viremia in the Berlin patient was in a large volume of blood (17–24 ml) and detected in 1/24 replicates (70 copies/ml) and 2/6 replicates (4 to 5 copies/ml).
EARLY cART RESTRICTS LATENT RESERVOIR ESTABLISHMENT
HIV reservoirs remain the critical barrier to remission. One major reservoir forms following infection of activated CD4+ T cells and subsequent differentiation into stable, quiescent memory cells that are unaffected by cART and unrecognized by immune surveillance. Perinatal HIV infection has additional implications for HIV reservoir development, however. Although the low abundance of circulating memory CD4+ T cells in neonates [9–11] suggests fewer activated CD4+ T cells are available for HIV infection, high levels of CD4+ CCR5+ T cells in the gut mucosa provide ample targets for productive virus replication and spread through an infant’s gastrointestinal tract [12]. With in-utero transmission, the more tolerogenic fetal immune system may create an environment that promotes HIV reservoir establishment [13]. The identification of a memory-like T cell phenotype in cord blood indicates susceptible neonatal target cells may be more prevalent than previously thought [14■■].
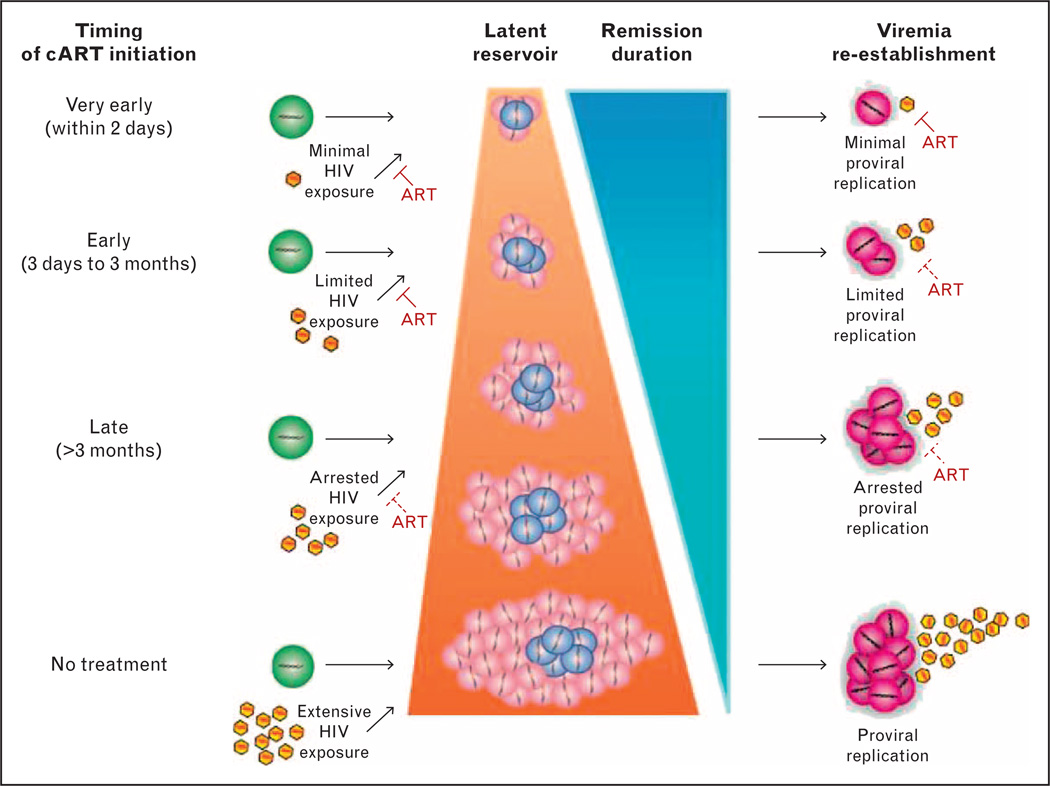
Standard clinical approaches to perinatal infection are to begin cART in all HIV-infected infants irrespective of CD4+ T cell levels [15], as early cART is life-saving for HIV-infected children [16]. This typically occurs around 2 or 3 months of age in settings with access to early infant diagnosis and treatment. By this time, HIV reservoirs are fully established. Very early treatment (VET), or the ability to initiate cART at the time of birth or within a few days thereafter, is unique to perinatal infection and may avert or attenuate HIV reservoirs (Fig. 1). Studies of youth who began effective cART within months of birth also suggest the size and composition of the latent HIV reservoir in perinatal infection is related to the duration between HIV infection and virologic suppression. Long-term follow-up of children who suppressed HIV replication for over 14 years following cART initiation prior to 3 months of age demonstrated extremely low levels of circulating provirus [17■■]. Of 144 perinatally infected adolescents on long-term effective cART, virologic control before 1 year of age was significantly associated with smaller proviral burdens than virologic control between 1 and 5 years or after 5 years of age [18■]. Furthermore, 46% of 13 adolescents with virologic control before 1 year of age had proviral DNA concentrations below the limit of detection of four copies/106 peripheral blood mononuclear cells (PBMC) compared with only 11% of 101 adolescents with virologic control at older ages [18■]. This effect is also noted in infection with nonsubtype B HIV infections [19■■]. Among 15 HIV-infected Thai children who were virologically suppressed before 6 months of age, a median of seven copies of integrated HIV DNA/106 CD4+ T cells was identified on long-term cART [19■■]. The relationship between early effective cART and smaller reservoir size is also noted in HIV-infected adults treated during acute infection, although this is approximately 10-fold higher (47 copies/106 PBMC) than that of early treated, perinatally infected youth [20■■]. By early arrest of HIV replication, expansion of latent HIV reservoirs is restricted and fewer cells harboring infectious viral genomes are capable of reactivating productive infection (Fig. 1). Indeed, we have previously reported marked restriction in replication-competent reservoirs following early cART [21].
FIGURE 1.
Reservoir size and duration of HIV remission is directly affected by the time from HIV infection to initiation of cART. Susceptible CD4+ T cells (green) become infected with HIV (yellow) and transition to either productively infected CD4+ T cells (pink) or latently infected CD4+ T cells (blue). Reactivated CD4+ T cells (purple) from latency re-establish viremia. ART, antiretroviral therapy; cART, combination antiretroviral therapy.
EARLY cART DECREASES LATENT RESERVOIR SIZE
Effective early cART in perinatal HIV infection also appears to modify long-term decay of circulating proviral reservoirs. Early hope for HIV cure was created by initial estimates suggesting 7–10 years of continuous suppressive cART would permit adequate natural turnover of latently infected cells to eradicate the reservoir [22]. Unfortunately, additional studies dampened this enthusiasm by demonstrating a slow and negligible decay of latently infected resting CD4+ T cells, necessitating life-long cART [23–25]. In a study of five perinatally infected adolescents who initiated cART at about 2.5 months of age [17■■], the median proviral burden was seven copies/106 PBMC, considerably smaller than the 182 copies/106 PBMC in four adolescents who achieved virologic control at a median of 12.9 years of age. Long-term maintenance of small reservoir size reinforced the notion that early virologic control prevented ongoing seeding of HIV reservoirs. Additionally, longitudinal measures of proviral burdens from four of these adolescents showed decreased concentrations of circulating proviral reservoirs and undetectable proviral burdens after 14–25 years of effective cART, indicating a lifetime of cART may not be necessary for extensive reservoir reduction.
The time frame for reservoir attenuation with VET that creates a state of permanent remission in which cART can be stopped without viremic rebound is unclear (Fig. 2). Although minimizing reservoir size may lengthen the duration of virologic remission [26■■,27■■], the question remains: how early is early enough to achieve permanent HIV remission and cure? The Mississippi child provides proof-of-concept that VET may influence HIV reservoir size and sustain long-term virologic remission. After receiving cART 30 h after birth and for approximately 18 months, virologic rebound did not occur for 27 months [2■■]. In a second neonate from Long Beach, California, USA, who initiated cART 4h after birth, standard diagnostic tests for HIV nucleic acid became negative after 6 days and coculture assays of CD4+ T cells failed to show replication-competent reservoirs through 9 months of age [3]. A recent report of four Canadian children who initiated cART within 24 h of birth indicates replication-competent HIV and HIV DNA remained undetectable after 2.5–7 years on cART [28■■,29]. Therefore, understanding why the Mississippi child achieved virologic remission with VET is critical. The VET cases described above acquired HIV in utero and therefore were infected for an unknown duration before cART initiation. The potential to achieve long-term HIV remission and cure through VET of neonates may be more likely in infants with more recently acquired infection through peripartum or breast-milk transmission routes. Regardless of transmission route, however, minimizing the size of the reservoir will inform new strategies aimed at achieving virologic remission, such as latency reversing agents, broadly neutralizing antibodies, or therapeutic vaccines.
FIGURE 2.
Summary of potential HIV remission biomarkers in perinatally infected children who initiated very early cART. Solid lines represent time since cART initiation with duration in text. Dotted line connected to time line indicates age at cART initiation. Dashed line for Mississippi case indicates period off combination antiretroviral therapy. * No replication-competent HIV detected. c/m, copies per million cells; c/ml, copies per milliliter blood; cART, combination antiretroviral therapy; ELISA, enzyme immunoassay; IUPM, infectious units per million resting CD4+ T cells; Neg, negative; np, assay not performed; Undet, undetectable; WB, western blot.
ABSENT HIV-SPECIFIC IMMUNE RESPONSES
Perinatal HIV infection results in decreased immune responses to not only HIV but also routine childhood immunizations. Many studies have documented HIV-infected children with waning immunity to vaccinations received prior to cART initiation or with poor cART adherence [30,31], suggesting revaccination may be necessary. Limited or absent HIV-specific immune responses are a hallmark of restricted HIV replication following VET in infants (Fig. 2) [2■■,3,17■■,18■,19■■,28■■,32■■] and were associated with low proviral reservoir size in early treated, perinatally infected youth [17■■,18■]. Of those in whom T cell responses to HIV peptides have been measured, HIV-specific cellular responses are also absent [28■■,32■■]. Thus, restricted reservoir size occurs in the setting of absent or restricted HIV-specific immunity. Factors contributing to this may include low levels of HIV antigen exposure, decreased efficiency of HIV-specific memory response formation in neonates and cytopathic effects of HIV on CD4+ T cells with consequent loss of CD4+ T cell help [33]. Importantly, the absence of HIV-specific immune responses does not signify lack of replication-competent latent reservoirs or cure as the Mississippi child and one Canadian child lacked both humoral and cellular immune responses yet developed rebound viremia [8,29].
DEFINING HIV CURE AND BIOMARKERS
Definitions for HIV cure continually evolve with increased understanding of the latent reservoir and HIV persistence. The broadest definition of cure is the permanent remission of disease following cessation of therapy [8]. Although this definition is an overarching, long-termgoal for the HIV field, it lacks specific benchmarks from which we can evaluate whether an individual would be capable of longterm remission. As a field, we have yet to define what these benchmarks should be, however. Replication-competent HIV, as measured through the activation of latent CD4+ T cells and the resulting viral outgrowth, has long served as the standard for estimating latent reservoir size and the capacity for re-establishment of viral replication. Comparisons of potential biomarkers for remission between the Mississippi child, the Berlin patient, and the Boston patients demonstrate that the inability to detect replication-competent HIV in peripheral blood and circulating cells is insufficient to predict HIV remission (Table 1). Thus, the quantitative coculture assay lacks sensitivity for the purposes of deeming an infected individual as ‘cured’ [2■■,7,34]. Molecular approaches lack specificity as the detection of HIV DNA may simply represent defective viral genomes incapable of re-establishing productive infection. In a recent summary of the Berlin patient’s clinical follow-up [5■■], the authors suggest the lack of HIV-specific immune responses as a biomarker for cure. However, both Boston patients and the Mississippi child lacked HIV-specific immune responses during treatment cessation and later experienced virologic rebound, suggesting an absence of HIV-specific immune responses may be necessary but insufficient to define cure. Cell-associated RNA has yet to be vetted as a potential biomarker. Although it was the only HIV-associated biomarker detected in the four Canadian children treated within 24h of birth who remain on cART [28■■] and was undetectable in the Berlin patient [5■■], it was not measured in the Boston patients or the Mississippi child.
Bifurcation into functional and sterilizing cure definitions essentially represents the presence and absence of replication-competent HIV capable of reigniting viremia (Table 2) [35]. Functional cure describes a state in which HIV replication is controlled through host-mediated immune mechanisms in the absence of cART and encompasses the long-term nonprogressor status, whereas sterilizing cure requires eradication of replication-competent HIV from the host. These definitions can be broadly applied to both adult and pediatric HIV, but the differing pathogenesis in perinatal infection may necessitate a more specific pediatric definition that accounts for the developing immune system throughout childhood. Given the unpredictability of virologic rebound following cART cessation [26■■], the most appropriate terminology for the majority of HIV-infected individuals is not ‘cure’ but ‘remission’. In the context of perinatal infection, we propose HIV remission be defined as sustained virologic control to clinically undetectable levels in the absence of cART with maintenance of normal levels of CD4+ T cells and the capacity to maintain immune responses to routine immunizations. This definition provides specific benchmarks for measuring remission for perinatally infected individuals (Table 2).
Table 2.
Proposed definitions of HIV remission and cure
| Definition | Implications for clinical biomarkers | |
|---|---|---|
| Comprehensive [8] | Permanent remission of disease following cessation of therapy | |
| Adults | ||
| Functional cure [35] | Host-mediated control of HIV replication in the absence of cART and: | Detectable replication-competent HIV by viral outgrowth assay |
| Normalized, effective immune function | Normalization of inflammatory biomarkers | |
| Decreased HIV-related inflammation | ||
| Reduced risk of HIV transmission | ||
| Sterilizing cure [35] | Complete elimination of replication-competent HIV | Undetectable replication-competent HIV |
| Children | ||
| Pediatric remission | Sustained virologic control in the absence of cART that includes: | Undetectable replication-competent HIV |
| Normal CD4+ T cell levels | Normalization of CD4+ T cell percentages and absolute numbers | |
| Preservation of immune responses to routine immunizationa | Maintenance of vaccine-specific antibodies and CMI | |
cART, combination antiretroviral therapy; CMI, cell-mediated immunity.
Proposed by the IMPAACT Cure Committee.
CHALLENGES
Implementation of VET to achieve viral remission in perinatal infection is a challenge. First, current diagnostic tests have limitations. Diagnosing infection in HIV-exposed infants requires nucleic acid testing, for which there are no widely available rapid diagnostic tests. Low HIV DNA concentration at birth leads to delay in diagnosis [36■] and these infants are the most apt to benefit from VET. Also, long turn-around times for test results and the patient’s need to return for clinical follow-up can result in several months of delay before cART can be started in perinatally infected infants [37], at which point the latent viral reservoir is established [38]. Second, VET restricts viral spread and prevents immune responses. This can result in conflicting diagnostic test results. Confirming infection with the current state-of-the-art HIV DNA and RNA assays require a high concentration of HIV DNA to detect infection, which is limited in low blood volume samples. Third, exceedingly large blood volumes are required to comprehensively assess patients approaching near-negligible reservoir size for HIV cure research despite the high sensitivity of current methods, emphasizing the need for improved assays to detect persistent replication-competent HIV.
The presence of latent reservoirs in sites beyond the lymphocytic compartment of peripheral blood remains a fundamental challenge in moving patients to a state of HIV remission. Detection of replication-competent HIV in gut-associated lymphoid tissue or the central nervous system is likely important for assessing a child’s propensity for viremic rebound upon cART cessation [39,40]. Using simian immunodeficiency virus, intrarectally infected rhesus monkeys initiating cART 3 days postinfection harbored infectious SIV in lymph nodes and the gastrointestinal tract [41■■], highlighting the rapidity with which the reservoir develops following infection. How to measure gut-associated lymphoid tissue and central nervous system reservoirs in a pediatric population is a challenge as rectal biopsy and lumbar puncture procedures are invasive. Moreover, whether a small sampling of gut cells or a few milliliters of spinal fluid are the relevant sample types for accurately assessing replication-competent HIV capable of reestablishing productive infection in children are unknown but important for moving the field forward.
Controversy ensues over the ethical implications surrounding treatment cessation to assess virologic remission in the absence of clear biomarkers heralding long-term HIV remission for cure research. The planned P1115 clinical trial by the IMPAACT Network aims to conduct closely monitored treatment cessation among HIV-infected infants who initiated cART within 48h of age [42] Frequent, careful evaluation for potential virologic rebound and detailed safety monitoring are of the utmost importance to maintain the principle of beneficence [43■■]. Moreover, understanding risk factors from participants who experience virologic rebound will aid in future cure research efforts.
CONCLUSION
HIV-infected children face a lifetime of treatment because of the persistent nature of HIV. VET alone or in combination with immunotherapeutic approaches provides the first glimmer of hope toward virologic remission for perinatally infected children. The unique aspects of perinatal infection with respect to knowledge of timing of infection and a developing immune system set the pediatric cure agenda apart from that of adults. Global efforts to enhance earlier diagnosis and implement VET will greatly facilitate the overall goal of pediatric HIV cure. Other novel strategies that target gut-associated lymphoid tissue and central nervous system reservoirs or augment the immune system’s response may be necessary to achieve this goal. Even if not curative, VET yields substantially reduced reservoir size, which will place children in a better place for treatment with new approaches expected to emerge from this endeavor.
KEY POINTS.
The first case of long-term remission in a perinatally HIV-1-infected child is described in the context of biomarkers of long-term remission and cure in adult HIV infection.
Converging evidence suggests very early combination antiretroviral therapy is associated with very small proviral reservoirs and restricted HIV-specific immune responses in perinatal infection.
Unique immunological aspects of perinatal HIV infection indicate that HIV remission in pediatric populations be defined as sustained virologic control to clinically undetectable levels in the absence of cART with maintenance of normal levels of CD4+ T cells and the capacity to maintain immune responses to routine immunizations.
Very early therapy, novel immunotherapeutics, and strategies that target latent HIV reservoirs in gut-associated lymphoid tissue and the central nervous system will advance the potential for pediatric HIV cure.
Acknowledgements
We thank the IMPAACT Cure Committee for contributing to the definition for pediatric HIV remission.
Financial support and sponsorship
All authors are supported by the National Institute of Allergy and Infectious Disease and the National Institute of Child Health and Human Development (R01 HD080474). K.R.L. and D.P. are also supported by The Johns Hopkins Center for AIDS Research (P30 AI094189).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.UNAIDS. AIDS by the numbers. [Accessed 4 December 2013]; http://www.unaids.org/en/resources/documents/2013/name,88096,en.asp.
- 2. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. This case report describes the first known case of sustained absence of viremia without combination antiretroviral therapy in a perinatally infected child, the ‘Mississippi Child’ likely by very early antiretroviral treatment.
- 3.Persaud D, Deveikis A, Gay H, et al. Very early combination anti-retroviral therapy in perinatal HIV infection: two case studies. 21st Conference on Retroviruses and Opportunistic Infections; 3–6 March 2014; Boston, MA, USA. 2014. abstract 75LB. [Google Scholar]
- 4.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yukl SA, Boritz E, Busch M, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. This study highlights the extensive blood and tissue sampling required for cure-related research and challenges in interpretation of the findings.
- 6.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 7.Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207:1694–1702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauci AS, Marston HD, Folkers GK. An HIV cure: feasibility, discovery, and implementation. JAMA. 2014;312:335–336. doi: 10.1001/jama.2014.4754. [DOI] [PubMed] [Google Scholar]
- 9.Schatorje EJ, Gemen EF, Driessen GJ, et al. Paediatric reference values for the peripheral T cell compartment. Scand J Immunol. 2012;75:436–444. doi: 10.1111/j.1365-3083.2012.02671.x. [DOI] [PubMed] [Google Scholar]
- 10.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 11.van GR, van Tilburg CM, Nibbelke EE, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133:95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Bunders MJ, van der Loos CM, Klarenbeek PL, et al. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood. 2012;120:4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 13.Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang X, Mozeleski B, Lemoine S, et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med. 2014;6:238ra72. doi: 10.1126/scitranslmed.3008748. This recent article challenges the notion that the fetal immune system is ‘dedicated to tolerance’ with the identification of fetally-derived effector memory T cells displaying a large variety of inflammatory effector functions associated with CD4 TH cells at birth.
- 15.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [Accessed 10 July 2013]; http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html. [PubMed]
- 16.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants with HIV: results from the children with HIV antiretroviral (CHER) randomized trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luzuriaga K, Tabak B, Garber M, et al. HIV Type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis. 2014;210:1529–1538. doi: 10.1093/infdis/jiu297. This recent case series is the first to identify ongoing decay of proviral reservoirs through adolescence despite an absence of HIV-specific immune responses during long-term effective combination antiretroviral therapy in perinatal infection.
- 18. Persaud D, Patel K, Karalius B, et al. Age at virologic control influences peripheral blood HIV reservoir size and serostatus in perinatally-infected adolescents. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2014.1560. [Epub ahead of print] This study highlights that extremely low proviral reservoir size can be achieved and maintained for over a decade when virologic control occurs in infancy; the association of low proviral burdens and restricted HIV-antibody responses are also identified.
- 19. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28:1015–1020. doi: 10.1097/QAD.0000000000000178. This article shows that early treatment before age six months is associated with restricted proviral reservoir size and HIV-specific immune responses in nonsubtype B infection.
- 20. Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. This study provides a comprehensive analysis of biomarkers of HIV persistence in adult patients treated during acute and chronic infection, and therefore provides important benchmarks for the field.
- 21.Persaud D, Palumbo PE, Ziemniak C, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS. 2012;26:1483–1490. doi: 10.1097/QAD.0b013e3283553638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 23.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 24.Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24:1598–1601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 25.Zanchetta M, Walker S, Burighel N, et al. Long-term decay of the HIV-1 reservoir in HIV-1-infected children treated with highly active antiretroviral therapy. J Infect Dis. 2006;193:1718–1727. doi: 10.1086/504264. [DOI] [PubMed] [Google Scholar]
- 26. Hill AL, Rosenbloom DI, Fu F, et al. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A. 2014;111:13475–13480. doi: 10.1073/pnas.1406663111. This recent article uses mathematical modeling to illustrate that time to virologic rebound will differ between patients undergoing interventions aimed at HIV cure and will be influenced by log-fold reduction in the latent reservoir size and that the stochastic nature of the latent reservoir is a function of the rate at which resting cells reactivate from latency and the probability that these cells re-establish viremia.
- 27. Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Posttreatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. A landmark study showing that a subset of adult patients can achieve sustained virologic control without combination antiretroviral therapy or known HLA alleles.
- 28. Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59:1012–1019. doi: 10.1093/cid/ciu432. This retrospective case series identified four Canadian children who received combination antiretroviral therapy shortly after birth, like the Mississippi Chld, and highlights the effects of very early treatment on restricting biomarkers of HIV persistence in perinatal infection.
- 29.Brophy J, Chun TW, Samson L, et al. Impact of early initiation of combination antiretroviral therapy on measure of virus in peripheral blood of vertically HIV-1 infected children. 20th International AIDS Conference; 19–25 July 2014; Melbourne, Australia. 2014. abstract PE47LB. [Google Scholar]
- 30.Cotugno N, Douagi I, Rossi P, et al. Suboptimal immune reconstitution in vertically HIV infected children: a view on how HIV replication and timing of HAART initiation can impact on T and B-cell compartment. Clin Dev Immunol. 2012;2012:805151. doi: 10.1155/2012/805151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? Lancet Infect Dis. 2010;10:630–642. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 32. Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol. 2000;74:6984–6991. doi: 10.1128/jvi.74.15.6984-6991.2000. This paper was the first to demonstrate an absence of HIV-specific immune responses in early treated infants who achieved durable control of HIV replication.
- 33.Scott ZA, Chadwick EG, Gibson LL, et al. Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8(+) T cell responses in young HIV-1-infected infants. J Immunol. 2001;167:7134–7140. doi: 10.4049/jimmunol.167.12.7134. [DOI] [PubMed] [Google Scholar]
- 34.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katlama C, Deeks SG, Autran B, et al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013;381:2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitchell C, Dross S, Beck IA, et al. Low concentrations of HIV-1 DNA at birth delays diagnosis, complicating identification of infants for antiretroviral therapy to potentially prevent the establishment of viral reservoirs. Clin Infect Dis. 2014;58:1190–1193. doi: 10.1093/cid/ciu068. This recent study highlights that some in-utero-infected infants can have very low concentration of HIV-infected cells making diagnosis a challenge; such infants may be ideal candidates for very early treatment toward virologic remission.
- 37.Sutcliffe CG, van Dijk JH, Hamangaba F, et al. Turnaround time for early infant HIV diagnosis in rural Zambia: a chart review. PLoS One. 2014;9:e87028. doi: 10.1371/journal.pone.0087028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persaud D, Palumbo P, Ziemniak C, et al. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis. 2007;195:1402–1410. doi: 10.1086/513871. [DOI] [PubMed] [Google Scholar]
- 39.Hatano H, Somsouk M, Sinclair E, et al. Comparison of HIV DNA and RNA in gut-associated lymphoid tissue of HIV-infected controllers and noncontrollers. AIDS. 2013;27:2255–2260. doi: 10.1097/QAD.0b013e328362692f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrido C, Margolis DM. Translational challenges in targeting latent HIV infection and the CNS reservoir problem. J Neurovirol. 2014 doi: 10.1007/s13365-014-0269-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whitney JB, Hill AL, Sanisetty S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. An important study showing quantitative and qualitative differences in viral dynamics when cART is started at 3, 7, 10 and 14 days postinfection, with rapid seeding of gut reservoirs.
- 42.Dolgin E. New, intensive trials planned on heels of Mississippi HIV ’cure’. [Accessed 14 April 2013]; doi: 10.1038/nm0413-380. http://www.nature.com/nm/journal/v19/n4/full/nm0413-380.html. [DOI] [PubMed] [Google Scholar]
- 43. Shah SK, Persaud D, Wendler DS, et al. Research into a functional cure for HIV in neonates: the need for ethical foresight. Lancet Infect Dis. 2014;14:893–898. doi: 10.1016/S1473-3099(14)70766-2. This is the first report on the ethical considerations for cure research in pediatric populations.