Abstract
Plants have a high capacity to transform and thereby detoxify deleterious or poisonous compounds, like mycotoxins. The formation of glucose conjugates has a central role in this process. Mammals, however, are able to (partly) release the precursor substances during digestion, reactivating the mycotoxins. This short review provides a brief summary about the metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone in plants. Two examples are discussed in greater detail. First, the formation of deoxynivalenol-3-glucoside in wheat is linked to a quantitative trait locus that is often used for Fusarium head blight resistance breeding. Secondly, the metabolism of zearalenone in Arabidopsis thaliana results in at least 17 different metabolites, all of which are potentially hazardous for humans and animals.
Keywords: deoxynivalenol, zearalenone, conjugated mycotoxins, masked mycotoxins, metabolism, mass spectrometry
Metabolic transformations of xenobiotics in plants
Plants can metabolize and detoxify xenobiotics by a variety of enzymatic reactions and with extraordinary diversity among species. This phenomenon is widely exploited in agriculture to control competing weeds in a field of crop plants that are tolerant to a particular herbicide (1). The primary mechanism of herbicide selectivity in plants is their different ability or kinetics of metabolic detoxification. The same processes used by plants to detoxify pesticides or herbicides hold true for all other xenobiotics, including mycotoxins.
The metabolism of xenobiotics in plants can be divided in three phases. In phase I, also called transformation phase, reactive groups are introduced into the molecules for instance by oxidation or dealkylation. In many cases activation of the molecules through phase I biotransformation is necessary for conjugation, but if the reactive groups are already present in the parent molecule they may undergo direct conjugation. In phase II, also called conjugation phase, polar substances like sugars (often glucose) are coupled to the reactive groups of the respective metabolites. With the formation of conjugates, the foreign substances very often get inactivated and detoxified. In phase III, or compartmentation phase, metabolites formed in phase II are either transported to the vacuole and stored there, or further modified and deposited in the cell wall.
Regarding food safety, the major problem is that such conjugated mycotoxins are usually not detected by analytical methods commonly used for mycotoxin determination, but can be reactivated in the digestion tract of human and animals. Phase II metabolites can potentially be hydrolyzed by acid in the stomach, by enzymes in the gut or by intestinal bacteria, releasing the toxic precursor as has been shown for zearalenone-4-β-D-glucopyranoside (Z4G) by Gareis et al. (2).
Conjugated Fusarium mycotoxins
In 1983, it was shown that the DON concentration of Fusarium graminearum infected wheat reached a maximum and then declined until harvest (3). A year later it was reported that the DON content of yeast fermented food products was higher than that of the contaminated flour used for their production (4). The authors explained these findings with the presence of an unknown DON metabolite. Without knowing the exact structures of the possible plant metabolites, glucose and fatty acids conjugates of DON were chemically synthesized (5). Deoxynivalenol-3-β-D-glucopyranoside (D3G) was identified by NMR experiments as the main DON metabolite after treatment of maize cell suspension cultures with DON in 1992 (6). Also Arabidopsis thaliana, and in particular its glucosyltransferase DOGT1, was shown to produce D3G upon treatment with DON (7). Finally, D3G was found co-occurring along with DON in naturally contaminated wheat and maize (8). In our laboratory, D3G was so far found in all of the wheat and maize samples that also had DON concentrations of at least 100 μg/kg. The relative concentrations of D3G in these samples were up to 12% of the DON concentrations in wheat and up to 50% of the DON concentrations in maize.
Back in 1988, zearalenone-4-β-D-glucopyranoside (Z4G) was shown to be a metabolite of plants, after zearalenone (ZON) was transformed by maize cell suspension cultures to its glucoside (9). A mini-survey of 24 wheat samples – 22 of them were contaminated with ZON above the limit of quantification – resulted in 10 samples (42%) also positive for Z4G (10). Approximately 10-20% of the total ZON content of these samples were detected as Z4G. Several papers describe the metabolism of mycotoxins in plants, especially the transformation of ZON to α-Zearalenol (αZOL), β-Zearalenol (βZOL) and their corresponding glucosides (11, 12, 13). Furthermore, the hydrolysis-labile Zearalenone-4-sulfate (ZON-4-Sulf) was identified after microbial transformation of ZON by Rhizopus arrhizus (14), as a natural Fusarium metabolite (15) and as a conjugation product of ZON in the urine of mammals, but – so far – not yet as a plant metabolite.
Example 1: Formation of D3G in wheat
D3G is a detoxification product of DON in plants and can also by formed by conjugation of DON in wheat. Lemmens et al. (16) investigated whether differences in D3G formation exist in wheat lines that show different levels of resistance towards DON. Moreover, it was tested whether this resistance to DON also correlates with the resistance to the disease Fusarium Head Blight (FHB) of wheat.
Double haploid lines originating from a cross between the susceptible cultivar “Remus” and the resistant cultivar “CM-82036” were treated with high doses of DON at flowering. Whole wheat ears were harvested, ground and extracted. Raw extracts were purified and analyzed by LC-MS/MS for DON and D3G. The DON treated ears showed bleaching, a typical FHB symptom, although the plants were not exposed to fungi. All wheat lines were tested for DON and D3G concentrations by LC-MS/MS, and D3G concentrations in the resistant lines were found to be higher than in FHB sensitive lines. A close relation between the D3G/DON ratio and the bleaching resistance was found. Lines with high DON resistance (very little DON-induced symptoms as indicated by a low area under the disease progress curve (AUDPC) value) had most of the DON converted into D3G. It was proposed that the quantitative trait locus (QTL) Qfhs.ndsu-3BS affects the expression of a DON-glucosyltransferase in some – so far unknown – way. Positional cloning and sequencing of this chromosomal area of wheat is ongoing at the moment (J. Anderson, personal communication).
Example 2: ZON metabolites of Arabidopsis thaliana
In a recent Ph.D. thesis the plant Arabidopsis thaliana was used as a model plant to explore the mode of action of ZON in plants (17). The potential of plants to metabolize mycotoxins is shown with ZON and Arabidopsis thaliana in (18) and discussed further here.
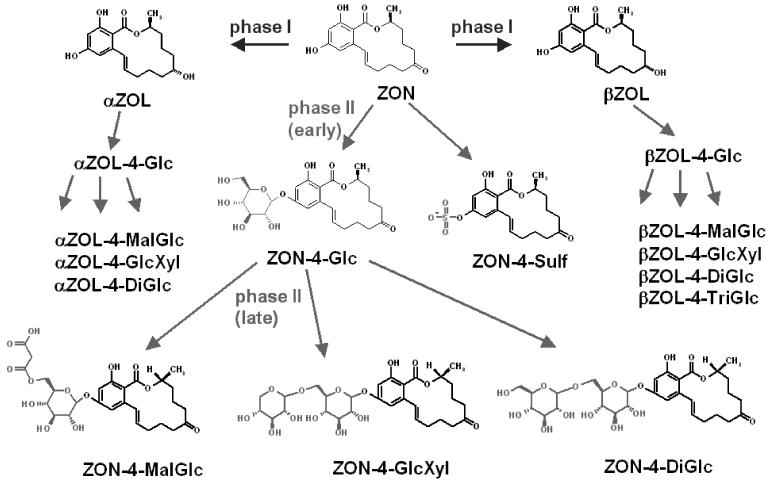
Arabidopsis thaliana seedlings were cultivated on Murashige and Skoog (M&S) agar at 22 °C under continuous light. 10 days after germination, the plants were transferred in sterile liquid M&S medium and treated with 50 μM ZON after further 3 days. After 0 h, 0.5 h, 2 h, 5 h, 12 h, 24 h, 36 h and 48 h the plants were collected, washed and extracted. Finally, all plant extracts and liquid media were analyzed by LC-MS/MS. In the liquid media (supernatant) ZON, Z4G, ZON-4-Sulf and in low concentrations αZOL and βZOL were identified. In the extracts of the plant material also αZOL-4-glucoside, βZOL-4-glucoside, ZON-4-malonylglucoside, αZOL-4-malonylglucoside, βZOL-4-malonylglucoside, ZON-dihexoside, αZOL-dihexoside, βZOL-dihexoside, βZOL-trihexoside, ZON-hexosyl-pentoside, αZOL-hexosyl-pentoside and a βZOL-hexosyl-pentoside were identified. The di- and trihexosides are most likely glucosides (Di- and TriGlc), the hexosyl-pentosides presumeably glucosyl-xylosides (GlcXyl). A total of 17 different metabolites of ZON in Arabidopsis thaliana were detected. As a result of this study, a metabolic scheme of ZON in Arabidopsis thaliana has been proposed, which is shown in Figure 1.
Figure 1.
Proposed metabolism of ZON in Arabidopsis thaliana
A time course revealed that after 24 hours very little (<3% of the added) ZON could be found remaining in the cultivation medium. Phase I metabolism led to the formation of α-ZOL and β-ZOL, which were further conjugated to phase II metabolites, in the same way as ZON.
Conclusion
D3G was found to occur in naturally contaminated cereals (8). The QTL Qfhs.ndsu-3BS shows great influence on the formation of D3G from DON in highly Fusarium-resistant wheat lines (16). DON is a known virulence factor of Fusarium, especially aiding the fungi to spread within wheat ears from spikelet to spikelet. Due to the higher DON resistance, lines carrying this QTL are also more resistant towards DON producing Fusarium species. The increased use of such resistant wheat lines in agriculture would be favorable, as the total DON amount (DON, acetylated forms and D3G) is most likely far lower as compared to susceptible lines. It has to be kept in mind, however, that the relative amounts of D3G compared to DON would increase. The bioavailability and stability of D3G in the digestive tract of mammals are currently not known and will be the topic of further studies.
Z4G is also naturally occurring in Fusarium contaminated cereals (10) and swine can cleave it completely to ZON and glucose (2). The aim of our study (18) was to identify further plant metabolites of ZON using the model plant Arabidopsis thaliana. More than 97% of the added ZON was metabolized by the plant within a day. The occurrence of 17 different substances is a further proof for the remarkable ability of plants to metabolize xenobiotics. Besides derivatives of ZON, also α-ZOL, β-ZOL and conjugates thereof were found. The estrogenic activity of α-ZOL is even higher than of ZON. Studies to prove the supposed structures with NMR and to check the occurrence of these metabolites in agriculturally relevant plants are planned.
With respect to food safety it would be desirable to screen cereals not only for known mycotoxins, but also for in planta formed metabolites (masked mycotoxins), which escape current routine detection methods but may contribute significantly to toxicity after reactivation in the digestive tract of man and animals. While this actually would mean higher costs for analyzing food samples, some justification is given by the occurrence of D3G of up to 50% of the DON concentration in maize (manuscript in preparation). To give the community easier access to the analytical standard of D3G, required for quantitative analysis, it has been made available through a local commercial supplier of mycotoxins. Also, more and more multimycotoxin methods, most of them utilizing LC-MS/MS equipment, arise that are capable of determining multiple (classes of) mycotoxins at once. D3G can also be easily included in such methods, as shown by Sulyok et al. in 2006 (19). Regarding the metabolites of ZON, except for Z4G the occurrence in cereals is still unclear. However, Z4G concentrations decreased after a while in Arabidopsis thaliana and further metabolizetion took place. After several days, also these di- and tri-hexosides declined in concentration and ZON practically “vanished”, most likely by incorporation of the metabolites into the insoluble “bound residue”. Next to nothing is known about the bioavailability of these bound residues of mycotoxins. Clearly, more research in that area is necessary to grant increased food safety to the consumers.
Acknowledgments
Financial support: Christian Doppler Society, the Austrian Genome Research Initiative GEN-AU, the Lower Austrian government, the Austrian Science Fund FWF
Footnotes
Presented at the 28th Mykotoxin-Workshop, Bydgoszcz, Poland, May 29-31, 2006
References
- 1.Kreuz K, Tommasini R, Martinoia E. Old Enzymes for a New Job: Herbicide Detoxification in Plants. Plant Physiol. 1996;111:349–353. doi: 10.1104/pp.111.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gareis M, Bauer J, Thiem J, Plank G, Grabley S, Gedek B. Cleavage of zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. J Vet Med. 1990;37:236–240. doi: 10.1111/j.1439-0450.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller JD, Young JC, Trenholm HL. Fusarium toxins in field corn. I. Time course of fungal growth and production of deoxynivalenol and other mycotoxins. Can J Bot. 1983;61:3080–3087. [Google Scholar]
- 4.Young CJ, Fulcher GR, Hayhoe JH, Scott PM, Dexter JE. Effect of milling and baking on deoxynivalenol (vomitoxin) content of eastern canadian wheats. J Agric Food Chem. 1984;32(3):659–664. [Google Scholar]
- 5.Savard ME. Deoxynivalenol fatty acid and glucoside conjugates. J Agric Food Chem. 1991;39:570–574. [Google Scholar]
- 6.Sewald N, Lepschy von Gleissenthall J, Schuster M, Müller G, Aplin RT. Structure elucidation of a plant metabolite of 4-desoxynivalenol. Tetrahedron. 1992;3(7):953–960. [Google Scholar]
- 7.Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glössl J, Luschnig C, Adam G. Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem. 2003;278(48):47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- 8.Berthiller F, Dall’Asta C, Schuhmacher R, Lemmens M, Adam G, Krska R. Masked Mycotoxins: Determination of a Deoxynivalenol Glucoside in Artificially and Naturally Contaminated Wheat by LC-MS/MS. J Agric Food Chem. 2005;53:3421–3425. doi: 10.1021/jf047798g. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt G, Zill G, Wohner B, Wallnöfer PR. Transformation of the Fusarium mycotoxin zearalenone in maize cell suspension cultures. Naturwissenschaften. 1988;75:309–310. doi: 10.1007/BF00367324. [DOI] [PubMed] [Google Scholar]
- 10.Schneweis I, Meyer K, Engelhardt G, Bauer J. Occurrence of zearalenone-4-β-D-glucopyranoside in wheat. J Agric Food Chem. 2002;50(6):1736–1738. doi: 10.1021/jf010802t. [DOI] [PubMed] [Google Scholar]
- 11.Gareis M. Maskierte Mykotoxine. Übers Tierernährung. 1994;22(1):104–113. [Google Scholar]
- 12.Wallnöfer PR, Preiß U, Ziegler W, Engelhardt G. Konjugatbildung organischer Schadstoffe in Pflanzen. Z Umweltchem Ökotox. 1996;8(1):43–46. [Google Scholar]
- 13.Engelhardt G, Ruhland M, Wallnöfer PR. Metabolism of mycotoxins in plants. Adv Food Sci. 1999;21(3/4):71–78. [Google Scholar]
- 14.Plasencia J, Mirocha CJ. Isolation and characterization of zearalenone sulfate. Appl Environ Microbiol. 1991;57(1):146–150. doi: 10.1128/aem.57.1.146-150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sharkawy S, Selim MI, Afifi MS, Halaweish FT. Microbial transformation of zearalenone to a zearalenone sulfate. Appl Environ Microbiol. 1991;57(2):549–552. doi: 10.1128/aem.57.2.549-552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemmens M, Scholz U, Berthiller F, Koutnik A, Dall’Asta C, Schuhmacher R, Adam G, Mesterhazy A, Krska R, Buerstmayr H, Ruckenbauer P. A major QTL for Fusarium head blight resistance in wheat is correlated with the ability to detoxify the mycotoxin deoxynivalenol. Mol Plant Microbe Interact. 2005;18:1318–1324. doi: 10.1094/MPMI-18-1318. [DOI] [PubMed] [Google Scholar]
- 17.Werner U. Characterisation of the effect of the Fusarium mycotoxin zearalenone in Arabidopsis thaliana. University of Natural Resources and Applied Life Sciences; Vienna: 2006. pp. 1–128. Ph.D. thesis. [Google Scholar]
- 18.Berthiller F, Werner U, Sulyok M, Krska R, Hauser MT, Schuhmacher R. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Addit Contam. 2006;23(11):1187–1193. doi: 10.1080/02652030600778728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulyok M, Berthiller F, Krska R, Schuhmacher R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom. 2006;20(18):2649–2659. doi: 10.1002/rcm.2640. [DOI] [PubMed] [Google Scholar]