Abstract
Uric acid (UA) is produced from purines by the enzyme xanthine oxidase, and elevated levels may cause arthritis and kidney stones. Conversely, UA also appears to function as an antioxidant and may protect against the oxidative stress associated with aging and disease. We performed a prospective fracture case-cohort study to understand the relation of UA and fracture risk in older men enrolled in the Osteoporotic Fractures in Men (MrOS) study. In the cohort of 5994 men aged 65 years and older attending the baseline MrOS examination, we evaluated a subgroup 1680 men in a case-cohort study design. The analytic group included 387 men with incident nonspine fractures (73 hip) and a random sample of 1383. Serum UA was measured in baseline serum samples. Modified proportional hazards models that account for case-cohort study design were used to estimate the relative hazards (RH) of hip and nonspine fracture in men for serum UA. Models were adjusted for age, race, clinic site, body mass index, vitamin D, parathyroid hormone, walking speed, Physical Activity Scale for the Elderly (PASE) score, frailty, and total. Subjects with incident nonspine fractures were older, had lower total hip bone mineral density (BMD), and higher serum phosphorus. There was an 18% decreased risk of nonspine fractures (95% confidence interval [CI] 0.71–0.93; p = 0.003) per 1 SD increase of baseline serum and 34% decreased risk of nonspine fractures in quartile 4 of UA versus quartiles 1, 2, and 3 (95% CI 0.49–0.89; p = 0.028) compared with nonfracture cases after multivariate adjustment. Hip fractures were not significantly associated with UA. Total hip BMD was significantly higher in the group of men with high UA levels compared with lower UA levels and increased linearly across quartiles of UA after multivariate adjustment (p for trend = 0.002). In summary, higher serum UA levels were associated with a reduction in risk of incident nonspine fractures but not hip fractures and higher hip BMD.
Keywords: AGING, DXA < ANALYSIS/QUANTITATION OF BONE, EPIDEMIOLOGY
Introduction
Elevated serum uric acid (UA) level, or hyperuricemia, is a metabolic disorder that is often associated with acute and chronic attacks of gouty arthritis resulting from the deposition of monosodium urate crystals in joints. Elevated serum UA is also associated with an increased risk of nephrolithiasis. The prevalence of gout is 1% of the general population; however, the prevalence is not similar across all age groups because it increases with age and is higher in middle-aged and older men and older (postmenopausal) women compared with younger individuals.(1) Hyperuricemia is the most significant predisposing factor for the development of gout, and it results from either increased production or reduced excretion of UA, or both.(1,2)
UA is the end product from the breakdown of purines in humans. Normal serum levels of UA are generally below 6.8 mg/dL. Recent studies have reported that an elevated serum level of UA is a risk factor for both hypertension and cardiovascular disease, renal disease, type 2 diabetes mellitus, and metabolic syndrome. The proposed mechanism is through direct interaction of the UA with the vascular endothelial cells.(2-7) However, serum UA is also a potent antioxidant and is reported to account for nearly 50% of the antioxidant activity in the plasma.(8,9) As a result of this antioxidant effect, investigators have examined whether higher levels of UA were associated with lower risk of disorders characterized by high oxidative states including neurodegenerative disorders like Parkinson’s disease and osteoporosis. A strong inverse relationship has been reported for serum UA andthe risk of Parkinson’s disease, bone mineral density (BMD) and prevalent fractures in older men, bone mass in perimenopausal women, and postmenopausal bone loss in women.(10-15) The relation of UA and bone density is hypothesized to be through its antioxidant effects. Both observational and epidemiologic studies have reported an association of oxidative stress or low levels of antioxidants with reduced bone mineral density and osteoporosis.(15-17) In a large population-based study of older men, higher serum UA levels were associated with significantly higher BMD at the lumbar spine and hip and prevalent fractures at the lumbar spine and hip even after adjustments for covariates.(2) Ahn and colleagues reported that higher serum UA was associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in postmenopausal women.(12) Also, a longitudinal study of peri- and postmenopausal women reported that higher serum UA levels reduced the rate of bone loss by an average of 9.7 years later for lumbar spine, forearm, and whole body bone mass after adjustments for possible confounders including lean mass and fat mass.(15)
Although the relation of serum UA and bone mass and prevalent fractures has been reported, there is little to no information about the association of serum UA with incident fracture risk. Therefore, the purpose of our study was to perform a prospective case-cohort study to understand the relation of serum UA and fracture risk in older men enrolled in the Osteoporotic Fractures in Men (MrOS) study.
Materials and Methods
Study Population
A total of 5994 community-dwelling men at six clinical centers in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA) enrolled in the MrOS (from March 2000 through April 2002), a study of osteoporosis and fractures in elderly men.(18,19) Eligible men were aged 65 years or older, without bilateral hip replacements, and able to walk without the assistance of another person. Details of the MrOS design and cohort have been published elsewhere.(18,19) The Institutional Review Board (IRB) at each center approved the study protocol, and written informed consent was obtained from all participants.
Follow-up and outcome ascertainment
Tri-annual questionnaires were sent to the men to report any fractures. All nonspine fractures were verified by medical records and confirmed by blinded central adjudicators.(20) Pathologic fractures were excluded.
Case-cohort study design
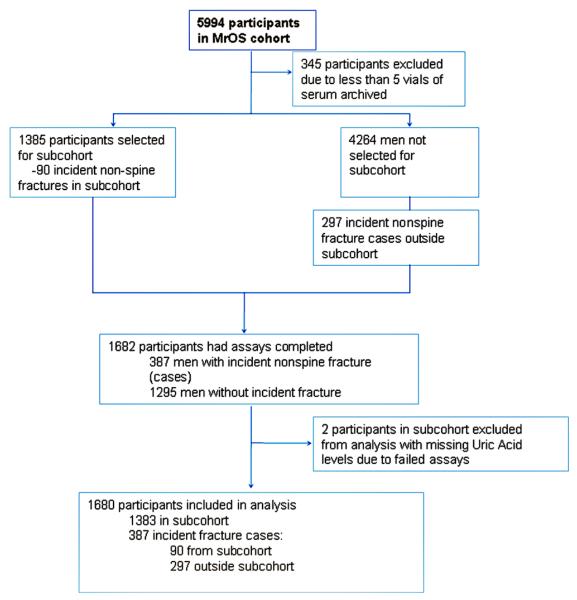
The current study is a prospective case-cohort study of men nested within MrOS. Men with 5 or more vials of archived serum were eligible for inclusion in the study. Hence, of the 5994 MrOS participants enrolled in the study, 5649 had sufficient serum archived. Of these men, the first 1385 samples of serum collected were selected to serve as the subcohort. Over 5.3 years of follow-up, 90 nonspine fractures (including 16 hip fractures) arose from the randomly selected subcohort. Among the 4264 participants not selected for the random cohort, there were 297 nonspine fractures (including 57 hip fractures) that occurred over 5.3 years of follow-up in the remaining MrOS cohort. Hence, a total of 1682 participants including 387 nonspine fracture cases and 1295 non-cases were selected for UA measures. Two participants with missing UA levels owing to invalid assays were excluded, resulting in 1383 men in the subcohort and 297 fracture cases outside of the cohort for this study. The total study sample for the nonspine fracture analyses was 1680 men (Fig. 1). Similarly, there were 73 hip fractures with 16 occurring in the subcohort and 57 hip fracture cases outside the cohort resulting in 1440 men for the hip fracture analysis.
Fig. 1.
Case-cohort design for the MrOS Uric Acid and Fracture Study.
Vitamin D, parathyroid hormone (PTH), and cystatin C assays
Fasting morning blood was collected; sera was protected from sunlight, prepared immediately after phlebotomy, and was stored at −70°C until first thawed for vitamin D, PTH, and cystatin C and creatinine assays. Measures for 25(OH) vitamin D2 (derived from ergocalciferol) and 25(OH) vitamin D3 (derived from cholecalciferol) were performed at the Mayo Clinic using mass spectrometry as previously described.(21) Total 25(OH) vitamin D was calculated by summing the values of 25(OH)vitamin D2 and 25(OH)vitamin D3. Total intact PTH was assessed at Columbia University using the 115 immunoradiometric assay from Scanti-bodies (3KG600). Serum cystatin C levels were determined using a BN100 nephelometer (Dade Behring Inc., Deerfield, IL, USA) using a particle-enhanced immunonepholometric assay.(22) Serum creatinine, calcium, and phosphate concentrations were measured using a Roche COBAS Integra 800 analyzer (Roche Diagnostic Corporation, Indianapolis, IN, USA) at the Portland VA Medical Center using an enzymatic method calibrated with materials assayed by isotope-dilution mass spectrometry (IDMS). A combined formula using standardized cystatin C and creatinine-based estimated glomerular filtration rate (eGFRcysC) was computed using a CKD-EPI creatinine-cystatin C equation given that this was shown to be the most accurate estimate of renal function in a recent publication.(23-25)
UA assays
UA assays were conducted in the clinical pathology laboratory at University of California at Davis Medical Center using serum collected at the baseline visit. Samples were thawed and the assays performed on a UnicalDxC 800 auto-analyzer (Beckman Coulter, Fullerton, CA, USA). The interassay and intra-assay reproducibility of this measurement is greater than 99%. The UA assay has a detection limit of 0.01 mmol/L, reference range of 0.18 to 0.41 mmol/L, and a combined measurement of uncertainty of 1.1% at 0.18 and 0.44 mmol/L in the University of California at Davis Medical Center clinical laboratory.
Other measures
All covariates were assessed at baseline. Participants completed questionnaires to ascertain information on date of birth, race/ethnicity, self-rated health, self-reported doctor-diagnosed gout, fracture history, alcohol intake, physical-diagnosed gout, and history of falls. Physical performance was assessed using the Physical Activity Scale for the Elderly (PASE).(26) Physical function was assessed using walking speed (time in seconds to walk 6 m at usual pace expressed as m/s). BMD (g/cm2) of the total hip was measured using fan-beam dual-energy X-ray absorptiometry (DXA) (QDR 4500W, Hologic Inc., Bedford, MA, USA).(18,27,28) Height (cm) was measured on Harpenden stadiometers, and weight (kg) was measured on standard balance beam or digital scales using standard protocols, with participants wearing light clothing without shoes. Body mass index (BMI) was calculated as kg/m2.
Frailty status was defined using a slightly modified criteria proposed by Fried and colleagues using data collected in the Cardiovascular Health Study.(29,30) Frailty was identified by the presence of three or more of the following five components: 1) shrinking/sarcopenia defined by appendicular lean mass (adjusted for height and total body fat) in lowest quintile; 2) weakness defined by grip strength in lowest quintile stratified by BMI quartile; 3) exhaustion defined by an answer of “a little or none” to the question “How much of the time during the past 4 weeks did you have a lot of energy?” from the Medical Outcomes Study SF-12;(31) 4) slowness defined by a walking speed in lowest quintile stratified by standing height (median); 5) low physical activity level defined by a PASE score in lowest quintile.(26)
A modified Block Food Frequency questionnaire was administered to assess usual dietary and supplement intake over the past year (Block Dietary Data Services, Berkeley, CA, USA). Vitamin D and calcium intake were examined in these analyses. Values for participants who reported a total of <400 kcal per day were recorded as missing. At baseline, participants were asked to bring in all medications used within the last 30 days. All prescription medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA, USA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA, USA).
Statistical methods
Baseline characteristics were compared in the subcohort across quartiles of UA using chi-square tests for categorical variables, ANOVA for normally distributed continuous variables, and Wilcoxon nonparametric tests for nonparametric distributed covariates. Baseline characteristics were also compared between fracture cases and nonfracture cases (data not shown). Baseline characteristics that were associated with nonspine fracture and UA at p < 0.1 were identified as confounders. Other covariates known to be confounders from the literature were also selected such as physical activity, vitamin D, and PTH. Associations were first examined in our base model, which included adjustment for age, clinic, race, and BMI and total hip BMD in fracture models. Models were further adjusted for vitamin D, PTH, walking speed, PASE score, and frailty. To determine if the association between UA and fractures and hip BMD was independent of renal function, models were then further adjusted for eGFR.
Associations between UA and BMD levels were assessed in the random cohort by generalized linear models. Adjusted least square means of total hip BMD across quartiles of UA and p for linear trend by expressing quartiles of UA as an ordinal variable were calculated.
Hazard ratios (HR) and 95% confidence intervals were calculated from the Cox proportional hazards models modified for case-cohort analysis to test the association of nonspine fractures and UA across quartiles and per standard deviation increase in UA. Serum UA levels were divided into quartile categories defined on the basis of the distribution in the random cohort. The lowest quartile formed the referent group. Similar models were performed for the association of hip fractures and UA.
Results
The mean age was 73 years and 91% of the subjects were white. BMI and femoral neck BMD were higher in men with high levels of UA (Table 1, p < 0.001) compared with lower UA levels. Frailty status and walking speed were significantly associated with UA, although the directions of associations appeared nonlinear across quartiles. There were no significant differences in 25(OH) vitamin D, phosphorus levels, PASE score, prevalent fractures, or history of falls across the quartiles of UA. However, serum creatinine, cystatin C, and PTH were higher and eGFR was lower in the men in the highest UA quartile compared with those in the other quartiles of UA (p < 0.001). In addition, men in the highest quartile of UA were more likely to use thiazide, diuretics, and oral or inhaled corticosteroids compared with men with lower UA levels. There were no significant differences in smoking, alcohol drinks per week, history of falls, or bisphosphonate use across UA quartiles (data not shown in Table 1). Men with nonspine fracture were older, mostly white, were more likely to have a history of fracture after age 50 years and falls, had slower walking speed, were more frail, and had less alcoholic drinks per week compared with those who did not fracture (data not shown). In addition, men with nonspine fractures had lower hip BMD and higher serum creatinine and phosphorous levels than those who did not fracture (data not shown). There were no significant differences in 25(OH) vitamin D, serum calcium, and eGFR between nonspine fracture cases and non-cases.
Table 1.
Baseline Characteristics Across Quartiles of Uric Acid (mg/dL) in the Random Cohort
| Q1: 2.6 to 5.0 |
Q2: 5.1 to 5.8 |
Q3: 5.9 to 6.6 |
Q4: 6.7 to 13.6 |
||
|---|---|---|---|---|---|
| Characteristics | (n = 297) | (n = 379) | (n = 337) | (n = 370) | p Value |
| Mean ± SD | |||||
| Age (years) | 73.8 ± 5.8 | 73.7 ± 5.6 | 73.6 ± 6 | 74.1 ± 6.1 | 0.598 |
| BMI (kg/m2) | 26.7 ± 3.6 | 27.3 ± 3.6 | 27.1 ± 3.3 | 28.3 ± 4.0 | <0.0001 |
| Total hip BMD (g/cm2) | 0.929 ± 0.146 | 0.943 ± 0.139 | 0.948 ± 0.134 | 0.981 ± 0.144 | 0.0003 |
| Uric acid mg/dL | 4.4 ± 0.5 | 5.5 ± 0.2 | 6.2 ± 0.2 | 7.6 ± 1.0 | <0.0001 |
| 25(OH) total vitamin D (ng/mL) | 25.5 ± 7.6 | 25.1 ± 8.1 | 25.5 ± 7.6 | 24.8 ± 8.6 | 0.590 |
| Total intact PTH (pg/mL) | 29.1 ± 12.3 | 30.7 ± 11.7 | 32.7 ± 13.2 | 40.9 ± 46.1 | <0.0001 |
| eGFR (mL/min/1.73m2) | 81.3 ± 13.9 | 76.8 ± 14.2 | 75.6 ± 14.3 | 64.1 ± 17.9 | <0.0001 |
| Serum phosphate (mg/dL) | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.5 | 0.228 |
| Creatinine (mg/dL) | 9.3 ± 0.3 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.4 ± 0.4 | 0.0022 |
| Serum calcium (mg/dL) | 0.9 ± 0.2 | 1.0 ± 0.2 | 1 ± 0.2 | 1.2 ± 0.4 | <0.0001 |
| Serum cystatin C (mg/L) | 0.86 ± 0.18 | 0.91 ± 0.18 | 0.91 ± 0.18 | 1.09 ± 0.33 | <0.0001 |
| PASE score | 151.4 ± 70.8 | 149.1 ± 68.1 | 149.4 ± 67.3 | 139.8 ± 69.6 | 0.1117 |
| 6-Meter usual pace (m/sec) | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | 0.0008 |
| Vitamin D supplements (IU) | 425.2 ± 256.7 | 396.2 ± 244.25 | 389.2 ± 249.4 | 344.3 ± 238.5 | 0.0003 |
| Calcium supplements (IU) | 1206.9 ± 634.3 | 1201.5 ± 592.4 | 1159.5 ± 587.5 | 1035.7 ± 568.0 | 0.0001 |
| White, n (%) | 269 (90.6) | 352 (92.9) | 313 (92.9) | 324 (87.6) | 0.0378 |
| Self-reported health status, n (%) | 256 (86.2) | 329 (86.8) | 296 (87.8) | 293 (79.4) | 0.006 |
| History of gout, n (%) | 13 (4.4) | 31 (8.2) | 13 (3.7) | 50 (13.5) | <0.0001 |
| History of fracture after age 50 years, n (%) | 83 (28.1) | 76 (20.1) | 75 (22.3) | 86 (23.3) | 0.2915 |
| Thiazide use, n (%) | 16 (5.5) | 30 (8.3) | 44 (14.0) | 89 (24.9) | <0.0001 |
| Loop diuretic use, n (%) | 7 (2.4) | 6 (1.67) | 15 (4.8) | 39 (10.9) | <0.0001 |
| Frail status | 0.0126 | ||||
| Robust, n (%) | 133 (44.8) | 169 (44.6) | 171 (50.7) | 148 (40) | |
| Pre-frail, n (%) | 137 (46.1) | 185 (48.8) | 143 (42.3) | 175 (47.3) | |
| Frail, n (%) | 27 (9.1) | 25 (6.6) | 23 (6.8) | 47 (12.7) | |
Association of total hip BMD and UA
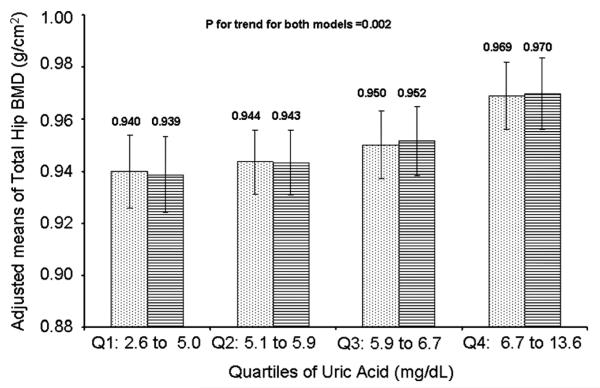
The mean total hip BMD in the random cohort was 0.95 g/cm2 (SD 0.14). Total hip BMD increased linearly across quartiles of UA after multivariate adjustment for age, race, clinic status, BMI, walking speed, vitamin D, PTH, fracture history, and frailty (p for trend = 0.002). The linear trend remained after adjustment for eGFR (p for trend = 0.0023) (Fig. 2).
Fig. 2.
Adjusted means and 95% CI for total hip BMD across quartiles of uric acid (mg/dL). Dotted bars = models adjusted for age, race, clinic site, BMI, vitamin D, PTH, walking speed, frailty status, and PASE score. Dashed bars = models adjusted for age, race, clinic site, BMI, vitamin D, PTH, walking speed, frailty status, PASE score, and eGFR.
Association of fracture and uric acid
The mean serum UA in the study population was 6.0 pg/dL (range 2.6–13.59 pg/dL). There was an 18% decrease in nonspine fracture risk per SD increase in UA (hazard ratio [HR] = 0.82, 95% confidence interval [CI] 0.72–0.93) in base models adjusted for age, race, BMI, clinic status, and total hip BMD (Table 2). Results remained significant after further adjustments for vitamin D, PTH, walking speed, PASE score, and frailty (HR = 0.81, 95% CI 0.71–0.93). A graded association with nonspine fractures and increase in UA quartiles was observed (p for trend in MV models = 0.003) (Table 2). Men in the highest quartile of UA had a significantly lower risk of nonspine fracture compared with men in Q1 (HR = 0.59, 95% CI 0.41–0.84). Results were slightly attenuated but remained significant when models were further adjusted for eGFR (HR for Q4 versus Q1 = 0.61, 95% CI 0.41–0.91, p for trend 0.014) (Table 2).
Table 2.
Association of Fractures With Uric Acid
| Nonspine fractures |
Hip fractures |
|||||
|---|---|---|---|---|---|---|
| Uric acid (mg/dL) | Base model1 | MV2 | MV + eGFR | Base model | MV | MV + eGFR |
| Per SD increase | 0.82 (0.72–0.93) | 0.81 (0.71–0.93) | 0.82 (0.7–0.95) | 0.79 (0.57–1.09) | 0.78 (0.56–1.09) | 0.83 (0.56–1.24) |
| Quartiles of uric acid | ||||||
| Q1: 2.6 to 5.0 (ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 5.1 to 5.8 | 0.85 (0.62–1.16) | 0.88 (0.64–1.21) | 0.88 (0.63–1.21) | 0.93 (0.46–1.86) | 0.99 (0.49–2.00) | 0.97 (0.47–2.01) |
| Q3: 5.9 to 6.6 | 0.78 (0.56–1.08) | 0.81 (0.58–1.13) | 0.82 (0.59–1.14) | 0.92 (0.47–1.81) | 0.97 (0.49–1.93) | 1.01 (0.51–1.99) |
| Q4: 6.7 to 13.6 | 0.60 (0.43–0.85) | 0.59 (0.41–0.84) | 0.61 (0.41–0.91) | 0.48 (0.21–1.09) | 0.44 (0.18–1.06) | 0.49 (0.18–1.33) |
| p for trend | 0.0038 | 0.0032 | 0.014 | 0.0883 | 0.069 | 0.213 |
| Q4 versus Q1, Q2, Q3 | 0.69 (0.52–0.93) | 0.66 (0.49–0.89) | 0.69 (0.49–0.96) | 0.5 (0.25–1.03) | 0.45 (0.21–0.97) | 0.49 (0.21–1.18) |
eGFR = estimated glomerular filtration rate.
Base model adjusted for age, race, clinic site, BMI, and total hip BMD for fracture models.
MV (multivariate) model adjusted for covariates in base model and vitamin D, PTH, PASE score, walking speed, and frailty status.
There was a significant association of hip fracture in men with the highest UA compared with men with lower UA levels (HR for Q4 versus Q1, 2, 3 = 0.45, 95% CI 0.21–0.97) in multivariable models. However, this association was attenuated after adjustment for eGFR (HR Q4 versus Q1, 2, 3 = 0.49, 95% CI 0.21–1.18) (Table 2).
Exclusion of men using allopurinol medication or having gout disease
After excluding 144 men with gout disease and/or allopurinol medication use, there was no significant change in the association between nonspine fractures or hip fractures and UA levels. Similarly, there was no difference in the association between total hip BMD and nonspine or hip fractures after exclusion of 108 men in the random cohort with gout disease or using allopurinol medication.
Discussion
Serum UA is frequently measured in clinical medicine as a biochemical parameter of purine metabolism, and elevated levels are associated with gout (a crystalline arthritis), renal disease, and recently cardiovascular outcomes.(3-7) We have studied a large population of older mostly white men and found that elevated serum UA was associated with both increased BMD at the total hip and reduced risk of incident nonspine fractures. The results remained statistically significant even after adjustment for renal disease.
Our results are similar to those reported by Nabipour and colleagues,(2) who found higher serum UA levels defined as above the median values of 0.36 mmol/L were also associated with higher BMD at all skeletal sites evaluated. Also, serum UA values above the median level reduced the odds of prevalent vertebral and nonspine fractures by 35% to 49%. Although the serum UA accounted for 1.0% to 1.44% of the variance in the BMD with R2 values between 0.10 to 022, in multivariate regression models serum UA levels above the median reduced the odds of osteoporosis defined by T-score of ≤2.5 at the lumbar spine to 0.44, and for the femoral neck to 0.42. The characteristics of the CHAMP cohort of elderly men that was studied is quite similar to the MrOS cohort including the range of the age of the participants (73 to 76 years), total hip BMD, eGFR, average serum UA levels, and PASE levels.(2)
The association of serum UA and BMD has also been observed in women. Markovey and colleagues performed both cross-sectional and longitudinal analyses of serum UA and bone density and bone loss in peri- and postmenopausal women.(15) The cross-sectional analyses revealed nearly a 6% difference from quartile 4 to quartile 1 for lumbar spine BMD, 2% for femoral neck, 2% total hip (p < 0.01) and 1% for total body BMD (p < 0.05).(15) A multiple regression analysis was performed to adjust for the differences in body weight across the quartiles and the results still remained significant. In addition, higher serum UA levels were associated with less annual loss of BMD at the lumbar spine, forearm, and total body but not at the hip. The observed reduction in protection of hip BMD by serum UA over time may be explained by the hip BMD influence of both body composition and weight-bearing activities.(15) However, the lumbar spine differs from the hip in that it contains a significantly higher percentage of the more metabolically active trabecular bone so that it may be more affected by elevation of serum UA.
Recently, Ahn and colleagues reported that serum UA was positively correlated with bone mass at the lumbar spine and at the hip in a cohort of healthy postmenopausal women.(12) They also found serum UA was inversely related to serum C-terminal telopeptide of type I collagen and osteocalcin levels. The subjects with prevalent vertebral fractures had significantly lower serum UA compared with subjects without fractures. The investigators performed in vitro studies of osteoclastogenesis and reported that exposure to increasing doses of uric acid decreased osteoclastogenesis by reducing reactive oxygen species production. These investigators confirmed the strong association with serum UA and bone mass and bone strength and further investigated the mechanism for this association by concluding that the mechanism appears to be through reduction in number and activity of osteoclasts that resorb bone. Additional work to further characterize this observation is warranted.(12)
Patients with elevated serum UA levels frequently have reduced renal function. The reduction in renal function alters the conversion of 25 vitamin D to 1,25 vitamin D as the 1 hydroxylase enzyme levels are reduced, and this can lead to an increase in serum PTH. In our study, increasing levels of serum UA was significantly associated with increasing serum creatinine and PTH levels and decreasing levels of eGFR. There was a nonlinear association with increasing UA levels and calcium. Interestingly, Nabipour and colleagues(2) observed that serum UA was positively correlated with serum PTH and calcium concentrations, and Yoneda and Valdemarsson observed that patients with hyperparathyroidism had higher serum UA and lower UA clearance that subjects with idiopathic hypoparathyroidism; however, the serum UA returned to normal with successful parathyroidectomy.(32,33) Serum UA has been reported to increase in osteoporotic patients with daily injections of recombinant human PTH (1-34).(34) These results suggest that renal handling of UA is somewhat dependent on serum PTH values. However, the association between UA with fracture and BMD was not explained by PTH levels in our analysis.
The mechanism for the association between serum UA and BMD is not clear. UA is the final breakdown product of purine metabolism and it crystalline state has inflammatory properties,(2,15) whereas the soluble form within the normal physiologic levels may have antioxidant properties.(8,15,31-33) UA accounts for nearly half of the antioxidant properties in the circulation,(3) and studies have associated oxidative stress or low serum levels of antioxidants to reduced BMD and osteoporosis.(12-15) Investigators have hypothesized that the antioxidant effects of elevated UA might protect against oxidative stress, such as oxidative injury to cardiac, vascular, and neural cells.(35-37) Support for these hypotheses has come from studies that show elevated serum UA levels protect against the development of Parkinson’s disease.(10,11) UA has a strong antioxidant effect at physiologic concentrations, and oxidative stress has been proposed as amajor predictor of Parkinson’s disease.(10,11) An in vitro study demonstrated that administration of UA reduced the adverse effects of homocysteine, whereas pesticides increased oxidative stress, mitochondrial dysfunction, and apoptosis on human dopaminergic cells.(38) However, it may be the precursors of serum UA that could be responsible for the protective associations including a nucleotide, inosine, that is deribosylated and oxidized to urate and has been demonstrated to have neuroprotective actions in patients with both stroke and multiple sclerosis.(10,39–41)
However, elevated serum UA has also been associated with endothelial dysfunction, and UA-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP that may contribute to the pathogenesis of hypertension and vascular disease. It is possible that a mitochondrial dysfunction may be along the causal pathway of UA and diabetes mellitus.(42)
This study has a number of strengths including a well-characterized longitudinal cohort of elderly men with information on both risk factors and central validation of fractures; more than 97% of the enrolled subjects have complete follow-up. Also, our cohort of men was recruited from the community and not clinical practices such that the sample was not selected for comorbid diseases that would influence serum UA levels. However, there are a few shortcomings that include a single measure of serum UA levels at the baseline visit; the potential effects of change on UA levels on fracture risk were not examined. In addition, we performed our analysis on baseline serum that had been thawed and then refrozen, and this may have influenced our results. However, we utilized a standard clinical laboratory-based assay, and the mean values of serum UA were within the normal clinical range. Also, we only report results on older men; therefore, these results are not generalizable to older women or younger individuals.
In conclusion, in this large community-based cohort of elderly men, serum UA levels within the physiologic range were significantly associated with a reduction in incident nonspine fractures and increased BMD. These data confirm other reports that serum UA protects bone mass older adults. Based on these data, we hypothesize that serum UA may have an antioxidant activity that may prevent the loss of bone mass and nonspine fractures. Additional studies to understand the mechanism for the protective effects of UA on bone mass and strength are warranted.
Acknowledgments
This work was supported by grants K24-AR04884 and R01AR052000 MrOS Genetics (NEL) and other grants for MrOS. The Osteoporotic Fractures in Men Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, UL1 TR000128, and R01AR052000.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75:S5–8. doi: 10.3949/ccjm.75.suppl_5.s5. [DOI] [PubMed] [Google Scholar]
- 2.Nabipour I, Sambrook PN, Blyth FM, et al. Serum uric acid is associated with bone health in older men: a cross-sectional population based study. J Bone Miner Res. 2011;26(5):955–64. doi: 10.1002/jbmr.286. [DOI] [PubMed] [Google Scholar]
- 3.Edwards NL. The role of hyperuricemia in vascular disorders. Curr Opin Rheumatol. 2009;21:132–7. doi: 10.1097/BOR.0b013e3283257b96. [DOI] [PubMed] [Google Scholar]
- 4.Holme I, Aasteveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke, and congestive heart failure in 417,734 men and women in the Apolipoprotein Mortality Risk study (AMORIS) J Intern Med. 2009;266:558–60. doi: 10.1111/j.1365-2796.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Guevera JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:885–92. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31:361–2. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 7.Oda E, Kawai R, Sukumaran V, Watanabe K. Uric acid is positively associated with metabolic syndrome but negatively associated with diabetes in Japanese men. Intern Med. 2009;48:1785–91. doi: 10.2169/internalmedicine.48.2426. [DOI] [PubMed] [Google Scholar]
- 8.Glantzounic GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:724–8. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 9.Ames BN, Cathart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso A, Garcia-Rodriguez LA, Logroscino G, Herman MA. Gout and risk of Parkinson’s disease: a cohort study. Neurology. 2007;69:1696–700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson’s disease. Am J Epidemiol. 2009;169:1064–9. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn SH, Lee SH, Kim B-J, et al. Higher serum uric acid is associated with higher bone mass, lower bone turnover and lower prevalence of vertebral fracture in health postmenopausal women. Osteoporos Int. 2013;24:2961–70. doi: 10.1007/s00198-013-2377-7. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura M, Nakamura M, Ogawa K, Iloma Y, Ando F, Yano M. Bone mineral density in post-menopausal female subjects is associated with serum antioxidants carotenoids. Osteoporos Int. 2008;19:211–9. doi: 10.1007/s00198-007-0457-2. [DOI] [PubMed] [Google Scholar]
- 14.Sritara C, Ongphiphadhanakui B, Chailurkit L, Yamwong S, Ratanachaiwong W, Sritara P. Serum uric acid levels in relation to bone related phenotypes in men and women. J Clin Densitom. 2013;16:336–40. doi: 10.1016/j.jocd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Makovey J, Macara M, Chen JS, et al. Serum uric acid plays a protective role for bone loss in peri and postmenopausal women: a longitudinal study. Bone. 2013;52:400–6. doi: 10.1016/j.bone.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women; results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–7. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 17.Sendur OF, Turan Y, Tastaban E, Serter M. Antioxidant status in patients with osteoporosis: a controlled study. Joint Bone Spine. 2009;76:514–8. doi: 10.1016/j.jbspin.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of nonspine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–9. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 21.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. JCEM. 2006;91(8):3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 22.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–4. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;5(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–40. [PubMed] [Google Scholar]
- 27.Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-hydroxyvitamin D levels,rate of hip bone loss in older men. JCEM. 2009;94:2773–80. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CG, Boyko EJ, Nielson CM, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–40. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 30.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–23. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Kosinski M, Keller SD. SF-12: How to score the SF-12 physical and mental health summary scores. 3rd ed. QualityMetric, Inc; Lincoln, RI: 1998. [Google Scholar]
- 32.Yoneda M, Takatsuki K, Tomita A. Parathyroid function and uric acid metabolism. Nippon Naibunpi Gakkai Zasshi. 1983;59:1738–51. doi: 10.1507/endocrine1927.59.11_1738. [DOI] [PubMed] [Google Scholar]
- 33.Valdemarsson S, Lindblom P, Bergenfelz A. Metabolic abnormalities related to cardiovascular risk in primary hyperparathyroidism: effects of surgical treatment. J Intern Med. 1998;244:241–9. doi: 10.1046/j.1365-2796.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 34.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 35.Masseoud D, Rottt K, Liu-Bryan R, Agudelo C. Overview of hyperuricemia and gout. Curr Pharm Des. 2005;11:4117–24. doi: 10.2174/138161205774913318. [DOI] [PubMed] [Google Scholar]
- 36.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–51. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 37.Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392:1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80:101–10. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 39.Spitsin S, Hooper DC, Leist T, et al. Inactivation of peroxynitrite in multiple sclerosis patients after oral administration of inosine may suggest possible approaches to therapy of the disease. Mult Scler. 2001;7:313–9. doi: 10.1177/135245850100700507. [DOI] [PubMed] [Google Scholar]
- 40.Scott GS, Spitsin SV, Kean RB, et al. Therapeutic intervention in experimental allergic encephalitis by administration of uric acid precursors. Proc Natl Acad Sci USA. 2002;99:16303–8. doi: 10.1073/pnas.212645999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen H, Chen GJ, Harvey BK, et al. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–9. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121(3-4):71–8. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]