Abstract
Purpose
Vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) play a significant role in glioblastoma angiogenesis and proliferation making tyrosine kinase (TK) receptors logical targets for treatment. We evaluated AEE788, a reversible TK inhibitor that inhibits EGFR and VEGFR, in recurrent glioblastoma patients.
Methods
In this dose-escalation, phase-I study, patients with recurrent glioblastoma received AEE788 once daily in 28-day cycles in stratified subgroups: those receiving 1) non–enzyme-inducing anticonvulsants drugs or no anticonvulsants (Group A) and 2) enzyme-inducing anticonvulsant drugs (EIACDs) (Group B). A dose-expansion phase stratified patients by surgical eligibility. Primary objectives were to determine dose limiting toxicity (DLT) and maximum tolerated dose (MTD); secondary objectives included evaluating 1) safety/tolerability, 2) pharmacokinetics, and 3) preliminary antitumor activity.
Results
Sixty-four glioblastoma patients were enrolled. Two Group A patients experienced DLTs (proteinuria and stomatitis) at 550 mg; 550 mg was, therefore, the highest dose evaluated and dose limiting. One Group B patient receiving 800 mg experienced a DLT (diarrhea). The initially recommended dose for dose-expansion phase for Group A was 400 mg; additional patients received 250 mg to assess hepatotoxicity. Most frequently reported adverse events (AEs) included diarrhea and rash. Serious AEs, most commonly grade 3/4 liver function test elevations, were responsible for treatment discontinuation in 17% of patients. AEE788 concentrations were reduced by EIACD. The best overall response was stable disease (17%).
Conclusions
Continuous, once-daily AEE788 was associated with unacceptable toxicity and minimal activity for the treatment of recurrent glioblastoma. The study was, therefore, discontinued prematurely.
Introduction
Glioblastoma, the most common primary malignant brain tumor in adults, is associated with a high degree of morbidity and mortality. The median survival time from diagnosis is approximately 1 year, even in patients who undergo aggressive treatment.1,2 For patients with recurrent glioblastoma, salvage therapies have been of limited value historically. However, recent studies have shown that therapies targeting vascular endothelial growth factor (VEGF), or its cognate receptor (VEGFR), can achieve durable antitumor benefit in some patients with recurrent malignant glioma.3–8 Based on these findings, the Food and Drug Administration (FDA) recently granted bevacizumab, a humanized monoclonal antibody against VEGF, accelerated approval for patients with recurrent glioblastoma based on durable radiographic response.6,7,9 Compared with historical benchmarks, however, only modest improvements in OS were noted in these studies. Nonetheless, rationally designed combinatorial strategies may further enhance the antitumor benefit of VEGF/VEGFR-targeted therapeutics and show an improvement in OS.3–7
Results of several genomic studies have enhanced the characterization of the complex molecular composition of glioblastoma tumors.10–13 In particular, ErbB tyrosine kinase (TK) receptors, such as epidermal growth factor receptor (EGFR), have been shown to be significantly upregulated in most glioblastoma tumors and play a significant role in glioblastoma tumor survival, proliferation, and angiogenesis. Additionally, EGFR gene amplification occurs in approximately 40% of glioblastoma tumors.14–17 Indeed, 50% of tumors with the amplified EGFR gene undergo intragene rearrangements responsible for an overexpression of mutant EGFR receptors (ie, EGFRvIII), which demonstrate constitutive TK activity.18–20 Based on these findings, several studies have evaluated inhibition of EGFR activity as a treatment modality for glioblastoma. The results of studies evaluating EGFR-targeted therapies (eg, erlotinib, gefitinib), however, have demonstrated minimal and/or mixed efficacy results in glioblastoma patients, most likely because of various factors, such as the ability of glioma cells to develop compensatory mechanisms through other uninhibited pathways.20–22 More effective therapies may, therefore, be those that target several pathways. In preclinical glioblastoma models, combined targeting of the EGFR and VEGF pathways has demonstrated significant antitumor activity.23 AEE788, an orally active TK inhibitor (TKI), potently inhibits EGFR/ErbB-1 and HER-2/neu (ErbB-2) as well as the VEGF receptor KDR (VEGFR-2) making it a logical potential treatment for glioblastoma.24,25
This 2-arm, multicenter, dose-escalation, phase I study evaluated the safety, tolerability, pharmacokinetics (PK), and preliminary antitumor activity of AEE788 in adults with recurrent or relapsed glioblastoma. To determine the effects of cytochrome P450 (CYP 450) enzyme inducers on the PKs of AEE788, patients were stratified into those receiving nonenzyme (cytochrome 3A4 [CYP3A4])-inducing anticonvulsants drugs (nonEIACDs) or no anticonvulsants drugs (ACDs) and those receiving enzyme (CYP3A4)-inducing anticonvulsants drugs (EIACDs) (eg, phenytoin, phenobarbitol, carbamazepine, oxcarbazepine, primidone).
Materials and Methods
Study Objectives
The primary objectives of the study were to assess dose-limiting toxicity (DLT) and to determine the maximum tolerated dose (MTD) of continuous, once-daily oral AEE788 as a single agent in patients with recurrent or relapsed glioblastoma who were receiving either non-EIACDs or no ACDs (dose-escalation Group A) or EIACDs (dose-escalation Group B). Secondary objectives included determining the safety, tolerability, and PK profiles of AEE788 and evaluating preliminary efficacy of AEE788 in patients with recurrent glioblastoma.
Patient Eligibility
The study enrolled adults (≥ 18 yr of age) with histologically confirmed glioblastoma who were experiencing a first or second recurrence or relapse and had at least one measurable or evaluable enhancing lesion on baseline gadolinium-magnetic resonance imaging (Gd-MRI) (standard brain magnetic resonance imaging that included precontrast and postcontrast images; postcontrast images obtained nondynamically using gadolinium chelate as contrast agent) performed within 3 weeks of study entry. Table 1 outlines additional eligibility criteria. Inclusion criteria: a Karnofsky Performance Status ≥ 70; life expectancy ≥ 12 weeks; an absolute neutrophil count ≥ 1.5 × 109/L; hemoglobin level ≥ 9 g/dL, platelet count ≥ 100 × 109/L; serum bilirubin level ≤ 1.5 x upper limit of normal (ULN); serum creatinine level ≤ 1.5 x ULN (or 24-hour creatinine clearance ≥ 50 mL/min/1.73m2); and potassium, magnesium, calcium, phosphorus all within normal limits. In the dose expansion phase, patients treated with 250 mg were required to have normal liver function tests. Patients with a history of polifeprosan with carmustine intracranial wafer implantation were eligible at the discretion of the investigator and study sponsor.
Table 1.
Study Arms and AEE788 Dosing Schedule
| Study Group or Arm | Eligibility Criteria | AEE788 Schedule | No. of Patients Enrolled/MTD Evaluable |
|---|---|---|---|
| Dose-escalation phase | |||
| Group A | Patients receiving non-EIACD or no ACD | Continuous, once daily |
Total: 26/23 50 mg: 2/2 100 mg: 6/6 200 mg: 1/1 400 mg: 3/3 450 mg: 6/5 550 mg: 8/6 |
| Group B | Patients receiving EIACD | Continuous, once daily |
Total: 14/13 300 mg: 2/2 600 mg: 6/5 800 mg: 6/6 |
| Dose-expansion phase | Dose Received: No. of Patients | ||
| Arm 1 | Patients at 1st or 2nd recurrence or relapse and eligible for surgerya | Group A (Patients receiving non-EIACD or no ACD): AEE788 x 5–9 consecutive days before surgery; then, once daily starting within 15–21 d postoperatively | 250 mg: 6b |
| Group B (Patients receiving EIACD): Once daily beginning 15–21 d postoperatively | 600 mg: 1 | ||
| Arm 2 | Patients at 1st recurrence or relapse with measurable disease and ineligible for surgery | Groups Ad and Bf: continuous, once daily | 250 mg: 12 (Group A) 400 mg: 4 (Group A) 1 Group-B patient/600 mg |
Tumor biopsy or surgical resection to confirm recurrence or debulk tumor.
Pharmacokinetic data was available for only 4/6 patients.
ACD, anticonvulsant drug; EIACD, enzyme-inducing anticonvulsant drug; Gd-MRI, gadolinium-magnetic resonance imaging; nonEIACD, nonenzyme-inducing anticonvulsant drug.
Exclusion criteria included the presence of greater than grade 1 peripheral neuropathy or unresolved diarrhea, impaired cardiac function or other significant cardiovascular disease (eg, uncontrolled hypertension, recent history of myocardial infarction), uncontrolled diabetes, an active or uncontrolled infection (including human immunodeficiency virus), a gastrointestinal condition or disease that could alter the absorption of AEE788, or another active malignancy. Patients were excluded if they had received any of the following: hematopoietic colony-stimulating factor or immunotherapy ≤ 2 weeks before study entry; chemotherapy, investigational drugs, or radiation therapy ≤ 4 weeks before study entry; prior EGFR/ErbB-2- or VEGF/VEGFR-directed therapies. Patients receiving warfarin, digoxin for congestive heart failure, or verapamil for cardiac arrhythmias were also excluded as were patients who had undergone surgery within 2 weeks of enrollment (1 week for stereotactic biopsy). Pregnant and breastfeeding women or adults with reproductive potential who did not employ effective birth control were also deemed ineligible. All study participants provided informed consent before study entry.
Study Design
The dose was escalated according to a modified accelerated titration design for phase I studies described by Simon and colleagues26, which includes single-patient cohorts treated at each dose level until the second occurrence of a National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (version 3.0) grade 2 toxicity or first occurrence of a ≥ CTCAE grade 3 toxicity in cycle 1 where 3–6 patients will be enrolled in all subsequent cohorts. Although there was no ≥ CTCAE grade 2 toxicity up to 200 mg, the 100 mg cohort was expanded after 1 patient reported grade 4 transaminases elevation outside of the DLT observation period (28 days after the first AEE788 dose). The MTD was defined as the dose at which 0 or 1 out of 6 patients experienced DLT, with at least 2 patients experiencing DLT at the next higher dose. DLT was defined 1) as an AE or abnormal laboratory value unrelated to disease progression, intercurrent illness, or concomitant medications that occurred during the first 28 days after the first dose of AEE788 during cycle 1, and 2) according to other predetermined criteria (Table 2). If no DLT occurred during a cycle, the patient continued treatment with AEE788 at the same dose as the previous cycle, unless the criteria for AEE788 dose interruption or modification were met.
Table 2.
Dose-Limiting Toxicity Criteria/Definition
| Toxicity | Criteria |
|---|---|
| Hematologic | ≥ CTCAE grade-3 neutropenia: ANC (including bands) < 1.0 × 109/L |
| ≥ CTCAE grade-3 thrombocytopenia: platelets < 50 × 109/L | |
| Neutropenic fever: ANC (including bands) < 1.0 × 109/L, fever ≥ 38.5°C | |
| Renal | ≥ CTCAE grade-2 proteinuria |
| ≥ CTCAE grade-2 hematuria | |
| Serum creatinine ≥ 2.0 x ULN | |
| Hepatic | CTCAE grade-3 AST/SGOT or ALT/SGPT for > 7 d |
| CTCAE grade-4 AST/SGOT or ALT/SGPT | |
| Total bilirubin ≥ 2 x ULN | |
| Cardiac | |
| Hypertension | CTCAE grade 4 (hypertensive crisis) |
| CTCAE grade 2 or 3 (only if diastolic blood pressure does not stabilize to within 20 mmHg [or clinically acceptable range for that patient] of pretreatment [baseline] diastolic blood pressure, despite concomitant antihypertensive treatment for ≤ 7 d) | |
| Other | ≥ CTCAE grade 3 |
| Neurotoxicity | > 1 CTCAE grade-level increase |
| Skin | Any skin toxicity requiring interruption of AEE788 for > 7 d |
| Other | ≥ CTCAE grade-3 AEs (excluding ≥ CTCAE grade-3 elevation in alkaline phosphatase) requiring interruption of AEE788 for > 7 d |
| ≥ CTCAE grade-3 vomiting or nausea despite antiemetic use | |
| ≥ CTCAE grade-3 diarrhea despite optimal antidiarrheal treatment | |
AEs, adverse events; ALT/SGPT, alanine aminotransferase/serum glutamic-pyruvic transaminase; ANC, absolute neutrophil count; AST/SGOT, aspartate aminotransferase/serum glutamic-oxaloacetic transaminase; CTCAE, Common Toxicity Criteria for Adverse Events; ULN, upper limit of normal.
Based on the results of other phase I AEE788 studies, the starting dose of AEE788 for Group A patients was 50 mg.27,28 Because Group B patients were receiving anticonvulsants, which are expected to lower the exposure of AEE788 due to CYP 450-enzyme induction, enrollment for Group B began with the second occurrence of a CTCAE grade 2 or first occurrence of a ≥ CTCAE grade 3 toxicity in Group A. The starting dose for Group B, however, was dependent on the aggregate toxicities observed in Group A and was one dose level below the dose that induced the AE. Upon establishing an MTD in Groups A or B patients, 2 additional cohorts (arms 1 and 2) of glioblastoma patients were to be enrolled in the dose-expansion phase of the study to receive the MTD so that safety, tolerability, biologic activity, systemic and intratumoral PK, and pharmacodynamic activity of AEE788 could be further evaluated (see Table 1). Arm 1 included patients experiencing a first or second recurrence/relapse who were surgical candidates; patients experiencing their first relapse/recurrence who were ineligible for surgery were included in Arm 2. (Table 1)
Pharmacokinetics
Single and multiple-dose PKs (trough concentration, maximum concentration of drug [Cmax], and area under the curve [AUC]) were assessed in all patients who received at least one dose of AEE788. Plasma PK data were evaluated at all dose levels. AEE788 tumor levels (intratumoral PK) were assessed in Group-A, Arm-1 patients during dose-expansion.
During cycle 1, PK blood sampling was performed before therapy administration on days 1, 8, 15, 22, and 28; multiple postadministration samples were obtained on days 1 and 15 (at 1, 2, 3, 4, 7, 10, 24 hr) and day 28 (at 1, 3, 5, 9, 24 hr). Preadministration samples were obtained on day 15 during cycle 2 and day 1 during cycle 3. A PK sample was collected within 2 hours before surgery in the dose expansion Arm 1, Group-A patients who had been on AEE788 once daily for 5 to 9 consecutive days. Intratumoral PKs were determined using 60- to 70-mg tumor samples.
A validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay with a lower limit of quantification (LLOQ) of 0.5 ng/mL was used to assay serum samples for concentrations of AEE788 and its metabolite, AQM674. To determine intratumoral PK, tumor tissue samples were homogenized after they were mixed with phosphate buffered saline. The homogenate was extracted for the analyte(s), and the extract was analyzed using LC-MS/MS. The LLOQ for tissue samples was 5 ng/g.
Intratumoral Biomarker Analyses
Unstained slides were prepared from formalin-fixed paraffin-embedded archived tumor blocks collected at initial glioblastoma diagnosis or recurrence. Each slide was stained for EGFR, p-4EBP1, p-AKT, p-KDR, p-MAPK, p-p70S6K, p-S6, or PTEN using the method described by Simmons and colleagues.31 Slides were stained for EGFRvIII and p-EGFR only for EGFR-positive tumors. Samples were evaluated by immunohistochemistry (IHC). The IHC staining was scored on a scale from 0 to 200 according to the intensity of the stain and categorized by a 3-tiered system (tier 1: 0=negative staining; tier 2: > 0 and ≤ 100=light to light–moderate staining; tier 3: > 100=moderate-strong to strong staining). This exploratory analysis included at least 10 patients per group; Kaplan-Meier analyses were performed between tiers 1 and 2 versus tier 3 for p-4EBP1, p-AKT, p-KDR, p-MAPK, p-p70S6K, p-S6, and PTEN, and tier 1 versus tiers 2 and 3 for EGFR, p-EGFR and EGFRvIII. Degree of staining were grouped as negative to light-moderate staining (tiers 1 and 2) versus moderate-strong to strong (tier 3) for PTEN, p-AKT, p-S6, p-p70S6K, p-4EBP1, p-KDR and p-MAPK, and negative (tier 1) versus light-strong (tiers 2 and 3) for EGFR, p-EGFR and EGFRvIII.
Safety
Safety assessments were performed on: days 1, 2, 3, 8, 15, 22, and 28 during cycle 1; days 1, 8, 15, 22, and 28 during cycle 2; days 1, 15, and 28 during subsequent cycles; and at study completion. These evaluations included: monitoring AEs and serious AEs (SAEs) and hematology, blood chemistry, and urine values; measuring vital signs, weight, and performance status; performing a physical and neurologic examination; and/or assessing cardiac function. A chest radiograph was performed when clinically indicated.
AEs were graded according to the CTCAE version 3.0. If CTCAE grading did not exist for a particular AE, the AE was graded according to severity (mild [grade 1], moderate [grade 2], severe [grade 3], life threatening [grade 4]). Monitoring AEs continued for at least 28 days after the last dose of study drug was administered.
Efficacy
Evaluations based on the Macdonald32 criteria, including a neurologic examination and Gd-MRI to assess tumor response was used to evaluate efficacy at baseline, the end of cycle 1, the end of each subsequently even-numbered cycle, and at the end of study. Objective responses had to be confirmed on consecutive Gd-MRI scans performed at least 1 month apart. Patients without measurable disease were not evaluable for radiographic response.
Changes in uptake of the positron emission tomography (PET) probe [18F]-fluoro-L-thymidine (FLT) was used to assess drug-induced changes in tumor cell proliferation at clinical centers where FLT was available. FLT scans were performed in dose-escalation and dose-expansion Arm 2 patients at baseline, on cycle 1 day 15 (optional), and on cycle 1 day 28. Cycle 1 day 28 parameters for all images were quantitated and compared to their respective baseline measurement. Dose expansion Arm 1, Group A patients underwent FLT scans at baseline (≤ 4 days before first AEE788 dose) and day 1 (or within 48 hr) before surgery. Dynamic contrast-enhanced (DCE)-magnetic resonance imaging (MRI) and dynamic susceptibility contrast (DSC)-MRI was performed to assess antiangiogenic effects of the AEE788, and diffusion-weighted imaging (DWI)-MRI was performed to assess changes in tumor cellularity/necrosis in dose-expansion (Arm 2) patients.
Statistical Analyses
All patients who received at least one dose of AEE788 were included in the intent-to-treat (ITT) population. The safety population included all patients who received at least one dose of AEE788 and underwent at least one post-baseline safety assessment. The MTD population included all patients from the safety population who either met the minimum exposure criteria (ie, ≥ 75% planned cycle-1 doses) and underwent sufficient safety assessments (ie, received AEE788 for ≥ 21 days, was observed for ≥ 28 days after the first dose, and completed all safety evaluations) or discontinued the study before end of cycle 1 due to DLT. Sample size estimates relied on the Shuster minimax 2-stage design for Arm 1 (based on six month progression-free survival [PFS] rate) and the multinomal 2-stage design for Arm 2, (based on response rates [RRs] and PFS rate at 8 weeks) because these patients had measurable disease and, using an early-stopping design, may be useful to allow for other treatment options in patients who are not responding.29,30 Patients were followed for disease progression (ie, PFS) while on study only. Patients who did not have documented tumor progression before discontinuation had a censored PFS at the date of the last tumor assessment before study discontinuation. Descriptive statistics were used to summarize baseline patient characteristics and patient disposition. Safety data was summarized according to type and frequency of AE. Efficacy data (ie, RRs) were summarized according to treatment schedule and group; Kaplan-Meier estimates were performed to determine PFS and overall survival (OS) times. Summary statistics for the PK parameters AUC0–24, Cmax, time to maximum concentration (tmax), and accumulation index (RA) were calculated on days 1, 15, and 28 of cycle 1. PK parameters were determined using noncompartmental methods using WinNonlin Pro (Version 3.2).
Three exploratory analyses were performed on the intratumoral biomarker data: 1) Spearman rank correlation analyses were conducted to determine the correlation structure among p-KDR, p-S6, p-AKT, and PTEN and 6 other biomarkers (all as continuous variables); 2) univariate Kaplan-Meier analyses and log-rank tests were applied to each of the 10 biomarkers comparing IHC staining score ≤ 100 vs > 100 with respect to PFS; and 3) Cox proportional hazards models for PFS were applied to the 10 continuously measured biomarkers to get a parsimonious set of predictive biomarkers. Consistency of model selection was examined by using both stepwise and backward elimination variable selection methods. A 5% level of significance was used as the cutoff for selection. No adjustments were made for the multiple comparisons.
Results
Patient Characteristics
Between January 2004 and November 2005, 64 glioblastoma patients were enrolled in the study including 40 patients in the dose-escalation phase (26 patients, Group A; 14, Group B) and 24 in the dose-expansion phase (7, Arm 1; 17, Arm 2). The dose-escalation cohorts and the number of patients within each cohort are listed in Table 1. Table 3 presents baseline patient demographics and patient study disposition. All patients had PD after having undergone surgery and received radiation therapy and/or systemic chemotherapy before enrolling to the study.
Table 3.
Patient Demographics
| Variable | Group Aa n=48 |
Group Bb n=16 |
|---|---|---|
| Study Populations, n (%) | ||
| ITT | 48 (100) | 16 (100) |
| Safety | 48 (100) | 16 (100) |
| Dose escalation | 26 (54.2) | 14 (87.5) |
| MTD evaluation | 23 (47.9) | 13 (81.3) |
| Dose expansion | 22 (45.8) | 2 (12.5) |
| Gender, n (%) | ||
| Male | 28 (58.3) | 12 (75) |
| Female | 20 (41.7) | 4 (25) |
| Age (Yr) | ||
| Mean (SD) | 52.1 (11) | 48.3 (12.7) |
| Median (range) | 53 (24–70) | 48 (29–68) |
| Race, n (%) | ||
| Black | 3 (6.3) | 0 (0) |
| White | 41 (85.4) | 15 (93.8) |
| Asian | 0 (0) | 1 (6.3) |
| Other | 4 (8.3) | 0 (0) |
| KPS (%), n (%) | ||
| 100 | 5 (10.4) | 2 (12.5) |
| 90 | 21 (43.8) | 6 (37.5) |
| 80 | 14 (29.2) | 6 (37.5) |
| 70 | 7 (14.6) | 2 (12.5) |
| < 70 | 1 (2.1) | 0 (0) |
| Prior relapse, n (%) | ||
| 1st | 40 (83.3) | 7 (43.8) |
| 2nd | 8 (16.7) | 8 (50) |
| Prior treatment, n (%) | ||
| Surgery or biopsy | ||
| Yes | 48 (100)c | 16 (100) |
| No | 0 (0) | 0 (0) |
| Radiation therapy | ||
| Yes | 48 (100) | 16 (100) |
| No | 0 (0) | 0 (0) |
| Chemotherapy | ||
| Adjuvant | ||
| Yes | 39 (81.3) | 12 (75) |
| No | 9 (18.7) | 4 (25) |
| Therapeutic | ||
| Yes | 13 (27.1) | 9 (56.3) |
| No | 35 (72.9) | 7 (43.7) |
| Reason for treatment discontinuation, n (%) | 26 (100) | 14 (100) |
| Abnormal lab value | 2 (7.7) | 3 (21.4) |
| Adverse event | 1 (3.8) | 0 (0) |
| Progressive disease | 18 (69.2) | 11 (78.6) |
| Death | 2 (7.7) | 0 (0) |
| Patient withdrawal | 3 (11.5) | 0 (0) |
Patients receiving non-enzyme-inducing anticonvulsant drugs or no anticonvulsant drugs.
Patients receiving enzyme-inducing anticonvulsant drugs.
One patient with prior biopsy only.
ITT, intent-to-treat; KPS, Karnofsky Performance Status; MTD, maximum tolerated dose; SD, standard deviation.
Pharmacokinetics
Serum concentrations of both AEE788 and its primary metabolite AQM674 were highly variable. By day 15 of cycle 1, the mean coefficient of variation in AUC0–24 was 67% (range, 47%–102%) for AEE788. AEE788 and AQM674 exposure increased with dose and dose duration (number of doses). In Group A, an 11-fold range in doses (50–550 mg) yielded a 52-fold range in exposure to the parent drug, suggesting the exposure of AEE788 exposure increases overproportionately with increased dose while AQM674 exposure increased 20 fold (Table 4). On average, the maximum serum concentrations of AEE788 and AQM674 occurred at 5 and 4.6 hours after administration, respectively. The AQM674 serum concentration profile appears to reflect relative changes in AEE788, suggesting rapid metabolite formation and elimination equal to or faster than those with AEE788. Parent and metabolite exposure was approximately 4.5- and 3-fold, respectively, greater on day 15 than day 1. Exposure of AEE788 and AQM674 after 15 and 28 days of dosing were similar, suggesting PK steady state was reached on or before day 15. Accumulation of both AEE788 and AQM674 was 2 to 8 times greater after daily (q 24 h) administration rather than a single dose, with a Cmax to minimum concentration (Cmin) ratio (ie, Cmax:Cmin) of approximately 2.
Table 4.
AEE788 and AQM674 mean (CV) pharmacokinetic parameters
| AEE788 [n] | AQM674 [n] | |||||
|---|---|---|---|---|---|---|
| Dose mg/day | Cmax ng/mL | tmax h | AUC 0–24 h ng/mL | Cmax ng/mL | tmax h | AUC 0–24 h ng/mL |
| Group A | ||||||
| 50 | 7.7 (40.4) [2] | 3.0 (94.3) [2] | 82.0 (−) [1] | 6.1 (21.1) [2] | 1.0 (0.0) [2] | 63.0 (−) [1] |
| 100 | 12.7 (29.0) [3] | 4.3 (25.8) [3] | 168.0 (8.4) [2] | 6.0 (8.3) [3] | 4.3 (25.8) [3] | 88.5 (4.0) [2] |
| 200 | 8.2 (−) [1] | 9.0 (−) [1] | 189.0 (−) [1] | 3.9 (−) [1] | 5.2 (−) [1] | 79.0 (−) [1] |
| 250 | 74.5 (41.0) [5] | 5.9 (49.8) [5] | 1438.0 (36.3) [5] | 20.1 (53.7) [5] | 4.3 (23.6) [5] | 392.4 (55.1) [5] |
| 400 | 77.1 (32.5) [3] | 6.7 (48.4) [3] | 1154.0 (53.1) [3] | 19.2 (25.7) [3] | 6.7 (48.4) [3] | 298.3 (54.1) [3] |
| 450 | 147.7 (48.2) [4] | 11.0 (72.4) [4] | 3005.0 (35.3) [2] | 35.7 (16.3) [4] | 6.8 (27.0) [4] | 648.5 (11.7) [2] |
| 550 | 234.3 (52.8) [4] | 12.5 (62.5) [4] | 4300.5 (50.1) [4] | 68.4 (64.9) [4] | 12.5 (62.5) [4] | 1284.8 (59.6) [4] |
| Group B | ||||||
| 300 | 55.2 (−) [1] | 4.9 (−) [1] | 628.0 (−) [1] | 16.0 (−) [1] | 4.9 (−) [1] | 198.0 (−) [1] |
| 600 | 71.8 (100.0) [3] | 3.7 (63.0) [3] | 730.0 (86.6) [3] | 25.8 (73.1) [3] | 3.7 (63.0) [3] | 268.3 (75.5) [3] |
| 800 | 95.2 (56.3) [2] | 3.0 (0.0) [2] | 1464.0 (73.5) [2] | 30.1 (36.5) [2] | 4.0 (34.0) [2] | 452.0 (56.3) [2] |
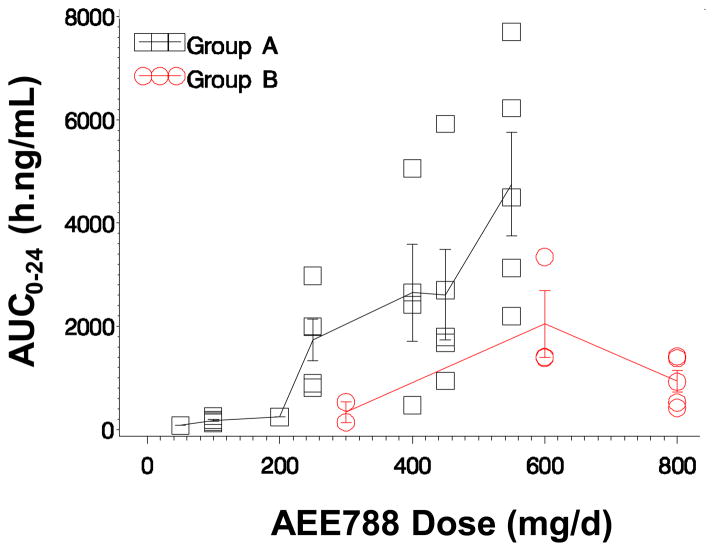
Because oral clearance is confounded by bioavailability, clearance is instead based on the exposure profile of AEE788. Neither the nonlinear dose-exposure relationship of AEE788 nor the drug accumulation could be explained by a decrease in total systemic clearance, indicated by an increase in apparent terminal phase slope. The exposure patterns, however, were consistent with a first-pass metabolism process by the gut and/or liver that can be saturated at high drug concentrations. The AEE788 dose-exposure relationship of groups A and B exhibited nonlinear patterns for both AEE788 and AQM674 (Figs 1 and 2). Overall exposure of parent and metabolite were reduced in patients receiving EIACD (Group B). For example, the exposure of Group B patients who received 600 mg was half the exposure observed in Group-A patients who received 550 mg. This suggests metabolism of AEE788 is susceptible to enzyme induction by EIACD. The effective half-life, estimated from accumulation index with a 24-hour dose interval, exceeds 24 hours.
Fig. 1.
Dose-exposure relationship. Mean (± 1 SD) AEE788 exposure (AUC0–24) versus AEE788 dose
SD, standard deviation
Fig. 2.
Dose-exposure relationship. Mean (± 1 SD) AQM674 exposure (AUC0–24) versus AEE788 dose
SD, standard deviation
Parent and metabolite reach pharmacologically active concentrations (ie, concentration at which EGFR and VEGFR pathways are inhibited) in glioma tumor tissue (Table 5). Four patients had their tumor surgically removed approximately 12 hours after the eighth dose of AEE788 (250 mg). AEE788 and AQM674 tumor concentrations ranged from 2.9 to 7.1 μM and 0.21 to 0.92 μM, respectively, while serum concentrations collected at the same time ranged from 0.10 to 0.32 μM and 0.05 to 0.07 μM. The concentration of AEE788 in patient glioblastoma tumor samples was therefore 14–59-fold higher than in plasma. This is consistent with findings in a NCI-H596 lung carcinoma-bearing nude mice model (data on file). Consequently, AEE788 tumor concentrations achieved in this study exceeded concentrations required for tyrosine kinase- and cell growth-inhibition that has been observed in various in vitro models (data on file).
Table 5.
AEE788 Concentration (μM) in Tumor versus Seruma
| Patient | AEE788 | AQM674 | ||
|---|---|---|---|---|
|
| ||||
| Tumor ng/mL | Serum ng/mL | Tumor ng/mL | Serum ng/mL | |
| 1 | 1277.7 | 57.3 | 196.7 | 20.6 |
| 2 | 2467.2 | 41.9 | 411.2 | 22.8 |
| 3 | 3128.1 | 141.0 | 299.5 | 31.3 |
| 4 | 1586.1 | 110.1 | 93.9 | 22.8 |
Sample taken approximately 12 hours after 8th AEE788 (250 mg) dose.
Intratumoral Biomarker Analyses
A total of 47 archival tumor samples obtained from enrolled patients, were analyzed for all biomarkers. Of these 47 samples, 39 were collected at initial glioblastoma diagnosis and 8 at disease recurrence. Correlations between p-KDR, p-S6, p-AKT, and PTEN and others biomarkers are summarized in Table 6. IHC staining greater than 100 (ie, tier 3) for p-KDR and PTEN was found in 19 of 28 (68%) and 10 of 28 (36%) patients, respectively. Expression of p-EGFR was negative in 24 of 28 patients and correlated poorly with other markers. EGFRvIII mutation was present in 2 patients. PFS was inversely correlated with p-S6 (P=.04) and p-KDR (P=.03) expression according to log-rank analyses. However, the Cox proportional hazards analysis found that only p-KDR was a significant predictor of PFS (inverse relationship, P=.01, both variable selection methods).33
Table 6.
Correlations Between p-KDR, p-S6, p-AKT, and PTEN and Other Biomarkers (5% Level of Significance) (n=47)
| Biomarker | Comparison Biomarkers | Spearman Correlation (P value) |
|---|---|---|
| p-AKT | p-KDR | 0.52 (P = 0.00) |
| p-MAPK | 0.47 (P = 0.00) | |
| p-S6 | 0.45 (P = 0.00) | |
| p-p70S6K | 0.41 (P = 0.00) | |
| PTEN* | 0.39 (P = 0.01) | |
|
| ||
| p-KDR | p-MAPK | 0.57 (P < 0.00) |
| p-p70S6K | 0.54 (P < 0.00) | |
| p-AKT | 0.52 (P = 0.00) | |
| p-S6 | 0.49 (P = 0.00) | |
| p-S6 | p-p70S6K | 0.56 (P < 0.00) |
| p-KDR | 0.488 (P = 0.00) | |
| p-MAPK | 0.45 (P = 0.00) | |
| p-AKT | 0.45 (P = 0.00) | |
| p-EGFR | 0.29 (P = 0.0464) | |
| PTEN | p-AKT* | 0.39 (P = 0.00) |
| p-MAPK | 0.34 (P = 0.02) | |
| p-p70S6K | 0.33 (P = 0.03) | |
Dose-Limiting and Other Toxicities
Initial dose-escalation cohorts for Group A included 50, 100, 200, 400, and 550 mg. DLTs occurred in 2 of 6 patients (grade-4 stomatitis [1 patient]; grade-2 proteinuria, grade-3 fatigue, and grade-3 atrial fibrillation [1 patient each]) at 550 mg. Because no patients experienced a DLT at the 400 mg dose level, a cohort of 6 patients then received 450 mg. One of these 5 patients experienced a DLT (grade 2 seizure); however, because 1 of the initial 6 patients of this cohort withdrew consent and, therefore, was deemed ineligible for inclusion in the MTD-determining population, a MTD could not be defined. Enrollment of Group B patients initiated when a grade 3 skin rash was reported in a Group A patient treated at 400 mg. The starting dose level of Group B was, therefore, 300 mg with subsequent cohorts receiving 600 mg and 800 mg. One of 6 patients experienced a DLT (grade 3 diarrhea resistant to antidiarrheal treatment) at 800 mg.
The dose-expansion phase included 24 patients. Because 550 mg was dose limiting in 2 of 6 evaluable patients and 450 mg was dose limiting in 1 of 5 evaluable patients in Group A of the dose-escalation phase, 400 mg was the initially selected dose for the dose expansion phase. Four patients with baseline AST or ALT levels of up to CTCAE grade 1 toxicity levels received 400 mg. An additional 18 patients with normal AST or ALT levels at baseline received 250 mg to further assess hepatoxicity as initial safety data suggested less frequent liver function test abnormalities at dose levels of 250 mg or less. In Group B, 2 patients received 600 mg. Enrollment for the dose-expansion phase of the study was not completed because the sponsor terminated the study early after review of the PK, safety, and efficacy data.
All patients reported at least one AE. Diarrhea was the most commonly reported, occurring in 72% of patients (grade 3, 9.4%; grade 4, none) and at similar rates in groups A and B. Table 7 lists the most frequently occurring AEs by dose level. Twenty eight percent (28%) of patients discontinued treatment because of AEs.
Table 7.
Most Frequent (≥ 15%) AEs by Dose Levela
| Adverse Event | Number of Patients (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non EIACD 50 mg/d (n=2) | Non EIACD 100 mg/d (n=6) | Non EIACD 200 mg/d (n=1) | Non EIACD 250 mg/d (n=18) | Non EIACD 400 mg/d (n=7) | Non EIACD 450 mg/d (n=6) | Non EIACD 550 mg/d (n=8) | EIACD 300 mg/d (n=2) | EIACD 600 mg/d (n=8) | EIACD 800 mg/d (n=6) | |
| ALT elevation | 0 | 9 (18.8) | 0 | 2 (25) | 2 (33.3) | 4 (25) | 0 | 0 | 2 (25) | 2 (33.3) |
| Confusion | 0 | 1 (16.7) | 0 | 4 (22) | 2 (28.6) | 0 | 1 (12.5) | 0 | 1 (12.5) | 1 (16.7) |
| Seizure | 1 (50) | 0 | 0 | 5 (27.8) | 1 (14.3) | 1 (16.7) | 1 (12.5) | 0 | 1 (12.5) | 2 (33.3) |
| Diarrhea | 1 (50) | 2 (33.3) | 1 (100) | 12 (66.7) | 4 (57.1) | 6 (100) | 8 (100) | 1 (50) | 6 (75) | 5 (83.3) |
| Fatigue | 0 | 2 (33.3) | 0 | 8 (44.4) | 1 (14.3) | 4 (66.7) | 5 (62.5) | 1 (50) | 3 (37.5) | 2 (33.3) |
| Headache | 1 (50) | 2 (33.3) | 0 | 3 (16.7) | 3 (42.9) | 1 (16.7) | 1 (12.5) | 0 | 1 (12.5) | 0 |
| Hemiparesis | 0 | 3 (50) | 0 | 5 (27.8) | 4 (57.1) | 1 (16.7) | 1 (12.5) | 0 | 0 | 3 (50) |
| Insomnia | 0 | 0 | 0 | 6 (33.3) | 0 | 0 | 2 (25) | 0 | 3 (37.5) | 0 |
| Muscle weakness | 1 (50) | 2 (33.3) | 0 | 1 (5.6) | 1 (14.3) | 0 | 2 (25) | 1 (50) | 1 (12.5) | 1 (16.7) |
| Nausea | 0 | 2 (33.3) | 0 | 3 (16.7) | 1 (14.3) | 4 (66.7) | 5 (62.5) | 1 (50) | 2 (25) | 2 (33.3) |
| Rash | 2 (100) | 2 (33.3) | 0 | 3 (16.7) | 1 (14.3) | 4 (66.7) | 6 (75) | 1 (50) | 0 | 2 (33.3) |
| UTI | 0 | 0 | 0 | 5 (27.8) | 2 (28.6) | 2 (33.3) | 1 (12.5) | 1 (50) | 0 | 0 |
| Vomiting | 0 | 1 (16.7) | 0 | 2 (11.1) | 1 (14.3) | 3 (50) | 2 (25) | 1 (50) | 0 | 0 |
Includes AEs observed during all treatment cycles.
AE, adverse event; ALT, alanine aminotransferase; UTI, urinary tract infection.
SAEs were more frequent in Group A than Group B (42% vs 25%), causing 17% of patients to discontinue the study. Of the 64 patients enrolled in the study, 14 (22%) experienced grade 3/4 AST or ALT elevations, including 2 patients who had concurrent ≥ grade 2 total bilirubin elevation. Grade 2 and 3 AST or ALT abnormalities were observed in more Group B patients; while grade 3/4 AST or ALT elevations with concurrent ≥ grade 2 total bilirubin elevations and grade 4 AST or ALT elevations occurred in 2 Group A patients and 0 Group B patients. No relationship between higher AEE788 dose levels and AST or ALT abnormalities was observed. Three deaths (Group A: disease progression, 2 patients; Group B: disease progression, 1 patient) occurred during AEE788 therapy, and 4 deaths occurred within 28 days of last dose of AEE788 (Group A: disease progression, 1 patient; pulmonary failure, 2 patients; pneumonia, 1 patient).
Efficacy
No CR or PR was reported. The overall best response was SD for > 3 cycles in 6 of 36 patients (17%); 4 Group A patients treated at doses 50 mg to 200 mg had SD for 6 to 10 months. Median PFS for patients treated in the dose expansion phase were 2.7 (90% CI: 1.9, 2.8) and 1.6 (90% CI: 0.2, 2.6) months for Groups A and B, respectively. Disease progression was reported (and caused AEE788 treatment discontinuation) for most patients in both dose-escalation groups (all dose levels) (non-EIACD, 18 patients [69.2%]; EIACD, 11 [78.6%]) and both dose-expansion arms (all dose levels) (Arm 1, 5 patients [71.4%]; Arm 2, 10 [58.8%]).
FLT-PET was performed in 6 patients (3 at 250 mg; 1 at 400 mg; 2 at 600 mg). Ten patients (8 at 250 mg; 2 at 400 mg) underwent DWI-, DCE-, and/or -MRI. Gd-MRI was performed on all patients. Overall, one patient experienced a significant decrease in PET parameters, and no significant pharmacodynamic effects based on FLT-PET and/or MRI analyses were noted for patients at any dose level.
Discussion
The role of EGFR and VEGF in tumor cell development and progression and the potential benefit of concomitantly targeting these cellular pathways to treat various tumor types are well described.24,34–38 Hence, we hypothesized that AEE788, a dual inhibitor of both EGFR- and VEGF-mediated pathways, would prove effective for the treatment of glioblastoma, a tumor typically presenting with high VEGF concentrations, EGFR gene amplification, and an overexpression of EGFR proteins. Our findings, however, demonstrate an unfavorable AE profile and minimal activity of AEE788 in this population. The most frequently occurring AEs during all cycles (all dose levels) were diarrhea, rash, fatigue, nausea, hemiparesis, and ALT elevations. Grade 3–4 AST or ALT elevations occurred in 14 patients (22%). Although most cases occurred in patients receiving ≥ 250 mg, a relationship between AEE788 dose and AST or ALT abnormalities was not observed. DLTs were defined as proteinuria, stomatitis, fatigue, and seizures. Whether the seizures experienced by 1 patient receiving AEE788 450 mg was truly an AEE788-related DLT verses an AE of the glioblastoma tumor itself is unknown; however, the investigator did suspect the event was related to AEE788.
These safety and efficacy findings are similar to those observed in other recently completed studies evaluating multitargeted TKIs (eg, erlotinib, sorafenib, vandetanib, sunitinib), either alone or in combination with chemotherapeutic agents, for the treatment of glioblastoma as well as other tumor types (eg, breast, kidney, thyroid).39–42 As with the current study, many of these studies demonstrated “off-target” or unexpected toxicities coupled with poor efficacy outcomes. For example, in a recent phase I/II study evaluating erlotinib in combination with the mammalian target of rapamycin (mTOR) inhibitor temsirolimus in patients with recurrent glioblastoma or anaplastic gliomas (phase I, N=22; phase II, N=56), toxicities, particularly rash and mucositis, requiring significant temsirolimus dose reductions were observed; furthermore, antitumor activity was minimal (glioblastoma patients: SD, 30% of patients; 6-month PFS, 12.5%; PR, none).42 Likewise, when temsirolimus was combined with sorafenib in patients with recurrent glioblastoma, several patients experienced grade 3, off-target toxicities with the most common being thrombocytopenia (9 of 19 patients [47%]) and tumor RRs did not merit further study.41 Although some off-target toxicities, such as hypothyroidism and cardiotoxicity, which are often observed in patients receiving the multitargeted TKIs sorafenib or sunitinib for the treatment of renal cell and other carcinomas, are considered manageable with optimal patient monitoring and treatment, many off-target toxicities are unmanageable and, thus, limit the use of many multitargeted therapies.43,44 For example, we observed dose-independent hepatotoxicity (ie, at least grade 2 AST or ALT elevations) in 27% of Group A and 44% of Group B patients.
Collectively, the data from these and other studies highlight potential gaps in current methodologies evaluating multitargeted therapies. First, identifying the optimal biological dose (OBD) and/or minimally active dose (as opposed to the MTD) will be important to help define dose-escalation methods. For targeted therapies, the OBD, however, takes into consideration the unconventional characteristics of targeted therapies, such as wider therapeutic indexes and differing mechanisms causing therapeutic versus toxic effects (ie, mechanism-dependent vs mechanism-independent [ie, off-target] AEs) and may often be lower than the MTD for a given drug.45 Determining the OBD requires that biologic markers (eg, circulating VEGF levels, tumor vasculization, PK/pharmacodynamic relationships) predictive of optimal inhibition of the drug’s target and, theoretically, tumor response be identified and validated in preclinical trials.45 Additionally, proactively identifying subgroups of patients most likely to benefit from a particular multitargeted therapy for inclusion in phase I/II clinical trial could optimize trial design and outcomes, while sparing patients unlikely to benefit from a given therapy unnecessary drug exposure and associated toxicities.45 Lastly, optimizing drug combinations is necessary to minimize additive toxicities and/or drug interactions that may require dose adjustments that can result in inadequate target drug exposure, particularly in glioblastoma patients who often require EIACDs.42
In conclusion, continuous, once-daily AEE788 was associated with minimal activity for the treatment of recurrent glioblastoma and unexpected, off-target toxicities. After review of the PK, safety, and efficacy data, the study was discontinued prematurely, and further study of AEE788 for the treatment of glioblastoma has been terminated.
Acknowledgments
Research support: Novartis Pharmaceuticals Corporation, East Hanover, NJ
We are indebted to the patients who participated in this study and study coordinators. We thank Syntaxx Communications, Inc. for assistance with manuscript development and editing.
Footnotes
Study results, in part, previously presented at:
Proceedings of the 2005 ASCO Annual Meeting, May 13–17 2005, Orlando, FL. Proceedings of the AACR-NCI-EORTC Symposium on “Molecular Targets and Cancer Therapeutics,” November 14–18, 2005, Philadelphia, PA
Bibliography
- 1.Stupp R, Mason WP, van den Bent MJ, et al. for the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Krex D, Klink B, Hartmann C, et al. for the German Glioma Network. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 4.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 5.Desjardins A, Reardon DA, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14:7068–7073. doi: 10.1158/1078-0432.CCR-08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 7.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a Pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avastin [package insert] South San Francisco, CA: Genentech, Inc; 2009. [Google Scholar]
- 10.Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66:11502–11513. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 11.Bredel M, Scholtens DM, Harsh GR, et al. A network model of a cooperative genetic landscape in brain tumors. J Amer Med Assoc. 2009;302:261–275. doi: 10.1001/jama.2009.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons DW, Jones D, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 15.Steck PA, Gallick GE, Maxwell SA, et al. Expression of epidermal growth factor receptor and associated glycoprotein on cultured human brain tumor cells. J Cell Biochem. 1986;32:1–10. doi: 10.1002/jcb.240320102. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey PA, Wong AJ, Vogelstein B, et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res. 1988;48:2231–2238. [PubMed] [Google Scholar]
- 17.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 18.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagane M, Coufal F, Lin H, Bögler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 20.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial oferlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96:1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 23.Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–112. [PubMed] [Google Scholar]
- 24.Traxler P, Allegrini PR, Brandt R, et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 25.Reardon D, Cloughesy T, Conrad C, et al. A phase I study of AEE788, a novel multitargeted inhibitor of ErbB and VEGF receptor family tyrosine kinases, in recurrent GBM patients. J Clin Oncol. 2005;23:16s. doi: 10.1007/s00280-012-1854-6. (suppl; abstr 3063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 27.Martinelli E, Takimoto CH, van Oosterom AT, et al. AEE788, a novel multitargeted inhibitor of ErbB and VEGF receptor family tyrosine kinases: preliminary phase 1 results. J Clin Oncol. 2005;23:201s. (suppl; abstr 3039) [Google Scholar]
- 28.Xiong HQ, Takimoto C, Rojo F, et al. A phase I study of AEE788, a multitargeted inhibitor of ErbB and VEGF receptor family tyrosine kinases, to determine safety, PK and PD in patients (pts) with advanced colorectal cancer (CRC) and liver metastases. J Clin Oncol. 2007;25:179s. (suppl; abstr 4065) [Google Scholar]
- 29.Shuster J. Optimal two-stage designs for single arm phase II cancer trials. J Biopharm Statist. 2002;12:39–51. doi: 10.1081/bip-120005739. [DOI] [PubMed] [Google Scholar]
- 30.Dent S, Zee B, Dancey J, et al. Application of a new multinomial phase II stopping rule using response and early progression. J Clin Oncol. 2001;19:785–791. doi: 10.1200/JCO.2001.19.3.785. [DOI] [PubMed] [Google Scholar]
- 31.Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–1128. [PubMed] [Google Scholar]
- 32.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 33.Aldape K, Cloughesy T, Reardon D, et al. Analyses of EGF and VEGF signaling pathways in recurrent glioblastoma patients in a Phase I study of single agent AEE788. Presented at the Proceedings of the AACR-NCI-EORTC International Conference on “Molecular Targets and Cancer Therapeutics,”; November 14–18, 2005; Philadelphia, PA. [Google Scholar]
- 34.Younes MN, Park YW, Yazici YD, et al. Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases reduces growth and metastasis of human salivary adenoid cystic carcinoma in an orthotopic nude mouse model. Mol Cancer Ther. 2006;5:2696–2705. doi: 10.1158/1535-7163.MCT-05-0228. [DOI] [PubMed] [Google Scholar]
- 35.Wu W, Onn A, Isobe T, et al. Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther. 2007;6:471–483. doi: 10.1158/1535-7163.MCT-06-0416. [DOI] [PubMed] [Google Scholar]
- 36.Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546–1556. [PubMed] [Google Scholar]
- 37.Yokoi K, Thaker PH, Yazici S, et al. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res. 2005;65:3716–3725. doi: 10.1158/0008-5472.CAN-04-3700. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T, Kitadai Y, Nakamura T, et al. Inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation on tumor-associated endothelial cells leads to treatment of orthotopic human colon cancer in nude mice. Neoplasia. 2007;9:1066–1077. doi: 10.1593/neo.07667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr Med Chem. 2008;15:422–432. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 40.Prados M, Gilbert M, Kuhn J, Lamborn K, Cloughesy T, Lieberman F, Puduvalli V, Robins HI, Lassman A, Wen PY. Phase I/II study of sorefenib and erlotinib for patients with recurrent glioblastoma (GBM) (NABTC 05-02) J Clin Oncol 2009. 2009;27(88s) (suppl; abstr 2005) [Google Scholar]
- 41.Wen PY, Cloughesy T, Kuhn J, Lamborn K, Abrey LE, Lieberman F, Robins HI, Wright J, Prados MD, Gilbert M. Phase I/II study of sorafenib and temsirolimus for patients with recurrent glioblastoma (GBM) (NABTC 05-02) J Clin Oncol. 2009;27:88s. (suppl; abstr 2006) [Google Scholar]
- 42.Chang SM, Kuhn J, Lamborn K, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas (MG) (NABTC 04-02) J Clin Oncol. 2009;27:88s. doi: 10.1093/neuonc/not247. (suppl; abstr 2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 44.Torino F, Corsello SM, Longo R, et al. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez ME, Kummar S, Giaccone G. Next generation oncology drug development: opportunities and challenges. Nat Rev Clin Oncol. 2009;6:259–265. doi: 10.1038/nrclinonc.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]