Abstract
Platinum(IV) complexes are promising candidates as prodrugs for oral application in anticancer chemotherapy. However, only a few Pt(IV) compounds entered (pre)clinical trials, e.g. satraplatin, while most of the others were only tested in vitro. Aim of the study was investigation of the in vivo pharmacological behavior as well as the anticancer activity of two novel platinum(IV) complexes vs. satraplatin. The drugs were selected due to significantly different in vitro cytotoxicity while sharing some physicochemical properties (e.g. lipophilicity). Initial experiments indicated that the highly in vitro cytotoxic compound 1 ((OC-6-33)-dichloridobis((4-ethoxy)-4-oxobutanoato)-bis(ethylamine)platinum(IV)) was also characterized by high drug absorption and tissue platinum levels after oral application. Interestingly, analysis of serum samples using SEC-ICP-MS revealed that the administered drugs have completely been metabolized and/or bound to proteins in serum within 2 h after treatment. With regard to the activity in vivo, the outcomes were rather unexpected: although potent anticancer effect of 1 was observed in cell culture, the effects in vivo were rather minor. Nevertheless, 1 was superior to 2 ((OC-6-33)-diammine(cyclobutane-1,1-dicarboxylato)-bis((4-cyclopentylamino)-4-oxobutanoato)platinum(IV)) after i.p. administration, which was, at least to some extent, in accordance to the cell culture experiments. After oral gavage, both compounds exhibited comparable activity. This is remarkable considering the distinctly lower activity of 2 in cell culture as well as the low platinum levels detected both in serum and tissues after oral application. Consequently, our data indicate that the prediction of in vivo anticancer activity by cell culture experiments is not trivial, especially for orally applied drugs.
Keywords: Platinum(IV) complexes, Anticancer activity, ICP-MS, Oral administration
Introduction
Platinum-based complexes are established drugs as part of standard therapy in cancer treatment with usage in 50 % of chemotherapeutic regimens in clinics [1]. However, application of the three worldwide approved platinum(II)-based cytostatics (cisplatin, carboplatin and oxaliplatin) are limited by their severe side effects (e.g. nephrotoxicity, neurotoxicity, ototoxicity, myelosuppression, emesis and alopecia), intrinsic and/or acquired therapy resistance and the inconvenient way of intravenous administration [1-5]. These limitations together with the goal of expanding the therapeutic spectrum have led to an intense search for improved metal-based anticancer agents over the past decades [6-8]. One promising approach has focused on the development of platinum(IV) complexes featuring an octahedral geometry and consequently two additional ligand sites [9, 10]. Essential for the anticancer activity of Pt(IV) compounds is their in vivo activation by reduction (preferentially in the tumor cell) to the corresponding platinum(II) analogs, followed by aquation and induction of apoptotic cell death (prodrug-concept). In particular, the increased possibilities for tuning of pharmacological properties via modification of axial ligands combined with the high kinetic inertness to substitution reactions have turned platinum(IV) complexes into promising anticancer drug candidates [11, 12]. So far, three Pt(IV) compounds (tetraplatin, iproplatin and satraplatin) underwent clinical trials, but none of them has gained clinical approval yet. Tetraplatin ((OC-6-22)-tetrachlorido(trans-1,2-cyclohexanediamine)platinum(IV)) was abandoned due to severe neurotoxicity, while iproplatin ((OC-6-33)-dichloridodihydroxi dobis(isopropylamine)platinum(IV)) demonstrated lower activity compared with carboplatin and cisplatin [1]. Satraplatin ((OC-6-43)-bis(acetato)amminedichlorido(cyclohexylamine)platinum(IV)) was the first orally administered platinum complex with documented efficacy and acceptable safety in patients with hormone-refractory prostate cancer and small-cell lung cancer [13]. Despite the positive outcome after completion of the satraplatin and prednisone against refractory cancer (SPARC) phase III clinical trials, satraplatin was not approved by the FDA as it did not show a convincing benefit in terms of overall survival. However, clinical studies with satraplatin are still ongoing [1].
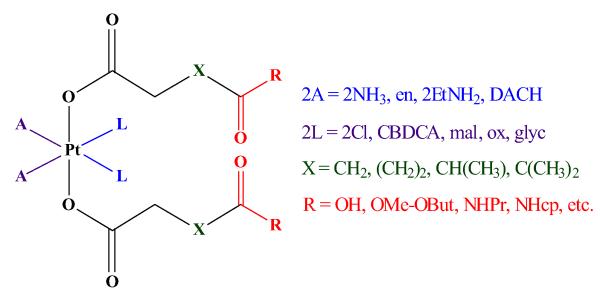
Recently, our group has reported a convenient synthetic procedure for preparation of novel bis(carboxylato)-platinum(IV) complexes and modulation of their physicochemical properties of interest (e.g. solubility, lipophilicity and redox behavior) [14]. Consequently, a plethora of diam(m)inebis-, tris- and tetrakis(carboxylato) platinum(IV) complexes featuring prodrugs of cisplatin, its bis(ethylamine) and ethylenediamine analogs, carboplatin, and its malonato analog, oxaliplatin, and nedaplatin, has been developed utilizing this approach (Fig. 1) [14-20]. The set of compounds covers a broad range of in vitro cytotoxicity (IC50 values from 0.009 to >500 μM) in the cancer cell lines tested.
Fig. 1.
General formula of diam(m)inebis-, tris- and tetrakis(carboxylato)platinum(IV) complexes developed, in our group (DACH 1R,2R-diaminocyclohexane, CBDCA 1,1-cyclobutanedicarboxylate, mal malonate, ox oxalate, glyc glycolate)
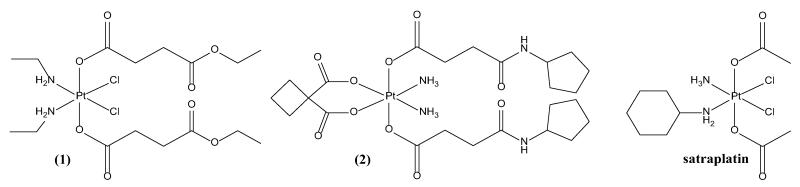
The analytical and biochemical, as well as theoretical and QSAR studies performed in the series [20] allowed us to select the most interesting compounds for further in vivo investigations (i.e. pharmacokinetic behavior and anticancer activity). (OC-6-33)-dichloridobis((4-ethoxy)-4-oxobutanoato)bis(ethylamine)platinum(IV) (Fig. 2, (1)) is one of the most active compounds in our set of over 60 investigated complexes, with higher or comparable potency to cisplatin in all the tested cell lines and adequate solubility and lipophilicity for further in vivo experiments [17, 20]. (OC-6-33)-diammine(cyclobutane-1,1-dicarboxylato)bis((4-cyclopentylamino)-4-oxobutanoato)platinum(IV) (Fig. 2, (2)) is a carboplatin prodrug representative, characterized with high kinetic stability towards biological reducing agents (e.g. ascorbate). This compound was chosen with respect to its solubility, lipophilicity and in vitro cytotoxicity in the sub-series [18].
Fig. 2.
Structural formulas of the platinum(IV) complexes under investigation
Herein, we have investigated the anticancer activity in murine cancer cell models as well as the platinum distribution in tissues by means of ICP-MS of the selected drug candidates in comparison to satraplatin (Fig. 2). The predictability of (1) cell culture experiments, (2) platinum accumulation in mice tissue samples and (3) serum platinum levels for the in vivo anticancer activity of Pt(IV) complexes has been assessed.
Experimental
Chemicals
Milli-Q water (18.2 MΩ cm, Milli-Q Advantage, Darmstadt, Germany) was used for RP-HPLC and SEC-ICP-MS experiments as well as for all dilutions for ICP-MS measurements. Nitric acid (≥65 %, p.a., Fluka, Buchs, Switzerland) was further purified in a quartz sub-boiling point distillation unit (Milestone-MLS GmbH, Leutkirch, Germany). Platinum and rhenium standards for ICP-MS measurements were derived from CPI International (Amsterdam, The Netherlands). All other reagents and solvents were obtained from commercial sources and were used without further purification. Synthesis and characterization of the novel Pt(IV)-based drug candidates (1 and 2) were described previously [17, 18] Satraplatin was prepared according to Ref. [21].
ICP-MS measurements
Quantification of platinum in liquid samples (including on-line sample introduction via SEC) was carried out with an ICP-quadrupole MS instrument Agilent 7500ce (Agilent Technologies, Waldbronn, Germany) equipped with a CETAC ASX-520 autosampler (Nebraska, USA) and a MicroMist nebulizer at a sample uptake rate of approx. 0.25 ml/min. The instrument was tuned on a daily base to achieve maximum sensitivity. Rhenium served as internal standard for platinum to account for instrumental fluctuations and matrix effects. The Agilent MassHunter software package (Workstation Software, version B.01.01, 2012) was used for data processing. The experimental parameters for ICP-MS are summarized in Table 1.
Table 1.
Instrumental parameters for quantification of platinum with ICP-MS
| ICP-MS | |
|---|---|
| RF power (W) | 1500 |
| Cone material | Nickel |
| Carrier gas (l/min) | 0.92-0.96 |
| Make up gas (l/min) | 0.16-0.19 |
| Plasma gas (l/min) | 15 |
| Monitored isotopes | 185Re, 194Pt, 195Pt |
| Dwell time (s) | 0.1 |
| Number of replicates | 10 |
Digestion of tissue samples (approx. 10–30 mg) was performed with 2 ml of 32.5 % subboiled nitric acid using a microwave system Discover SP-D (CEM Microwave Technology, Germany). The following microwave parameters were used: temperature, 200 °C; ramp time, 4 min; hold time, 6 min; maximal power, 300 W. Digested samples were diluted with Milli-Q water resulting in nitric acid concentrations lower than 3 % and platinum concentrations lower than 15 μg/g. In the case of serum and whole blood, approx. 50 μl were weighed and digestion was done under the same conditions as the organs. The total platinum content was determined with ICP-MS using the experimental parameters given in Table 1.
Determination of solubility
Solubility was determined using the conditions described in the OECD guidelines [22] with slight modifications. Saturated solutions of the investigated compounds were prepared in Milli-Q water at room temperature. Undissolved particles, remaining after repeated steps of ultrasonication, were removed using a 0.45 μm filter. Solubility of the complexes was calculated after dilution of the samples with 1 % HNO3 and determination of the platinum concentration by means of ICP-MS (using the instrumental parameters given in Table 1).
Determination of lipophilicity and stability
Lipophilicity was determined as the partition coefficient between n-octanol and water (log Po/w) and as the chromatographic retention factor (log kw). The log Po/w determination was carried out according to the OECD guidelines for the classical shake flask method [23] with slight modifications. The complexes were dissolved in Milli-Q water (presaturated with 1-octanol) and the platinum concentration was determined by ICP-MS. The remaining stock solution was mixed with an equal volume of 1-octanol (pre-saturated with Milli-Q water) and shaken for 1 h. After phase separation, the platinum concentration was again determined in the aqueous phase by ICP-MS and the partition coefficients were calculated assuming the missing quantity (compared to the stock solution) in the organic phase. ICP-MS measurements were carried out after dilution of the samples with 1 % HNO3 using the instrumental parameters given in Table 1.
The chromatographic retention factor (log kw) was assessed by means of RP-HPLC as described previously [17]. The analysis was carried out on a Dionex Summit system (Dionex, Germering, Germany) controlled by the Dionex Chromeleon 6.80 software. The following chromatographic conditions were used: Agilent ZORBAX SB aq C18 column (4.6 mm × 250 mm, 5 μm); injection volume: 20 μl; flow rate: 1 ml/min; isocratic elution; temperature of the column: 25 °C; UV-Vis detection set up at 210 nm; uracil was used as an internal reference to determine the column dead time (t0); 0.1 % TFA aqueous solution/MeOH-based mobile phases were employed. The chromatograms for each complex were run in triplicates with at least three different mobile phase compositions and the respective capacity factors (k = (tR - t0)/t0) were calculated. The extrapolated retention factor to 0 % MeOH (log kw) was determined, using the linear relationship between log k and the concentration of MeOH in the mobile phase. The same chromatographic conditions were used to determine the stability of the compounds in water solution. The complexes proved to be stable within 24 h of incubation at 37 °C.
SEC-ICP-MS experiments
Serum obtained from mice 2 h after single dose oral administration of compound 1, 2 and satraplatin was diluted in phosphate-buffered saline (PBS) and subjected to SEC-ICP-MS. For comparison reasons, the compounds were dissolved in PBS, incubated for 1 and 24 h at 37 °C and also analysed by SEC-ICP-MS. A size exclusion column (10 mm × 300 mm, 13 μm) (Superdex™ 200, 10/300 GL, GE Healthcare) with an approximate bed volume of 24 ml and a linear separation range of 10-600 kDa was used. Analysis was carried out on a Dionex Ultimate 3000 RS system (Dionex, Germering, Germany) controlled by the Dionex Chromeleon 6.80 software. Injection volume was 20 μl and elution was performed in isocratic mode with an aqueous solution of ammonium acetate (100 mmol/l, pH = 7.4) as mobile phase at a flow rate of 0.7 ml/min. The column was calibrated with a mixture of ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), ribonuclease (13.7 kDa) and aprotinin (6.5 kDa) using UV-Vis detection at 280 nm. The retention times (t) were plotted against the logarithm of the molecular mass (y) and showed linear correlation (y = −0.150t + 7.849, R2 = 0.993). The column was coupled via a PEEK tubing directly to a MiraMist nebulizer of the ICP-MS. The platinum trace was recorded using the Agilent MassHunter Chromatography software package (Workstation Software, version B.01.01, 2012). The instrumental parameters of ICP-MS are given in Table 1.
Cell cultures
The following cell lines were used in this study: the murine leukemia model L1210 (generously provided by Dr. Ganapathi, Cleaveland Clinic Foundation, Ohio, USA) and the murine colon cancer cell model CT-26 (from American Type Culture Collection, Manassas, VA, USA). All cells were grown in a humidified atmosphere with 5 % CO2 at 37 °C in medium supplemented with 10 % fetal bovine serum (FCS). L1210 cells were grown in RPMI 1640 and CT-26 cells in DMEM/F12 medium. Cultures were regularly checked for Mycoplasma contamination.
Cytotoxicity assay
The above described cells were seeded (2 × 103 cells/well) in 100 μl per well in 96 well plates. After a recovery period of 24 h, stock solutions (10 mM) of the drugs (freshly prepared in DMSO) were serially diluted with growth medium and added to the samples in volumes of 100 μl per well. Final DMSO concentrations never exceeded 0.5 %. At this concentration, DMSO neither impacted cell viability nor drug stability (confirmed by 1H NMR) of the test compounds, which is in accordance to the work of Hall et al. [24]. After exposure for 72 h, the cell viability was determined by MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay following the manufacturer’s recommendations (EZ4U, Biomedica, Vienna, Austria).
Animal experiments
All animal experiments were approved by the local ethics commission and carried out according to the Austrian and FELASA guidelines for animal care and protection. 6- to 8-week-old Balb/c or DBA/2J mice (weighing 25–30 g) were purchased from Harlan Laboratories, San Pietro al Natisone, Italy. The animals were kept in a pathogen-free environment and every procedure was done in a laminar airflow cabinet.
Kinetic experiments with Balb/c mice
Mice which received the drugs as an oral single dose (40 mg/kg, solutions freshly prepared in water with 1 % DMSO), were anesthetized after 2 h and blood was collected by heart punctuation. Serum was isolated by centrifugation of the blood samples at 3000 rpm for 10 min for two times and stored at −20 °C for SEC-ICP-MS experiments. In addition, samples of organs (kidney, liver, lung) were collected and stored at −20 °C for quantitative determination of platinum by means of ICP-MS after microwave-assisted digestion. For kinetic experiments, the compounds were administered orally at single dose of 0.05 mmol/kg (i.e. 34.1, 40, and 25.8 mg/kg for compound 1, 2, and satraplatin, respectively; solutions freshly prepared in water with 1 % DMSO; Table S1) and total blood was collected after 2, 4 and 6 h by tail vain puncture. Mice were sacrificed after 24 h and blood as well as organs was collected for platinum determination without prior perfusion of the animal.
Anticancer activity against CT-26 cells in vivo
Murine CT-26 cells (5 × 105) were injected subcutaneously into the right flank of female Balb/c mice. Animals were treated with the drug either intraperitoneally (solutions freshly prepared in water) or orally (solutions freshly prepared in water with 1 % DMSO) at indicated drug concentrations on days 4, 7, 11, and 14 (Table S1). Animals were controlled for distress development every day and tumor size was assessed regularly by caliper measurement. Tumor volume was calculated using the formula: length × width2/2. At day 15, tissue and blood samples from anesthetized animals were collected for quantitative determination of platinum as described above.
Antileukemic activity in vivo
L1210 murine leukemia cells (1 × 105) were injected intraperitoneally in a volume of 0.2 ml into male DBA/2 J mice on day 0. The test compounds (dissolved in water) were administered intraperitoneally at indicated drug concentrations on day 1, 5 and 9 (Table S1). Toxicity was monitored by daily observation of animals and registration of their body weight. Therapeutic efficacy of the investigated compounds was monitored by recording the lengths of survival of experimental mice compared to untreated control animals.
Results and discussion
Overview of in vitro cytotoxicity and pharmacologically relevant physicochemical properties
Physicochemical properties, relevant for the pharmacological behavior of the novel platinum(IV) anticancer drug candidates and satraplatin, together with their in vitro cytotoxicity in three human tumor cell lines, are summarized in Table 2. The new compounds have a lipophilicity being in an optimal range (log Po/w 0.5–3.5) for oral application which should in both cases allow a sufficient absorption from the intestinal lumen into the bloodstream [25]. Our approach for modification of the axial ligands of platinum(IV) complexes [18, 26] allows adjusting the lipophilicity to optimal values without compromising the solubility. As a consequence, compound 1 and 2 possess advantageous (20- to 100-fold higher) water solubility compared to satraplatin. This is also of importance as aqueous solubility is often a limiting factor for drugs when administered intraperitoneally (i.p.). Although being less critical for p.o. administration, it also impacts on the drug absorption efficiency via this route, as only the dissolved fraction can be absorbed in the digestive tract [25]. Regarding the Lipinski’s rule of five criteria for evaluation of drug likeness [27], all tested compounds satisfy the criteria for lipophilicity (log P o/w < 5), but not for a maximum molecular weight (MW < 500 Da). Compound 1 and 2 (646.4 and 757.7 Da, respectively) have a higher molecular weight, while satraplatin is exactly on the border of the limit (MW = 500.3 Da). The above-mentioned approach for tuning the lipophilicity via modification of the axial ligands also results in a higher number of hydrogen bond acceptors for compound 1 and 2, compared to satraplatin. However, complex 1 and satraplatin still satisfy Lipinski’s requirement for the maximal number of hydrogen bond donors and acceptors. Nevertheless, it should be mentioned that Lipinski’s rules of five were postulated for organic molecules and should be considered for metal-based drugs more carefully. For example, the heavy metal platinum (with a molecular weight of 195 Da) already contributes to approximately 40 % of the molecular weight limit.
Table 2.
In vitro cytotoxicity in three human cancer cell lines and pharmacologically relevant physicochemical properties of compound 1, 2 and satraplatin
| Compound | 1 | 2 | Satraplatin |
|---|---|---|---|
| Formula | C16H32N2O8PtCl2 | C24H40N4O10Pt·H2O | C10H22N2O4PtCl2 |
| MW (g/mol) | 646.4 | 757.7 | 500.3 |
| Aqueous solubility (mg/ml) | 1.94 ± 0.02 | 10.2 ± 0.04 | 0.10 ± 0.01 |
| Lipophilicity (log kw) | 2.87 ± 0.01 | 2.99 ± 0.06 | 1.49 ± 0.03 |
| Lipophilicity (log Po/w) | 0.72 ± 0.03 | 0.77 ± 0.06 | 0.23 ± 0.01 |
| IC50 (CH1), μMa | 0.061 ± 0.015b | 15 ± 5c | 0.10 ± 0.02d |
| IC50 (SW480), μMa | 0.30 ± 0.05b | >500c | 1.5 ± 0.1d |
| IC50 (A549), μMa | 1.0 ± 0.4b | >500c | 6.4 ± 0.4d |
With regard to the anticancer activity, compound 1 demonstrated pronounced in vitro cytotoxicity, slightly higher than satraplatin in the cell lines tested. Despite featuring similar lipophilicity, compound 2 proved to be far less potent than complex 1, most probably due to its slower rate of activation (reduction and subsequent aquation) [18].
Tissue distribution of platinum in mice after single dose oral administration of the investigational drugs
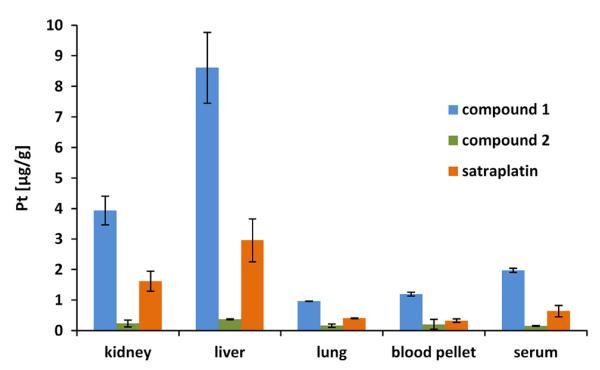
As a first approach to test the bioavailability of the novel platinum(IV) drugs, healthy mice were treated orally with the test compounds (Table S1). After 2 h, blood and organs were collected and the total platinum concentrations were determined by ICP-MS measurements. In these preliminary experiments, a dose of 40 mg/kg was chosen as this represents a frequently used oral dose for satraplatin [29]. Overall, a similar platinum distribution pattern could be observed for all three compounds with highest platinum levels in liver, followed by kidney and lung tissue (Fig. 3). The mice were not perfused after sacrificing, thus the high platinum concentrations in liver and kidney can be explained by their high blood supply as well as their biological functions. Liver is responsible for drug metabolism and especially for orally administered drugs the first-pass metabolism can be extensive [25]. In renal tissues frequently high drug levels occur due to the high blood flow to the kidneys (~25 % of cardiac output) together with the enormous concentrating ability of the organ [30]. This often results in high concentrations of parent compounds as well as their metabolites. Lung tissue showed platinum concentrations similar to serum and blood clot, containing the total cellular fraction of the blood samples (blood pellet), which can be also explained by the high blood perfusion of the organ [31].
Fig. 3.
Total platinum levels of compound 1, 2 and satraplatin in mice organs, blood pellet and serum 2 h after single dose oral application (40 mg/kg), corresponding to Pt doses of 12.1, 10.32 and 15.63 mg/kg, respectively
Notably, although the relative platinum distribution patterns of the three drugs were very similar, the total platinum concentrations differed distinctly (Fig. 3, Table S2). Thus, 2 h after drug application highest platinum levels were observed in mice treated with compound 1 (~9 μg/g in liver > ~4 μg/g in kidneys > ~2 μg/ml in serum > ~1 μg/g in blood pellet = ~1 μg/g in lungs). Satraplatin exhibited 2-3 times lower platinum concentrations in the investigated tissues. In contrast, complex 2 showed the lowest platinum accumulation with less than 0.5 μg/g in all organ samples. This might be based on the comparably low platinum levels in the blood serum (~0.15 μg/ml), indicating lower oral bioavailability and/or faster excretion of 2 than of compound 1 and satraplatin. Ranking the complexes according to the percentage of platinum amount found in serum after 2 h relative to the administered platinum dose, was as follows: ~16 % Pt for compound 1 > ~4 % Pt for satraplatin > ~1.5 % Pt for compound 2.
SEC-ICP-MS study in mice serum after single dose oral administration of the investigational drugs
In addition to the absolute quantification of platinum in tissues, the interaction with protein binding partners in serum in vivo can provide further information on the fate of the drug in the organism. For this purpose, size-exclusion chromatography (SEC) was hyphenated to ICP-MS, allowing the separation and detection of metal-containing species in serum according to their molecular weight. Overall, all three compounds demonstrated high affinity to serum proteins and appeared to have binding partners with similar size as the general pattern of chromatograms was comparable (Figure S1). The main peak observed at ~20 min (corresponding to approx. 66 kDa) represents most likely platinum bound to serum albumin (molecular weight 66.5 kDa), the most abundant serum protein which has been identified as the main binding partner in serum for several metal-based drugs (e.g. cisplatin, oxaliplatin and KP1019) [32-35]. Thus, all three drugs seem to be predominately bound to albumin and to lesser extent to serum proteins and protein complexes corresponding to fractions eluting after ~17 min (approx. 170 kDa) and ~12 min (> 600 kDa). In addition, a peak at 42 min corresponding to the void volume of the column was found in all samples investigated. Thus, small platinum-containing molecules, which do not interact with the column material, are present in the serum samples.
To assign these peaks to metabolites or parent drug molecules, the compounds were dissolved in phosphate-buffered saline (PBS) and injected into the SEC column, either freshly prepared or after 1 and 24 h of incubation at 37 °C. The different species of the metal-based drugs are separated due to unspecific interactions with the stationary phase, since these molecules are too small to be separated based on their molecular weights [36]. Subsequently, the obtained serum-free chromatograms were compared with those of the serum samples to identify unbound platinum-containing species (Figure S1, arrows indicate metabolites and the parent drug). For all spectra, the above-mentioned peak at 42 min was assigned to metabolites of the respective drugs. Satraplatin exhibits two more metabolites at ~29 and ~32 min, while compound 2 was the only one showing the presence of a small amount (~5 %) of the free intact platinum(IV) complex. The absence of intact satraplatin in serum samples is in accordance with literature reports for satraplatin [37, 38]. Thus, it was demonstrated in clinical trials that already 15 min after oral application of satraplatin, six new species were found in patients’ plasma ultrafiltrate whereas no parent drug was detected [38].
Kinetics of whole blood platinum following single p.o. administration
Platinum levels in whole blood (collected after vein puncture using a capillary) upon single oral administration of novel drug candidates and satraplatin were determined by means of ICP-MS at 2, 4, 6 and 24 h. For all three compounds, highest levels of platinum were found at the first time point investigated (2 h) with subsequent decreasing amounts of platinum in blood (up to 24 h) (Figure S2). According to literature reports on the pharmacokinetics of satraplatin in rats, the maximum concentration of platinum in plasma after p.o. application of satraplatin is found between 1.5 and 4 h [39]. Similar results were observed in clinical trials where patients were treated with satraplatin [40]. Based on these reports and the platinum concentration–time curves obtained in our experiments, it can be assumed that the highest platinum levels in blood are reached ≤2 h upon oral administration of the investigated complexes. Main pharmacokinetic parameters for compound 1, 2 and satraplatin are summarized in Table 3. The calculated AUC2–24 indicate that the total systemic platinum exposure (between the 2nd and 24th hour) after single oral administration of compound 1 and satraplatin is rather similar and more than two times higher than that for complex 2. The lower C2h and AUC2–24, observed for complex 2 are probably connected with its lower oral bioavailability and/or fast excretion before the 2nd hour after oral administration.
Table 3.
Preliminary pharmacokinetic parameters of blood platinum, following a single oral dose of 0.05 mmol/kg of 1, 2 or satraplatin, corresponding to approx. 0.2 mg of administered platinum
| Compound | C2h (μg/ml)a | Kel (1/h)b | t1/2 (h)b | AUC2–24 (μg h/ml)c |
|---|---|---|---|---|
| 1 | 1.89 | 0.058 | 12 | 17.36 |
| 2 | 0.82 | 0.065 | 11 | 8.66 |
| Satraplatin | 1.36 | 0.037 | 19 | 19.40 |
Values were calculated using the trapezoidal rule
Platinum concentration after 2 h is set to be close to Cmax
Elimination rate constants (Kel) and half-lives (t1/2) were determined using the ln C/t plot
Area under the curve between the 2nd and 24th hour after administration (AUC2–24)
Evaluation of in vivo anticancer activity of novel platinum(IV) compounds in comparison to satraplatin
As a first step, the cytotoxic potential of the compounds was assessed against the murine leukemia L1210 and the colon carcinoma model CT-26 in cell culture experiments. As shown in Table 4, compound 1 and satraplatin displayed potent activity in the low μM range in both cell lines tested. Comparable to the experiments on human cell models (see Table 2), complex 1 showed slightly higher cytotoxicity than satraplatin in L1210 cells, while in CT-26 cells satraplatin was ~2-fold more active than 1. In contrast, compound 2 was inactive in both cell lines, with no signs of cytotoxicity up to the highest tested concentration of 50 μM.
Table 4.
In vitro cytotoxicity of complex 1, 2 and satraplatin in two murine cancer cell lines
| Compound | IC50 (μM)a mean ± SD |
|
|---|---|---|
| L1210 | CT-26 | |
| 1 | 1.5 ± 0.4 | 5.7 ± 0.2 |
| 2 | >50 | >50 |
| Satraplatin | 2.5 ± 0.2 | 2.9 ± 0.3 |
50 % inhibitory concentrations (means ± standard deviations from at least three independent experiments), as obtained by the MTT assay, 72 h exposure
Subsequently, the in vivo anticancer activity of the novel platinum(IV)-based drug candidates was evaluated in CT-26-bearing mice in comparison to satraplatin. For these experiments, the drugs were applied four times (on days 4, 7, 11, and 14) and mice were sacrificed 24 h after the last application (Table S1). For oral application, a dosage of 40 mg/kg was used for satraplatin and compound 2. In case of compound 1, two dosages were tested orally: 51.7 mg/kg (equimolar to 40 mg/kg satraplatin) and 34.1 mg/kg (equimolar to 40 mg/kg of complex 2). In addition, complex 1 and 2 were tested intraperitoneally at equimolar concentration (8.5 mg/kg for 1 and 10 mg/kg for 2). In all experiments, the compounds were well tolerated with no signs of toxicity such as fatigue or weight loss.
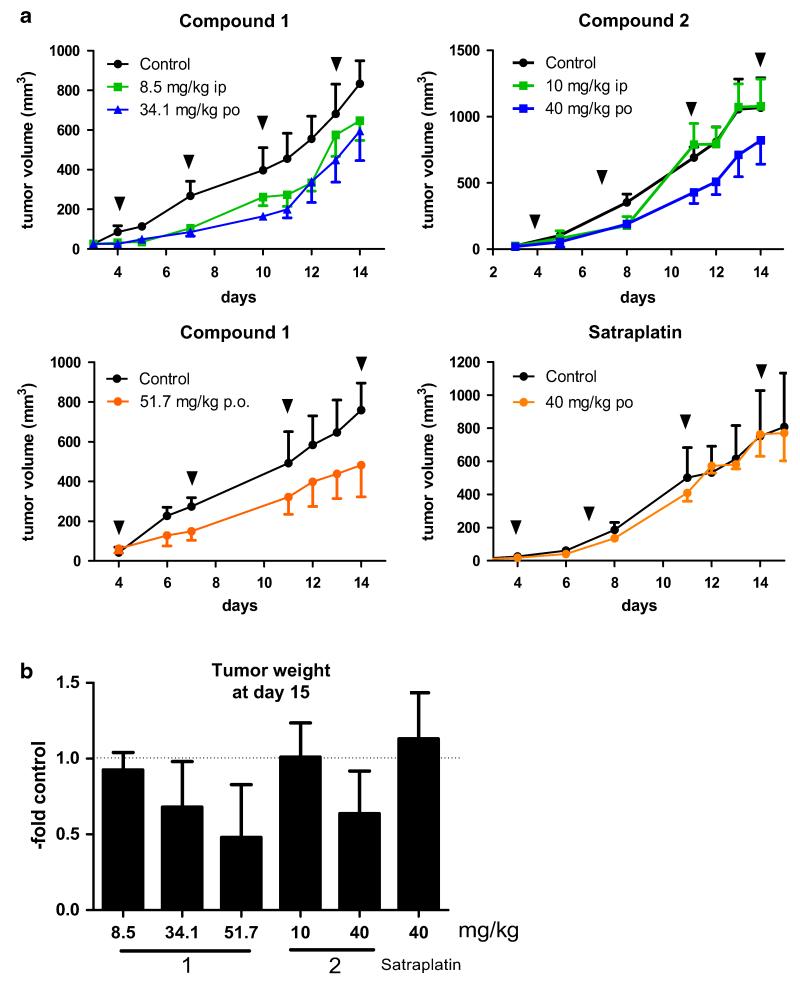
The anticancer activity of the three platinum(IV) compounds, expressed as tumor volume (mm3), is shown in Fig. 4a. Upon oral administration, treatment with complex 1 and 2 led to a slight tumor growth delay to similar extent. In case of compound 1, this effect was independent from the drug dose used (34.1 or 51.7 mg/kg). Unexpectedly, despite its potent anticancer activity in vitro, satraplatin was completely inactive against CT-26 cells in vivo. To the best of our knowledge, this might also explain the lack of reports on experiments with satraplatin in CT-26-bearing mice, although CT-26 is a known model for in vivo tests of platinum drugs [41-44]. In addition, the results obtained upon oral treatment with 1 and 2 are unexpected considering the in vitro IC50 values shown in Tables 2 and 4. Although complex 1 was more than tenfold more active against CT-26 cells in MTT assays, it did not show convincingly higher antitumor activity upon oral application in vivo and the experimental setup used (Fig. 4).
Fig. 4.
In vivo anticancer activity of compound 1, 2 and satraplatin. CT-26 cells were injected subcutaneously into the right flank of BALB/c mice. Mice were treated on day 4, 7, 11, and 14 (indicated by black triangles) with either compound 1 at concentrations of 8.5 mg/kg (i.p.), 34.1 mg/kg (p.o.) and 51.7 mg/kg (p.o.), compound 2 at concentrations of 10 mg/kg (i.p.) and 40 mg/kg (p.o.) or satraplatin at 40 mg/kg (p.o.). Data visualized in the same color correspond to equimolar concentrations. a Tumor volumes were calculated as described in the “Experimental” part. Each experimental group contained four animals. Data are mean ± SEM. b Animals were sacrificed on day 15 and tumors were collected and weighed. Data are expressed as fold change to the untreated control group of the respective experiment
With regard to the intraperitoneal application, complex 1 displayed anticancer activity comparable to the p.o. experiments, while complex 2 was inactive when given i.p. at equimolar concentrations. Similar results were obtained upon intraperitoneal application of the novel Pt(IV) anticancer drugs in mice bearing L1210 leukemia cells (Figure S3).
Tissue distribution of platinum after multiple dose administration of investigational drugs in CT-26 tumor-bearing mice
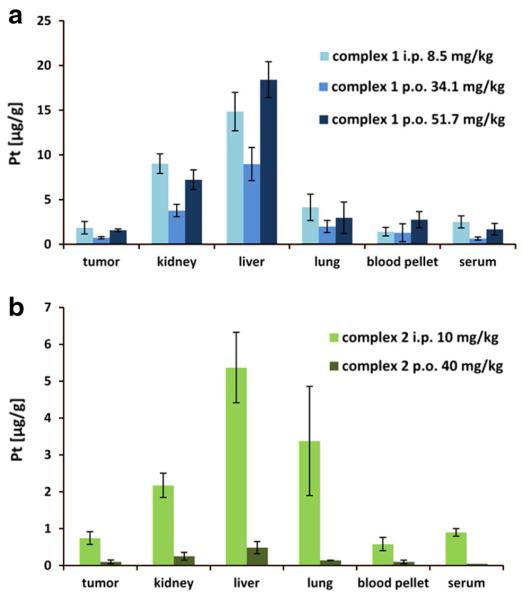
To better understand the pharmacology and toxicology of the novel complexes, tissue samples, collected from CT-26-bearing mice (compare Fig. 4) were analyzed by ICP-MS. The platinum levels in tumor, kidney, liver, lung, and fractions of whole blood (serum and blood pellet) are shown in Fig. 5 and Table S2. As expected, the route of administration had a distinct impact on the quantitative platinum accumulation in all tissues. Platinum concentrations in tissue upon i.p. application were higher, despite the four times lower dosages administered, compared to the p.o. application. In general, this is not unexpected as usually not the full amount of the orally applied compound is bioavailable. The difference between oral and intraperitoneal treatment was most pronounced for complex 2, where tenfold higher amounts of platinum were measured in liver, kidney and serum after i.p. administration. Nevertheless, the same trend of platinum distribution as for the single dose experiments could be observed independently from the route of administration (compare with Fig. 3). Thus, compound 1 displayed again the highest platinum levels in all samples, followed by satraplatin and compound 2 (Fig. 5, Table S2 and Figure S4) and the highest platinum concentrations were found in liver followed by kidney and lung tissue.
Fig. 5.
Platinum accumulation after multiple dose administration (i.p. and p.o.) of complex 1 (a) and complex 2 (b) in different tissues, blood pellet and serum originating from CT-26-bearing mice. Data are based on four animals per group, average concentrations and corresponding standard deviations are given
The repeated oral administration over 2 weeks resulted in an overall increase of platinum content in the tissues in comparison to the single dose experiments over 24 h indicating a cumulative effect (Table S2). Complex 1 was administered orally in two doses (Fig. 5a) leading to correspondingly elevated platinum levels in the investigated samples: a two times higher platinum concentration in kidney and liver was observed, whereas in tumor the platinum level increased up to 30 %. This indicates that although the total body platinum levels increased in accordance to the applied higher dose, this had a minor impact on the amount of platinum delivered to the malignant tissue. For compound 2, platinum concentrations <0.5 μg/g could be observed for all tissues after oral application, corresponding to 10- to 50-fold lower platinum amounts compared to compound 1. Interestingly, despite the overall significantly higher platinum levels, complex 1 did not demonstrate distinctly better in vivo anticancer activity in the CT-26 model after oral treatment, compared to complex 2.
Overall, the outcome of the in vivo experiments was rather unexpected considering the results of the cell culture tests. Although a potent anticancer activity of compound 1 against both CT-26 and L1210 cells was observed in cell culture, the effects in vivo were rather minor. Nevertheless, compound 1 was superior to 2 after i.p. administration, which is, at least to some extent, in accordance to the cell culture experiments. In contrast, both compounds were similarly active after oral gavage and at least in the case of 1 also widely independent of the given dose. This is remarkable, especially considering the almost complete lack of activity in cell culture as well as the low platinum levels detected both in serum and tissues after oral drug treatment in the case of complex 2. Noteworthy, compound 2 is a prodrug of the clinically used platinum(II) drug carboplatin. We have previously reported that the rate of reduction by biological reducing agents (e.g. ascorbate) of tetracarboxylatoplatinum(IV) complexes, prodrugs of carboplatin are slower than for diaminedichloridobis(carboxy lato)platinum(IV) complexes (e.g. compound 1 and satraplatin) [18]. In addition, carboplatin is much less reactive than cisplatin (and its dichlorido analogs), which is reflected in slower metabolism (in vitro and in vivo) and rather low IC50 values in cell culture experiments. Moreover, the therapeutic dose of carboplatin is approx. four times higher than for cisplatin, as nearly 90 % of the injected dose is recovered in urine [45]. The slow metabolism of compound 2 could be an explanation for the low platinum levels detected both in serum and tissues after treatment with 2. Our SEC-ICP-MS experiments showed that nearly no free parental species of the investigated platinum(IV) compounds could be detected in blood serum already 2 h after oral application. It may be speculated that the tested complexes in their native forms are excreted faster than their metabolites, which tend to bind to serum protein and to be retained in tissues. In addition, especially the first-pass effect, also reported for many other orally applied drugs [25] has to be considered.
Our data suggest that after oral gavage only a fraction of the test drugs is taken up into the body and thus is bio-available. This is a frequent observation when using oral application and the reasons, why only part of the drug reaches the systemic circulation are complex and can be influenced by various factors [46]: (1) drugs might have insufficient stability in the gastrointestinal tract leading to degradation before drug uptake. (2) Several physicochemical factors (e.g. lipophilicity) can influence the drug absorption. This effect was minimized in our study as the compounds possess similar lipophilicity. Nevertheless, it cannot be completely ruled out that some other physicochemical parameters might impact on their bio-availability. Finally, (3) there might be insufficient drug absorption from the intestinal lumen, for example, based on drug efflux back into the gastrointestinal tract by cellular transport proteins, e.g. P-glycoprotein (ABCB1) or the breast cancer resistance protein (ABCG2). These efflux proteins are strongly expressed at the membranes of the gut epithelium and are known to impact on multiple drugs [3, 46]. To gain insights into this topic, the role of ABC transporter expression on the anticancer activity of our two test drugs (especially compound 2) is matter of ongoing investigations.
Conclusions
The results from this study indicate that pre-experiments using cell culture can only be taken as a first indication to estimate the in vivo anticancer activity of platinum (IV)-based cytostatics. Thus, i.p. experiments were found to be in some agreement with the cell culture experiments, predicting the reduced antitumor activity of compound 2 compared to compound 1. In contrast, the data from cell culture experiments were not at all predictive for the in vivo activity after oral gavage. Despite the low IC50 values demonstrated in the viability tests and the high amount of platinum detected in blood and tumor tissue, compound 1 did not show significantly enhanced activity against CT-26 cells in vivo in comparison to compound 2. However, the mechanisms leading to this discrepancy between in vivo and cell culture experiments are so far speculative. One explanation might be rapid metabolization indicated by the serum analysis, which showed that already 2 h after oral treatment the administered drugs have been completely metabolized and/or bound to proteins in serum. In addition, cytotoxicity tests conducted solely with human cancer cell lines do not consider the complex interactions between various tissue types and their impact on pharmacokinetics and pharmacodynamics of the test compounds. Further investigations of the role and activity of diverse drug metabolites is topic of ongoing studies and will help to better understand the fate of anticancer drugs in vivo. Moreover, such detailed knowledge can improve the prediction of in vivo anticancer activity and, thus, allow selecting the best drug candidates for further (pre)clinical development.
Supplementary Material
Acknowledgments
Matthias Klose and Ricarda Bugl are acknowledged for their assistance in sample preparation and ICP-MS measurements. In addition, we are thankful to G. Zeitler and S. Van Schoonhoven for animal care. This work was supported by the Austrian Science Fond grant P26603 (to P.H.). This work was performed in the surrounding of COST action CM1105.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00775-014-1214-6) contains supplementary material, which is available to authorized users.
Contributor Information
Sarah Theiner, Institute of Inorganic Chemistry, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria; Research Platform ‘Translational Cancer Therapy Research’, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Hristo P. Varbanov, Institute of Inorganic Chemistry, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria
Mathea Sophia Galanski, Institute of Inorganic Chemistry, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria.
Alexander E. Egger, ADSI-Austrian Drug Screening Institute GmbH, Innrain 66a, 6020 Innsbruck, Austria
Walter Berger, Research Platform ‘Translational Cancer Therapy Research’, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria; Department of Medicine I, Comprehensive Cancer Center of the Medical University, Institute of Cancer Research, Medical University of Vienna, Borschkegasse 8a, 1090 Vienna, Austria.
Petra Heffeter, Research Platform ‘Translational Cancer Therapy Research’, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria; Department of Medicine I, Comprehensive Cancer Center of the Medical University, Institute of Cancer Research, Medical University of Vienna, Borschkegasse 8a, 1090 Vienna, Austria.
Bernhard K. Keppler, Institute of Inorganic Chemistry, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria; Research Platform ‘Translational Cancer Therapy Research’, University of Vienna, Waehringer Strasse 42, 1090 Vienna, Austria
References
- 1.Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 2.Yao X, Panichpisal K, Kurtzman N, Kenneth N. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 3.Heffeter P, Jungwirth U, Jakupec M, Hartinger C, Galanski M, Elbling L, Micksche M, Keppler B, Berger W. Drug Resist Updat. 2008;11:1–16. doi: 10.1016/j.drup.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Kelland LR. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 5.Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK. Dalton Trans. 2008;2:183–194. doi: 10.1039/b712656p. [DOI] [PubMed] [Google Scholar]
- 6.Galanski M, Arion VB, Jakupec MA, Keppler BK. Curr Pharm Des. 2003;9:2078–2089. doi: 10.2174/1381612033454180. [DOI] [PubMed] [Google Scholar]
- 7.van Rijt SH, Sadler PJ. Drug Discov Today. 2009;14:1089–1097. doi: 10.1016/j.drudis.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambley TW. Dalton Trans. 2007:4929–4937. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]
- 9.Hall MD, Mellor HR, Callaghan R, Hambley TW. J Med Chem. 2007;50:3403–3411. doi: 10.1021/jm070280u. [DOI] [PubMed] [Google Scholar]
- 10.Hall MD, Hambley TW. Coord Chem Rev. 2002;232:49–67. [Google Scholar]
- 11.Gibson D. Dalton Trans. 2009:10681–10689. doi: 10.1039/b918871c. [DOI] [PubMed] [Google Scholar]
- 12.Wexselblatt E, Gibson D. J Inorg Biochem. 2012;117:220–229. doi: 10.1016/j.jinorgbio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Choy H, Park C, Yao M. Clin Cancer Res. 2008;14:1633–1638. doi: 10.1158/1078-0432.CCR-07-2176. [DOI] [PubMed] [Google Scholar]
- 14.Reithofer MR, Valiahdi SM, Jakupec MA, Arion VB, Egger A, Galanski M, Keppler BK. J Med Chem. 2007;50:6692–6699. doi: 10.1021/jm070897b. [DOI] [PubMed] [Google Scholar]
- 15.Reithofer M, Galanski M, Roller A, Keppler BK. Eur J Inorg Chem. 2006;2006:2612–2617. [Google Scholar]
- 16.Reithofer MR, Schwarzinger A, Valiahdi SM, Galanski M, Jakupec MA, Keppler BK. J Inorg Biochem. 2008;102:2072–2077. doi: 10.1016/j.jinorgbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Varbanov H, Valiahdi SM, Legin AA, Jakupec MA, Roller A, Galanski M, Keppler BK. Eur J Med Chem. 2011;46:5456–5464. doi: 10.1016/j.ejmech.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varbanov HP, Valiahdi SM, Kowol CR, Jakupec MA, Galanski M, Keppler BK. Dalton Trans. 2012;41:14404–14415. doi: 10.1039/c2dt31366a. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmeister BR, Adib-Razavi MS, Jakupec MA, Galanski M, Keppler BK. Chem Biodivers. 2012;9:1840–1848. doi: 10.1002/cbdv.201200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varbanov HP, Jakupec MA, Roller A, Jensen F, Galanski M, Keppler BK. J Med Chem. 2012;56:330–344. doi: 10.1021/jm3016427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giandomenico CM, Abrams MJ, Murrer BA, Vollano JF, Rheinheimer MI, Wyer SB, Bossard GE, Higgins JD. Inorg Chem. 1995;34:1015–1021. doi: 10.1021/ic00109a004. [DOI] [PubMed] [Google Scholar]
- 22.OECD Guideline for Testing of Chemicals, 105 OECD. Paris: 1995. [Google Scholar]
- 23.OECD guideline for testing of chemicals, 107 OECD. Paris: 1995. [Google Scholar]
- 24.Hall MD, Telma KA, Chang K-E, Lee TD, Madigan JP, Lloyd JR, Goldlust IS, Hoeschele JD, Gottesman MM. Cancer Res. 2014;74:3913–3922. doi: 10.1158/0008-5472.CAN-14-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrick GL. An introduction to medicinal chemistry. Oxford University Press; Oxford: 2008. [Google Scholar]
- 26.Reithofer MR, Bytzek AK, Valiahdi SM, Kowol CR, Groessl M, Hartinger CG, Jakupec MA, Galanski M, Keppler BK. J Inorg Biochem. 2011;105:46–51. doi: 10.1016/j.jinorgbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Lipinski CA. Drug Discov Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Varbanov HP, Göschl S, Heffeter P, Theiner S, Roller A, Jensen F, Jakupec MA, Berger W, Galanski M, Keppler BK. J Med Chem. 2014;57:6751–6764. doi: 10.1021/jm500791c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelland LR, Abel G, McKeage MJ, Jones M, Goddard P, Valenti M, Murrer BA, Harrap KR. Cancer Res. 1993;53:2581–2586. [PubMed] [Google Scholar]
- 30.Perazella MA. Clin J Am Soc Nephrol. 2009;4:1275–1283. doi: 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
- 31.Wallace GC, Ramsden DB, Grant HM. Encyclopedia of Drug Metabolism and Interactions. Wiley; New York: 2012. [Google Scholar]
- 32.Sulyok M, Hann S, Hartinger CG, Keppler BK, Stingeder G, Koellensperger G. J Anal At Spectrom. 2005;20:856–863. [Google Scholar]
- 33.Hartinger CG, Zorbas-Seifried S, Jakupec MA, Kynast B, Zorbas H, Keppler BK. J Inorg Biochem. 2006;100:891–904. doi: 10.1016/j.jinorgbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Groessl M, Hartinger CG, Polec-Pawlak K, Jarosz M, Keppler BK. Electrophoresis. 2008;29:2224–2232. doi: 10.1002/elps.200780790. [DOI] [PubMed] [Google Scholar]
- 35.Allain P, Heudi O, Cailleux A, Le Bouil A, Larra F, Boisdron-Celle M, Gamelin E. Drug Metab Dispos. 2000;28:1379–1384. [PubMed] [Google Scholar]
- 36.Groessl M, Terenghi M, Casini A, Elviri L, Lobinski R, Dyson PJ. J Anal At Spectrom. 2010;25:305–313. doi: 10.1039/B922701F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell DN, Liu JJ, Tingle MD, Rattel B, Meyer TU, McKeage MJ. Clin Exp Pharmacol Physiol. 2008;35:1440–1446. doi: 10.1111/j.1440-1681.2008.05017.x. [DOI] [PubMed] [Google Scholar]
- 38.Raynaud FI, Mistry P, Donaghue A, Poon GK, Kelland LR, Barnard CFJ, Murrer BA, Harrap KR. Cancer Chemother Pharmacol. 1996;38:155–162. doi: 10.1007/s002800050464. [DOI] [PubMed] [Google Scholar]
- 39.Sova P, Mistr A, Kroutil A, Semerád M, Chlubnová H, Hrusková V, Chládková J, Chládek J. Cancer Chemother Pharmacol. 2011;67:1247–1256. doi: 10.1007/s00280-010-1411-0. [DOI] [PubMed] [Google Scholar]
- 40.Vouillamoz-Lorenz S, Buclin T, Lejeune F, Bauer J, Leyvraz S, Decosterd L. Anticancer Res. 2003;23:2757–2765. [PubMed] [Google Scholar]
- 41.Guo H-F, Huang J, Shi HS, Chen X-C, Wang Y-S. PLoS One. 2014 doi:10.1371/journal.pone.0085789. [Google Scholar]
- 42.Jungwirth U, Xanthos DN, Gojo J, Bytzek AK, Koerner W, Heffeter P, Abramkin S, Jakupec MA, Hartinger CG, Windberger U, Galanksi M, Keppler BK, Berger W. Mol Pharamcol. 2012;81:719–728. doi: 10.1124/mol.111.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge R, Ahn J-C, Shin J-I, Bahk CW, He P, Chung P-S. Photomed Laser Surg. 2011;29:155–160. doi: 10.1089/pho.2009.2750. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Lee HJ, Lee HJ, Choi WC, Yoon SW, Ko SG, Ahn KS, Choi SH, Ahn KS, Lieske JC, Kim SH. Phytomedicine. 2009;16:188–197. doi: 10.1016/j.phymed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Lokich J, Anderson N. Ann Oncol. 1998;9:13–21. doi: 10.1023/a:1008215213739. [DOI] [PubMed] [Google Scholar]
- 46.Stuurman FE, Nuijen B, Beijnen JH, Schellens JH. Clin Pharmacokinet. 2013;52:399–414. doi: 10.1007/s40262-013-0040-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.