Abstract
Purpose
Cilengitide is a cyclic pentapeptide that is a specific inhibitor of the αvβ3 and αvβ5 integrins. Preclinical studies demonstrate antiangiogenic activity and anti-invasive activity in a number of glioma models. This study was designed to evaluate the efficacy and tumor delivery of cilengitide in patients with recurrent glioblastoma.
Experiment design
Patients with recurrent glioblastoma who require a surgical resection for optimal clinical care received 3 intravenous doses of cilengitide at either 500 or 2000 mg (day -8, -4, -1) prior to undergoing tumor resection with corresponding blood samples for plasma to tumor comparisons. After recovery from surgery, patients were treated with cilengitide (2000 mg i.v. twice weekly, maximum of 2 years of treatment).
Results
The study accrued 30 patients with recurrent glioblastoma, 26 were evaluable for efficacy. The 6-month progression free survival rate was 12%. Cilengitide was detected in all tumor specimens with higher levels in the group receiving 2000 mg dosing while corresponding plasma concentrations were low, often below the lower limit of detection. These results confirm drug delivery and possibly retention in tumor.
Conclusions
This study provides evidence that with established dosing, cilengitide is adequately delivered to the tumor, although as a single agent, efficacy in recurrent glioblastoma is modest. However, these results demonstrating drug delivery to tumor do support continued investigation of this agent as preliminary results from recent studies combining cilengitide with cytotoxic therapies are promising.
Keywords: glioblastoma, pharmacokinetics, tumor pharmacokinetics, integrins
Introduction
The prognosis for patients with recurrent glioblastoma remains poor and most series report a median survival of 4 months or less and a 6 month progression free rate of less than 20 percent1. The treatment choices are limited as most chemotherapy agents are limited by poor delivery through the blood-brain-barrier and intrinsic tumor resistance to these drugs. Agents that target specific signal transduction pathways vital to the survival or progression of the tumor have become the focus of recent trials, and in particular anti-angiogenic strategies have received significant attention because these tumors are highly angiogenic, anti-tumor activity of anti-angiogenic agents has been established, and it is presumed that the likelihood that tumor-associated endothelial cells would develop resistance is low2, 3.
Vascular endothelial growth factor (VEGF) promotes both angiogenesis and invasion, and the two processes are closely linked in glioma. “Invasion” of endothelial cells into the tumor is an important component of the angiogenic process. In addition to growth factors such as VEGF, integrins, which modulate the extracellular matrix, are an essential component of both angiogenesis and tumor cell migration4. The αβ integrin family has been shown to be of particular importance5. In particular, αvβ3 and αvβ5, expressed on endothelial cells, promote invasion of endothelial cells into a wide variety of tissues, and maintain endothelial cell viability suggesting an important role in development (reviewed in6). Additional studies support the role of αvβ3 in selectively preventing endothelial cell apoptosis in newly formed blood vessels7. The αvβ5 integrin is an important component of the VEGF-mediated angiogenic response8. The αvβ3 integrin has been found on endothelial cells of small blood vessels in glioblastoma (9 of 12) and some anaplastic astrocytoma (2 of 4), but not on normal brain vessels9. A direct correlation between the grade of tumor and level of expression of integrins was reported by measuring expression of both the αvβ3 and αvβ5 integrins in brain tumor specimens at on the growing edge of the tumor.10.
Cilengitide is a cyclic pentapeptide that targets the RGD sequence on vitronectin and as a consequence is a specific inhibitor of both the αvβ3 and αvβ5 integrins. Preclinical studies using both in vitro and in vivo glioma models have demonstrated efficacy in tumor cell lines that express the αvβ3 integrin11 and in orthotopic (intracranial) but not subcutaneous animal models12. Clinical trials of cilengitide demonstrate that the agent is well tolerated with equal tolerance at either 500 or 2000 mg delivered intravenously twice weekly in adults13. There are no apparent drug-drug interactions or pharmacokinetic alterations with concurrent use of enzyme-inducing antiepileptic agents. Preliminary phase II studies suggest that the higher dose schedule in recurrent malignant gliomas may be associated with greater anti-tumor activity; nevertheless, this activity is modest at best14.
The pre-clinical studies suggest that cilengitide may have at least two mechanisms of action. First, cilengitide inhibits the endothelial cell integrin interaction with ligands, thereby permitting tumor-associated endothelial apoptosis. Second, in tumor cells expressing αvβ3, inhibition of integrin interaction with the extracellular matrix may promote tumor cell apoptosis, a phenomenon seen most potently at the leading edge of the tumor. These findings suggest that the possibility that the optimal clinical setting for using cilengitide in malignant gliomas could be patients with minimal tumor burden. Therefore, we performed a clinical trial to assess efficacy and tumor delivery of cilengitide in patients with recurrent GBM. We included only those patients who required tumor resection as part of their optimal clinical management. Patients received cilengetide both pre- and post-operatively at dose levels of 500 and 2000mg respectively. Following recovery from surgery a standard efficacy evaluation at the 2000mg dose level was then performed. This protocol design allowed assessment of intra-tumoral drug uptake and by continuing treatment after the surgical procedure, determination of efficacy in a select patient population undergoing extensive tumor resection.
Patients and Methods
Patient population
Eligible patients were ≥ 18 years of age and had a histologically confirmed diagnosis of progressive glioblastoma or gliosarcoma, and were at least 4 weeks from the completion of radiotherapy prior to study registration. A maximum of 2 prior tumor relapses were allowed and the patient must have recovered from the toxic effects of prior therapy. Patients were required to be a candidate for a tumor gross- or near-total resection as a component of clinical care, and this would occur after pre-operative administration of cilengitide. Patients had to have a Karnofsky performance score ≥ 60, acceptable hematologic (WBC ≥ 3,000/μl, ANC ≥ 1,500/mm3, platelet count of ≥ 100,000/mm3, and hemoglobin ≥ 10 gm/dl), and biochemical studies including liver function (SGOT and bilirubin < 2 times ULN), and renal function (creatinine < 1.5 mg/dL) before starting therapy.
Protocol design (Figure 1)
Figure 1.
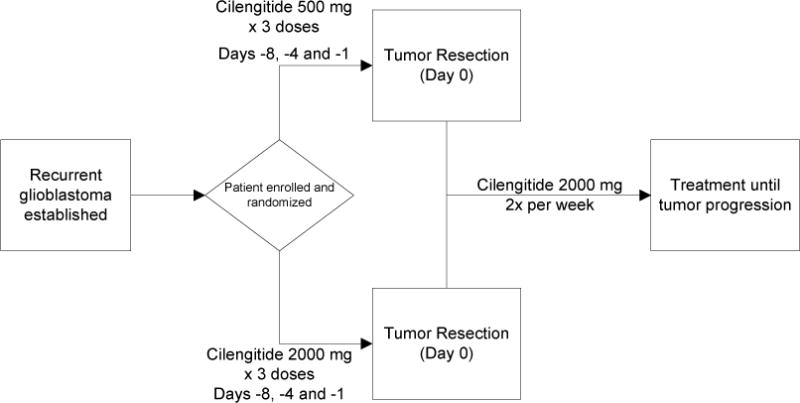
Schema for the protocol depicting the randomization of the Cilengitide dosing prior to surgery, then a single dosing regimen after recovery from the surgical procedure.
Patients were randomized to receive either high dose (2000 mg) or low dose (500 mg) cilengitide on days -8, -4 and -1 prior to the planned tumor resection. Blood samples were obtained in this preoperative period for concordant plasma pharmacokinetic analysis. Samples were drawn at the initiation of the final infusion of cilengitide, at the conclusion of this final infusion and at the time of tumor removal. After the patient recovered from the surgical procedure and a minimum of 2 post-operative weeks, treatment with cilengitide was recommenced at a dose of 2000 mg twice weekly as an hour-long intravenous infusion, with a cycle defined as 4 weeks of delivery. Treatment was continued until tumor progression or development of serious treatment-related toxicity.
Patient evaluation
Pretreatment evaluation included a complete medical history and physical and neurologic examination, performed prior to initiation of the presurgical component of the study and repeated prior to the initiation of the post-surgical treatment with cilengitide. Laboratory studies including hematologic, renal and hepatic function analysis was performed every 2 weeks. Clinical and imaging evaluations were performed every 8 weeks (2 cycles). Imaging was performed using either contrast enhanced magnetic resonance imaging or computed tomography, maintaining the same modality throughout the study.
Objective response assessments were not included as enrolled patients had either a complete or near complete resection of tumor. Imaging studies were therefore evaluated for progressive disease which was defined as the appearance of any new lesion or site of tumor, enlarging residual disease, or clinical worsening (unless unrelated to the cancer), and clinical deterioration precluding further evaluation or death.
Pharmacokinetic analysis
A limited pharmacokinetic study was incorporated in this trial. Blood samples were obtained in the preoperative period to have concordant plasma pharmacokinetics. Samples were drawn at the initiation of the final infusion of cilengitide, at the conclusion of this final infusion and at the time of tumor removal. All blood samples were drawn in sodium heparin 7 ml tubes. At surgical resection, tumor tissue and a blood sample were obtained. The tumor tissue was snap frozen and the tissue and plasma were stored frozen (≤−20° C) until the analysis was performed.
Samples were analyzed using a validated high performance liquid chromatography coupled with tandem mass spectrometry (Dr. SabineWittemer, Merck KGaA). The lower limit of quantification (LLOQ) with a precision of 20% and accuracy of 80 to 125% was a cilengitide concentration of 200 ng/mL for plasma and 5 ng/g for tumor tissue. The intent was to measure, the Cmax (end of infusion; EOI) and tissue concentration (ng/g) of cilengitide along with concurrent plasma (trough) for each patient.
Statistical considerations
The primary endpoint for this study was the determination of the 6-month progression free survival rate. Based on historical values from a database of 225 patients with recurrent GBM undergoing treatment with ineffective therapies, the 6-month PFS was 15%15. A review of 220 patients with recurrent GBM treated at M.D. Anderson Cancer Center determined that although the median time to progression was prolonged in those patients undergoing gross total resection at recurrence compared with those undergoing biopsy or subtotal resection, there was no difference in the 6-month PFS. (M. Gilbert, personal communication). The study was intended to enroll 44 patients, which would provide over 90% power to detect an improvement in 6-month PFS from 15% to 38%,. The sample size was also designed to provide adequate pharmacokinetic data for each of the two presurgery dose groups.
Results
Patient Characteristics
A total of 30 patients were enrolled onto the study. The study was closed due to slow accrual. Patient demographics are provided in Table 1. All patients were confirmed to have GBM by central review and all underwent a tumor resection after receiving pre-operative dosing with cilengitide. One patient was deemed ineligible due to a protocol violation. Three patients did not restart treatment after the tumor resection and are not included in the efficacy analysis. One patient had an extended hospitalization after initiating the first post-operative treatment and an additional patient chose to stop treatment during the first post-operative treatment cycle.
Table 1.
Patient Demographics
| EVALUABILITY | ||||
|---|---|---|---|---|
| TOXICITY ONLY | 4 | (13.3%) | ||
| TOXICITY&EFFICACY | 26 | (86.7%) | ||
| NUMBER OF PATIENTS ENTERED | 30 | |||
| AGE MEDIAN (RANGE) | ||||
| 54 | (42–68) | |||
| PERFORMANCE STATUS | ||||
| 100 | 5 | (16.7%) | ||
| 90 | 10 | (33.3%) | ||
| 80 | 10 | (33.3%) | ||
| 70 | 5 | (16.7%) | ||
| SEX | ||||
| FEMALE | 18 | (60.0%) | ||
| MALE | 12 | (40.0%) | ||
| RACE | ||||
| ASIAN | 1 | (3.3%) | ||
| WHITE | 29 | (96.7%) | ||
| HISTOLOGY | ||||
| GLIOBLASTOMA MULTIFORME | 30 | (100.0%) | ||
| PRIOR THERAPY | ||||
| CHEMOTHERAPY | YES | 30 | (100.0%) | |
| IMMUNOTHERAPY | NONE | 27 | (90.0%) | |
| IMMUNOTHERAPY | YES | 3 | (10.0%) | |
| RADIOTHERAPY | YES | 30 | (100.0%) | |
| BIOPSY ONLY | NONE | 1 | (3.3%) | |
| SURGERY | YES | 29 | (96.7%) | |
| NUMBER OF PRIOR RX REGIMENS | ||||
| 1 | 19 | (63.3%) | ||
| 2 | 8 | (26.7%) | ||
| 3 | 3 | (10.0%) |
Efficacy analysis
Of the twenty six patients evaluable for efficacy, two patients were censored within the first month of treatment. Three patients were confirmed to have exceeded the 26 week progression-free threshold after the initiation of post-operative chemotherapy, with progression times of 44, 48 and > 100 weeks. The final patient had treatment stopped at 100 weeks, without evidence of progression. Overall, the 6-month PFS rate was 12%. From the Kaplan-Meier curve, the median PFS was 8 weeks (95% confidence interval 4–16 weeks).
Safety and Toxicity
Cilengitide was very well tolerated with only 9 grade 3–4 toxicities, mainly lymphopenia that was likely related to prior cytotoxic chemotherapy (Table 2). There were no dose reductions for toxicity and no treatment related deaths.
Table 2.
Treatment-related Toxicity
| ADVERSE EVENT | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|---|---|---|---|---|
| Elevated ALT | 2 | 0 | 0 | 0 |
| Anorexia | 2 | 0 | 0 | 0 |
| Elevated AST | 1 | 0 | 0 | 0 |
| Bruising | 2 | 0 | 0 | 0 |
| Constipation | 0 | 1 | 0 | 0 |
| Diarrhea | 2 | 0 | 0 | 0 |
| Erythema multiforme | 1 | 0 | 0 | 0 |
| Fatigue | 5 | 3 | 0 | 0 |
| Fatigue/intermittent | 1 | 0 | 0 | 0 |
| Hemoglobin | 8 | 0 | 0 | 0 |
| Hot flashes | 0 | 1 | 0 | 0 |
| Hyperglycemia | 3 | 0 | 0 | 0 |
| Hypoalbuminemia | 2 | 1 | 0 | 0 |
| Hypocalcemia | 1 | 0 | 0 | 0 |
| Hypokalemia | 1 | 0 | 0 | 0 |
| Hyponatremia | 2 | 0 | 0 | 0 |
| Infection with normal ANC – Wound | 0 | 1 | 0 | 0 |
| Leukocytes | 6 | 4 | 0 | 0 |
| Lymphopenia | 1 | 3 | 5 | 2 |
| Nausea | 3 | 0 | 0 | 0 |
| Neuropathy-motor | 1 | 0 | 0 | 0 |
| Neutrophils | 1 | 2 | 0 | 0 |
| Non-Cardiogenic Pulmonary Edema | 0 | 0 | 1 | 0 |
| Platelets | 5 | 0 | 1 | 0 |
| Rash | 1 | 0 | 0 | 0 |
| Rash/echymotic | 1 | 0 | 0 | 0 |
| Speech impairment | 0 | 1 | 0 | 0 |
| Vitreous hemorrhage | 1 | 0 | 0 | 0 |
| Vomiting | 1 | 0 | 0 | 0 |
| Grand Total | 54 | 17 | 7 | 2 |
Pharmacokinetic analyses
A summary of the end of infusion (EOI) plasma concentrations at the 500 and 2000 mg dose levels, and the brain tumor concentrations following the third dose of cilengitide are shown in Table 3. The plasma and tissue concentration at the time of the tumor resection represents the 24 hour post-dose trough level. Individually, for the group receiving the 500 mg dose, plasma concentrations at the 24 hour post-dose were below the lower limit of quantitation (LLOQ = 200 ng/ml) in 4 of 6 patients. Similarly, for patients receiving the 2000 mg dose, plasma concentrations at the 24 hour post-dose were below the LLOQ in 5 of 9 patients. However, all of the tumor samples in both dose groups had detectable levels of cilengitide ranging from 224 – 4210 ng/g of tissue. In the cases where there was measurable Cilengitide in plasma, the tissue to plasma ratio ranged from from 1.83 to 12.10. At the time of tumor removal, there were on average, three-fold (500 mg dose) or four-fold (2000 mg dose) higher concentrations in the tumor tissue than time-matched plasma concentrations. Furthermore, the intra-tumor concentrations were higher on average with the higher (2000 mg) dose of cilengitide. The ratio could not be calculated in the 9 patients where the plasma concentrations were LLOQ. These results demonstrate that there was clear evidence of drug delivery to the tumor.
Table 3.
Cilengitide Peak/Trough Plasma and Tumor Concentrations
| Plasma (ng/mL) | Tumor Tissue Concentrations (ng/g) | |||||
|---|---|---|---|---|---|---|
| End of infusion (ng/ml) | At time of resection (ng/ml)* | |||||
| Cilengitide dose | 500 mg | 2000 mg | 500 mg | 2000 mg | 500 mg | 2000 mg |
| Number of patients | 8 | 11 | 2 | 4 | 5 | 7 |
| Mean conc. | 25,000 | 123,000 | 333 | 386 | 919 | 1,413 |
| (± SD) | (±3,000) | (±24,000) | (±16.97) | (±184) | (±1,235) | (±1,335) |
represents a 24 hour post dose concentration. Six of 8 and 7 of 11 plasma samples at the 500 mg & 2000 mg dose level, respectively, were below the lower level of quantitation (LLOQ)
Molecular analyses evaluating alterations in αvβ3 and αvβ5 integrin receptors after Cilengitide treatment were planned as a component of this clinical trial. Unfortunately, the majority of tumor samples were either too small to do both measures of drug and molecular analysis or the sample was inadequate for both after removal of areas of necrosis and gliosis.
Discussion
Targeting angiogenesis as a treatment strategy for malignant gliomas is an area of active investigation. Most of these approaches have targeted the vascular endothelial growth factor pathway, either by antibody (bevacizumab) or antibody-like (VEGF-trap) molecule sequestering the VEGF ligand or use a tyrosine kinase small molecule inhibitor (i.e. cedirinib). These strategies have demonstrated clinical efficacy but are all associated with significant toxicities, treatment resistance and in some cases, development of a more invasive tumor phenotype16. For these reasons, there has been interest in developing alternative antiangiogenesis strategies as either alternatives or complementary treatments to the VEGF pathway targeted approaches.
The αvβ3 and αvβ5 integrin receptors are logical therapeutic targets. Preclinical studies demonstrate the impact of successful inhibition of the integrin receptors on tumor growth, demonstrating cytostasis when the tumor-related endothelial cells are targeted and tumor cell apoptosis in the subpopulation of malignant gliomas that express these integrin receptors. Cilengitide, a cyclic RGD-containing peptide that specifically targets the αvβ3 and αvβ5 integrin receptors was shown to have efficacy in several of the preclinical studies that demonstrated the potential of integrin inhibition in gliomas.
Cilengitide has also undergone phase I testing in the general oncology population and in patients with malignant gliomas and phase II testing in patients with both recurrent and newly diagnosed malignant gliomas17–20. In this study, the average plasma concentration of cilengitide at the end of infusion and half-life are consistent with the published literature17, 20. Plasma concentrations increased in a dose – dependent manner, whereas the increases in tumor concentrations were less than dose proportional. Cilengitide was also observed to distribute into the CSF in a less than dose-dependent manner possibly related to the polymorphic ABC transporter, ABCB120, 21. Reportedly, cilengitide’s IC50 for the inhibition of the integrins αvβ3 and αvβ5 receptor binding to vitronectin are l nM (0.6 ng/mL) and 140 nM (82 ng/mL), respectively19. In contrast, in vitro concentrations of 1000 to 100,000 nM were necessary to inhibit growth of a variety of glioma cell lines, unrelated to inhibition of αvβ3 and αvβ5 integrin expression22.
This study does demonstrate some of the challenges associated with attempting to do post-treatment assessments of drug delivery and tumor pharmacodynamics. Some tissue specimens were inadequate because of the predominance of necrosis or handling. Unanticipated events such as freezer failure also compromised sample collection. Furthermore, this study does underscore the importance in instituting multidisciplinary approach to these types of studies, involving neurosurgical and neuropathology colleague in the planning of the protocol. However, our study clearly demonstrates that intravenous administration of cilengitide does result in therapeutic concentrations in tumor tissue. However, despite this effective treatment delivery, the 6-month PFS of 12%, similar to that reported from a recent phase II trial, suggests that as a single agent, cilengitide has, at best, modest activity in recurrent glioblastoma. Nevertheless, there are encouraging results using cilengitide in patients with newly diagnosed glioblastoma18, 23. In these studies, cilengitide was added to the current standard chemoradiotherapy for patients with newly diagnosed glioblastoma. These results warrant further investigation. The results from our study demonstrate that there is adequate delivery of the drug to the tumor tissue and the higher concentrations of drug in tumor compared to time-comparable plasma further suggest that cilengitide is sequestered in tumor, although the duration of drug retention could not be determined by this study.
Cilengitide represents a novel class of agents, a small molecule that specifically targets integrins involved in both angiogenesis and tumor invasion. Our study provides evidence that drug is delivered to the tumor and given preliminary results from recent studies combining cilengitide with cytotoxic therapies, additional investigations are warranted.
Acknowledgments
The Cilengitide was generously provided by Merck KgaA and the National Cancer Institute, NIH.
Grant support: NIH Grant U01 CA-062412
Footnotes
Conflict of Interest Statements
Mark R. Gilbert, MD: Research support from Genentech, Advisory Affiliations with Merck, Genentech
John Kuhn, Pharm D.: none
Kathleen R. Lamborn, PhD: none
Frank Lieberman, MD: Advisory affiliations with Roche/Genentech. Paid consulting with Roche/Genentech and EMD Merck.
Patrick Y. Wen, MD: none
Minesh Mehta, MD: Consultant to Adnexus, Bayer, Genenteh, Merck, Schering Plough, YM BioSciences and Tomotherapy; on the Board of Directors of Pharmacyclics, an advisor to Colby and Stemina, and is on the DSMB for Apogenix. He holds stock options in Pharmacyclics and Tomotherapy.
Timothy Cloughesy, MD: none
Andrew B. Lassman, MD: Research support from Schering Plough, Sigma Tau, Genentech, Keryx, Astra Zeneca, Exelixis. Advisory affiliations with Bristol-Myers Squibb, Campus Bio, Cephalon, Eisai, Enzon, Genentech, Imclone, Schering-Plough, Merck. Paid consulting with Schering Plough, Merck
Lisa M. DeAngelis, MD: none
Susan Chang, MD: none
Michael Prados, MD: none
References
- 1.Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–70. doi: 10.1215/15228517-2007-062. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18356283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groot JF, Gilbert MR. New molecular targets in malignant gliomas. Curr Opin Neurol. 2007;20(6):712–8. doi: 10.1097/WCO.0b013e3282f15650. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17992095. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. ANGIOGENESIS. Annual Review of Medicine. 2006;57(1):1–18. doi: 10.1146/annurev.med.57.121304.131306. Available from http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 4.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149(1):293–305. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8686754. [PMC free article] [PubMed] [Google Scholar]
- 5.Tonn JC, Wunderlich S, Kerkau S, Klein CE, Roosen K. Invasive behaviour of human gliomas is mediated by interindividually different integrin patterns. Anticancer Res. 1998;18(4A):2599–605. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9703915. [PubMed] [Google Scholar]
- 6.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103(9):1227–30. doi: 10.1172/JCI6869. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10225964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byzova TV, Rabbani R, D’Souza SE, Plow EF. Role of integrin alpha(v)beta3 in vascular biology. Thromb Haemost. 1998;80(5):726–34. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9843163. [PubMed] [Google Scholar]
- 8.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270(5241):1500–2. doi: 10.1126/science.270.5241.1500. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7491498. [DOI] [PubMed] [Google Scholar]
- 9.Gladson CL. Expression of integrin alpha v beta 3 in small blood vessels of glioblastoma tumors. J Neuropathol Exp Neurol. 1996;55(11):1143–9. doi: 10.1097/00005072-199611000-00005. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8939197. [DOI] [PubMed] [Google Scholar]
- 10.Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–9. doi: 10.1097/00006123-200108000-00022. discussion 90. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11504114. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Matsumura A, Schradermeier J, Gillespie GY. Human malignant glioma therapy using anti-alpha(v)beta3 integrin agents. J Neurooncol. 2000;46(2):135–44. doi: 10.1023/a:1006444300504. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10894366. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48(1):151–7. doi: 10.1097/00006123-200101000-00026. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11152340. [DOI] [PubMed] [Google Scholar]
- 13.Nabors L, Rosenfeld S, Mikkelsen T, et al. NABTT 9911: a phase I trial of EMD 121974 for treatment of patients with recurrent malignant gliomas. Neuro-oncology. 2004;6(4):379. [Google Scholar]
- 14.Reardon D, Fink K, Nabors B, Cloughesy T, Plotkin S, Schiff D, et al. Phase IIa trial of cilengitide (EMD121974) single-agent therapy in patients (pts) with recurrent glioblastoma (GBM): EMD 121974–009. J Clin Oncol. 2007;25(Suppl. 18):2002. [Google Scholar]
- 15.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572. doi: 10.1200/JCO.1999.17.8.2572. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10561324. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–6. doi: 10.1212/WNL.0b013e3181bc0184. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19822869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25(13):1651–7. doi: 10.1200/JCO.2006.06.6514. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17470857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stupp R, Goldbrunner B, Neyns B, Schlegel U, Clement P, Grabenbauer G, et al. Phase I/IIa trial of cilengitide (EMD121974) and temozolomide with concomitant radiotherapy, followed by temozolomide and cilengitide maintenance therapy in patients (pts) with newly diagnosed glioblastoma (GBM) J Clin Oncol. 2007;25(Suppl. 18):2000. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 19.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39(7):917–26. doi: 10.1016/s0959-8049(03)00057-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12706360. [DOI] [PubMed] [Google Scholar]
- 20.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–7. doi: 10.1200/JCO.2008.16.7510. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18981465. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Phillips P, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol. 2008;26(6):919–24. doi: 10.1200/JCO.2007.14.1812. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18281665. [DOI] [PubMed] [Google Scholar]
- 22.Maurer GD, Tritschler I, Adams B, Tabatabai G, Wick W, Stupp R, et al. Cilengitide modulates attachment and viability of human glioma cells, but not sensitivity to irradiation or temozolomide in vitro. Neuro Oncol. 2009;11(6):747–56. doi: 10.1215/15228517-2009-012. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19221171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabors LB, Mikkelsen T, Batchelor TT, Lesser G, Rosenfeld M, Ye X, et al. NABTT 0306: A randomized phase II trial of EMD 121974 in conjunction with concomitant and adjuvant temozolomide with radiation therapy in patients with newly diagnosed glioblastoma multiforme (GBM) J Clin Oncol. 2009;27(15s) abstr. 2001. [Google Scholar]


