Abstract
Angiogenesis is required for tumour growth and is induced principally by VEGF-A. VEGF-A pre-mRNA is alternatively spliced at the terminal exon to produce two families of isoforms, pro- and anti-angiogenic, only the former of which is upregulated in prostate cancer. In renal epithelial cells and colon cancer cells, the choice of VEGF splice isoforms is controlled by the splicing factor SRSF1, phosphorylated by SRPK1. Immunohistochemistry staining of human samples revealed a significant increase in SRPK1 expression both in prostate intra-epithelial neoplasia lesions as well as malignant adenocarcinoma compared to benign prostate tissue. We therefore tested the hypothesis that the selective upregulation of pro-angiogenic VEGF in prostate cancer may be under the control of SRPK1 activity. A switch in the expression of VEGF165 towards the anti-angiogenic splice isoform, VEGF165b, was seen in PC-3 cells with SRPK1 knock-down (KD). PC-3 SRPK1-KD cells resulted in tumours that grew more slowly in xenografts, with decreased microvessel density. No effect was seen as a result of SRPK1-KD on growth, proliferation, migration and invasion capabilities of PC-3 cells in vitro. Small molecule inhibitors of SRPK1 switched splicing towards the anti-angiogenic isoform VEGF165b in PC3 cells and decreased tumour growth when administered intraperitoneally in an orthotopic mouse model of prostate cancer. Our study suggests that modulation of SRPK1 and subsequent inhibition of tumour angiogenesis by regulation of VEGF splicing can alter prostate tumour growth and supports further studies into the use of SRPK1 inhibition as a potential anti-angiogenic therapy in prostate cancer.
Keywords: prostate cancer, angiogenesis, VEGF alternative splicing, SRPK1
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed non-skin cancer and the 3rd most common cause of cancer death in men in the Western world (1-4). While many men have indolent disease that can be cured with localized therapies, a significant minority of men will relapse and eventually progress to castration-resistant PCa. The majority of lethal PCa is due to spread through castration-resistant metastasis (5, 6).
Alongside the long-established treatments for PCa including prostatectomy, radiotherapy and androgen-deprivation there is currently a list of therapies that have been approved recently or are still in development that target various pathogenic processes shown to be important for PCa progression – e.g androgen-related, targeting several signaling pathways (PI3K-Akt-mTOR, IGFIR, hedgehog), immunomodulators and vaccines, epigenetic modifiers as well as bone-specific treatments (7-9).
Angiogenesis has been described as being essential for tumour growth (10). It is today recognized as one of the hallmarks of cancer (11) and subject to intense investigations for therapeutic control. There are many studies that have suggested angiogenesis to be important in PCa: VEGF is expressed at high levels in human PCa cell lines (12, 13); urine and plasma levels of VEGF are increased in advanced stages of PCa (14-16) and microvessel density correlates with PCa metastasis and Gleason score (17). However, all of these studies show correlations and do not demonstrate causality i.e. is angiogenesis driving the prostate cancer progression and spread or is it, for example, an associated phenomenon resulting from the hypoxic environment of tumour growth? The most definitive answer to this question may come only from studies assessing the efficacy of anti-angiogenic therapy in PCa patients.
Based on this rationale several multi-centre studies have been initiated using combinations of adjuvant anti-angiogenic therapies that target angiogenesic molecules e.g VEGF or VEGFRs, angiopoetins [reviewed in (18)]. To date, the results are discouraging for angiogenesis specific agents - e.g a phase III study recently completed that used bevacizumab (humanized antibody against VEGF) in addition to docetaxel and prednisone, failed to show a significant improvement in overall survival (19). Several other phase III studies of anti-VEGF based strategies have failed to improve survival in unselected patients with metastatic castration-resistant prostate cancer (mCRCP) including sunitinib (a VEGF/PDGF tyrosine kinase inhibitor) and aflibercept (VEGF trap) (18). Several explanations have been advanced for the failure of these studies including excess treatment-related toxicities, lack of patient selection and dilution of treatment effect given the notorious heterogeneity of PCa at every level (clinical, histopathological or molecular)(19).
More than 10 years ago a 3′ alternative splicing event in the terminal exon of VEGF-A pre-mRNA was described that does not change the number of amino-acids in the VEGF protein but rather the sequence of the last six amino-acids (20). This gives rise to a family of VEGF isoforms (VEGFxxxb, with the dominant isoform VEGF165b) that act as antagonist/partial-agonist to the main VEGF receptor (VEGFR2) (21). VEGF165b has been shown in several studies to be anti-angiogenic (22-24). An alternative hypothesis for the failure of anti-angiogenic therapies in PCa which has not been considered thus far, is that the above-mentioned studies use treatments that inhibit all VEGF isoforms regardless of their pro/anti-angiogenic potential and/or do not select patients based on these VEGF isoforms. Indeed, it has been shown, for example, that pro/anti-angiogenic VEGF isoform balance can predict response to anti-VEGF antibodies in animals (25) and humans in colon carcinoma (26).
We recently described an essential pathway that regulates the choice of pro/anti-angiogenic splicing isoforms that involves the serine-arginine protein kinase 1 (SRPK1) and splicing factor SRSF1 (27, 28) in renal epithelial cells and in colon carcinoma. We have shown that knock-down of SRPK1 in colon adenocarcinoma cells (LS174t) increases the levels of anti-angiogenic splicing isoform VEGF165b and inhibits tumour growth in a xenograft model. SRPK1 is a kinase, a class of proteins that have proven recently to be exceptional targets for oncology therapeutic development. Our previous findings suggested that selective SRPK inhibitors could act as anti-angiogenic agents and novel cancer treatments in cancers where SRPK1 was active.
The aim of this study was therefore to assess whether the SRPK1-SRSF1 axis is upregulated in PCa human samples and cell lines and, if so, test the hypotheses that i) manipulation of VEGF165b levels and SRPK1 axis in PCa cells is able to decrease tumour growth in xenografts models and ii) that this is primarily due to an effect on VEGF splicing. The ultimate goal was to provide basic proof-of-principle studies for use of SRPK1 inhibitors in PCa treatment.
Results
SRPK1 and SRSF1 expression in human PCa
In a previous study (23) we reported that the anti-angiogenic VEGF165b splice isoform is strongly down-regulated in malignant human prostate tissue compared to benign tissue, based on transurethral sections of the prostate (TURP) samples. The choice of pro/anti-angiogenic VEGF isoforms has been shown in colonic and renal epithelial cells to be under the control of SRSF1 and SRPK1 and knockdown of SRPK1 switched splicing from VEGF165 to VEGF165b in colon carcinoma cells (LS174t)(27). Here we show that in a mouse subcutaneous xenograft model of prostate cancer using human PC-3 cells, intraperitoneal administration of rhVEGF165b is also able to decrease prostate tumour growth in a similar manner to the anti-VEGF antibody bevacizumab and this is associated with a decrease in microvessel density in tumours (Supplementary Figure 1). We therefore considered that the expression level of SRPK1 and SRSF1 in human prostate cancer tissue may be able to control VEGF-A165b expression and thus be a potential target for therapy.
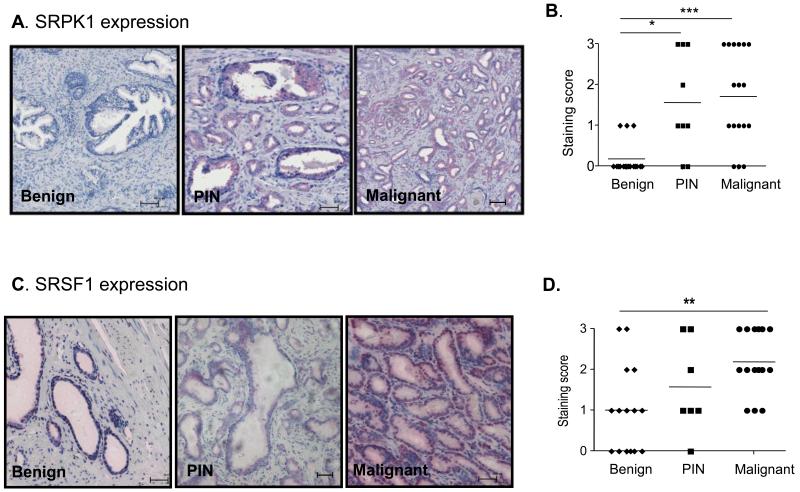
Samples from 17 patients with radical prostatectomy were immuno-stained for SRPK1 and SRSF1 (Figure 1). Staining intensities were scored by a urological pathologist in benign areas, PIN lesions, as well as malignant parts of the gland. SRPK1 staining (Figure 1A) was significantly increased in both PIN and malignant regions compared to benign areas of the samples (Figure 1B). SRSF1 staining (Figure 1C) was increased in malignant but not PIN lesions (Figure 1D).
Figure 1. SRPK1 and SRSF1 expression are altered in PIN and malignant prostatectomy samples.
A. Immunohistochemical staining for SRPK1 in benign, PIN and cancerous prostate tissue sections. B. Quantification of the staining intensity from 17 patients (*=p<0.05, ***=p<0.001, Kruskal–Wallis and Dunn’s post–test). C. Examples of SRSF1 staining for benign, PIN and malignant prostate samples. D. Quantification of the staining intensity from 17 patients.
SRPK1 knock-down results in a splicing switch from pro- to anti-angiogenic VEGF isoforms in PCa cells
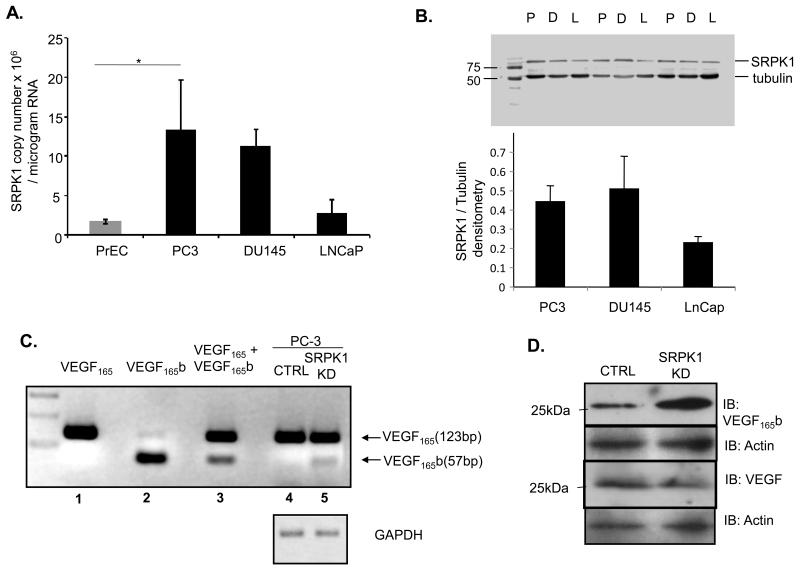
SRPK1 expression was assessed in several PCa cell lines (PC-3, DU145 and LNCaP) in comparison with normal prostate epithelial cells by qRT-PCR (Figure 2A). The highest expression was present in PC-3 cells. At the protein level SRPK1 was also higher in PC-3 and DU145 cells when compared to LNCaP (Figure 2B). This heterogeneity was reflective of PCa samples where SRPK1 is increased in ~ 50% of patients (Figure 1B). We therefore used PC-3 in further studies to understand the role of SRPK1 in those prostate cancers with increased expression.
Figure 2. SRPK1 is increased in prostate cancer cell lines and SRPK1 knockdown switches expression of VEGF165 into VEGF165b in PC-3 cells.
A. SRPK1 expression quantified by qRT-PCR in several PCa cell lines compared to primary prostate epithelial cells (*=p<0.05 PreC vs PC-3, One-way ANOVA). B. Western blot analysis of SRPK1 in PC3 (P), DU145 (D) and LNCaP (L); upper panel – duplicate examples of extracts; lower panel – quantification from three replicates with normalization on tubulin signal for equal loading. C. RT-PCR analysis shows presence of VEGF165b splicing isoforms in PC3 cells with SRPK1-KD (lanes 1, 2, 3 – plasmid controls; lanes 4,5 – RT-PCRs). D. Effect of SRPK1-KD on VEGF165b protein expression in PC3 cells
To establish whether the SRPK1-VEGF splicing regulation was present in PC-3 cells we generated a stable knock-down of SRPK1. PC-3 cells were transduced with lentivirus containing shRNAi to SRPK1 or scrambled shRNAi, selected in puromycin for 3 weeks and mRNA and protein extracted. The extent of knock-down was assessed both by qRT-PCR and Western blotting (Supplementary Figure 2). RT-PCR analysis, Western blot and ELISA demonstrated that there was a switch towards the VEGF165b isoform in cells with stable SRPK1-KD (Figure 2 C,D and Supplementary Figure 3). Interestingly, the fold-increase in VEGF165b at protein level (2D) seems to be higher than at the RNA level (2C) suggesting a possible additional post-transcriptional layer of regulation (see discussion).
To determine whether SRPK1-KD in PC3 cells influenced SR protein expression and/or phosphorylation, western blot analysis was performed. Supplementary Figure 4 shows that expression of different SR proteins was not affected but there was a pronounced decrease in phosphorylation in SRSF 1, 2 and 5 in KD cells compared to controls.
SRPK1 knock-down does not affect cell growth, proliferation, invasion and migration of PC-3 cells in vitro
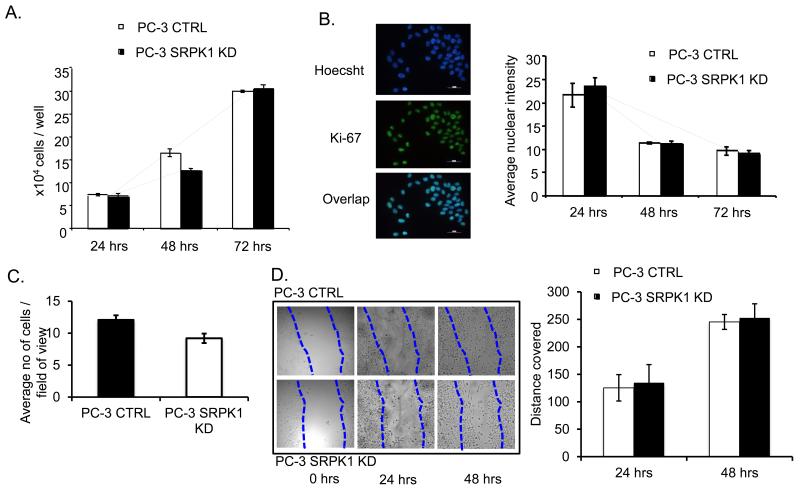
SRPK1 is a kinase with multiple SR-proteins as targets, which in turn affect splicing of several genes (28). Moreover, PC-3 cells express VEGFR2 and 1 as well as neuropilin 1 (Supplementary Figure 5) which potentially could respond to different VEGF ligands. Thus SRPK1-KD could result in differential autocrine effects of VEGF isoforms on PC-3 cells that may affect their ability to proliferate, invade and migrate. Cell counting assays showed no significant difference at any time point between the growth rates of PC-3 SRPK1-KD compared with control cells at the same time point (p>0.05, two-way ANOVA, n=3 experiments of three independent replicates) (Figure 3A). Cell proliferation was also assessed with Ki-67 staining, and again there was no significant difference between the proliferation rates of PC-3 SRPK1-KD and control cells (p>0.05, two-way ANOVA, n=2 experiments of three independent replicates). (Figure 3B). The potential of cells to migrate and invade was determined using a Matrigel invasion assay. There was no significant difference in the number of cells migrating between PC-3 SRPK1-KD and control (CTRL) groups (p=0.06, unpaired t-test) (Figure 3C). The same experiment was repeated using a different migration set-up – Fluoroblock membranes (BD Biosciences) with the same result (data not shown). Furthermore, no difference in migration velocities between PC-3 cells with SRPK1-KD and control cells were observed in a scratch-wound assay (p>0.05, two-way ANOVA, n=3) (Figure 3D).
Figure 3. SRPK1-KD has no effect on proliferation, migration and invasion of PC-3 cells in vitro.
A. Growth of PC-3 cells stably transfected with control and SRPK1 shRNA at 24, 48 and 72 hours after plating equal numbers; B. Ki-67 staining; left panels - examples of microscopic fields of PC-3 cells double-stained with Hoechst and Ki-67; right panels - quantification of Ki-67 fluorescence in control and SRPK1-KD cells at 24, 48 and 72 hours after plating equal numbers; C. Matrigel migration-invasion assay. Quantification of cells migrated on the bottom part of membranes after 24h. D. Scratch-wound assay. Migration potential of cells was calculated as a measure of the distance (mm) covered by the cells to the middle of the scratch wound, 24 and 48 hours after the initial scratch.
These data taken together suggest that SRPK1-KD does not result in an effect on PC-3 cells, by regulating VEGF or other genes splicing, that would influence their rate of growth, proliferation, migration or invasion in vitro.
SRPK1 knock-down reduces subcutaneous PC-3 tumour growth in vivo through inhibition of angiogenesis in a manner dependent on VEGF splicing
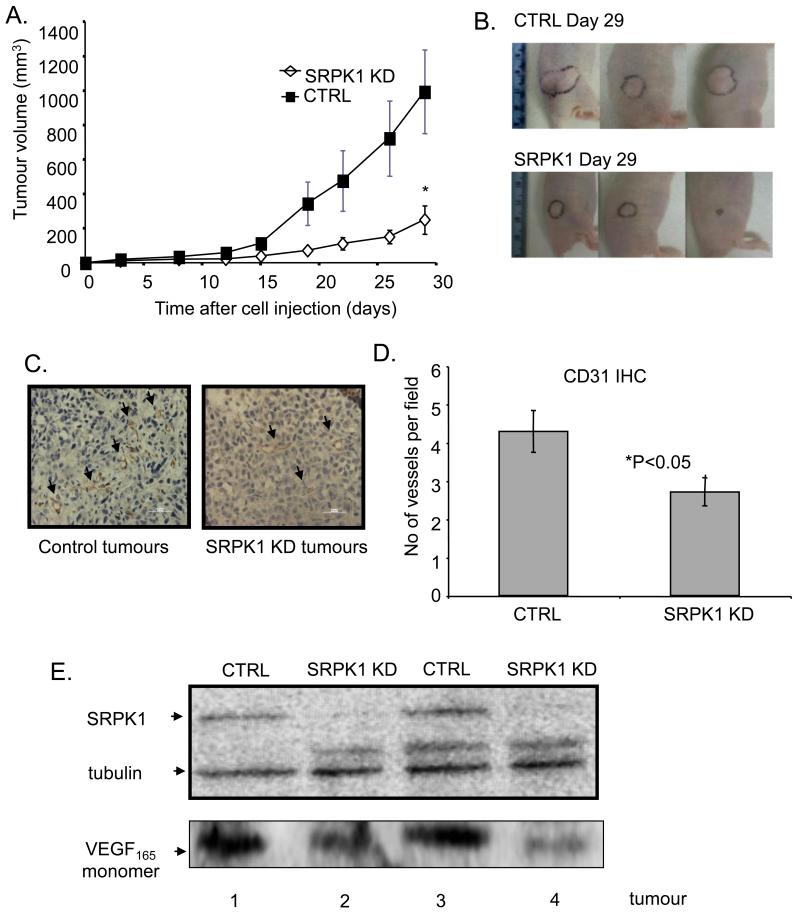
Since SRPK1-KD induced a splicing switch towards VEGF anti-angiogenic isoforms we investigated whether this would affect the rate of tumour growth in vivo. 1×106 PC-3 SRPK1-KD or control cells were injected subcutaneously in the flank of male nude mice and tumour volume was monitored. There was a significant decrease in tumour growth rate in PC-3 SRPK1-KD mice compared with control (n=9), *=p<0.05, two-way ANOVA (Figure 4A and examples of tumour growth in mice in Figure 4B).
Figure 4. SRPK1-KD reduces tumour growth in PC3 xenografts in nude mice.
A. Quantitations of the tumour volumes in control and SRPK1-KD mice. B. Examples of tumour growth in both mice groups (tumours outlined in black). C. Examples of microscopic fields of control and SRPK1-KD tumours stained for CD31. Arrows indicate blood vessels. D. Quantitation of microvessel density in control and SRPK1-KD tumours. E. Western blot analysis of SRPK1 and VEGF in duplicate tumours from control and SRPK1-KD groups
At day 29 the mice were culled and RNA from the tumour samples was subjected to qRT-PCR to examine SRPK1 expression (normalized to total amount of RNA). There was a significant difference in the mean SRPK1 expression between the two groups (***p<0.001 t-test) and there was a significant positive correlation of SRPK1 copy number and tumour volume (***p<0.001, r=0.7377 Spearman Rank Correlation test) (Supplementary Figure 6).
PC-3 SRPK1-KD or control tumour samples were stained for CD31 endothelial cell marker and the blood vessel density counted. There was a significant reduction in blood vessel density in PC-3 SRPK1-KD mice compared with control. (*p<0.05 unpaired t-test) (Figure 4 C,D). Western blot analysis of protein extracts from tumours shows SRPK1-KD corresponds to a decrease in VEGF levels (see examples in Figure 4E).
These data taken together suggest that the main effect through which SRPK1 causes a decrease in tumour growth is by affecting angiogenesis through a switch in VEGF splicing isoforms from pro-angiogenic to anti-angiogenic. To more clearly test this hypothesis we designed a VEGF-rescue experiment in vivo in which we asked whether VEGF165 cDNA overexpression driven by a VEGF-promoter (which would mimic endogenous VEGF but be insensitive to alternative splicing) could rescue the tumour growth in SRPK1-KD cells.
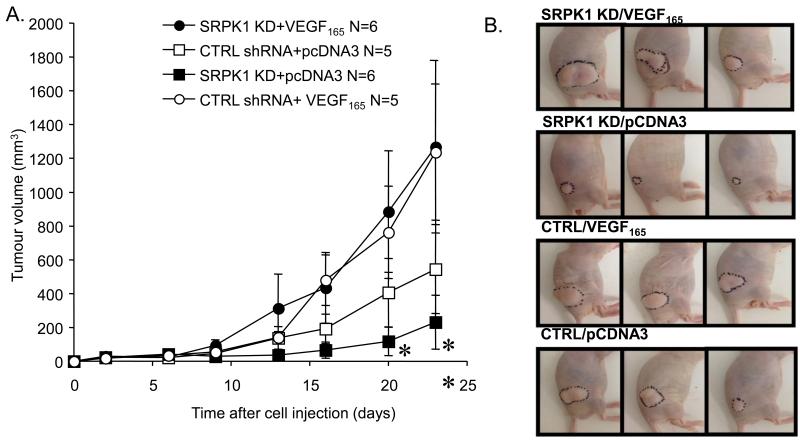
SRPK1-KD or control cells were transfected with a plasmid containing the VEGF165 cDNA under the control of the VEGF promoter. SRPK1-KD did not affect VEGF promoter activity in PC3 cells, as assessed in vitro using a luciferase reporter plasmid driven by the endogenous VEGF promoter sequence (Supplementary Figure 7). One million PC-3 SRPK1-KD/VEGF165 and CTRL KD/VEGF165 cells were injected subcutaneously in the flank of male nude mice and tumour volume was monitored. As a control, 1×106 PC-3 SRPK1-KD/pCDNA3 and CTRL/pCDNA3 cells (transfected with empty plasmid) were injected in parallel. The ability of the cell to generate VEGF165 (circles) significantly rescued the inhibition of tumour growth in the presence of SRPK1-KD (filled symbols, p<0.01, two-way ANOVA). SRPK1-KD thus had no effect on cells that could express VEGF165 under control of the VEGF promoter (circles, p>0.1, two way ANOVA) but did in the cells expressing multiple isoforms of VEGF (squares, p<0.05 two-way ANOVA). *=p<0.05, **=p<0.01 compared with SRPK1 KD-VEGF165 (Figures 5A and B).
Figure 5. Exogenous expression of VEGF cDNA from a VEGF promoter rescues the effect of SRPK1-KD on tumour growth in vivo.
A. Tumour growth curves for four groups of mice injected s.c with the following stably-transfected cells: open circles - control shRNA and VEGF165 plasmid; filled circles - SRPK1-KD and VEGF165 plasmid; open squares - control shRNA and empty vector; filled circles - SRPK1-KD and empty vector; B. Examples of tumour growth in all mice groups (tumours outlined in black).
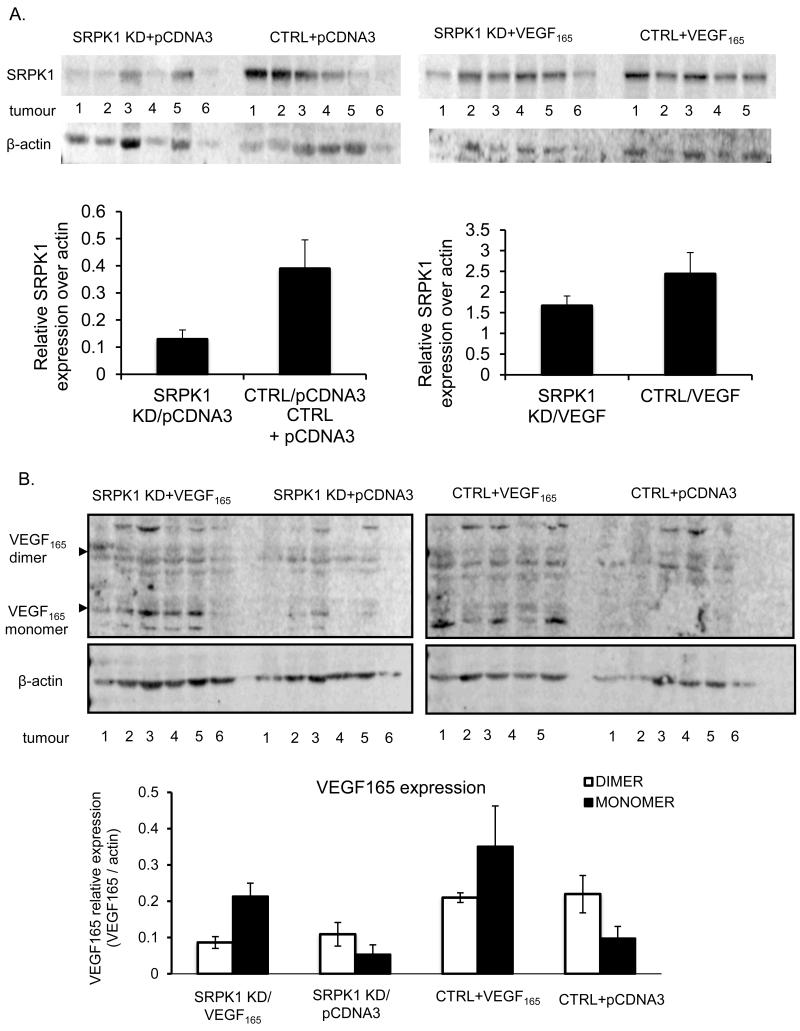
SRPK1 levels were decreased in tumours grown from SRPK1-KD cells compared with control cells in both sets with either expression of empty vector plasmid (Figure 6A, left panel) or VEGF plasmid (Figure 6A, right panel). Also, VEGF levels were increased in tumours grown from cells over-expressing VEGF cDNA plasmid compared to cells transfected with empty vector, both in the presence of SRPK1-KD (Figure 6B, left panel) or absence (Figure 6B, right panel).
Figure 6.
Expression analysis by Western blot of SRPK1 (A.) and VEGF (B.) levels in the four sets of tumours from the rescue experiment
Small molecule inhibitors of SRPK1 are able to switch VEGF splicing in vitro in PC-3 cells and decrease tumour growth in vivo in an orthotopic PCa mouse model
We have previously shown that several SRPK1 inhibitors are able to switch VEGF splicing towards the anti-angiogenic isoforms in vitro and have anti-angiogenic effects in vivo in different systems (27, 29, 30). Since SRPK1-KD is able to slow tumour growth in PC-3 xenografts we enquired whether chemical inhibition of SRPK1 may have similar results in a therapeutic proof-of-principle experiment.
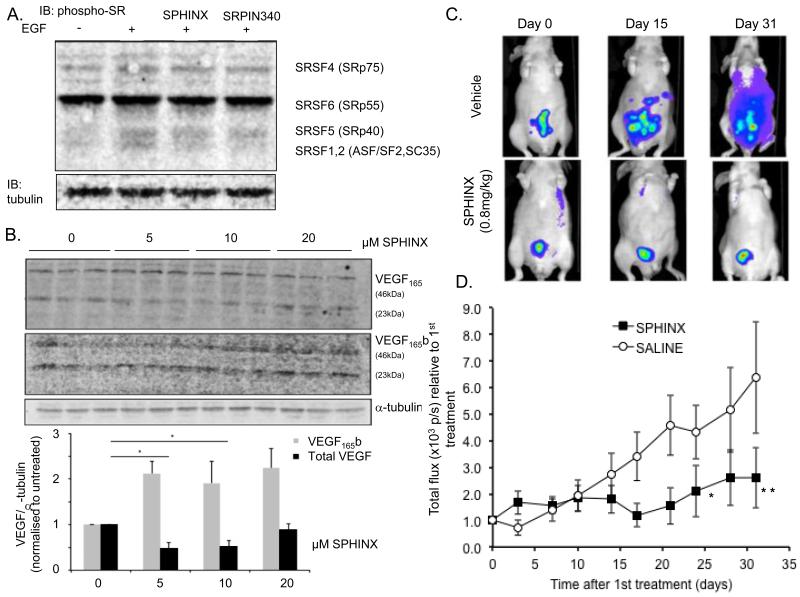
To examine changes in SR protein phosphorylation, PC-3 cells were treated with SRPK1 inhibitors followed by EGF, previously shown to induce SRPK1-dependent SR activation (31). Two different inhibitors were used at 10 μM; SPHINX, and SRPIN340 which have different selectivities for SRPK1 (30). After 30 min pre-treatment with the inhibitors, cells were incubated with 100 ng/ml EGF for an hour and protein extracted. Immunoblot analysis for phosphorylated SR proteins revealed a 115% up-regulation of phosphoSRSF1/2 and 90% upregulation of pSRSF5, but no change in pSRSF6 upon EGF treatment, compared with control treated cells (DMSO 0.02%). However, prior treatment with the inhibitors resulted in reduction of EGF-dependent SRSF1/2 phosphorylation (to 14 and 1% by SRPIN340 and SPHINX respectively) but not SRSF5 (60% and 50% increase respectively) (Figure 7A). This was confirmed in a different experimental design where phospho-SR proteins were immunoprecipitated with mAb 104 antibody followed by immunoblotting with SRSF1 antibody (Supplementary figure 8).
Figure 7. Small molecule inhibitors of SRPK1.
A. SPHINX and SRPIN340 are able to block phosphorylation of SR proteins induced by EGF in PC3 cells and (B.) to switch VEGF terminal exon splicing. C. Intravital imaging of RFP-PC3 tumours in mice treated with saline or SPHINX (i.p. 50μg, triweekly) by fluorescence imaging. D. Intraperitoneal administration of SPHINX significantly decreases tumour growth in orthotopic xenografts of PC3 cells (p<0.05, two-way Anova).
The effect of SPHINX on VEGF protein isoform expression was also examined (Figure 7B). There was a significant down-regulation of VEGF165 after treatment with 5 and 10μM SPHINX compared with control (p=0.020; One-way ANOVA, *=p<0.05). However, at 20μM treatment, the reduction of the pro-angiogenic isoform was lost and no difference was observed compared to control group (p>0.05, One-way ANOVA). Subsequently, the expression of VEGF165b isoform was examined using an antibody against all VEGFxxxb isoforms. As shown in Figure 7B, there was a significant effect on VEGF165b expression upon treatment with the small molecule inhibitor.
Based on these results and to examine the hypothesis that SRPK1 is a therapeutic target for PCa, we used the orthotopic PCa model to assess the effect of SPHINX on tumour growth. 1×106 PC-3 RFP-tagged cells, resuspended in 20 μl of sterile PBS, were injected in the prostate gland of male nude mice. The use of PC-3 RFP expressing cells enabled the detection and quantification of tumours, beneath the skin, using the IVIS Lumina system (examples of fluorescent detection are shown in Figure 7C). Once tumours reached approximately 4-5 ×1010 photons per second (p/s), mice were treated. Two treatment groups were used: intra-peritoneal injections of 20 μg SPHINX or saline three times a week. In the control treated groups, the fluorescence signal increased. The fluorescence was quantitated as shown in figure 7D, It should be noted that the images shown in figure 7B show both direct and reflected light collected from the cone emitting from the fluorescent source, and so do not reflect metastasis, but an increased size of primary tumour. This was confirmed when the mice were killed and dissected and no dissemination as seen but a large primary growth. Treatment with SPHINX resulted in a significant inhibition of tumour growth compared with saline group (p<0.05 and p<0.01, two-way ANOVA, Figure 7D).
Discussion
Angiogenesis is an essential process through which growing tumours are able to form blood vessels and obtain necessary nutrients. However, tumour cells can be very heterogeneous in their metabolism and therefore their dependence on oxygen and nutrients, with some of the most aggressive cancer cells being able to survive and proliferate with poor energy supplies (11).
Whilst correlation of tumour growth and progressive phenotype with microvessel density may provide clues for the importance of angiogenesis in cancers, including PCa, the ultimate proof of how reliant cancer growth is on angiogenesis comes from studies in which angiogenesis inhibitors are used. The discouraging results of recent trials using anti-angiogenic therapies on patients with advanced PCa may be explained, at least in part, by an incomplete knowledge of the regulation and biology of VEGF (the main determinant of angiogenesis) which most of these studies are set to inhibit. These studies use compounds that inhibit all forms of VEGF (either directly e.g bevacizumab, or indirectly through receptor inhibition e.g sunitinib) without taking into account the diversity of VEGF molecules and their functions (i.e some of them are naturally anti-angiogenic) acquired through numerous levels of gene regulation, including alternative splicing. Only recently a few studies on patients with various cancers (but not PCa) have taken into account the response to bevacizumab therapy depending on various VEGF isoforms [reviewed in (32)].
We have shown previously that the kinase SRPK1 is one of the most important regulators of the VEGF165/VEGF165b splicing switch (through modulation of the SR protein family splicing factor SRSF1) and that SRPK1-KD in colon carcinoma cell line LS174t inhibits tumour growth by inhibiting angiogenesis (27). Several small molecules inhibitors for SRPK1 exist [e.g SRPIN340 (33) and SPHINX (30)].
SRPK1 has been reported to be up-regulated in several cancers: for example breast, colon and pancreas (34); ovarian (35); non-small cell lung carcinoma (36) or hepatocellular carcinoma (37). Here we show that SRPK1 expression was much higher in samples from patients with malignant PCa compared to normal prostate tissue (Figure 1A). This correlates with data previously reported that malignant prostate tissue expresses the pro-angiogenic VEGF isoform while normal tissue shows a combination of both isoforms (23). Interestingly, SRPK1 expression was also significantly higher in PIN lesions when compared to benign tissue. This may suggest that activation of SRPK1 and therefore promotion of pro-angiogenic splicing in VEGF might be an early event in development of prostate cancer and moreover, indicates that angiogenesis may be a causal phenomenon that drives PCa progression and not a consequence of an increasing hypoxic environment during tumourigenesis. However, it was also interesting that SRPK1 was not raised in all prostate cancers (Figure 1B), and that some prostate cancer lines (e.g. LNCaP) do not have high SRPK1 expression. These cells may have other splicing regulatory pathways that are altered. Furthermore, SRSF1 expression (which is downstream of SRPK1) was significantly increased in malignant but not PIN lesions (Figure 1C), which may underlie the progressive and sequential activation of SRPK1-SRSF1 axis in PCa. It is not yet known whether SRSF1 expression follows aggressiveness in cancer cell lines, but it would be interesting to compare this across prostate cancer cell lines. Moreover, other splice factors like SRSF5 and SRSF6 have been shown to be involved in splicing of VEGF at the terminal exon and future assessments of expression of these factors in human samples will hopefully provide a comprehensive picture of SRPK1-SR axis in PCa.
We further show that SRPK1-KD switches the balance of the main VEGF splicing isoform towards the anti-angiogenic one in PC3 cells (Figure 2) and inhibits tumour growth in PC3 xenografts with associated decrease in microvessel density (Figure 4). It did this by reducing the amount of VEGF-A165a, i.e. switching from active to repressed proximal splicing. Of interest the switch in splicing at the protein level indicated a higher level of VEGF-A165b protein than that suggested by the PCR. This could be due to differential translation, but is likely also to be due to competition in amplification when two transcripts are generated from the same target sequence. VEGF-A165 competes with VEGF-A165b for primer annealing, resulting in over-representation of the most common isoform. Therefore a small increase in the PCR signal could result from a large increase in the relative abundance (38). SRPK1 has multiple targets beside SRSF1 and its knock-down has been shown to affect other hallmarks of cancer in different cancers (see references above). However, SRPK1-KD in PC-3 cells does not have any autocrine effect on cell growth, proliferation, migration and invasion in vitro (Figure 3), a feature that is different from cell lines representative for other types of cancers. Moreover, expression in SRPK1-KD cells of splicing-insensitive VEGF cDNA driven by VEGF endogenous promoter, rescues tumour growth (Figure 5). These data taken together strongly suggest that the main reason for inhibition of tumour growth in PC-3 cells with SRPK1-KD is the effect on angiogenesis and is unlikely to be related to other hallmarks of cancer. In addition, SRPK1-KD has no influence on the VEGF promoter (see Supplementary Figure 7) providing additional support for the hypothesis that the SRPK1-KD effect is mediated through the switch in VEGF splicing isoforms.
Finally, in a therapeutic proof-of-principle experiment we show that SRPK1 inhibitors are able to inhibit phosphorylation of relevant SR proteins and switch VEGF splicing in vitro (Figure 7A, B). One of the inhibitors, SPHINX, decreases tumour growth when administered repetitively intraperitoneally in a mouse model of orthotopic prostate cancer using PC3 cells (Figure 7C).
In conclusion, our study identifies SRPK1 as a key molecule in regulating the balance of VEGF splice isoforms in PC-3 cells whose inhibition results in decrease in tumour growth due to the effect on angiogenesis. This encourages novel studies into the potential use of small molecule inhibitors of SRPK1 in PCa as anti-angiogenic therapy.
MATERIALS AND METHODS
Cell Culture
PC-3 and DU145 cell lines were cultured in DMEM and LNCap cells in RPMI1640 with 10% FBS and antibiotics. PC3, LNCaP and DU145 cells were purchased from ATCC; cells were authenticated by Short Tandem Repeat Profiling at Identicell, Denmark (08/2011). Primary prostate epithelial cells were purchased from Lonza, not authenticated and cultured in PrEBM medium (Lonza). SRPK1-KD cell sublines were generated by transduction of cell lines with lentivirus encoding shRNAi to SRPK1 (Dharmacon). Transduced PC-3 cells were transfected with VEGF165 plasmid or empty pcDNA3 using Fugene HD and selected in Geneticin.
Proliferation assay
105 cells seeded in 6-well plates were counted after 24, 48 and 72 hours using a haemocytometer. For Ki67 proliferation assay, 105 cells were serum starved for 8 hours. Twenty-four, 48 and 72 hours after starvation, cells were fixed with 4% PFA and incubated with anti-Ki67 antibody 1:100 (Abcam), overnight at 4°C. Alexa-fluor 488 goat anti-rabbit antibody (1:750) was used as secondary. The proliferation potential of cells was measured on Image J, by calculating the total nuclear intensity of Ki67 and normalising to total nuclear area.
Migration-invasion assays
Scratch wound assay - 1mm thick line was scratched off the cell monolayer using a sterile tip. The migration potential of cells was determined by measuring the distance covered by cells (Image J) during 24 or 48 hours to the middle of the scratch wound. For the migration-invasion assay, 50μl of matrigel (Sigma) were added into each chamber (Millipore) prior to plating the cells. 5×104 cells, re-suspended in DMEM containing 2% FBS, were then plated onto the chambers. DMEM with 10% FBS was added in the wells of the plates to generate a serum gradient. After incubation inserts were fixed in 4% PFA and stained with Hoechst. To assess invasion potential, 5 fields of view were taken at ×20 magnification and cells counted.
RNA extraction, reverse transcription, PCR
TRIzol (Invitrogen) was used to extract RNA from all cells and tumours. One-two μg of RNA was used for first-strand cDNA synthesis. PCR was performed using primers in Supplementary Table 1.
Protein Studies
Cell lines and tumor samples were lysed in RIPA buffer, containing protease inhibitors. For VEGF ELISA the Pan-VEGF and VEGFxxxb Elisa kits (R&D) were used. For Western blotting mouse anti–SRPK1 (BD Biosciences), rabbit anti–panVEGF, mouse anti–VEGFxxxb (A56/1) or goat anti–β-actin (Santa Cruz) were used.
In vivo models
For heterotopic xenografts, 1×106 transduced and/or transfected PC-3 cells resuspended in 100μl of PBS were injected subcutaneously in male nude mice. Tumours were measured with a caliper every 3 days and tumour volume was calculated according to the formula: [(length+width)/2]*length*width. When the first tumour reached 16mm in diameter, mice were culled, tumours were excised, half homogenised in Trizol for RNA extraction and the other half embedded in paraffin for staining. For orthotopic implantation RFP-tagged PC-3 cells (AntiCancer Inc., San Diego)(39) were surgically injected into the prostate of nude mice and tumour growth monitored using a Xenogen IVIS device.
Immunohistochemistry
Paraffin embedded samples were cut in 5-7mm sections and standard IHC protocols were used. For vessel density rabbit polyclonal CD31 antibody (Abcam) and DAB kit (Vector Laboratories) was used for colour development. For SRPK1 immunohistochemistry, rabbit anti-SRPK1 primary antibody (Sigma) was used at 1μg/ml concentration and Fast-Red solution (Sigma) for colour development.
Blood vessel density
Two sections from each tumour were analysed using a Nikon E400 microscope (×40 objective). Blood vessels were identified and counted based on CD31 positive staining, and mean number of blood vessels per field-of-view (3 fields-of-view per section) was calculated.
Human samples scoring
Scoring was done blindly by a histopathologist (JO) according to the intensity of staining – 0 for no staining; 1 for weak; 2 for moderate and 3 for strong staining.
Supplementary Material
Acknowledgements
We wish to thank Dr Andrew Armstrong (Duke University) for critical reading of the manuscript and helpful suggestions and the staff from AntiCancer Research Ltd (San Diego) for training Athina Mavrou in the orthotopic prostate cancer model.
Financial support: Supported by Prostate Cancer Research UK, BBSRC (BB/J007293/1), the Medical Research Council (G10002073), Cancer Research UK (C11392/A10484) and Richard Bright VEGF Research Trust (North Bristol Cancer Research Projects Fund no. 96464)
Footnotes
Conflict of interest: The authors declare no conflict of interest. Dave O Bates and Steve Harper are inventors on patents related to control of splicing of VEGF
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, Mallone S, van der Zwan JM, Trama A, Siesling S, Capocaccia R. Cancer prevalence estimates in Europe at the beginning of 2000. Ann Oncol. 2013 Jun;24(6):1660–6. doi: 10.1093/annonc/mdt030. [DOI] [PubMed] [Google Scholar]
- 3.Quaglia A, Lillini R, Crocetti E, Buzzoni C, Vercelli M. Incidence and mortality trends for four major cancers in the elderly and middle-aged adults: An international comparison. Surg Oncol. 2013 Mar 24; doi: 10.1016/j.suronc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013 Mar;24(3):792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 5.Whang YE, Armstrong AJ, Rathmell WK, Godley PA, Kim WY, Pruthi RS, et al. A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patients with castration-resistant prostate cancer. Urol Oncol. 2013 Jan;31(1):82–6. doi: 10.1016/j.urolonc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012 Sep 27;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Clarke JM, Armstrong AJ. Novel therapies for the treatment of advanced prostate cancer. Curr Treat Options Oncol. 2013 Mar;14(1):109–26. doi: 10.1007/s11864-012-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013 Dec 5;32(49):5501–11. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake CG, Sharma P, Gerritsen W. Metastatic castration-resistant prostate cancer: new therapies, novel combination strategies and implications for immunotherapy. Oncogene. 2013 Nov 25; doi: 10.1038/onc.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–88. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, et al. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997 Jun;157(6):2329–33. [PubMed] [Google Scholar]
- 13.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, et al. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998 Jan;51(1):161–7. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 14.Duque JL, Loughlin KR, Adam RM, Kantoff P, Mazzucchi E, Freeman MR. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006 Oct;61(5):401–8. doi: 10.1590/s1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 15.Duque JL, Loughlin KR, Adam RM, Kantoff PW, Zurakowski D, Freeman MR. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999 Sep;54(3):523–7. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 16.Bok RA, Halabi S, Fei DT, Rodriquez CR, Hayes DF, Vogelzang NJ, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res. 2001 Mar 15;61(6):2533–6. [PubMed] [Google Scholar]
- 17.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993 Aug;143(2):401–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherji D, Temraz S, Wehbe D, Shamseddine A. Angiogenesis and anti-angiogenic therapy in prostate cancer. Crit Rev Oncol Hematol. 2013 Aug;87(2):122–31. doi: 10.1016/j.critrevonc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012 May 1;30(13):1534–40. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002 Jul 15;62(14):4123–31. [PubMed] [Google Scholar]
- 21.Cebe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006 Sep;63(17):2067–77. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004 Nov 1;64(21):7822–35. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 23.Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008 Apr 8;98(7):1250–7. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008 Nov;8(11):880–7. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008 Apr 22;98(8):1366–79. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates DO, Catalano PJ, Symonds KE, Varey AH, Ramani P, O’Dwyer PJ, et al. Association between VEGF splice isoforms and progression-free survival in metastatic colorectal cancer patients treated with bevacizumab. Clin Cancer Res. 2012 Nov 15;18(22):6384–91. doi: 10.1158/1078-0432.CCR-12-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin EM, Oltean S, Hua J, Gammons MV, Hamdollah-Zadeh M, Welsh GI, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011 Dec 13;20(6):768–80. doi: 10.1016/j.ccr.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oltean S, Gammons M, Hulse R, Hamdollah-Zadeh M, Mavrou A, Donaldson L, et al. SRPK1 inhibition in vivo: modulation of VEGF splicing and potential treatment for multiple diseases. Biochem Soc Trans. 2012 Aug;40(4):831–5. doi: 10.1042/BST20120051. [DOI] [PubMed] [Google Scholar]
- 29.Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, et al. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010 Feb 19;285(8):5532–40. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gammons MV, Federov O, Ivison D, Du C, Clark TL, Hopkins C, et al. Topical anti-angiogenic SRPK1 inhibitors reduce choroidal neovascularization in rodent models of exudative-AMD. Invest Ophthalmol Vis Sci. 2013 Jul 25; doi: 10.1167/iovs.13-12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, Qiu J, Liu W, Zhou Y, Plocinik RM, Li H, et al. The Akt-SRPK-SR axis constitutes a major pathway in transducing EGF signaling to regulate alternative splicing in the nucleus. Mol Cell. 2012 Aug 10;47(3):422–33. doi: 10.1016/j.molcel.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013 Mar 20;31(9):1219–30. doi: 10.1200/JCO.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara T, Hosoya T, Shimizu S, Sumi K, Oshiro T, Yoshinaka Y, et al. Utilization of host SR protein kinases and RNA-splicing machinery during viral replication. Proc Natl Acad Sci U S A. 2006 Jul 25;103(30):11329–33. doi: 10.1073/pnas.0604616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes GM, Carrigan PE, Miller LJ. Serine-arginine protein kinase 1 overexpression is associated with tumorigenic imbalance in mitogen-activated protein kinase pathways in breast, colonic, and pancreatic carcinomas. Cancer Res. 2007 Mar 1;67(5):2072–80. doi: 10.1158/0008-5472.CAN-06-2969. [DOI] [PubMed] [Google Scholar]
- 35.Odunsi K, Mhawech-Fauceglia P, Andrews C, Beck A, Amuwo O, Lele S, et al. Elevated expression of the serine-arginine protein kinase 1 gene in ovarian cancer and its role in Cisplatin cytotoxicity in vitro. PLoS One. 2012;7(12):e51030. doi: 10.1371/journal.pone.0051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gout S, Brambilla E, Boudria A, Drissi R, Lantuejoul S, Gazzeri S, et al. Abnormal expression of the pre-mRNA splicing regulators SRSF1, SRSF2, SRPK1 and SRPK2 in non small cell lung carcinoma. PLoS One. 2012;7(10):e46539. doi: 10.1371/journal.pone.0046539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou B, Li Y, Deng Q, Wang H, Wang Y, Cai B, et al. SRPK1 contributes to malignancy of hepatocellular carcinoma through a possible mechanism involving PI3K/Akt. Mol Cell Biochem. 2013 Jul;379(1-2):191–9. doi: 10.1007/s11010-013-1641-7. [DOI] [PubMed] [Google Scholar]
- 38.Bates DO, Mavrou A, Qiu Y, Carter JG, Hamdollah-Zadeh M, Barratt S, et al. Detection of VEGF-A(xxx)b isoforms in human tissues. PLoS One. 2013;8(7):e68399. doi: 10.1371/journal.pone.0068399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M, Jiang P, Yamamoto N, Li L, Geller J, Moossa AR, et al. Real-time whole-body imaging of an orthotopic metastatic prostate cancer model expressing red fluorescent protein. Prostate. 2005 Mar 1;62(4):374–9. doi: 10.1002/pros.20125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.