Abstract
Intracellular vitamin C, or ascorbic acid, has been shown to prevent the apoptosis of cultured vascular pericytes under simulated diabetic conditions. We sought to determine the mechanism by which ascorbate is transported into pericytes prior to exerting this protective effect. Measuring intracellular ascorbate, we found that pericytes display a linear uptake over 30 minutes and an apparent transport Km of 21 μM, both of which are consistent with activity of the Sodium-dependent Vitamin C Transporter 2 (SVCT2). Uptake of both radiolabeled and unlabeled ascorbate was prevented by inhibiting SVCT2 activity, but not by inhibiting the activity of GLUT-type glucose transporters, which import dehydroascorbate to also generate intracellular ascorbate. Likewise, uptake of dehydroascorbate was prevented with the inhibition of GLUTs, but not by inhibiting the SVCT2, indicating substrate specificity of both transporters. Finally, presence of the SVCT2 in pericytes was confirmed by western blot analysis, and immunocytochemistry was used to localize it to the plasma membrane and intracellular sites. Together, these data clarify previous inconsistencies in the literature, implicate SVCT2 as the pericyte ascorbate transporter, and show that pericytes are capable of concentrating intracellular ascorbate against a gradient in an energy- and sodium-dependent fashion.
Keywords: SVCT2, transport kinetics, ascorbic acid, pericytes
1. Introduction
Pericytes surround the endothelium of venules, post-capillary venules, and capillaries [1]. They are smooth muscle-derived cells that interact with endothelial cells to regulate blood flow and to tighten endothelial barrier permeability [2-5]. Particularly in the brain and retina, pericytes help to maintain a tight blood-brain barrier and preserve vascular integrity. For example, dropout of pericytes is one of the earliest changes of diabetic retinopathy [6-8], leading to endothelial cell dysfunction and subsequent extravasation of serum proteins into the retinal interstitium [9-12].
We recently evaluated human brain pericytes exposed to a diabetic milieu of high glucose-induced oxidative stress, mediated largely by activation of the Receptor for Advanced Glycation End-products (RAGE). With the daily addition of 100 μM ascorbate, an increase in intracellular ascorbate from 0.8 mM to 2-3 mM was shown to prevent apoptosis in these cultured pericytes [13]. This suggests that intracellular ascorbate accumulated against a concentration gradient, but the mechanism was not evaluated.
In contrast, a previous study using primary bovine retinal pericytes did not find that 5 μM radioactive ascorbate was concentrated against a gradient [14]. This was surprising because most non-epithelial cultured cells transport ascorbate in a sodium- and energy-dependent manner using the Sodium-dependent Vitamin C Transporter 2 (SVCT2) [15,16]. This co-transporter imports ascorbate against a gradient by coupling its entry with sodium influx, thus maintaining electroneutrality and utilizing energy derived from the inward-to-outward sodium gradient generated by the trans-membrane Na/K ATPase [17,18]. The SVCT2 shows saturable, high-affinity ascorbate uptake (apparent Km 20-50 μM). It is inhibited by removal of extracellular sodium, by energy depletion with ouabain, and by several anion transport inhibitors, such as sulfinpyrazone [16]. Ascorbate uptake on the SVCT2 is not inhibited by D-glucose [19-21]. In contrast, pericyte ascorbate uptake was inhibited by D-glucose and its derivatives [14], which further brings into question how pericytes transport ascorbate.
Dehydroascorbate (DHA), the two-electron oxidized form of ascorbate, is a substrate for the ubiquitous GLUT-type facilitative transporters but not for the SVCT2 [22,23]. DHA uptake on GLUTs is rapid compared with that of ascorbate on the SVCT2 and is inhibited by glucose and its transported derivatives, but not by energy depletion or sodium removal [21]. Although not transported on the SVCT2, DHA has recently been shown to inhibit radioactive ascorbate uptake in several immortalized cell lines, an effect that is half-maximal at about 20 μM DHA [24]. The mechanism of this inhibition is unknown, but was also observed at low millimolar DHA concentrations in primary culture pericytes by Khatami [14]. Whether this effect persists at lower, physiologically relevant DHA concentrations remains to be determined.
To define the role of the SVCT2 in pericyte ascorbate transport, to resolve the discrepancy between Khatami's study and the established function of the SVCT2 in other cells, and to assess whether DHA inhibits ascorbate transport, we studied SVCT2 expression and ascorbate transport and accumulation in human brain microvascular pericytes.
2. Materials and methods
2.1 Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied 3-O-methylglucose, ascorbate, ascorbate oxidase, N-2-hydroxyethylpiperazine N’-2-ethanesulfonic acid (Hepes), ouabain and sulfinpyrazone. Perkin-Elmer Life and Analytical Sciences, Inc. (Boston, MA) supplied the [1-14C]ascorbic acid (4.8 μCi/mmol).
2.2 Cell Culture
Human brain vascular pericytes were obtained from ScienCell Research Laboratories (catalog #1200, Carlsbad, CA) and cultured in Pericyte Medium with included supplements (catalog #1201). Cells were cultured on plates coated with poly-L-lysine at 37 °C in humidified air containing 5% CO2. Cells were used within 3-10 passages.
2.3 Assay of intracellular ascorbate
To measure intracellular pericyte ascorbate, near-confluent cells in 6-well plates were rinsed with Krebs-Ringer Hepes buffer (KRH; 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4) and lysed with 25 % metaphosphoric acid (w/v). Following neutralization with 3 volumes of 100 mM Na2HPO4 and 0.05 mM EDTA (pH 8.0), cells were scraped from the plate with a rubber spatula. Lysates were centrifuged at 3 °C for 1 min at 13,000 × g, and the supernatant was taken for assay of ascorbate. Assay of ascorbate was performed in duplicate by ion-pair high-performance liquid chromatography with electrochemical detection as previously described [25]. Intracellular ascorbate concentrations were calculated based on the intracellular distribution space of 3-O-methylglucose in pericytes, measured as previously described in endothelial cells [26]. This pericyte distribution space was 6.1 ± 1.6 μl/mg protein (N = 6 determinations).
2.4 Radioactive ascorbate uptake
Pericytes cultured to confluence in 12-well plates were treated as described for 30 min, followed by addition of up to 10 μM [1-14C]ascorbate or [1-14C]DHA. [1-14C]DHA was generated by treating [1-14C]ascorbate with 2 unit/ml ascorbate oxidase for 5 min. Following incubation with radioactive ascorbate or DHA for 30 min at 37 °C, buffer was removed and the cell layer was rinsed with ice-cold KRH. Cells were then treated with 1 ml of 0.05 N NaOH, scraped from the plate, and the extract was added to 5 ml Ecolume liquid scintillation fluid (catalog #882470 ICN, Costa Mesa, CA) and mixed. After ≥ 1 h, sample radioactivity was measured in duplicate with a Packard CA-2200 liquid scintillation counter.
2.5 Immunoblotting of the SVCT2
Near-confluent pericytes were lysed with RIPA Buffer (catalog # R0278 Sigma/Aldrich), and immunoblotting was performed as described previously [27]. Briefly, protein yield was quantified using a BCA assay (catalog # 23225, Pierce Biotechnology, Rockford, IL). Normalized samples were prepared with Laemmli sample buffer [28] containing 5% β-mercaptoethanol and electrophoresed on a 4-20% sodium dodecyl sulfate-polyacrylamide gel. Following transfer to polyvinylidene difluoride membrane, binding of antibodies against SVCT2 (catalog # 9926, Santa Cruz Biotechnology, Santa Cruz, CA; 1:900) and actin (catalog # 1616-R; 1:10,000) was detected with enhanced chemiluminescence reagent (catalog # NEL105001EA, Perkin Elmer) using 1:5000 horseradish peroxidase-conjugated secondary antibodies (catalog # W4011, Promega Corporation, Madison, WI; catalog # A5420, Sigma). As a negative control, anti-SVCT2 was pre-incubated overnight with its immunizing peptide at 5x the antibody concentration (catalog # 9926 P, Santa Cruz) before probing the membrane. Immunoblots were also carried out using a primary antibody against SVCT1 (catalog # 9924, Santa Cruz; 1:900).
2.6 Immunofluorescence Microscopy
Cells were grown on glass coverslips coated with poly-L-lysine and fixed with 4% formaldehyde for 15 minutes. Cells were blocked with 10% donkey serum, permeabilized with 0.1% saponin, and probed for SVCT2 (Santa Cruz #9926; 1:200) and neural/glial antigen-2 (NG2, catalog # ab50009, Abcam, Cambridge, MA; 1:200). Following incubation with Alexa Fluor 488 and Alexa Fluor 555-conjugated secondary antibodies (catalog #s A11055 and A31570, Life Technologies, Carlsbad, CA; 1:500), nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI). Cells were visualized using an Olympus FV1000 inverted confocal microscope (Olympus Corporation, Tokyo, Japan; Vanderbilt Cell Imaging Shared Resource).
2.7 Data Analysis
Results are shown as mean + standard error. Statistical comparisons were made using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA). Differences between treatments were assessed by one-way ANOVA with replication with post-hoc testing using Tukey's or Dunnett's test, as appropriate.
3. Results
3.1 Transport of ascorbate and DHA by human brain microvascular pericytes
To determine whether ascorbate transport in pericytes reflects that expected of the SVCT2, transport kinetics and inhibitor studies were performed. The commercial culture medium initially contained 100 μM ascorbate, but this was depleted with storage of the medium for ~2 weeks at 3 °C before use in culture (cold-stored), resulting in low intracellular ascorbate concentrations at baseline (Fig. 1, A and B). Brain microvascular pericytes readily took up added ascorbate (100 μM) with a linear time course over 30 min (Fig. 1A, circles). In contrast, uptake and reduction of 100 μM DHA to ascorbate was much more rapid than uptake of ascorbate, reaching a plateau beginning after 30 min of incubation (Fig. 1A, squares). Using the 30 min time point, addition of increasing amounts of ascorbate resulted in saturable uptake, with an apparent Km of 21 ± 11 μM and a calculated maximal intracellular ascorbate concentration of 0.9 mM (Fig. 1B, circles). On the other hand, uptake and reduction of DHA to ascorbate was not saturable over the concentration range used (Fig. 1B, squares).
Figure 1. Ascorbate loading of human pericytes.
Panel A. Cells cultured with cold-stored medium on 6-well plates were treated with 100 μM ascorbate (circles) or DHA (squares) for the times indicated. Cells were rinsed with KRH before removal from the plate and assay of ascorbate as described in Methods. Panel B. Cells cultured for 6 days with cold-stored medium were treated for 30 min with the indicated concentrations of ascorbate or DHA and taken for assay of intracellular ascorbate as described in Panel A. The data were fit by nonlinear (ascorbate, circles) or linear (DHA, squares) regression, presented as solid lines through the data points. Panel C. Cells cultured in fresh medium were treated with the indicated concentrations of DHA, with (circles) or without (squares) 100 μM ascorbate, for 30 min and taken for assay of intracellular ascorbate as in Panel A. “*” indicates p < 0.05 compared to the corresponding treatment without ascorbate. Panel D. Pericytes cultured in cold-stored medium were rinsed with KRH and treated with 10 μM [1-14C]ascorbate, followed immediately by the indicated concentration of DHA. After 30 min, cells were rinsed with KRH and taken for radioactive counting as described under Methods. Results were expressed as a fraction of the control uptake. N = 6 for all data points.
To determine whether pericytes can concentrate ascorbate in cells with higher basal intracellular ascorbate, cells were cultured in fresh medium instead of cold-stored medium, and DHA was added to further increase the intracellular ascorbate concentration. The basal intracellular ascorbate concentration was 0.4 mM (Fig. 1C, square at zero DHA). Nonetheless, treatment of cells with 100 μM ascorbate alone significantly increased ascorbate (Fig. 1C, compare circle and square at zero DHA). Further, this effect persisted as intracellular ascorbate was increased by simultaneous treatment with 50 or 100 μM DHA (Fig. 1C, compare squares and circles with DHA treatment). These results show clearly that the cells can concentrate ascorbate even when intracellular concentrations of ascorbate are over 1.5 mM. It has been shown in several cell lines that low concentrations of DHA actually inhibit radioactive ascorbate uptake [24]. However, the results in Fig. 1C show that the increase in uptake due to 100 μM ascorbate is not prevented by 50 or 100 μM DHA. To determine whether DHA might inhibit uptake of a 10-fold lower concentration of radioactive ascorbate, we incubated cells with 10 μM [1-14C]ascorbate in the presence of increasing concentrations of DHA. As shown in Fig. 1D, DHA had no effect on ascorbate uptake in these cells.
3.2 Differential inhibition of ascorbate and DHA uptake
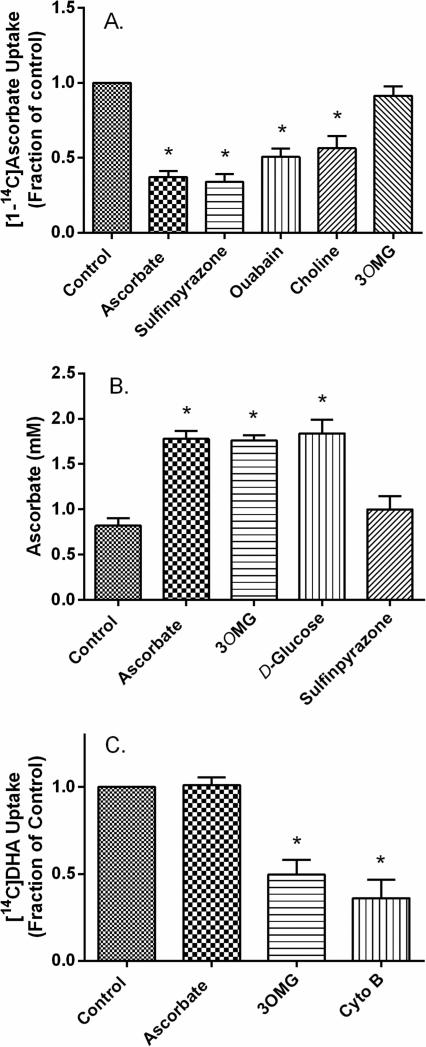
Effects of several known inhibitors of ascorbate and glucose uptake were evaluated on ascorbate and DHA uptake with the experiments shown in Fig. 2. Uptake of radiolabeled ascorbate (10 μM) was inhibited by ascorbate itself, by the anion channel inhibitor sulfinpyrazone, by inhibition of the Na/K ATPase with ouabain, and by substitution of choline for sodium in the KRH present during uptake (Fig. 2A). Radiolabeled ascorbate uptake was not inhibited by 30 mM 3-O-methylglucose. To confirm these findings, additional experiments were performed following the uptake of unlabeled ascorbate in cells cultured in fresh, ascorbate-containing medium. Although these cells had relatively high basal ascorbate concentrations (~0.8 mM), a 30 min incubation with 100 μM ascorbate doubled its intracellular concentration (Fig. 2B, first two bars). This uptake was not affected by treatment with 30 mM D-glucose or 3-O-methylglucose, but was prevented by 2 mM sulfinpyrazone (Fig. 2B). These results support the notion that uptake of either a low concentration of radiolabeled ascorbate or a 10-fold higher concentration of unlabeled ascorbate is mediated by the SVCT2 in these cells.
Figure 2. Inhibitor effects on radiolabeled ascorbate and DHA uptake.
Panel A. [1-14C]Ascorbate uptake. Using cultured pericytes, cold-stored medium was exchanged for KRH containing either 124 mM sodium chloride (first 5 bars) or 128 mM choline chloride instead of sodium (last bar). Cells in sodium-containing KRH were then incubated for 30 min with no additions (Control), with 1 mM ascorbate, with 2 mM sulfinpyrazone, with 300 μM ouabain, or with 30 mM 3-O-methylglucose (3OMG). After 30 min, 10 μM [1-14C]ascorbate was added to cells for an additional 30 minutes, and then cells were prepared for radioactive counting as described under Methods. Panel B. Uptake of unlabeled ascorbate. Cells cultured in fresh medium were untreated (first bar) or treated with 100 μM ascorbate, without or in addition to 3-O-methylglucose (3OMG, 30 mM), D-glucose (30 mM), or sulfinpyrazone (2 mM). After 30 min, cells were rinsed and harvested for assay of intracellular ascorbate. Panel C. [1-14C]DHA uptake. Cells cultured in cold-stored medium were treated for 30 min with the inhibitors and under the conditions noted in Panel A. Cytochalasin B (Cyto B, 10 μM) or 3-O-methylglucose (3OMG, 30 mM) were added where noted. [1-14C]DHA was prepared as described under Methods and its uptake at a final concentration of 10 μM was measured as described for [1-14C]ascorbate. Results in all panels are shown from 4-6 experiments, with those in A and C normalized in each experiment to an untreated control. “*” indicates p < 0.05 compared to the respective control.
When radiolabeled ascorbate was converted to radiolabeled DHA by the action of ascorbate oxidase before and during addition of ascorbate to the cells, its uptake was not inhibited by 300 μM ascorbate or by 1 mM sulfinpyrazone (Fig. 2C, first two bars). On the other hand, it was significantly decreased by the GLUT-type transport inhibitors 3-O-methylglucose and cytochalasin B at concentrations known to inhibit GLUT-type transporters (Fig. 2C, last 2 bars). Together with the radiolabeled ascorbate uptake data, these results extend those of Fig. 1, showing that the two forms of ascorbate were taken up by different transport mechanisms.
3.3 Pericytes express the SVCT2
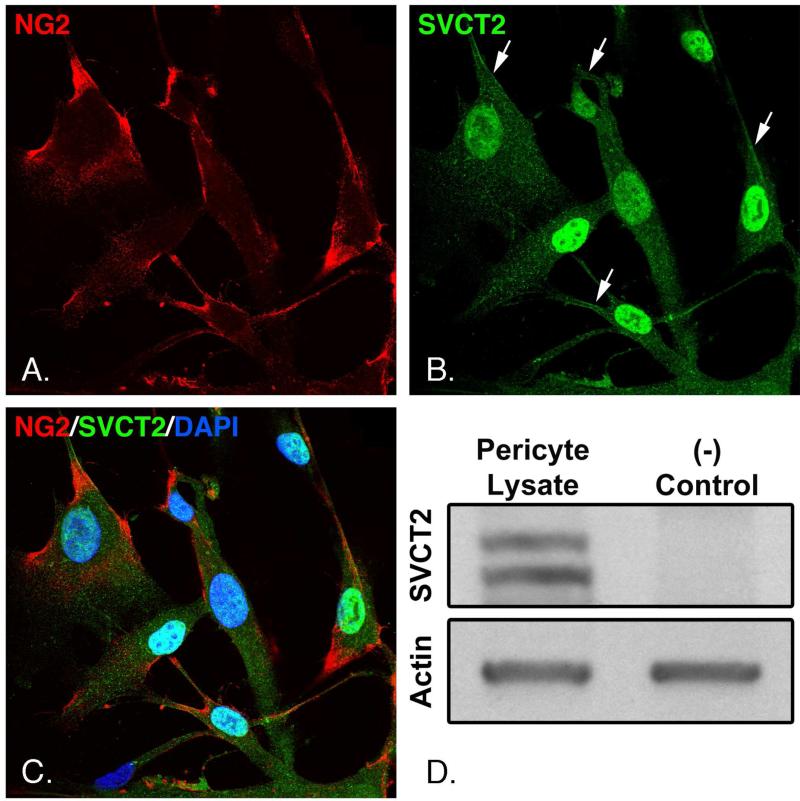
To assess the presence and cellular distribution of the SVCT2, cultured pericytes were fixed and immunofluorescently stained for the SVCT2, the pericyte marker NG2 [29] and the nuclear stain DAPI, as shown in Fig. 3. NG2 was present in the cell periphery and in proximal cell extensions (Fig. 3A). The SVCT2 was present in punctate areas throughout the cells, with areas of plasma membrane staining particularly evident in some cell extensions (Fig. 3B, arrows). Additionally, NG2 and SVCT2 appeared to overlap in some plasma membrane areas (Fig. 3C).
Figure 3. Presence of the SVCT2 in pericytes.
Panels A-C. Pericytes cultured on coverslips were fixed and probed with antibodies against the SVCT2 and NG2 as described under Methods. Cells were counterstained with DAPI to visualize nuclei and resolved by confocal microscopy at 600x magnification. Panel D. Pericyte lysates were subjected to gel electrophoresis and transferred to PVDF membranes as described under Methods. Membranes were probed with antibody against the SVCT2 (left lane), or with the same antibody pre-incubated with 5x its immunizing peptide as a negative control (right lane). The same membranes were probed for actin to confirm equal protein loading. Results shown are representative of 3 experiments.
Western blot analysis was performed to confirm the presence of SVCT2 and verify its antibody's specificity. When probed with anti-SVCT2, two bands were found between 50 and 75 kDa (Fig. 3D, left lane). Both bands completely disappeared when the SVCT2 antibody was incubated with its immunizing peptide before probing the membrane (Fig 3D, right lane). A blot for actin was performed on the same membrane to confirm equal protein loading between lanes. On the other hand, the alternative SVCT isoform, SVCT1, was not observed in pericytes (data not shown).
4. Discussion
The present studies suggest that ascorbate is taken up by human brain endothelial pericytes on the ascorbate transporter SVCT2. This is supported by the observed high-affinity transport kinetics, by transport inhibition with known inhibitors of the SVCT2, and by the demonstrated presence of the SVCT2 in these cells. Although ascorbate could enter pericytes after extracellular oxidation as DHA on pericyte GLUT-type glucose transporters, this seems unlikely based on several findings. First, uptake and reduction of DHA to ascorbate was much more rapid than observed for ascorbate, which would fit with an abundant high-capacity GLUT-type transporter. Second, DHA uptake and reduction was not saturated by increasing DHA concentrations as high as 300 μM, which would also be expected from the low-millimolar Km of the GLUT-type transporters for glucose (or DHA). Third, DHA uptake and reduction was inhibited by both 3-O-methylglucose and cytochalasin B, which are known inhibitors of GLUT-type transporters [30,31]. In contrast, ascorbate uptake was not inhibited by 3-O-methylglucose.
These findings contrast with several results reported by Khatami [14] in cultured bovine retinal pericytes. Although both studies found a relatively high affinity for ascorbate (Km = 76 μM in the Khatami study, 21 μM in the present study), Khatami found ascorbate uptake was inhibited by substrates for the GLUTs. We did not find 30 mM 3-O-methylglucose to inhibit either 10 μM radiolabeled or 100 μM unlabeled ascorbate uptake. The differing results between this study and that of Khatami could be due to use of bovine retinal versus human brain pericytes, but more likely relate to other factors. First, apparent inhibition of radiolabeled ascorbate uptake by glucose derivatives in the Khatami study could be due to oxidation of radioactive ascorbate to radioactive DHA outside the cells, making it susceptible to inhibition by glucose and its derivatives. This caveat was recognized by Khatami, who found that 25-30% of even a relatively high medium concentration of ascorbate (200 μM) was oxidized to DHA over 40 min [14]. At lower radioactive ascorbate concentrations (5-50 μM) used in most of the Khatami studies, this oxidation would have been relatively greater. Thus, glucose-inhibitable uptake of radiolabeled DHA on glucose transporters may have accounted for a significant fraction of apparent radioactive ascorbate uptake.
A second difference was failure of pericytes to accumulate 5 μM radioactive ascorbate against a concentration gradient after 40 min in the Khatami study. In contrast, we found unlabeled ascorbate to accumulate against a concentration gradient over 15 – 300 μM extracellular ascorbate. We have no explanation for this difference, but would note that steady-state intracellular ascorbate concentrations in culture were also higher than those known to be present in the media and depended on the medium ascorbate concentration.
A third difference we observed was the failure of DHA to inhibit uptake of both unlabeled and radiolabeled ascorbate. This inhibition was reported previously in bovine retinal pericytes [14] and immortalized cell lines [24]. Failure of DHA to inhibit ascorbate uptake in our studies cannot be explained by rapid decomposition of DHA, since uptake and reduction of the same lot of DHA over 30 min resulted in substantial intracellular ascorbate accumulation. On the other hand, commercial DHA is known to have impurities and to contain low amounts of ascorbate that could have contributed to inhibition of high affinity ascorbate uptake in the previous studies. Future studies in other cell types expressing the SVCT2 will need to clarify this issue further.
Human brain microvascular pericytes expressed the SVCT2 in culture, as assessed by both immunocytochemistry and immunoblotting. The SVCT1 was not found by immunoblotting, suggesting that the SVCT2 is the major pericyte ascorbate transporter. The SVCT2 appeared to be present in the plasma membrane, where it would account for the uptake features we observed in this study. However, much of it was intra-nuclear and in discreet particles, as shown previously in other primary cell types [32,33], which may be due to functional expression in mitochondria [34,35].
In conclusion, human brain microvascular pericytes import ascorbate primarily on the SVCT2, concentrating intracellular ascorbate against a gradient in an energy- and sodium-dependent fashion. Pericytes also import the less-abundant DHA using GLUTs, forming intracellular ascorbate after reduction. Both transporters function independently and are unaffected by the others’ substrates. Thus, in vivo, SVCT2 is likely a key player in protecting pericytes from diabetes-induced oxidative stress and RAGE activation that causes apoptosis.
Supplementary Material
HIGHLIGHTS.
Human brain pericytes accumulated ascorbate against a concentration gradient.
Ascorbate transport had kinetic features and inhibitor sensitivity of the SVCT2.
Pericyte ascorbate uptake was not inhibited by glucose transporter ligands.
Pericytes expressed the SVCT2 and not the SVCT1.
SVCT2 localized to pericyte plasma membranes, nuclei, and cytoplasmic organelles.
Acknowledgement
This work was supported by National Institutes of Health grant DK 50435.
Abbreviations
- DAPI
4',6-diamidino-2-phenylindole
- DHA
dehydroascorbate
- GLUT
facilitative glucose transporter
- Hepes
N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid
- KRH
Krebs-Ringer Hepes
- NG2
neural/glial antigen-2
- RAGE
receptor for advanced glycation end products
- SVCT
sodium-dependent vitamin C transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc. Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 2.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol. Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci. Methods. 2011;199:223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Wisniewska-Kruk J, Hoeben KA, Vogels IM, Gaillard PJ, Van Noorden CJ, Schlingemann RO, Klaassen I. A novel co-culture model of the blood-retinal barrier based on primary retinal endothelial cells, pericytes and astrocytes. Exp. Eye Res. 2012;96:181–190. doi: 10.1016/j.exer.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira F. Pericytes in diabetic retinopathy. Br. J Ophthalmol. 1966;50:134–143. doi: 10.1136/bjo.50.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Yanoff M, Liu X, Ye X. Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med. J (Engl.) 1997;110:659–663. [PubMed] [Google Scholar]
- 8.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimura K, Umeda F, Yamashita T, Kobayashi K, Hashimoto T, Nawata H. Effects of glucose and an aldose reductase inhibitor on albumin permeation through a layer of cultured bovine vascular endothelial cells. Horm. Metab Res. 1995;27:442–446. doi: 10.1055/s-2007-979998. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T, Mimura K, Umeda F, Kobayashi K, Hashimoto T, Nawata H. Increased transendothelial permeation of albumin by high glucose concentration. Metabolism. 1995;44:739–744. doi: 10.1016/0026-0495(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 11.Stauber WT, Ong SH, McCuskey RS. Selective extravascular escape of albumin into the cerebral cortex of the diabetic rat. Diabetes. 1981;30:500–503. doi: 10.2337/diab.30.6.500. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzi M, Healy DP, Hawkins R, Printz JM, Printz MP. Studies on the permeability of the blood-brain barrier in experimental diabetes. Diabetologia. 1986;29:58–62. doi: 10.1007/BF02427282. [DOI] [PubMed] [Google Scholar]
- 13.May JM, Jayagopal A, Qu ZC, Parker WH. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem. Biophys. Res. Commun. 2014 doi: 10.1016/j.bbrc.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatami M, Li WY, Rockey JH. Kinetics of ascorbate transport by cultured retinal capillary pericytes. Inhibition by glucose. Invest Ophthalmol. Vis. Sci. 1986;27:1665–1671. [PubMed] [Google Scholar]
- 15.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 16.May JM. The SLC23 family of ascorbate transporters: ensuring that you get and keep your daily dose of vitamin C. Br. J Pharmacol. 2011;164:1793–1801. doi: 10.1111/j.1476-5381.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X-Z, Wang YX, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 18.Hediger MA. New view at C. Nat. Med. 2002;8:445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 19.May JM, Li L, Hayslett K, Qu ZC. Ascorbate transport and recycling by SH-SY5Y neuroblastoma cells: Response to glutamate toxicity. Neurochem. Res. 2006;31:785–794. doi: 10.1007/s11064-006-9077-z. [DOI] [PubMed] [Google Scholar]
- 20.Talluri RS, Katragadda S, Pal D, Mitra AK. Mechanism of L-ascorbic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr. Eye Res. 2006;31:481–489. doi: 10.1080/02713680600693629. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JX. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 22.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 23.Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999;460:480–484. doi: 10.1016/s0014-5793(99)01393-9. [DOI] [PubMed] [Google Scholar]
- 24.Fiorani M, Azzolini C, Guidarelli A, Cerioni L, Cantoni O. A novel biological role of dehydroascorbic acid: Inhibition of Na(+)-dependent transport of ascorbic acid. Pharmacol. Res. 2014;84:12–17. doi: 10.1016/j.phrs.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 25.May JM, Qu Z-C, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 26.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 27.Meredith ME, Harrison FE, May JM. Differential regulation of the ascorbic acid transporter SVCT2 during development and in response to ascorbic acid depletion. Biochem. Biophys. Res. Commun. 2011;414:737–742. doi: 10.1016/j.bbrc.2011.09.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. J. Mol. Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 29.Schallek J, Geng Y, Nguyen H, Williams DR. Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Invest. Ophthalmol. Vis. Sci. 2013;54:8237–8250. doi: 10.1167/iovs.13-12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carruthers A. The glucose transporter reconsidered. Trends Biochem. Sci. 1988;13:426–428. doi: 10.1016/0968-0004(88)90213-7. [DOI] [PubMed] [Google Scholar]
- 31.Due AD, May JM. Kinetic features of the human GLUT1 transporter are retained when expressed in Xenopus oocytes. Diabetes. 1993;42:168A. [Google Scholar]
- 32.García ML, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005;50:32–47. doi: 10.1002/glia.20133. [DOI] [PubMed] [Google Scholar]
- 33.Gess B, Lohmann C, Halfter H, Young P. Sodium-dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of L-ascorbic acid into Schwann cells. Glia. 2009 doi: 10.1002/glia.20923. [DOI] [PubMed] [Google Scholar]
- 34.Azzolini C, Fiorani M, Cerioni L, Guidarelli A, Cantoni O. Sodium-dependent transport of ascorbic acid in U937 cell mitochondria. IUBMB. Life. 2013;65:149–153. doi: 10.1002/iub.1124. [DOI] [PubMed] [Google Scholar]
- 35.Munoz-Montesino C, Roa FJ, Pena E, Gonzalez M, Sotomayor K, Inostroza E, Munoz CA, Gonzalez I, Maldonado M, Soliz C, Reyes AM, Vera JC, Rivas CI. Mitochondrial ascorbic acid transport is mediated by a low-affinity form of the sodium-coupled ascorbic acid transporter-2. Free. Radic. Biol. Med. 2014;70:241–254. doi: 10.1016/j.freeradbiomed.2014.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.