Abstract
Many birds and mammals drastically reduce their energy expenditure during times of cold exposure, food shortage, or drought, by temporarily abandoning euthermia, i.e., the maintenance of high body temperatures. Traditionally, two different types of heterothermy, i.e., hypometabolic states associated with low body temperatures (torpor), have been distinguished: Daily torpor, which lasts less than 24 h and is accompanied by continued foraging, versus hibernation, with torpor bouts lasting consecutive days to several weeks in animals that usually do not forage but rely on energy stores, either food caches or body energy reserves. This classification of torpor types has been challenged however, suggesting that these phenotypes may merely represent the extremes in a continuum of traits. Here, we investigate whether variables of torpor in 214 species, 43 birds and 171 mammals form a continuum or a bimodal distribution. We use Gaussian-mixture cluster analysis as well as phylogenetically informed regressions to quantitatively assess the distinction between hibernation and daily torpor and to evaluate the impact of body mass and geographical distribution of species on torpor traits. Cluster analysis clearly confirmed the classical distinction between daily torpor and hibernation. Overall, heterothermic endotherms are small on average, but hibernators are significantly heavier than daily heterotherms and also are distributed at higher average latitudes (~35°) than daily heterotherms (~25°). Variables of torpor for an average 30-g heterotherm differed significantly between daily heterotherms and hibernators. Average maximum torpor bout duration was >30-fold longer, and mean torpor bout duration >25-fold longer in hibernators. Mean minimum body temperature differed by ~13°C, and the mean minimum torpor metabolic rate was ~35% of the BMR in daily heterotherms but only 6% of basal metabolic rate in hibernators. Consequently, our analysis strongly supports the view that hibernators and daily heterotherms are functionally distinct groups that probably have been subject to disruptive selection. Arguably, the primary physiological difference between daily torpor and hibernation, which leads to a variety of derived further distinct characteristics, is the temporal control of entry into and arousal from torpor, which is governed by the circadian clock in daily heterotherms, but apparently not in hibernators.
Keywords: daily torpor, hibernation, heterothermy, energy savings, hypothermia, hypometabolism, endotherms, thermoregulation, over-wintering
I. INTRODUCTION
Birds and mammals spend a large proportion of their energy expenditure on maintaining high euthermic1 body temperatures (Tb). This optimises many physiological functions, such as mobility, digestion, and brain function, but becomes costly during periods of cold exposure, which require substantial heat production that is impossible to sustain during food shortages. The only ‘logical solution’ (Schmidt-Nielsen, 1979) for animals that cannot escape harsh environmental conditions by migration is to suspend the maintenance of high body temperatures (Tb) and employ a mode of living that saves energy. This is the strategy used by many birds and mammals that employ hypometabolism, i.e., periods of profoundly reduced metabolic rate (MR) and Tb, which typically occur on a seasonal basis (Lyman et al., 1982). States of profound but controlled reductions of MR and Tb in endotherms are called torpor (cold-lethargy). Birds and mammals that use torpor are traditionally classified as either hibernators or species using daily torpor (i.e., daily heterotherms). The main distinguishing trait that is often invoked, is that species regarded as hibernators are capable of consecutive multiday torpor bouts, lasting on average for more than a week, whereas torpor in animals traditionally viewed as daily heterotherms usually lasts only between ~3 and 12 hours (Geiser & Ruf, 1995). Other traits that point to functional differences are the minimum metabolic rate (MR) during torpor (TMRmin), which in in animals categorized as hibernators appears to be much lower, as well as the minimum body temperature (Tb min) that is defended during torpor, which seems higher in species regarded as daily heterotherms, although the variation among species in Tb min is large. It appears that body mass also differs between the two categories with species conventionally viewed as hibernators being significantly bigger than species classified as daily heterotherms (Geiser & Ruf, 1995).
In recent years, the development of miniature temperature data loggers and transmitters has resulted in a large number of new data on torpor expression in free-living animals. Obviously, these species are subject to natural ambient conditions, especially to short-term fluctuations in ambient temperature (Ta) which may hamper the detection of the actual capabilities of species: (1) Variables usually measured in the field, such as Tb and torpor bout duration (TBD), are strongly affected by Ta (e.g., Twente & Twente, 1965; Geiser & Kenagy, 1988; Bieber & Ruf, 2009; Stawski & Geiser, 2010). During torpor, Tb decreases with Ta over a wide range of Ta until Tb min is reached (e.g., Buck & Barnes, 2000; Arnold et al., 2011). (2) Whereas species conventionally categorized as hibernators may remain torpid for more than a month at low Ta and thus Tb, the duration of torpor bouts shortens with increasing Ta. At high Ta between 20° and 30°C, where some of these species still express torpor, torpor bouts often last only for hours and superficially appear to be indistinguishable from the traditional category daily torpor (e.g., Song et al., 1997; Bieber & Ruf, 2009). (3) Many species are sensitive to fluctuating Ta as usually experienced in the wild and will not remain torpid if Ta is not stable (Henshaw, 1970). Thus, patterns of torpor observed in free-ranging animals are often not an indication of the animals’ physiological capability, but rather reflect its response to current ambient conditions. Consequently, these data may not be suitable for meaningful inter-specific comparisons of physiological capabilities. With regard to laboratory measurements, a commonly overlooked problem with quantitatively characterising torpor patterns is that species capable of very long torpor episodes often require a number of short torpor bouts before they express multiday bouts (Strumwasser et al., 1967; Geiser, 2007). Laboratory measurements of torpor, especially when respirometry is involved, typically last for around 1 day or less and even species traditionally classified as hibernators under these conditions usually express torpor that lasts only for hours rather than days (Song et al., 1997).
Despite these shortcomings, data from the field under varying thermal conditions and short-term measurements in the laboratory have been used to question whether “hibernators” and “daily heterotherms” are two distinct functional groups or simply a single group of heterotherms characterized by a continuum of variables (Wilz & Heldmaier, 2000; Lovegrove et al., 2001; Canale et al., 2012; Boyles et al., 2013). For instance, using indices of heterothermy in a recent comprehensive study, Boyles et al. (2013) found that, with the exception of permanent homoeotherms, Tb variation was otherwise largely continuously distributed, and concluded that the traditional classification of mammals as hibernators versus daily heterotherms is “clouded or possibly misleading”. Here, we re-address this controversy by analysing physiological variables characterising torpor in both birds and mammals. In contrast to Boyles et al. (2013), however, we focused on extremes of characteristics such as minimum Tb (Tb min) or maximum TBD (TBDmax) in order to assess species-specific physiological capabilities, rather than short-term responses to environmental conditions. Compared with previous studies on these variables (e.g., Geiser & Ruf, 1995) we investigated a much larger dataset now available for 214 heterothermic bird and mammal species.
We hypothesized that, variables characterizing each species’ capacity for torpor would support the classical distinction between daily torpor and hibernation. Specifically we hypothesized (1) That frequency distributions of TBDmax (and possibly also mean torpor bout duration; TBDmean) absolute TMR (TMRmin), as well as TMRrel, i.e., the relative reduction of TMR below basal metabolic rate (BMR) and Tb min would all show clearly bimodal patterns. (2) That statistical cluster analysis based on the above variables would support the existence of two distinct subgroups among heterotherms and show a high degree of coherence with the traditional classification. (3) That phylogenetically informed regression analysis within hibernators and daily heterotherms, if maintaining these categories was in fact justified by results of the above analyses, would support earlier findings (e.g., Geiser and Ruf 1995, Geiser 2004) suggesting that (a) Mean body mass of hibernators is higher than in daily heterotherms (b) TBDmax (and probably also TBDmean) is different between the two subgroups (c) Both TMR and Tb min show allometric relationships to body mass with distinctly different elevations in the two categories. 4) That similar to results for general heterothermy indices (Boyles et al., 2013), the maximum depth or duration of torpor may increase with latitude of the species’ geographical range.
II. METHODS
Data were collected using primary literature on 43 birds and 171 mammals. As a “working hypothesis” all species were initially classified according to the maximum torpor bout duration (TBDmax) as reported by the respective authors or determined by us (see below; TBDmax <1day, or TBDmax >1 day; Table 1) and preliminarily labelled “daily heterotherms” (42 birds, 78 mammals) or “hibernators” (1 bird, 93 mammals). The only exception to this rule was classifying Sminthopsis macroura as a daily heterotherm, despite a reported TBDmax of 25.9 h, which was observed only once (see Discussion). Our initial classification was identical to that of the original authors of the studies evaluated, except for Elephantulus myurus, as it showed a TBDmax of 39 h, but has been classified as a daily heterotherm previously (see sources in Table 1). If data for a species were available in more than one publication, the season in which torpor was most pronounced, or the reference with the most extensive data set was used. For TBD the maximum values and mean values as reported were used. However, TBDmean was often not reported. In these cases we either calculated TBDmean from raw data obtained during ‘mid-hibernation’ presented in figures, or, if only a range of torpor bout durations was provided, we estimated the mean from the average minimum and maximum given. TBDmax was difficult to define in a few species (e.g., Cheirogaleus medius) that maintained Tb slightly above fluctuating Ta for up to several weeks with no indications for active warm-ups (e.g., Dausmann et al., 2004). In these species we used two alternative methods to determine TBDmax: 1) We used the maximum time of passively fluctuating Tb observed and 2) we used TBDmax from conventional torpor bout patterns (with clear arousals) determined in the same species at lower and more constant mean Ta. Because using type 1 or type 2 TBDmax did not affect any of the conclusions, we maintained only type 2 TBDmax, because using maximum observation periods (as in type 1) seemed somewhat arbitrary. TBDmax was also difficult to assign in 3 carnivores (Ursus americanus, Ursus arctos and Meles meles), which - based on records of Tb, MR and behaviour - were initially classified as “hibernators” as they are capable of consecutive multiday torpor episodes (e.g., Tøien et al., 2011). Whereas these species show multiday Tb cycles, the peaks in these cycles are not considered homologous to arousal episodes in small hibernators (Tøien et al., 2011). Therefore, we assigned no values for TBD in these three species. For minimum TMR only values that were below the basal metabolic rate (BMR) of a species were included in our analyses. TMRmin that were not steady-state, according to visual inspection of graphs, usually because animals were not measured long enough (or if torpor was induced for brief periods at inappropriate times of day), were excluded. If only Tb measurements were available, we considered animals with a Tb reduction of >5°C below euthermic resting values to be torpid.
Table 1.
Torpor characteristics in birds and mammals. T: type - daily torpor (DT) or hibernation (HIB); BM: body mass (kg); Tbmin: minimum body temperature in torpor (°C); TMRmin: Minimum torpor metabolic rate (mL O2 g−1 h−1); TMRrei: Relative metabolic rate in torpor (TMRmin as % of BMR); TBDmax: Maximum torpor bout duration(h); TBD Ø: Mean torpor bout duration(h); IBE: Duration of inter-bout euthermia (h)1; LAT: Latitude of mid species range (>0: °N, <0: °S).
| Taxon | T | BM | Tb min | TMR min | TMR rel | TBD max | TBD ø | IBE | LAT | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| AVES | ||||||||||
| Coraciiformes | ||||||||||
| Dacelo novaeguineae | DT | 0.35 | 28.6 | 11.1 | 9.3 | −25 | Cooper et al. (2008) | |||
| Todus mexicanus | DT | 0.0062 | 22.4 | 2 | 62 | 18 | Merola-Zwartjes and Ligon (2000) | |||
| Coliiformes | ||||||||||
| Colius castanotus | DT | 0.058 | 18 | 0.49 | 21 | 11 | −10 | Prinzinger et al. (1981); Hoffmann and Prinzinger (1984) | ||
| Colius colius | DT | 0.035 | 26 | −27 | McKechnie and Lovegrove (2001b) | |||||
| Colius striatus | DT | 0.051 | 18.2 | 0.11 | 13 | 10 | −4 | Hoffmann and Prinzinger (1984); McKechnie and Lovegrove (2001a) | ||
| Urocolius indicus | DT | 0.053 | 0.68 | 28 | 10 | −23 | Hoffmann and Prinzinger (1984) | |||
| Urocolius macrourus | DT | 0.049 | 22 | 0.65 | 24 | 11 | 3 | Hoffmann and Prinzinger (1984); Schaub et al. (1999) | ||
| Apodiformes | ||||||||||
| Aeronautes saxatalis | DT | 0.0305 | 20 | 10 | 23 | Bartholomew et al. (1957) | ||||
| Apus apus | DT | 0.042 | 28 | 0.4 | 5 | 50 | Koskimies (1948) | |||
| Hirundapus caudacutus | DT | 0.085 | 28 | 10 | −25 | Pettigrew and Wilson (1985) | ||||
| Trochiliformes | ||||||||||
| Amazilia versicolor | DT | 0.0039 | 21.8 | 10.4 | 6.3 | −8 | Bech et al. (1997) | |||
| Archilochus alexandri | DT | 0.0032 | 13.5 | 0.2 | 5.7 | 35 | Lasiewski (1963); Lasiewski (1964) | |||
| Calypte anna | DT | 0.0034 | 16 | 0.17 | 4.4 | 39 | Lasiewski (1963) | |||
| Calypte costae | DT | 0.0032 | 9 | 0.38 | 12.7 | 37 | Lasiewski (1963); Lasiewski (1964) | |||
| Chrysuronia oenone | DT | 0.005 | 18 | 2.05 | −4 | Krüger et al. (1982) | ||||
| Clytolaema rubricauda | DT | 0.0077 | 18 | 0.5 | 16 | −22 | Bech et al. (2006) | |||
| Eugenes fulgens | DT | 0.008 | 10 | 0.5 | 27 | Wolf and Hainsworth (1972) | ||||
| Eulampis jugularis | DT | 0.008 | 18 | 1 | 33 | 15 | Hainsworth and Wolf (1970) | |||
| Eupetomena macroura | DT | 0.0085 | 22 | 9 | 5.8 | −15 | Bech et al. (1997) | |||
| Lampornis clemenciae | DT | 0.008 | 19.6 | 0.45 | 17 | 25 | Krüger et al. (1982) | |||
| Melanotrochilus fuscus | DT | 0.0069 | 22 | 11.2 | 9.6 | −26 | Bech et al. (1997) | |||
| Oreotrochilus estella | DT | 0.0085 | 6.5 | 0.75 | 10 | −17 | Carpenter (1974); Krüger et al. (1982) | |||
| Orthorhynchus cristatus | DT | 0.0029 | 20.8 | 1.25 | 20 | 16 | Krüger et al. (1982) | |||
| Panterpe insignis | DT | 0.005 | 10 | 0.5 | 9 | Wolf and Hainsworth (1972) | ||||
| Selasphorus platycercus | DT | 0.0035 | 6.5 | 0.54 | 37 | Calder and Booser (1973); Bucher and Chappell (1992) | ||||
| Selasphorus rufus | DT | 0.0033 | 13 | 0.43 | 12.8 | 10 | 5 | 48 | Lasiewski (1963); Hiebert (1990); Hiebert (1993) | |
| Selasphorus sasin | DT | 0.003 | 23 | 1.24 | 32.6 | 38 | Lasiewski (1963) | |||
| Strigiformes | ||||||||||
| Otus senegalensis | DT | 0.061 | 29 | 5 | 2.8 | −2 | Smit and McKechnie (2010) | |||
| Caprimulgiformes | ||||||||||
| Aegotheles cristatus | DT | 0.05 | 22.4 | 10.7 | 3 | −25 | Brigham et al. (2000); Doucette et al. (2012) | |||
| Caprimulgus europaeus | DT | 0.07 | 14 | 16 | 3 | −7 | Peiponen (1965); Schlegel (1969) | |||
| Caprimulgus guttatus (argus) | DT | 0.075 | 29.6 | 0.4 | 48 | −28 | Dawson and Fisher (1969) | |||
| Caprimulgus tristigma | DT | 0.073 | 10.5 | 15 | 6.4 | −11 | Smit et al. (2011); McKechnie and Mzilikazi (2011) | |||
| Caprimulgus vociferus | DT | 0.055 | 18.5 | 34 | Lane et al. (2004) | |||||
| Chordeiles acutipennis | DT | 0.05 | 15.7 | 33 | Marshall (1955) | |||||
| Chordeiles minor | DT | 0.073 | 18 | 43 | Lasiewski and Dawson (1964) | |||||
| Phalaenoptilus nuttallii | HIB | 0.035 | 3 | 0.05 | 6 | 120 | 35 | Withers (1977); Brigham (1992); Woods and Brigham (2004) | ||
| Podargus strigoides | DT | 0.5 | 29.1 | 11 | 7 | −25 | Körtner et al. (2000); Körtner et al. (2001) | |||
| Columbiformes | ||||||||||
| Drepanoptila holosericea | DT | 0.2 | 24.8 | 0.27 | 38 | 10 | 7 | 21 | Schleucher (2001) | |
| Scardafella inca | DT | 0.044 | 29 | 1 | 66 | 10 | 23 | MacMillen and Trost (1967) | ||
| Passeriformes | ||||||||||
| Artamus cyanopterus | DT | 0.035 | 29 | 12 | −30 | Maddocks and Geiser (2007) | ||||
| Delichon urbicum | DT | 0.022 | 26 | 0.75 | 59 | 12 | 50 | Prinzinger and Siedle (1986); Prinzinger and Siedle (1988) | ||
| Nectarinia famosa | DT | 0.017 | 25.4 | 10 | −12 | Downs and Brown (2002) | ||||
| Manacus vitellinus | DT | 0.0155 | 26.8 | 1.8 | 66 | 3.5 | 5 | Bartholomew et al. (1983) | ||
| MAMMALIA | ||||||||||
| Monotremata | ||||||||||
| Tachyglossus aculeatus | HIB | 2.8 | 4 | 0.03 | 20 | 648 | 271 | 25 | −22.26 | Augee and Ealey (1968); Grigg et al. (1989); Nicol and Andersen (2002) |
| Placentalia Rodentia | ||||||||||
| Acomys russatus | DT | 0.064 | 25 | 0.25 | 38 | 10 | 7.5 | 22.84 | Ehrhardt et al. (2005); Levy et al. (2011) | |
| Aethomys namaquenesis | DT | 0.046 | 18.9 | −23.16 | Withers et al. (1980) | |||||
| Allactaga euphratica | HIB | 0.09 | 336 | 96 | 33 | Çolak and Yiğit (1998) | ||||
| Allactaga williamsi | HIB | 0.15 | 144 | 96 | 39 | Çolak and Yiğit (1998) | ||||
| Apodemus peninsulae | DT | 0.026 | 20 | 6.5 | 2.1 | 48.26 | Masaki et al. (2005) | |||
| Baiomys taylori | DT | 0.0064 | 22 | 0.5 | 26 | 20 | 10 | 26.19 | Hudson (1965) | |
| Calomys musculinus | DT | 0.017 | 0.52 | 32 | 16 | 13 | −31.61 | Bozinovic and Rosenmann (1988) | ||
| Calomys venustus | DT | 0.05 | 16.4 | 0.96 | 67 | 8 | 7 | −37 | Caviedes-Vidal et al. (1990) | |
| Cricetus cricetus | HIB | 0.4 | 3.6 | 0.032 | 4 | 160 | 87 | 51.6 | Eisentraut (1933); Kayser (1964); Waßmer and Wollnik (1997); Siutz et al. (2012); Siutz C. pers. comm. | |
| Cynomys leucurus | HIB | 1.5 | 8 | 199 | 121 | 41.59 | Bakko and Nahornia (1986) | |||
| Cynomys ludovicianus | HIB | 1 | 15 | 214 | 141 | 39.25 | Lehmer et al. (2001) | |||
| Cynomys parvidens | HIB | 0.8 | 6 | 418 | 252 | 38.49 | Lehmer and Biggins (2005) | |||
| Eliomys quercinus | HIB | 0.07 | 1 | 0.034 | 3 | 480 | 336 | 12 | 42.91 | Kayser (1964); Pajunen (1984) |
| Fukomys damarensis | DT | 0.145 | 28.5 | −18.37 | Streicher (2010) | |||||
| Gerbillus pusillus | DT | 0.0126 | 16.7 | 0.38 | 43 | 20 | 9 | 0.57 | Buffenstein (1985) | |
| Glirulus japonicus | HIB | 0.025 | 380 | 254 | 34.25 | Otsu and Kimura (1993) | ||||
| Glis glis | HIB | 0.2 | 1 | 0.026 | 3 | 977 | 222 | 6.7 | 45.93 | Wyss (1932); Kayser (1939); Kayser (1961); Pengelley and Fisher (1961); Bieber and Ruf (2009); Bieber & Ruf unpublished |
| Graphiurus murinus | HIB | 0.028 | 1.5 | 192 | 33 | −10.12 | Mzilikazi et al. (2012) | |||
| Graphiurus ocularis | HIB | 0.068 | 312 | 176 | 16 | −30.24 | Perrin and Ridgard (1999) | |||
| Ictidomys tridecemlineatus | HIB | 0.14 | 1.5 | 456 | 336 | 14.4 | 41.11 | Kisser and Goodwin (2012) | ||
| Jaculus orientalis | HIB | 0.17 | 10 | 158 | 77 | 24 | 29.53 | El Ouezzani et al. (2011) | ||
| Marmota broweri | HIB | 3.094 | 480 | 334 | 18.4 | 68.5 | Lee et al. (2009) | |||
| Marmota flaviventris | HIB | 2.5 | 7.5 | 0.022 | 9 | 360 | 206 | 15 | 42.67 | Florant and Heller (1977); Florant et al. (2000); French (1985) |
| Marmota marmota | HIB | 3.1 | 2.4 | 0.013 | 4 | 353 | 280 | 27.8 | 46.15 | Arnold (1993); Ortmann and Heldmaier (2000); Ruf and Arnold (2000); Arnold et al. (2011); Ruf & Arnold unpublished. |
| Marmota monax | HIB | 3.4 | 5 | 0.014 | 5 | 185 | 364 | 28.3 | 48.68 | Lyman (1958); Armitage et al. (2000); Zervanos et al. (2010) |
| Mesocricetus auratus | HIB | 0.09 | 4 | 0.07 | 6 | 264 | 90 | 36.76 | Lyman (1948); Pohl (1961) | |
| Mesocricetus brandti | HIB | 0.15 | 144 | 108 | 38.08 | Goldman (1989) | ||||
| Microdipodops pallidus | HIB | 0.012 | 6 | 0.1 | 4.8 | 84 | 48 | 8.2 | 38.57 | Brown and Bartholomew (1969) Bartholomew and MacMillen (1961) |
| Mus musculus | DT | 0.037 | 16 | 0.7 | 47 | 11 | 5.9 | 50 | Hudson and Scott (1979) | |
| Muscardinus avellanarius | HIB | 0.0235 | 0 | 0.04 | 2 | 624 | 218 | 6 | 47.96 | Kayser (1939); Kayser (1964); Eisentraut (1956); Prezlaff and Dausmann (2012) |
| Perognathus californicus | DT | 0.021 | 15 | 0.15 | 15 | 15.4 | 11.1 | 31.11 | Tucker (1962); Tucker (1965) | |
| Perognathus fasciatus | DT | 0.04 | 11 | 0.15 | 12 | 17 | 9.7 | 44.7 | Wang and Hudson (1970) | |
| Perognathus longimembris | HIB | 0.008 | 4 | 112 | 35.77 | Bartholomew and Cade (1957); French (1977) | ||||
| Perognathus parvus | HIB | 0.024 | 2 | 0.05 | 3 | 192 | 120 | 43.39 | MacMillen (1983) | |
| Peromyscus boylii | DT | 0.033 | 18 | 8.8 | 4.3 | 32.94 | Morhardt (1970) | |||
| Peromyscus crinitus | DT | 0.02 | 17 | 9.5 | 4.6 | 36.88 | Morhardt (1970) | |||
| Peromyscus eremicus | DT | 0.017 | 16 | 0.3 | 19 | 11.2 | 6.3 | 29 | Macmillen (1965); Morhardt (1970) | |
| Peromyscus gossypinus | DT | 0.022 | 14 | 4.9 | 31.36 | Tannenbaum and Pivorun (1984) | ||||
| Peromyscus leucopus | DT | 0.02 | 16.8 | 0.47 | 28 | 13 | 4.6 | 33.49 | Hill (1975); Deavers and Hudson (1981); Tannenbaum and Pivorun (1988) | |
| Peromyscus maniculatus | DT | 0.018 | 13.4 | 0.55 | 28 | 10.8 | 7.3 | 40.92 | McNab and Morrison (1963); Morhardt (1970); Geiser (1991) | |
| Petromyscus collinus | DT | 0.019 | 18 | −24.17 | Withers et al. (1980) | |||||
| Phodopus sungorus | DT | 0.025 | 12.3 | 0.88 | 46 | 13.8 | 6.3 | 50.89 | Ruf et al. (1993); T. Ruf unpublished data | |
| Phyllotis darwini | DT | 0.036 | 17.5 | 0.19 | 15 | 12 | −30.74 | Bozinovic and Marquet (1991) | ||
| Reithrodontomys megalotis | DT | 0.008 | 13 | 0.3 | 12 | 10 | 4 | 33.28 | Thompson (1985) | |
| Saccostomus campestris | DT | 0.071 | 25 | 0.35 | 56 | 2.7 | 6.8 | −19.68 | Mzilikazi and Lovegrove (2002) | |
| Spermophilus armatus | HIB | 0.5 | 576 | 302 | 9.5 | 42.02 | Cranford (1986) | |||
| Spermophilus beecheyi | HIB | 0.6 | 6.1 | 72 | 48 | 38.32 | Strumwasser (1960); Pengelley and Kelley (1966) | |||
| Spermophilus beldingi | HIB | 0.4 | 400 | 9 | 41.29 | French (1985) | ||||
| Spermophilus citellus | HIB | 0.25 | −0.7 | 415 | 192 | 14 | 45.52 | Németh et al. (2009) | ||
| Spermophilus columbianus | HIB | 0.5 | 0 | 424 | 600 | 12 | 49.15 | Young (1990) | ||
| Spermophilus dauricus | HIB | 0.35 | −2.4 | 377 | 260 | 12.75 | 43.33 | Yang et al. (2011) | ||
| Spermophilus elegans | HIB | 0.3 | 450 | 338 | 24 | 43.96 | Harlow and Menkens (1986) | |||
| Spermophilus lateralis | HIB | 0.2 | −1 | 0.028 | 4 | 504 | 408 | 13.5 | 44.51 | Hammel et al. (1968); Healy et al. (2012) |
| Spermophilus mexicanus | HIB | 0.2 | 7 | 0.06 | 7 | 60 | 36 | 26.39 | Neumann and Cade (1965) | |
| Spermophilus parryii | HIB | 0.65 | −2.9 | 0.012 | 2 | 550 | 420 | 14.8 | 63.39 | Hock (1960); Barnes (1989); Barnes and Ritter (1993); Buck and Barnes (2000); Karpovich et al. (2009) |
| Spermophilus richardsonii | HIB | 0.4 | 2 | 0.02 | 4 | 456 | 10.3 | 49.39 | Hudson and Deavers (1973); Wang (1978) | |
| Spermophilus saturatus | HIB | 0.23 | 0.3 | 0.017 | 4 | 360 | 254 | 47.94 | Geiser et al. (1990) | |
| Spermophilus tereticaudus | HIB | 0.125 | 0.048 | 7 | 120 | 32.04 | Pengelley and Kelley (1966); Bickler (1984) | |||
| Spermophilus variegatus | HIB | 0.7 | 8 | 172 | 110 | 29.99 | Pengelley (1964); Pengelley and Kelley (1966) | |||
| Spermophilus xanthoprymnus | HIB | 0.3 | 4 | 468 | 199 | 38.76 | Kart Gür et al. (2009) | |||
| Steatomys pratensis | DT | 0.028 | 16.4 | 0.3 | 22 | 16.9 | 21.5 | −20 | Ellison (1995) | |
| Tamias amoenus | HIB | 0.054 | −0.2 | 0.026 | 2 | 312 | 211 | 45.07 | Kenagy and Vleck (1982); Geiser et al. (1990) | |
| Tamias striatus | HIB | 0.087 | 4.9 | 0.06 | 6 | 150 | 120 | 18 | 40.78 | Wang and Hudson (1971); Pivorun (1976); Levesque and Tattersall (2010) |
| Zapus hudsonius | HIB | 0.0226 | 0.043 | 3 | 451 | 48.34 | Muchlinski and Rybak (1978) | |||
| Zapus princeps | HIB | 0.0336 | 5.5 | 0.024 | 2 | 650 | 480 | 5 | 47.4 | Cranford (1983); French (1985) |
| Primates | ||||||||||
| Cheirogaleus crossleyi | HIB | 0.5 | 9 | 168 | 111 | 10.2 | −18.72 | Blanco and Rahalinarivo (2010) | ||
| Cheirogaleus medius | HIB | 0.25 | 9.3 | 0.044 | 8.3 | 1680 | 160 | 6 | −19.03 | Dausmann et al. (2000); Dausmann et al. (2004); Dausmann et al. (2005); Dausmann et al. (2009) |
| Galago moholi | DT | 0.18 | 21.8 | 0.09 | 10 | 6.5 | 5 | −13.46 | Nowack et al. (2010) | |
| Microcebus griseorufus | HIB | 0.05 | 6.5 | 1848 | 43.1 | −22.94 | Dausmann et al. (2012); Kobbe et al. (2011) | |||
| Microcebus murinus | DT | 0.06 | 7.8 | 0.16 | 19 | 17.6 | 9.3 | −18.95 | Perret (1998); Schmid (2000) | |
| Microcebus myoxinus | DT | 0.033 | 6.8 | 0.09 | 4.5 | 19.2 | 4.6 | −20.08 | Schmid et al. (2000) | |
| Microcebus ravelobensis | DT | 0.063 | 25 | 7 | 5 | −19.32 | Lovegrove et al. (2013) | |||
| Carnivora | ||||||||||
| Meles meles | HIB | 13 | 28 | 51 | Fowler and Racey (1988) | |||||
| Mephitis mephitis | DT | 2.88 | 26 | 20 | 7.8 | 43.15 | Hwang et al. (2007) | |||
| Proteles cristata | DT | 9 | 31 | −5.74 | Anderson (2004) | |||||
| Taxidea taxus | DT | 9 | 28 | 0.13 | 43 | 22 | 14 | 38.45 | Harlow (1981) | |
| Ursus americanus | HIB | 80 | 29.4 | 0.042 | 19 | 47.57 | Watts et al. (1981); Tøien et al. (2011) | |||
| Ursus arctos | HIB | 100 | 32.5 | 50.76 | Hissa (1997) | |||||
| Chiroptera | ||||||||||
| Barbastella barbastellus | HIB | 0.007 | 0.04 | 2 | 44.28 | Pohl (1961) | ||||
| Carollia perspicillata | DT | 0.018 | 22 | 1 | 53 | −4.14 | Audet and Thomas (1997) | |||
| Chalinolobus gouldii | HIB | 0.018 | 5 | 0.05 | 3 | −27.37 | Hosken and Withers (1997) | |||
| Corynorhinus rafinesquii | HIB | 0.01 | 13.9 | 58 | 32.8 | Johnson (2012) | ||||
| Eptesicus fuscus | HIB | 0.0147 | 1 | 0.03 | 3 | 600 | 488 | 2 | 31.15 | Kulzer (1965) French (1985); Willis et al. (2005a) |
| Glossophaga soricina | DT | 0.01 | 21 | 0.23 | 9 | 17.5 | 11.4 | −2.61 | Kelm and von Helversen (2007) | |
| Hipposideros terasensis | HIB | 0.057 | 13.8 | 0.046 | 7 | 456 | 185 | 1.8 | 17.13 | Liu and Karasov (2011); Liu and Karasov (2012) |
| Lasiurus borealis | HIB | 0.011 | 3 | 0.035 | 2 | 260 | 190 | 2.83 | 36.39 | Dunbar and Tomasi (2006) |
| Lasiurus cinereus | HIB | 0.033 | 2 | 135 | 105 | 12.6 | Cryan and Wolf (2003); Willis et al. (2006) | |||
| Macroglossus minimus | DT | 0.016 | 21.6 | 0.52 | 40 | 9.5 | 6.7 | −1.28 | Bartels et al. (1998) | |
| Megaloglossus woermanni | DT | 0.012 | 26.2 | 0.8 | 50 | −1.12 | Kulzer and Storf (1980) | |||
| Miniopterus schreibersii | HIB | 0.015 | 5 | 288 | 5.87 | Hall (1982); Brown and Bernard (1994) | ||||
| Mops condylurus | HIB | 0.029 | 13 | −5.77 | Vivier and van der Merwe (2011) | |||||
| Myotis adversus | HIB | 0.0078 | 9 | 192 | 4.84 | Kulzer et al. (1970) | ||||
| Myotis lucifugus | HIB | 0.0052 | 1.3 | 0.022 | 1 | 1152 | 314 | 2.4 | 43 | Hock (1951) Jonasson and Willis (2012) |
| Myotis myotis | HIB | 0.025 | 2 | 0.04 | 3 | 2352 | 989 | 47.44 | Pohl (1961); Harmata (1987); Koteja et al. (2001) | |
| Myotis nattereri | HIB | 0.009 | 7 | 490 | 160 | 46.05 | Hope and Jones (2012) | |||
| Myotis velifer | HIB | 0.012 | 0.6 | 0.04 | 3 | 27.11 | Tinkle and Patterson (1965); Riedesel and Williams (1976) | |||
| Nyctalus noctula | HIB | 0.029 | 3 | 0.036 | 2 | 192 | 39.16 | Ransome (1990); Arlettaz et al. (2000) | ||
| Nycteris thebaica | DT | 0.011 | 27 | 1.28 | Cory Toussaint and McKechnie (2012) | |||||
| Nyctimene albiventer | DT | 0.028 | 25.5 | 0.67 | 47 | −4.02 | Bartholomew et al. (1970) | |||
| Nyctophilus bifax | HIB | 0.01 | 7.3 | 0.046 | 3 | 129 | 27 | 3 | −16.48 | Stawski et al. (2009); Stawski and Geiser (2010); Stawski and Geiser (2011) |
| Nyctophilus geoffroyi | HIB | 0.007 | 1.4 | 0.037 | 3 | 362 | 106 | 3 | −27.37 | Geiser and Brigham (2000); Turbill and Geiser (2008) |
| Nyctophilus gouldi | HIB | 0.01 | 2.3 | 0.052 | 4 | 259 | 106 | 3 | −33.54 | Geiser and Brigham (2000); Turbill and Geiser (2008) |
| Pipistrellus pipistrellus | HIB | 0.0074 | 3 | 0.024 | 1 | 43.44 | Kayser (1964); Kulzer (1965) | |||
| Pipistrellus subflavus | HIB | 0.005 | 1800 | 607 | 1.5 | 31.01 | Brack and Twente (1985); French (1985) | |||
| Plecotus auritus | HIB | 0.01 | −2 | 44.65 | Eisentraut (1956) | |||||
| Rhinolophus ferrumequinum | HIB | 0.023 | 9 | 432 | 104 | 4.3 | 38.48 | Kulzer (1965); Park et al. (2000) | ||
| Rhinolophus hipposideros | HIB | 0.006 | 2064 | 427 | 31.68 | Harmata (1987) | ||||
| Rhinopoma microphyllum | HIB | 0.01 | 23 | 18.84 | Kulzer (1965); Levin et al. (2010) | |||||
| Scotophilus dinganii | DT | 0.029 | 18.5 | 19 | 15 | −8.02 | Jacobs et al. (2007) | |||
| Scotophilus mhlanganii | DT | 0.028 | 17.2 | 18.5 | 17.2 | −6 | Jacobs et al. (2007) | |||
| Sturnira lilium | DT | 0.016 | 22 | 0.5 | 25 | −2.47 | Audet and Thomas (1997) | |||
| Syconycteris australis | DT | 0.018 | 17.2 | 0.47 | 36 | 8.2 | 7.3 | −16.55 | Coburn and Geiser (1998) | |
| Tadarida aegyptiaca | HIB | 0.017 | 6 | 228 | 1.12 | Cory Toussaint et al. (2010) | ||||
| Tadarida brasiliensis | HIB | 0.01 | 9 | 0.06 | 3 | 1.23 | Herreid (1963); Herreid and Schmidt-Nielsen (1966) | |||
| Tadarida teniotis | HIB | 0.035 | 6.7 | 0.04 | 4 | 192 | 528 | 4.5 | 35.4 | Arlettaz et al. (2000); Marom et al. (2006) |
| Vespadelus vulturnus | HIB | 0.004 | 5 | 0.014 | 1.3 | −34.21 | Willis et al. (2005b) | |||
| Eulipotyphla | ||||||||||
| Atelerix algirus | HIB | 0.63 | 9.7 | 168 | 84 | 16 | 33.74 | Mouhoub-Sayah et al. (2008) | ||
| Atelerix frontalis | HIB | 0.4 | 1 | 116 | 22 | 12 | −23.03 | Hallam and Mzilikazi (2011) | ||
| Crocidura flavescens | DT | 0.032 | 19 | −30.94 | Baxter (1996) | |||||
| Crocidura leucodon | DT | 0.012 | 18.6 | 42.54 | Nagel (1985) | |||||
| Crocidura russula | DT | 0.01 | 17.9 | 0.9 | 38 | 3 | 40.48 | Nagel (1977); Nagel (1985) | ||
| Crocidura suaveolens | DT | 0.008 | 21.6 | 45 | Nagel (1985) | |||||
| Erinaceus europaeus | HIB | 0.7 | 5.4 | 0.01 | 2.5 | 288 | 213 | 22.1 | 53.78 | Kristoffersson and Soivio (1964); Thati (1978) |
| Notiosorex crawfordi | DT | 0.004 | 27.4 | 1.42 | 43 | 31 | Lindstedt (1980) | |||
| Sorex sinuosus | DT | 0.0078 | 1.3 | 28 | 1 | 31.46 | Newman and Rudd (1978) | |||
| Suncus etruscus | DT | 0.002 | 12 | 0.6 | 10 | 7.6 | 2 | 28.2 | Vogel (1974); Frey (1979); Frey (1980) | |
| Xenarthra | ||||||||||
| Zaedyus pichiy | HIB | 1.1 | 12.5 | 112 | 75 | −40.89 | Superina and Boily (2007) | |||
| Afrosoricida | ||||||||||
| Amblysomus hottentotus | HIB | 0.075 | 8.6 | 96 | 78 | −29.74 | Scantlebury et al. (2008) | |||
| Echinops telfairi | HIB | 0.085 | 11 | 0.026 | 2 | 264 | 162 | −22.58 | Dryden et al. (1974); Scholl (1974) | |
| Geogale aurita | DT | 0.006 | 16 | 0.15 | 13 | −22.55 | Stephenson and Racey (1993a) | |||
| Microgale dobsoni | DT | 0.045 | 20 | 0.22 | 24 | −18.56 | Stephenson and Racey (1993b) | |||
| Setifer setosus | HIB | 0.32 | 13 | 0.014 | 4 | 3600 | −18.96 | Kayser (1964); Hildwein (1970); Lovegrove et al. (2013) | ||
| Tenrec ecaudatus | HIB | 0.65 | 15 | 0.027 | 9 | 6480 | −18.96 | F. Lachiver cited in Kayser (1961); Kayser (1964); Hildwein (1970); Lobban and Lovegrove (2012) | ||
| Macroscelidea | ||||||||||
| Elephantulus edwardii | HIB | 0.045 | 9.3 | 44 | 17.3 | −31.59 | Geiser and Mzilikazi (2011) | |||
| Elephantulus myurus | HIB | 0.057 | 5.5 | 0.078 | 7 | 39 | 8.8 | −23.07 | Lovegrove et al. (2001); Mzilikazi and Lovegrove (2004); McKechnie and Mzilikazi (2011) | |
| Elephantulus rozeti | DT | 0.045 | 5.1 | 0.023 | 2 | 20.1 | 13.6 | 32.6 | Lovegrove et al. (2001) | |
| Elephantulus rupestris | DT | 0.06 | 12 | 12 | 5 | −25.59 | Oelkrug et al. (2012) | |||
| Macroscelides proboscideus | DT | 0.046 | 9.4 | 18 | 10.7 | −26.24 | Lovegrove et al. (1999) | |||
| Marsupialia Diprodontia | ||||||||||
| Acrobates pygmaeus | HIB | 0.011 | 1.6 | 0.056 | 5 | 192 | 85 | −24.91 | Fleming (1985); Geiser and Ferguson (2001) | |
| Burramys parvus | HIB | 0.063 | 1.8 | 0.025 | 2 | 480 | 342 | −36.29 | Geiser and Broome (1991) | |
| Cercartetus concinnus | HIB | 0.018 | 4.7 | 0.046 | 4 | 264 | 102 | −33.82 | Geiser (1987) | |
| Cercartetus lepidus | HIB | 0.012 | 5.9 | 0.052 | 3 | 144 | −38.97 | Geiser (1987) | ||
| Cercartetus nanus | HIB | 0.02 | 1.3 | 0.018 | 2.7 | 840 | 101 | −35.06 | Geiser (1993); Song et al. (1997); Turner et al. (2012) | |
| Petaurus breviceps | DT | 0.13 | 10.4 | 0.07 | 9.5 | 23 | 13 | −20.7 | Fleming (1980); Körtner and Geiser (2000b) | |
| Tarsipes rostratus | DT | 0.01 | 5.4 | 0.15 | 5 | 14.4 | 10.5 | −31.3 | Collins et al. (1987); Withers et al. (1990) | |
| Microbiotheria | ||||||||||
| Dromiciops gliroides | HIB | 0.0402 | 7.1 | 0.03 | 3.8 | 144 | 120 | −39.86 | Grant and Temple-Smith (1987); Bozinovic et al. (2004); Franco et al. (2012) | |
| Dasyuromorphia | ||||||||||
| Antechinomys laniger | DT | 0.027 | 11 | 0.14 | 13 | 16 | 11.5 | −27.09 | Geiser (1986) | |
| Antechinus flavipes | DT | 0.026 | 24.5 | 0.48 | 46 | 5.5 | 2 | −26.92 | Geiser (1988) | |
| Antechinus stuartii | DT | 0.026 | 19.9 | 0.66 | 62 | 9 | 4 | −26.74 | Geiser (1988) | |
| Dasycercus cristica uda/blythi | DT | 0.1 | 10.8 | 0.12 | 23 | 20.8 | 5.8 | −25.02 | MacMillen and Nelson (1969); Geiser and Masters (1994); Kortner et al. (2008) | |
| Dasykaluta rosamondae | DT | 0.027 | 21 | 16.4 | 12.3 | −22.58 | Kortner et al. (2010) | |||
| Dasyuroides byrnei | DT | 0.12 | 20.4 | 0.4 | 54 | 7.5 | 2.7 | −26.08 | Geiser and Baudinette (1987) | |
| Dasyurus geoffroii | DT | 1 | 23.1 | −33.17 | Arnold (1976) | |||||
| Dasyurus hallucatus | DT | 0.516 | 28.4 | −18 | Cooper and Withers (2010) | |||||
| Dasyurus viverrinus | DT | 1 | 25 | −41.61 | Moyle in Reardon (1999) | |||||
| Myrmecobius fasciatus | DT | 0.5 | 19.1 | 15.3 | 9.7 | −33.82 | Cooper and Withers (2004) | |||
| Ningaui yvonnae | DT | 0.011 | 15.3 | 0.3 | 23 | 12.3 | 7.5 | −31.98 | Geiser and Baudinette (1988) | |
| Planigale gilesi | DT | 0.008 | 14.3 | 0.36 | 25 | 15.3 | 8.8 | −29.48 | Geiser and Baudinette (1988) | |
| Planigale ingrami | DT | 0.0076 | 0.48 | 30 | 4 | −18.58 | Dawson and Wolfers (1978) | |||
| Planigale maculata | DT | 0.013 | 19.6 | 0.4 | 40 | 1.8 | −21.9 | Morton and Lee (1978) | ||
| Planigale tenuirostris | DT | 0.007 | 0.48 | 30 | 4 | −28.53 | Dawson and Wolfers (1978) | |||
| Pseudantechinus macdonnellensis | DT | 0.031 | 15.9 | 14.3 | 5.8 | −22.83 | Geiser and Pavey (2007) | |||
| Sminthopsis crassicaudata | DT | 0.017 | 10.8 | 0.27 | 22 | 19.5 | 15 | −28.79 | Geiser and Baudinette (1987); Warnecke et al. (2008) | |
| Sminthopsis douglasi | DT | 0.06 | 16.9 | 0.43 | 40 | 8.8 | 3.2 | −20.1 | Muller (1996) | |
| Sminthopsis macroura | DT | 0.024 | 11.3 | 0.3 | 29 | 25.9 | 11 | −24.29 | Geiser and Baudinette (1987); Kortner and Geiser (2009) | |
| Sminthopsis murina | DT | 0.019 | 15 | 0.25 | 22 | 8 | −27.47 | Geiser et al. (1984) | ||
| Sminthopsis ooldea | DT | 0.0111 | 0.77 | 48 | −24.7 | Tomlinson et al. (2012) | ||||
| Didelpimorphia | ||||||||||
| Gracilinanus agilis | DT | 0.0291 | 20 | 0.3 | 30 | −18.71 | Cooper et al. (2009) | |||
| Marmosa microtarsus | DT | 0.013 | 16 | 0.25 | 18 | 9 | 15.53 | Morrison and McNab (1962) | ||
| Thylamys elegans | DT | 0.032 | 14 | 0.4 | 47 | 20 | 14 | −30 | Opazo et al. (1999); Silva-Duran and Bozinovic (1999) |
The duration of euthermic intervals between torpor episodes (inter-bout euthermia, IBE) was taken from the literature for species traditionally classified as hibernators, but limited data on IBE were available for species traditionally considered daily heterotherms. To obtain at least a rough estimate of IBE in for this subgroup, we computed IBE from 24 hours – TBD.
Both euthermic and torpor MR were analysed as mass-specific MR. We are aware that this is a potential source of error and that computing allometric relationships based on absolute MR would be much preferable (e.g., Packard & Boardman, 1988; Hayes, 2001). However, the vast majority of MRs in the literature we cite were given as mass-specific MR and – also in the majority of cases – body masses were provided for the species or experimental animals in general, but not the actual individuals in which MR was measured (and typically, only mean body masses were given). Thus, estimating total MRs from these different sources (i.e., multiplying mass-specific MR from one sample of individuals by mean body mass from another set) would lead to the same potential error pointed out by Packard and Boardman (1988): assuming a linear isometric relationship when this assumption may not be valid. Balancing the possible error in using mass-specific MR versus omitting most MR data altogether, we decided to analyse MR as given, especially as there is no apparent source of bias that could lead to larger errors in any subgroup of species investigated.
To test if either single variables (e.g., TMRmin) or combinations of torpor characteristics would point to the existence of a grouping structure within heterotherms we used cluster analysis based on Gaussian mixture models as implemented in R-package “mclust” (Fraley & Raftery, 2002). This procedure determines the number of clusters (one, two or more) of normally distributed variables that minimize the variance in the dataset based on the Bayesian Information Criterion (BIC), which adds a penalty term on the number of parameters to the log-likelihood of each model. To obtain approximately equal variances between potential groups, all variables (except Tb min) were log-transformed. We did not attempt to include IBE duration into these cluster analyses, as this variable may be affected by prior torpor episodes, but is not a characteristic of torpor episodes as such. As a measure of the strength of clustering, we used χ2 and P-values from likelihood-ratio tests comparing the best model for each variable with the null-model (i.e., a model assuming no subgroups). To minimize multiplicity of P-values, we limited testing of combinations of variables to those unrelated to TBD (i.e., TMRmin, TMRrel and Tbmin). We did not discriminate between birds and mammals in these cluster analyses. For models resulting in more than one cluster, each data point can be assigned to one of the groups determined. We compared these independent, model-generated classifications to our initial categories that were based on TBDmax being greater or less than 24 h.
To investigate the relationship of variables characterizing torpor (e.g., TBDmax or Tb min) to body mass or latitude of the species’ geographical range we fitted phylogenetically-informed generalized least squares (PGLS) models. Models were computed using function “gls” from package “nlme” (Pinheiro et al., 2013) in R 3.0.2 (R Development Core Team, 2013). In these models, phylogenetic correlation between taxonomically related species is used for sample-weighting, as data-points obtained from closely related species cannot be considered entirely independent.
The bird phylogeny used was based on Sibley and Ahlquist (1990). Two families (the Artamidae and the Pipridae) were added to this tree using information on their phylogenetic position given by (Norman et al., 2009) and (Ericson et al., 2006) (Fig. 1). As no sufficient information on branch lengths was available for birds, all initial branch lengths in this tree were set to 1. We are aware that more recent, albeit controversial, phylogenies of birds are available (e.g., Hackett et al., 2008). However, we decided to use the phylogeny proposed by Sibley & Ahlquist (1990), mainly to allow for comparisons with McKechnie & Lovegrove (2002) who used the same phylogeny. For mammals we used an updated version (Fritz et al., 2009) of the mammalian supertree (Bininda-Edmonds et al., 2007). For each data set analysed, tips for unavailable data were trimmed from this tree. The mammalian tree (which includes different branch lengths) for all species investigated here is shown in Fig. 2.
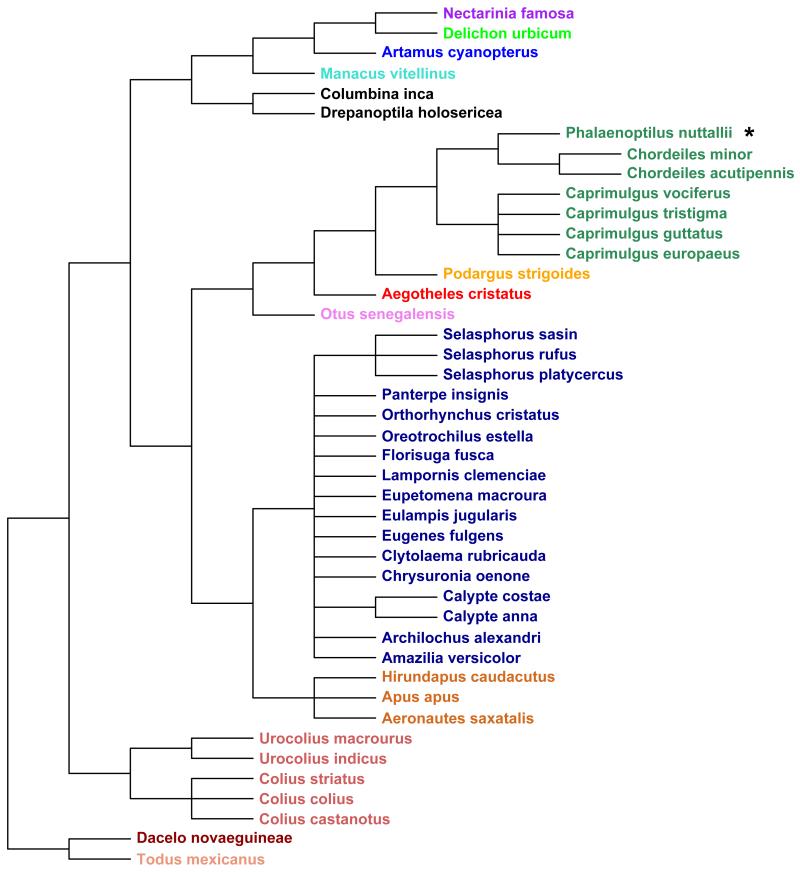
Fig. 1.
Phylogenetic tree of the bird species investigated. Different colours indicate the following families (from top to bottom): Nectariniidae, Hirundinidae, Artamidae, Pipridae, Columbidae, Podargidae, Aegothelidae, Strigidae, Trochilidae, Apodidae, Coliidae, Alcedinidae, Todidae. * The single hibernating species among birds was the Common Poorwill, Phalaenoptilus nuttallii.
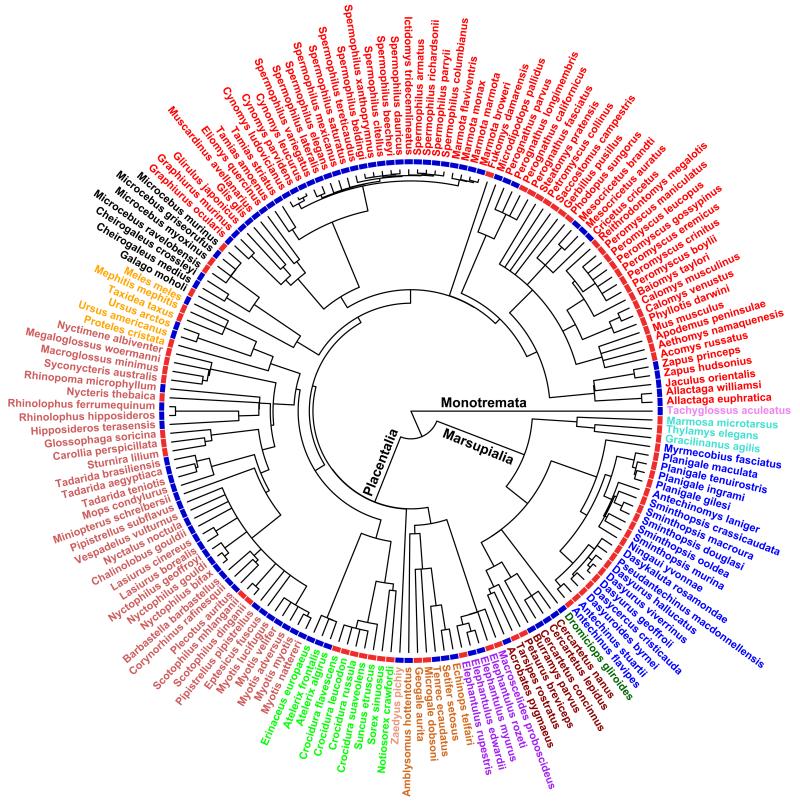
Fig. 2.
Phylogenetic tree of the mammal species investigated. Species names in different colours indicate different orders. The coloured blocks next to species names indicate the use of hibernation (blue) or daily torpor (red), according to the traditional definition of heterothermy types.
To compute phylogenetically informed regressions we used the evolutionary models and branch length transformation algorithms implemented in the R-library “ape” (Paradis et al., 2004). These included the Ornstein-Uhlenbeck model, the Brownian model, the ACDC model, Grafen’s method and Pagel’s algorithm. Initial trials showed that for all response variables investigated, using Pagel’s method (Pagel, 1999; Freckelton et al., 2002) led to much lower estimates of model AIC (Akaike’s information criterion) than any other algorihm. Hence, all PGLS models were computed using Pagel’s method. Pagels’ λ is expected to vary between 0 and 1 and can be determined by maximum likelihood fits. A λ of 0 indicates the absence of a phylogenetic signal, i.e., the trait under consideration is not more similar among closely related species. If λ equals 1 the trait distribution matches a Brownian model of evolution (i.e., “random walk” evolution). To determine 95% confidence limits for λ we used function “pgls” from the R-package “caper” (Orme et al., 2013), which also provides their probabilities of differing from 0 and 1 (which we give as Plower>0 and Pupper<1, respectively).
Parameter estimates (i.e., intercepts and slopes) were obtained from function “gls”, because this function allows the use of restricted maximum likelihood (REML), which returns unbiased estimates, and in this regard is preferable to full maximum likelihood (ML) as used by function “pgls” (e.g., Ives et al., 2007). Since the subset of species completely differed between the classical categories (i.e., avian and mammalian daily heterotherms and mammalian hibernators) separate statistical models were computed for each subgroup (please note that there was only a single bird species preliminarily classified as a hibernator). This separation of subgroups was justified by the results from cluster analysis, which confirmed the initial categories based on TBDmax.
The primary predictor variable to explain variation in torpor characteristics was body mass (c.f., Geiser & Ruf, 1995). Initially, we also included the absolute values of the latitude of the centre of species ranges as a predictor variable for all response variables. Species range latitudes (as a proxy for environmental harshness) were obtained from the PanTHERIA database (Jones et al., 2009) for 159 mammal species. For 12 additional mammals the latitude was estimated from visually locating the approximate centre of the species range in maps provided by the International Union for Conservation of Nature (www.iucn.org) and determining its latitude. This procedure was also used for all bird species. For migratory species with two ranges, we used the geographic range in which torpid animals had been observed. The latitude of the species range was indeed the best predictor of avian torpor bout duration (see Results). In all other cases, including latitude complicated the models without substantially decreasing the residual variance, as indicated by unchanged or strongly increased AIC values. At least partly, this was probably caused by multicollinearity, i.e., a correlation between body mass and latitude among hibernators (see Results). Therefore, latitude was omitted from these models. In models with TMR as the response variable and body mass as the predictor, we did not use Tb min as an additional covariate, because for many species Tb min and TMRmin were determined in different individuals and/or times.
To obtain approximately linear relationships and normally distributed residuals, body mass was log10 transformed, and in several cases, so was the response and other predictor variables (see Results). For significant linear PGLS regressions, we report R2 values as well as intercept, slope and the t- and corresponding P-values for the difference between the slope and 0. To compare slopes from separate regressions, we computed their 95% confidence intervals. It should be noted that regression lines in PGLS, due to sample weighting as derived from the phylogeny may substantially differ from “eye-fitted” lines, i.e. the relationship expected from the data scatterplot. Therefore, we show regression lines from PGLS models even if their slope was not significantly different from zero. As several torpor variables were affected by body mass, and mean masses considerably differed between subgroups, simple group means of variables investigated may reflect the combined effects of both torpor-type and body mass differences. Therefore, we additionally give variable values predicted from the regression equations for a 30 g animal of each subgroup (i.e., very close to the overall median body mass of 32 g for all species included in our analysis), which we also call “adjusted means”. Adjusted means, which arguably are better suited to assess the pure effects of torpor-type, are given together with 95% confidence intervals (95% CI) computed from the standard errors of model coefficients. Further 95% CI are also given for the arithmetic mean of all variables. These 95% CI were computed by bootstrapping the data (i.e., generating distributions of 1000 means by random sampling with replacement and determining their 0.025 an 0.975 quantiles; (for details see Efron & Tibshirani, 1993)). For body masses, which were skewed to the right, we also give geometric means. To allow for a comparison of body masses of heterothermic mammals with terrestrial mammals in general (both heterothermic and homeothermic species), we also computed mean and median, as well as their 95% CI, from adult body masses of 2636 terrestrial mammal species provided in the PanTHERIA database (Jones et al., 2009). All statistical analyses were all carried out using R 3.0.2 (R Development Core Team, 2013).
III. RESULTS
(1) Classification of torpid states
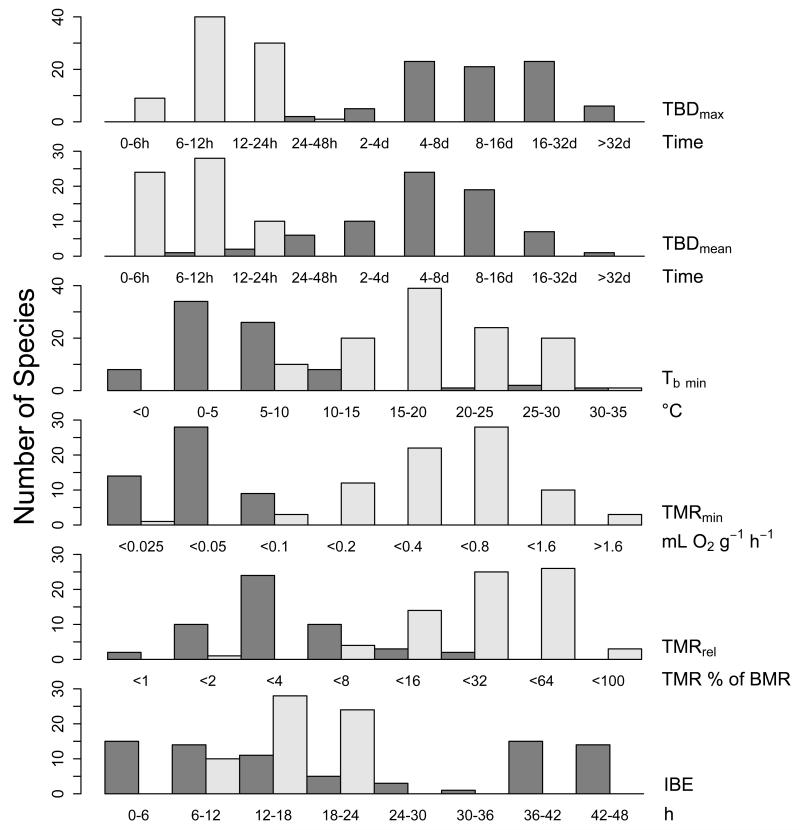
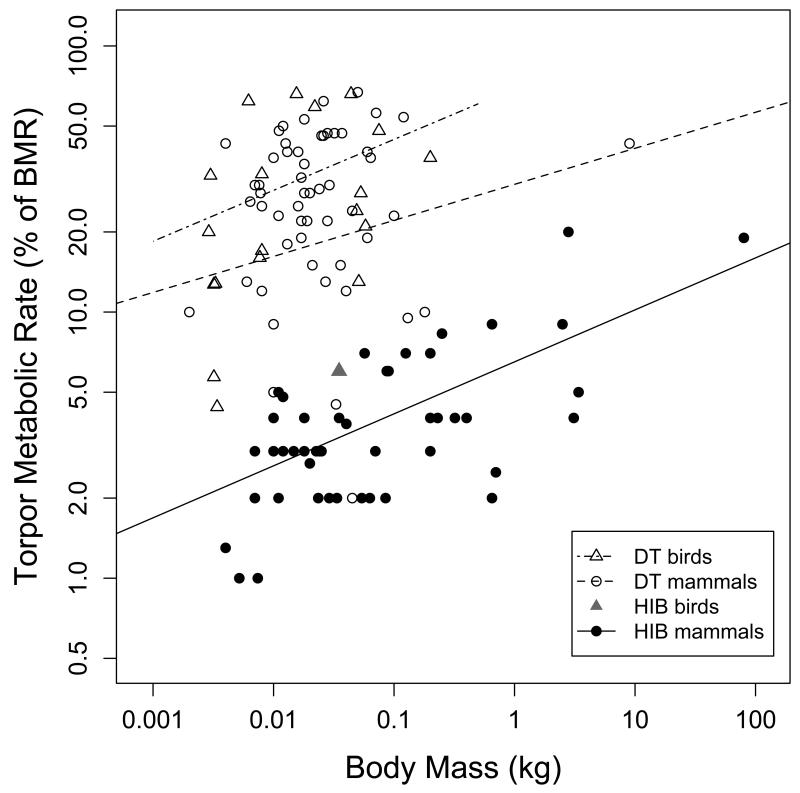
TBDmax was the variable that showed the clearest bimodal distribution (Fig. 3). All other variables, except for IBE, also showed distinctly bimodal distributions, with some overlap, however, between species previously classified as daily heterotherms and hibernators.
Fig. 3.
Frequency distributions of maximum torpor bout duration (TBDmax), mean torpor bout duration (TBDmean), minimum Tb in torpor (Tb min), inter-bout euthermia duration (IBE), minimum MR in torpor (TMRmin), and metabolic reduction below BMR (TMRrel). Dark bars show species traditionally classified (TBDmax<24 h) as hibernators, light bars show daily heterotherms (TBDmax>24 h). Sample size varied for different variables (see Table 2). Data from mammals and birds were combined.
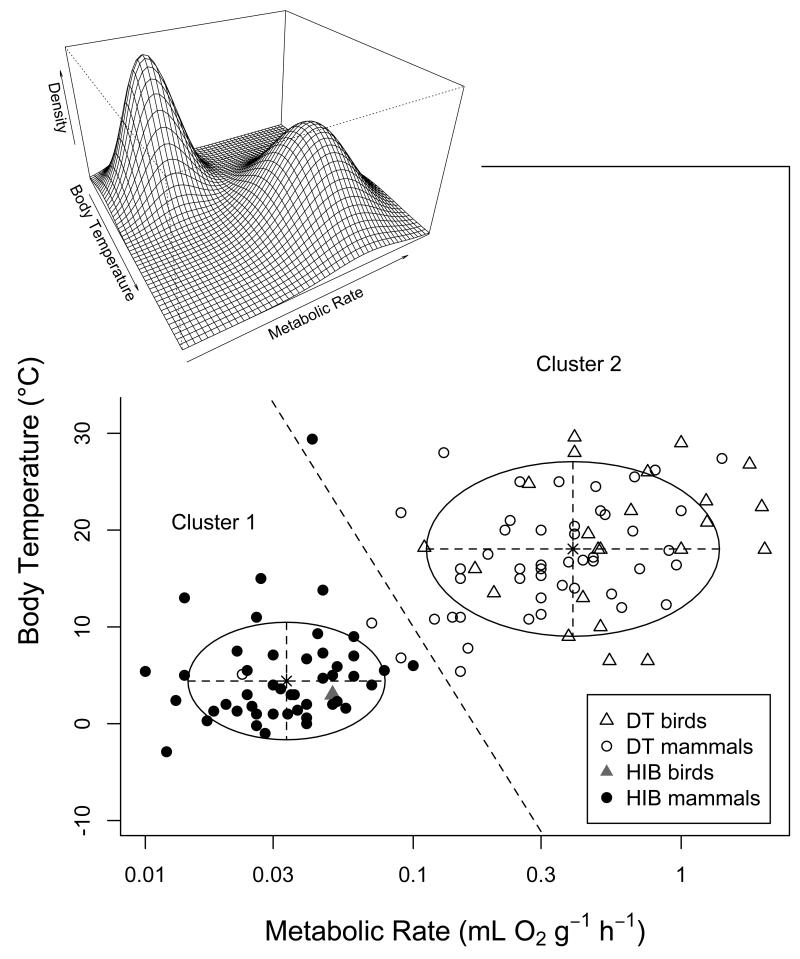
Results from Gaussian-mixture cluster analyses unambiguously pointed to the existence of two groups within the species investigated. A two-cluster structure was the best model for all single variables and variable combinations tested (P<0.0001 in all cases). The separation of clusters was strongest when based on TBDmax (χ2=91.3), followed by TBDmean (χ2=74.1), a combination of TMRmin and Tb min (Fig. 4; χ2=51.0), TMRrel (χ2=36.3), a combination of TMRrel and Tb min (χ2=32.9), and Tb min alone (χ2=22.5). The clusters suggested based on TBDmax were virtually identical to our initial classification (99% of species, with only two exceptions, i.e., Elephantulus edwardii and Elephantulus myurus). However, the agreement was also very high when based on other variables, i.e., 97% for TMRmin + Tb min, 96% for TBDmean, 95% for TMRmin, 93% for TMRrel, 92% for TMRrel + Tb min, and 84% for Tb min. Given that classifying species as daily heterotherms was confirmed by bimodal variable distributions (Fig. 3) as well as cluster analyses, and could be based largely on variables beyond TBDmax, such as TMRmin and Tb min alone (Fig. 4), we henceforth simply refer to these groups as “daily heterotherms” and “hibernators”, and maintain our preliminary classification of species.
Fig. 4.
Results from a cluster analysis based on the traits TMRmin and Tb min indicating the existence of two clusters within heterotherms. Circles represent 95% confidence ellipses for the estimated cluster centres (indicated by asterisks). All species on left of the dashed line were classified as belonging to cluster 1, which was identical to our initial category “hibernators” except for three species (Elephantulus rozeti, Microcebus myoxinus, Petaurus breviceps). Species on the right of the dashed line assigned to cluster 2, which was identical to the traditonal category “daily heterotherms” except for 1 species (Ursus americanus). Overall there was a high degree of agreement (117 of 121 species) between this cluster analysis and classical categories. The inset graph shows the density surface computed from the parameters of the Gaussian mixture model.
(2) Geographical and phylogenetic distribution of species
Centres of species geographic ranges were located in the northern hemisphere in 93 cases (mean latitude: 36.9 ± 1.3°N) and in the southern hemisphere in 78 cases (23.1 ± 1.1°S). The absolute mean latitude of species ranges was very similar for mammalian and avian daily heterotherms (Table 2; overall mean: 24.5 ± 1.1°). The mean latitude was significantly higher, i.e., 35.0 ± 1.4°, for mammalian hibernators (Table 2, and 35° for the single avian hibernator). Moreover, these mean latitudes of geographical ranges in heterothermic species were considerably higher than in mammals in general (17.5 ± 0.2°; no estimates were available for all birds). Among daily heterotherms, body mass was unaffected by the species range latitude among both mammals (log10BM=)1.66+0.003 Latitude, t=0.82, P=0.411) and birds (log10BM=−1.48+0.005 Latitude, t=0.18, P=0.858). However, body mass increased slightly with latitude in mammalian hibernators (log10BM=−0.901+0.007 Latitude, t=2.18, P=0.0031). Body mass contained a strong phylogenetic signal in all three subgroups. Pagel’s λ was 1.0 (95% CI: 0.93-1; Plower>0=<0.0001, Pupper<1=1) among avian daily heterotherms, 1.00 (95% CI: 00.86-1; Plower>0=<0.0001, Pupper<1=1) among mammalian daily heterotherms, and 0.99 (; 95% CI: 0.95-1; Plower>0=<0.0001, Pupper<1=0.39) among mammalian hibernators.
Table 2.
Central tendencies of variables of torpor as well as of body mass and the absolute value of latitude of species geographic ranges in mammals and birds. Adjusted means are values for a 30 g animal (the overall median body mass in the data set) predicted from the regression of variables against body mass. TBDmax: maximum torpor bout duration; TBDmean: mean torpor bout duration; IBE: inter-bout euthermia duration; TMRmin: minimum MR in torpor; TMRrei: metabolic reduction below BMR.
| Avian daily heterotherms | Mammalian daily heterotherms | Mammalian hibernators | |
|---|---|---|---|
| Body mass (kg) | |||
| Mean | 0.052 | 0.336 | 2.410 |
| 95% CI | 0.028-0.083 | 0.069 – 0.706 | 0.350 – 5.413 |
| Geometric Mean | 0.020 | 0.033 | 0.093 |
| 95% CI | 0.013 – 0.030 | 0.024 – 0.049 | 0.061-0.147 |
| Median | 0.026 | 0.026 | 0.068 |
| N | 42 | 78 | 93 |
| Latitude (°) | |||
| Mean | 23.1 | 25.3 | 35.0 |
| 95% CI | 19.1 – 27.1 | 22.5 – 27.8 | 32.3 – 37.4 |
| Median | 24.0 | 26.2 | 38.1 |
| N | 42 | 78 | 93 |
| TBDmax (h) | |||
| Adjusted mean | 10.1 | 11.2 | 266.6 |
| 95% CI | 9.7 – 10.6 | 10.6 – 11.8 | 111.7-636.6 |
| Mean | 10.1 | 12.9 | 391.9 |
| 95% CI | 9.0 – 11.2 | 11.4 – 14.5 | 303.9-479.9 |
| Median | 10 | 12.3 | 288 |
| N | 23 | 57 | 82 |
| TBDmean (h) | |||
| Adjusted mean | 6.1 | 6.0 | 123.9 |
| 95% CI | 3.0 – 12.4 | 3.0-12.4 | 51.7 – 297.2 |
| Mean | 6.3 | 8.2 | 198.0 |
| 95% CI | 4.9 – 7.6 | 7.0 – 9.3 | 158.2-233.8 |
| Median | 6.3 | 7.4 | 161 |
| N | 12 | 50 | 70 |
| Tbmin (°C) | |||
| Adjusted mean | 21.8 | 16.9 | 3.9 |
| 95% CI | 17.5 – 26.1 | 11.4 – 22.5 | --2.9 - 10.7 |
| Mean | 20.2 | 18.1 | 6.2 |
| 95% CI | 18.0 – 22.1 | 16.6 – 19.4 | 4.8 – 7.7 |
| Median | 20.8 | 17.9 | 5.0 |
| N | 41 | 73 | 79 |
| TMRmin (mL O2 g−1 h−1) | |||
| Adjusted mean | 0.585 | 0.237 | 0.039 |
| 95% CI | 0.302 – 1.134 | 0.100 – 0.600 | 0.036 – 0.040 |
| Mean | 0.740 | 0.430 | 0.037 |
| 95% CI | 0.557 – 0.951 | 0.352 – 0.509 | 0.032 – 0.043 |
| Median | 0.500 | 0.370 | 0.035 |
| N | 25 | 54 | 50 |
| TMRrel (% of BMR) | |||
| Adjusted mean | 35.3 | 18.8 | 4.3 |
| 95% CI | 16.0-78.0 | 7.5-47.2 | 2.1 – 8.7 |
| Mean | 30.5 | 29.9 | 4.4 |
| 95% CI | 22.0 – 39.8 | 26.1 – 34.4 | 3.5 – 5.6 |
| Median | 24.0 | 28.0 | 3.0 |
| N | 19 | 54 | 50 |
| IBE (h) | |||
| Adjusted mean | 17.2 | 14.1 | 6.9 |
| 95% CI | 13.9-21.4 | 10.1-19.7 | 3.5-13.4 |
| Mean | 17.7 | 15.8 | 12.0 |
| 95% CI | 16.4-19.0 | 14.6-16.8 | 9.8-14.4 |
| Median | 17.7 | 16.6 | 10.3 |
| N | 12 | 50 | 49 |
1) Note that inter-bout euthermia (IBE) in daily heterotherms was estimated from 24 h - TBDmean.
The mean body mass was significantly higher in mammalian hibernators than in both mammalian and avian daily heterotherms (Table 2). This was also true when geometric means were compared, to adjust for the skewness in the body mass data (Table 2). Body mass of the single avian hibernator was 0.035 kg. The mean and median body masses of mammalian heterotherms were significantly lower than those of terrestrial mammals in general (n=2636; mean: 24.4 kg, 95%CI: 17.3-32.4 kg; median: 0.134 kg, 95%CI: 0.111-0.165 kg).
Among mammals most orders represented here contained both daily heterotherms and hibernators (Fig. 2), with two exceptions: the carnivorous/omnivorous marsupial orders Dasyuromorphia and Didelphimorphia, for which daily torpor but not hibernation has been reported. In all other orders, the proportion of hibernators (overall 57%) and daily heterotherms (43%) was approximately the same (Chi2=11.4, df=10, P=0.325).
(3) Maximum torpor bout duration
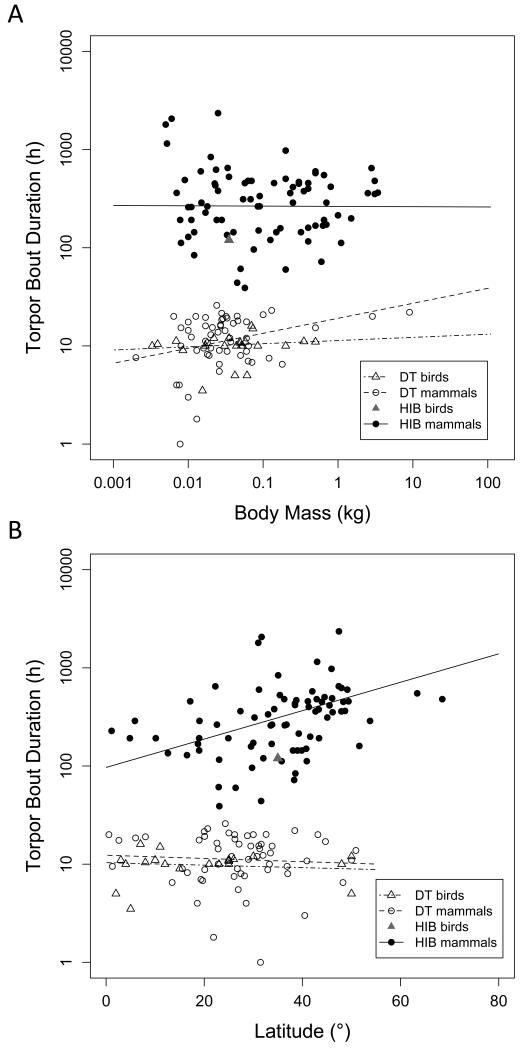
TBDmax slightly increased with body mass among mammalian daily heterotherms, but was independent of body mass among both avian daily heterotherms and mammalian hibernators (Fig. 5A); regression slopes did not differ between the three groups. Pagel’s λ was 0 (95% CI: 0-0.58; Plower>0=1, Pupper<1=<0.001) among mammalian daily heterotherms, indicating that a phylogenetic signal in this response variable was absent among mammals using daily torpor. This was also the case for avian daily heterotherms (λ=0, 95% CI: 0-0.67; Plower>0=1, Pupper<1=<0.0001). TBDmax did contain a significant phylogenetic signal, however, among hibernating mammals (λ=0.56, 95% CI: 0.13-0.82; Plower>0=0.018, Pupper<1=<0.0001). TBDmax significantly increased with latitude of the species’ distribution centre among hibernating mammals, but not among daily heterotherms (Fig. 5 B).
Fig. 5.
A) Maximum torpor bout duration in relation to body mass. In mammalian daily heterotherms TBD slightly increased with body mass (log10duration=1.28+0.152 log10BM, t=2.56, P=0.013, R2=0.10). In mammalian hibernators maximum TBD was independent of body mass (P=0.968) and this was also the case for avian daily heterotherms (P=0.55). B) Maximum torpor bout duration in relation to absolute latitude of the species distribution centre. For mammalian daily heterotherms the regression was not significant (t=−0.49, P=0.621). Among mammalian hibernators maximum torpor bout duration increased with latitude (log10duration=1.985+0.0144 Latitude, t=5.05, P<0.0001, R2=0.12). There was no significant relationship in avian daily heterotherms (t=−0.73, P=0.471).
Adjusted means of TBDmax (calculated for a body mass of 30 g) were ~10-11 h in both mammalian and avian daily heterotherms, and >200 h in hibernating mammals (Table 2). TBDmax was 120 h in the common poorwill.
(4) Mean torpor bout duration
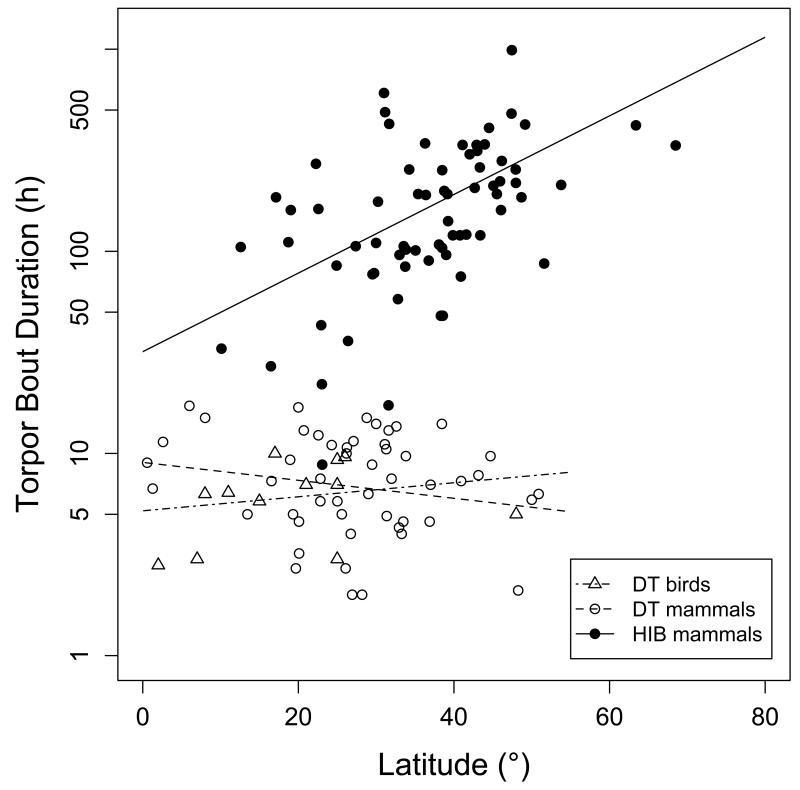
Mean torpor bout duration (TBDmean) was independent of body mass in all subgroups of birds and mammals (t<=1.04, P>=0302). TBDmean contained no significant phylogenetic signal among mammalian (λ=0, 95% CI: 0-1; Plower>0=1, Pupper<1=<0.0001) or avian (λ=0.53; 95% CI: 0-1; Plower>0=0.49, Pupper<1=0.21) daily heterotherms. TBDmean did, however, contain a slight phylogenetic signal in mammalian hibernators (λ=0.47, 95% CI: 0-0.79; Plower>0=0.070, Pupper<1=<0.0001). TBDmean increased with increasing latitude of the distribution range among hibernating mammals, but not for daily heterotherms (Fig. 6). The slope for this relationship in hibernators was steeper than that for TBDmax.
Fig. 6.
Mean torpor bout duration in relation to absolute latitude of the species distribution centre. There were no significant relationships in avian (t=0.73, P=0.487) or mammalian daily heterotherms (t=−1.49, P=0.140). Mean torpor bout duration increased with latitude in mammalian hibernators (log10duration=1.503+0.019 × Latitude, t=5.36, P<0.0001, R2=0.26).
Adjusted means (to 30 g body mass) of TBDmean were ~6-7 h in both avian and mammalian daily heterotherms, and >120 h (i.e., >17 times longer) in mammalian hibernators (Table 2). No mean torpor bout length was available for the single avian hibernator. Among mammals traditionally classified as hibernators the shortest TBDmean were recorded for Elephantulus myurus (8.8 h), Elephantulus edwardii (17.3 h) and Atelerix frontalis (22 h).
(5) Minimum body temperature
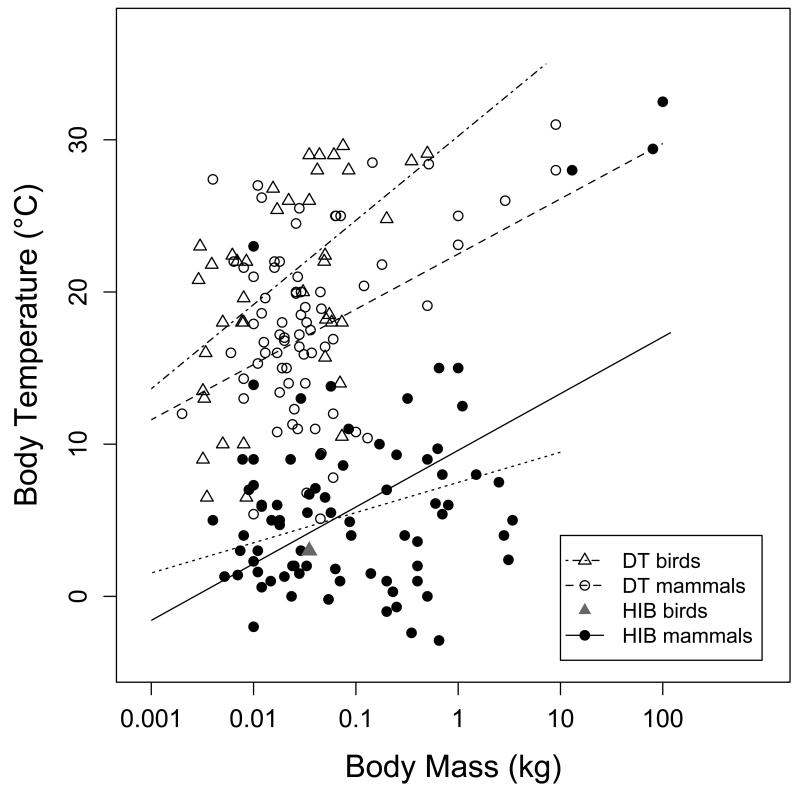
Tb min in daily heterotherms increased with increasing body mass among both mammals and birds (Fig. 7). The slopes of the regression lines did not differ significantly between avian daily heterotherms, mammalian daily heterotherms, and mammalian hibernators. Among hibernators, the regression was heavily influenced by data from 3 carnivores (Ursus americanus, Ursus arctos, Meles meles, all with Tb min >28°C). After removing these data points (as well as a Tb min of 23°C reported for the arid-zone bat Rhinopoma microphyllum) the regression slope was still significantly greater than zero, however. Tb min contained a strong significant phylogenetic signal among mammals, both among daily heterotherms (λ=0.68; 95% CI: 0.31-0.88; Plower>0=<0.001, Pupper<1=<0.0001) and among hibernators (λ=0.78; 95% CI: 0.55-0.91; Plower>0=<0.0001, Pupper<1=<0.0001). Among avian daily heterotherms however, the signal was weak (λ=0.35; 95% CI: 0-0.87; Plower>0=0.271, Pupper<1=0.004).
Fig. 7.
Tb min as a function of body mass. Tb min increased with mass among mammalian daily heterotherms (Tb min=22.5+3.63 log10BM, t=3.56, P<0.001, R2=0.14) and avian daily heterotherms (Tb min = 21.8 + 5.53 log10BM, t=2.84, P=0.007,R2=0.26). Tb min also increased with body mass among mammalian hibernators (Tb min=9.6+3.72 log10BM, t=3.98, P<0.001, R2=0.20). After removing data from hibernators with Tb min >20°C (n=4) the regression equation was Tb min= 7.5+1.98 log10BM, t=2.18, P=0.032, R2=0.02 (dotted line).
The predicted Tb min for a 30 g avian daily heterotherm was ~22°C, which was only slightly higher than in a mammalian daily heterotherm of the same body mass (17°C; Table 2). Adjusted mean Tb min in mammalian hibernators was ~4°C, i.e., significantly lower than in the other subgroups (Table 2). Interestingly, 8 mammalian hibernators had Tb min ≤ 0°C, and 3 of these had Tb min ≤−2°C.
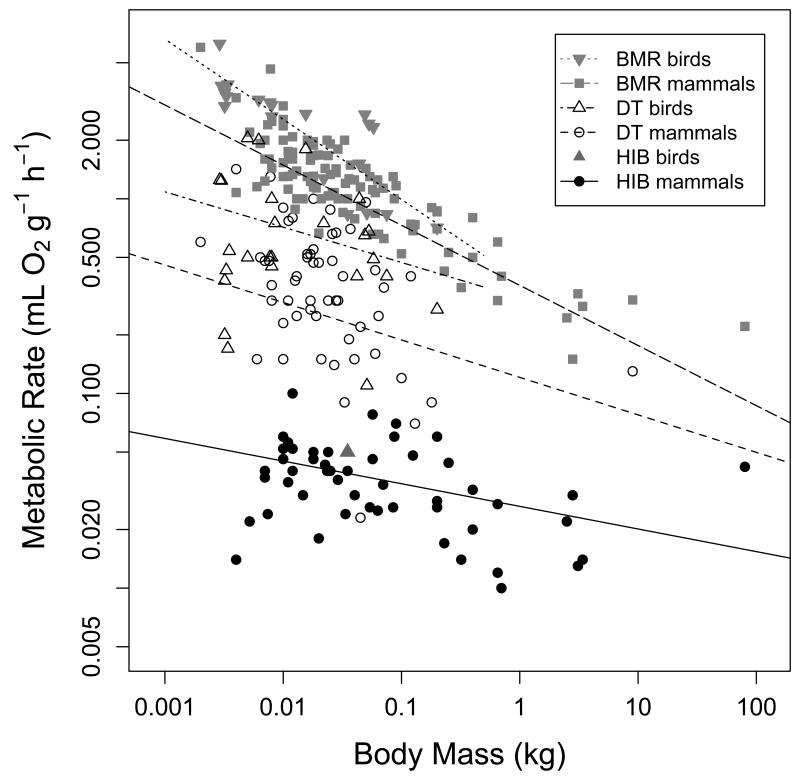
(6) Minimum metabolic rate
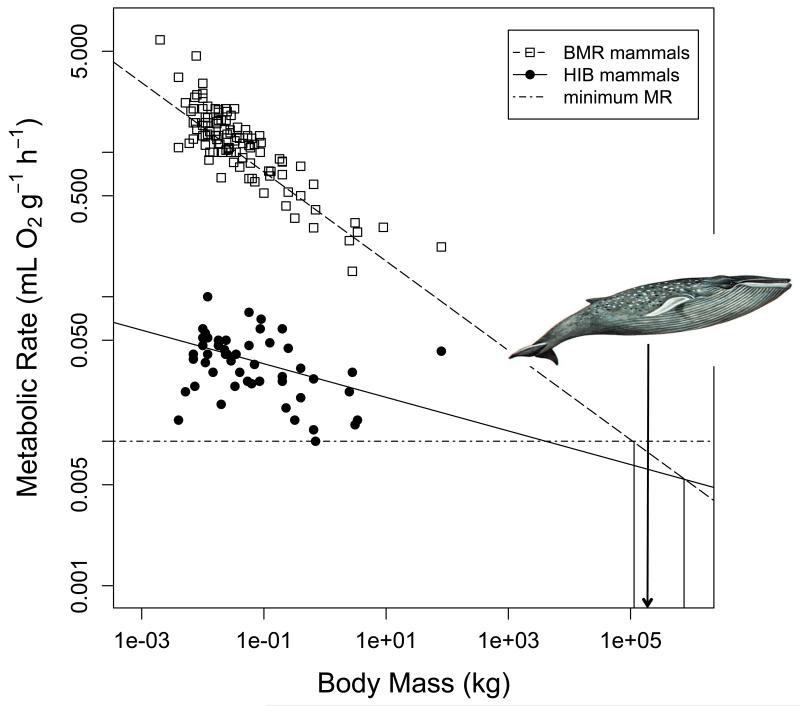
Mass-specific TMRmin decreased with increasing body mass in both daily heterotherms and hibernators (Fig. 8A). Among daily heterotherms the slopes of the regression lines for these relationships in both mammals and birds were not statistically different from that for BMR within the same group. In hibernating mammals, however, the slope of TMRmin as a function of body mass was significantly lower (P<0.05) than that for mammalian or avian BMR. Notably though, the decrease in TMRmin of hibernators with increasing body mass was statistically significant both with and without the largest hibernator (Ursus americanus; Fig. 8A). After excluding the black bear, the regression equation was TMR=−1.651-0.156 log10BM (t=−3.43, P=0.0012, R2=0.17).
Fig. 8.
A) Basal and minimum metabolic rate as a function of body mass. Regression equations for mass specific BMR were log10BMR= −0.444 – 0.308 log10BM (t= −13.9, P<0.0001,R2=0.73) among mammals and log10BMR= −0.415 – 0.412 log10BM (t= −4.33, P<0.001,R2=0.66) among birds. Minimum MR also decreased as body mass increased in mammalian daily heterotherms (log10MR= −0.917-0.192 log10BM, t=−2.30, P=0.025, R2=0.19). In avian daily heterotherms the slope of this regression was not significantly different from zero (t=−1.17, P=0.25). Among hibernating mammals the decrease of minimum metabolic rate with body mass was not pronounced but statistically significant (log10MR= −1.579 – 0.116 log10BM, t= −4.41, P=0.0001, R2=0.13). B) The relationship between minimum torpor metabolic rate and maximum torpor bout duration. TBDmax decreased with increasing TMRmin among mammalian hibernators (log10TBDmax=1.22-0.862 log10MR, t=−4.56, P<0.0001, R2=0.20). A weaker relationship in the same direction was also detectable among mammalian daily heterotherms (log10TBDmax=0.76-0.475 log10MR, t=−3.92, P<0.001, R2=0.27), but not in avian daily heterotherms (t=−1.37, P=0.205).
BMR contained a significant phylogenetic signal among mammals (λ=0.61; 95% CI: 0.18-0.87; Plower>0=<0.001, Pupper<1=<0.0001), but not among birds (λ=0; 95% CI: 0-1; Plower>0=1, Pupper<1=0.117). TMRmin during daily torpor in mammals was affected by phylogeny (λ=0.81; 95% CI: 0.44-0.96; Plower>0=0.003, Pupper<1=0.004). There was no evidence for a phylogenetic signal in TMRmin among hibernating mammals (λ=0; 95% CI: 0-0.48; Plower>0=1, Pupper<1=<0.0001) or birds using daily torpor (λ=52; 95% CI: 0-1; Plower>0=0.312, Pupper<1=<0.134).
The predicted, mass-specific BMR for 30-g animals was 1.060 mL O2 g−1 h−1 (95% CI: 0.757-1.48 mL O2 g−1 h−1) for mammals and 1.628 mL O2g−1 Fi. 10h−1 (95%CI 0.993-2.671 mL O2 g−1 h−1) for birds. In mammalian daily heterotherms the predicted TMRmin at a body mass of 30 g was 60% lower than in avian daily heterotherms, but the 95% CI of the estimates overlapped (Table 2). The adjusted mean of TMRmin in mammalian hibernators (~0.04 mL O2 g−1 h−1) was only 17% of that in mammalian daily heterotherms (~0.24 mL O2 g−1 h−1), and this difference was significant (Table 2).
There was a significant relationship between TMRmin and TBDmax among hibernators with short TBDmax being associated with high mass-specific torpor metabolic rate (Fig. 8B). A similar, but much weaker relationship between these traits was also detectable in mammalian, but not in avian daily heterotherms (Fig. 8B). At the median TMRmin across all subgroups (0.09 mL O2 g−1 h−1), the predicted TBDmax was significantly higher (133.2 h; 95%CI: 52.4-338.2 h) than among mammalian (13.2; 95% CI: 10.2-16.8 h) and avian daily heterotherms (13.0; 95% Ci: 7.0-23.9 h).
(7) Relative torpor metabolic rate
TMRrel, i.e., the TMRmin as % of BMR was variable among daily heterotherms and showed a tendency to increase with body mass, but not significantly so (Fig. 9). In mammalian hibernators the increase of TMRrel with body mass was statistically significant.
Fig. 9.
Metabolic reduction (TMRrel) as a function of body mass. Slight increases of TMRrel among daily heterothems were non-significant (birds: t= 1.12, P=0.275; mammals: t=1.59, P=0.117). Among hibernating mammals there was a significant relationship between TMRrel and body mass (log10TMRrel=0.81+0.20 log10BM, t=5.40, P<0.0001, R2=0.42).
There was evidence for a phylogenetic signal in TMRrel among mammalian daily heterotherms (λ=0.86; 95% CI: 0.49-0.99; Plower>0=0.002, Pupper<1=0.035) but only a tendency for a signal among avian daily heterotherms (λ=0.70; 95% CI: 0-1; Plower>0=0.104, Pupper<1=0.171). No phylogenetic signal was detectable among hibernating mammals (λ=0; 95% CI: 0-0.52; Plower>0=1, Pupper<1=<0.0001).
Adjusted means to 30 g body mass for TMRrel were ~40% and 30% for avian and mammalian daily heterotherms, respectively, but with overlapping 95% CI (Table 2). Among birds, the lowest TMRrel during daily torpor were observed in Calypte anna (4.4%) and Archilochus alexandri (5.5%); among mammals the lowest values for daily torpor were reported for Elephantulus rozeti (2.0%), Microcebus myoxinus (4.5%) and Tarsipes rostratus (5.0%). Among hibernators, the adjusted mean TMRrel was ~6% for all species (Table 2) and 4.0% (95%CI: 1.4-6.6) when the two largest values were excluded. The lowest TMRrel was found in a bat, Vespadelus vulturnus (1.3%). Relative TMR in the single avian hibernator, Phalaenoptilus nuttallii, was 6%.
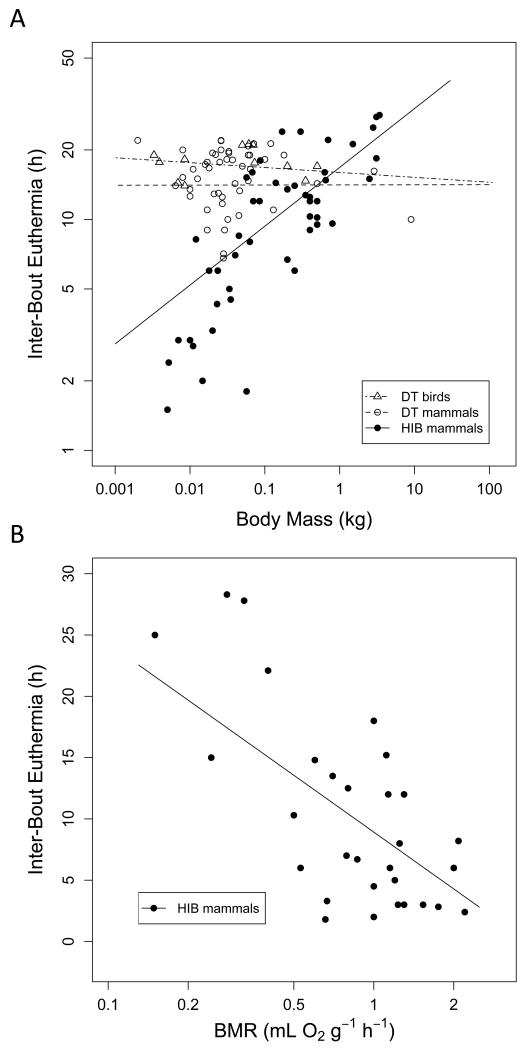
(8) Inter-Bout Euthermia
IBE was more variable in hibernators (range 1.5-44 h) than in daily heterotherms (6.8-22 h; Fig. 3, Table 2). IBE was not affected by body mass in either avian or daily heterotherms, but significantly increased with body mass in mammalian hibernators (Fig. 10A). IBE significantly decreased as mass-specific BMR increased among mammalian hibernators (Fig. 10B), but such a relationship was absent in daily heterotherms (not shown on graph). IBE contained a phylogenetic signal in mammalian hibernators (λ=0.77; 95% CI: 0.19-0.95; Plower>0=0.028, Pupper<1=<0.0001), but the signal was non-significant among mammalian (λ=0.63; 95% CI: 0-0.94; Plower>0=0.310, Pupper<1=<0.0001) and avian daily heterotherms (λ=0.53; 95% CI: 0-1; Plower>0=0.394, Pupper<1=0.182). There was no significant relationship between IBE and the latitude of the species’ geographical range (all t≤1.1, P≥0.275).
Fig. 10.
A) Duration of inter-bout euthermia as a function of body mass. There was no significant relationship to body mass in avian (t=−0.59, P=0.562) or mammalian (t=0.01, P=0.987) daily heterotherms, but the duration of euthermia episodes increased with body mass among mammalian hibernators (log10IBE=1.22+0.255 log10BM, t=4.59, P<0.0001, R2=0.66). B) The relationship between basal metabolic rate (BMR) and the duration of interbout euthermia in mammalian hibernators (IBE=8.92-15.39 log10BMR, t=−3.80, P<0.001, R2=0.50). There was no such relationship in daily heterotherms (data not shown for clarity).
IV. DISCUSSION
(1) The classification of types of heterothermy
Our results show that the classical distinction between hibernation and daily torpor is supported by clear differences in most torpor traits. Even traits that showed considerable overlap between the two groups (e.g. Tb min, Fig. 3), when combined with other characteristics such as TMRmin, can be used to predict whether a species uses daily torpor or hibernation to a reasonable degree of certainty (Fig. 4). Our results also show that allometric relationships of daily torpor and hibernation characteristics show distinct elevations and slopes if phylogeny is taken into account. Thus, previous analyses that pointed to the validity of this distinction of heterothermy types (e.g., Geiser & Ruf, 1995) were not caused by a lack of accounting for similar characteristics of closely-related species. The fact that Tb min was the variable least suited (using cluster analysis) to differentiate between daily torpor and hibernation may also explain why a recent comprehensive study (Boyles et al., 2013), using Tb-based indices of heterothermy, found only very weak evidence to support this classical division. In view of our current analysis, it seems clear that this outcome of the study by Boyles et al. (2013) does not indicate the absence of distinct types of torpor but merely underlines the problems inherent in focusing on Tb, or Tb-based indices, which -more than other variables- are affected by confounding factors such as body mass. Another reason why Boyles et al. (2013) found no clear subgroups among heterotherms was probably due to the fact that in that study, data were not restricted to extreme values, i.e., estimates of a species maximum physiological capability. Further, one of the indices used by Boyles et al. (2013), the Heterothermy Index, is intrinsically unable to distinguish between deep/short and long/shallow torpor bouts, which, given the high predictive value of torpor bout duration indicated by our analyses, questions is usefulness for the purpose of testing for the existence of physiologically distinct subgroups. This also applies to another recently suggested measure, the Thermoregulatory Index (Muñoz-Garcia et al., 2013) that requires simultaneous measurements of Tb, Ta, and MR, which currently limits its use largely to laboratory measurements. While this index may be a sensible measure of the degree of heterothermy at any point in time, it does not include the duration of torpor bouts, which, according to our analysis, was the most prominent distinctive characteristic of torpor types. For these reasons, the avoidance of arbitrary thresholds, which was the recommendable major rationale behind the development of the above indices of heterothermy, may also limit their usefulness, at least for classification purposes.
(2) Torpor bout duration and Interbout Euthermia
Our results show that classifying endotherms as daily heterotherms or hibernators based on their TBDmax (<>24 h) is highly reliable. The average TBDmax for a 30-g hibernator was >30 times greater than in a 30-g daily heterotherm. In our data set comprising 214 species, there was only one ambiguous species, Sminthopsis macroura, which had been classified as a daily heterotherm despite a TBDmax of 25.9 h. However, as pointed out by the authors (Körtner & Geiser, 2009) this maximum duration was taken from a highly unusual torpor bout for this species, and the species usually had TBDs of 11 h.
As mentioned before, hibernators may express bouts of torpor that last less than one day. Nevertheless, there are several reasons why hibernation and daily torpor should be considered as two distinct physiological states. Firstly, only one species previously classified as a daily heterotherm (Elephantulus myurus) has the capability to exhibit multiday torpor bouts (Fig. 3). Secondly, as demonstrated above, it is possible to separate the vast majority of hibernators from daily heterotherms based on a combination of Tb min and TMRmin alone. It remains to be seen whether those species with both low Tb min and low TMRmin (e.g., Elephantulus rozeti) were simply misclassified (based on TBDmax alone) and will display multiday torpor in future studies. On the other hand, reliance on extreme characteristics bears the danger of artefacts from unusual laboratory conditions, such as too low Ta (Tomlinson et al., 2007; Lovegrove, 2012a), which may have been the case in E. rozeti. However, only very few species were difficult to classify via cluster analysis, namely Elephantulus edwardii and E. myurus, both traditionally viewed as hibernators. At least in the latter species even free-ranging animals occasionally show torpor bouts lasting more than 24 hours (Mzilikazi & Lovegrove, 2004). Thus, these exptional cases seem insufficient to question the dichotomy between daily torpor and hibernation, or to postulate a third type of heterothermy (for which there was no evidence in our statistical analyses).
A third, and very important argument for differentiating between the two types of torpor is supported by studies showing that daily torpor is an extension of the circadian rhythm of Tb, and free-runs, i.e., continues with an endogenous period that slightly deviates from 24 h, under constant lighting conditions (Lynch et al., 1980; Ruf et al., 1989; Kirsch et al., 1991; Körtner & Geiser, 2000b). This is not the case, however, in hibernators, in which the circadian clock seems at least strongly suppressed, although it may still exert some influence on torpor/arousal cycles (e.g., Daan, 1973; Pohl, 1987; Grahn et al., 1994). Some studies suggest that the circadian clock actually is arrested and is restarted only after the hibernation season, at least when hibernators experience naturally low Ta (Hut et al., 2002a; Hut et al., 2002b; Ruby, 2003; Malan, 2010). Also, there is evidence that genes involved in the molecular mechanism of the circadian clock, such as Per1, Per2 and Bmal1, are expressed constantly, rather than rhythmically, during hibernation (Revel et al., 2007). Hence, although mechanisms of hypothalamic control of Tb or metabolic reduction may be similar in daily heterotherms and hibernators (while Tb set-points and the degrees of metabolic reduction differ), at least those physiological mechanisms that govern the temporal structure of heterothermy are clearly different between daily torpor and hibernation. Therefore, we concur with Sheriff et al. (2012) who suggested that short, shallow torpor bouts (<24 h), which hibernators often exhibit just prior to the hibernation season, physiologically resemble consecutive multiday torpor and should be called “short torpor”, but not “daily torpor”.
An early attempt to explain variation in TBD was a metabolism-dependent, so called “hourglass mechanism” that may control torpor/euthermia cycles. This hypothesis assumes the development of a metabolic imbalance during torpor (e.g., the accumulation or depletion of metabolites or the accretion of cellular damage) that can be eliminated only in the euthermic state (Fisher, 1964; French, 1985). This idea seemed to be supported by a decrease of TBD with body mass (and, by inference, MR), albeit in a very limited sample of mammals (French, 1985). Subsequent, more comprehensive comparisons showed no evidence for such a relationship between TBD and body mass (e.g., Geiser & Ruf, 1995; Malan, 2010), which was confirmed by the present study (Fig. 5A). The absence of an effect of body mass on TBD has been used to argue that torpor-arousal cycles are not governed by metabolism, and to dismiss the hypothesis that arousal from hibernation is driven by an hourglass mechanism (Malan, 2010). A problem with this argument is, however, that in torpid hibernators, the dependency of MR on body mass is drastically reduced (Fig. 8A), which renders body mass a very poor proxy for metabolic processes during deep torpor. Therefore, a more direct test of the hourglass hypothesis is evaluating the relationship between MR in torpor (rather than body mass) and the duration of torpor episodes. Our finding of a significant decrease of TBD with increasing TMR among hibernators (Fig. 8B), as well as a decrease of IBE with metabolic rate (Fig. 10), is fully compatible with the idea of an hourglass mechanism. If arousal is due to a metabolic imbalance, this imbalance will progress faster at high TMR, and can be eliminated more rapidly at high euthermic MR. Such a mechanism seems a more parsimonious explanation than the assumption of a specialized, non-temperature-compensated circadian torpor-arousal clock, as suggested by Malan (2010). However, the two mechanisms may not be entirely mutually exclusive, because any metabolism-driven hourglass may still be modulated by an endogeneous clock that influences the probability of actual arousal, once a certain metabolic imbalance is reached during torpor. In any case, it should be noted that such an endogenous clock modulating TBD in hibernators, if it exists, must differ anatomically and functionally from the central circadian clock controlling daily torpor (Ruby, 2003; Malan, 2010).
Arguably, the differences between clock mechanisms and respective temporal structures constitute the fundamental difference between daily torpor and hibernation, which has subsequently led to distinct levels of minimum Tb and degree metabolic reduction. They may explain the fact that most traits showed little overlap between daily heterotherms and hibernators (Fig. 3). As outlined in more detail before (Geiser & Ruf, 1995), the advantages of keeping entrained with the light-dark cycle should select against multiday torpor bouts in animals that continue to remain active and forage above ground, i.e., daily heterotherms. Species that opt to employ multiday torpor, on the other hand, should benefit from larger body mass facilitating higher body energy stores, and from reaching lower TMRmin to maximise energy savings. These differences may well have led to disruptive selection and hence to the absence of intermediate types of torpor.
Obviously, further research is necessary to clarify if it is indeed the clock mechanism governing the temporal control of torpor that separate hibernators from daily heterotherms. For instance, it would be would be interesting to see whether the constant, arrhythmic expression of clock genes in the central circadian pacemaker, which to our knowledge was demonstrated only in one mammal (Revel et al., 2007), is a general feature of hibernating mammals. We would predict that the massive suppression of clock genes in the SCN is a prerequisite of the capability for consecutive multiday torpor. However, a group of species that may be particularly important to study in this context, because some residual circadian clock activity may be adaptive for them, are those hibernators that occasionally show above ground activity during winter, such as hedgehogs (Morris, 1973) which may be exposed to light/dark cycles. Since our present analysis supports the hypothesis of an hourglass mechanism driving the torpor arousal cycles in hibernators, it points to a need for a renewed search for the nature of the “metabolic imbalance” that seems to accumulate during torpor (e.g., Fisher, 1964; French, 1985). It may be promising to concentrate this search on physiological functions that are paramount for survival at low Tb, and that have been previously suggested to play a role in the induction of arousals, namely brain and heart function (Daan et al., 1984; Carey et al., 2003; Ruf & Arnold, 2008; Giroud et al., 2013).
Advanced methods of genomics, transcriptomics and proteomics could help to identify molecular targets whose accumulation or depletion rates correlate with the durations of torpor bouts and inter-bout euthermia. Finally, another possible (albeit time-consuming) approach to better clarify the physiological differences between daily torpor and hibernation are artificial selection regimes designed to select either for or against long/deep torpor (again, along with genomics/transcriptomics or proteomics studies to identify molecular factors that may differ between selection lines). The most promising models for such a project may be those species with torpor characteristics that seem to be borderline between daily torpor and hibernation, such as Elephantulus spec.
There are further differences, apart from the circadian system functionality in winter, mainly concerning the seasonal control of torpor that appear to generally differ between hibernators and daily heterotherms. For instance, several hibernators make use of an endogenous circannual clock that drives the onset and termination of the hibernation season (Pengelley & Fisher, 1963; Pengelley & Asmundson, 1969; review in Körtner & Geiser, 2000a), whereas the seasonal occurrence of daily torpor is often triggered by short photoperiods (e.g., Lynch et al., 1978; Ruf et al., 1993), shortage of food or low ambient temperatures (e.g., Hainsworth et al., 1977; Hudson & Scott, 1979; Ruf et al., 1993; Silva-Duran & Bozinovic, 1999). Hibernation and daily torpor also differ in that the former typically relies on the availability of substantial energy reserves, either body fat or food stores, whereas daily torpor is accompanied by continued foraging. This factor likely contributed to our finding of a significant difference in body mass between daily heterotherms and hibernators. Although this variable showed the largest overlap between the two types of torpor, mean and median body mass was several times larger in hibernators. As pointed out earlier (Lindstedt & Boyce, 1985; Calder, 1996), small body mass constrains the size of body fat stores, not just absolute storage amounts but also in terms of the proportion of body fat. Hence hibernators, which seem to mostly rely on endogenous energy stores, i.e., body fat (Humphries et al., 2003b), will benefit from increased body size. Daily heterotherms, on the other hand, which continue to forage, should benefit from a functional circadian system that keeps them entrained with the light-dark cycle and serves to optimize times of daily activity and rest. The need to continue foraging also explains why both TBDmean and TBDmax among daily heterotherms peaked well below 24 h (Fig. 3; means: ~7 h for TBDmean and ~10 h for TBDmax; Table 2) as this average duration of daily torpor leaves sufficient time for foraging within the daily cycle.
Continued foraging versus reliance on energy stores would also help to explain why, on average, species using daily torpor have distribution ranges at lower latitudes, whereas hibernators extend geographic ranges closer to the poles. This result – which, to our knowledge, has not been reported previously – almost certainly reflects adaptations to increasing seasonality of habitats at higher latitudes and the absence of food resources in the environment during winter, favouring physiological responses that rely on energy reserves. The fact that among hibernators, and only in this subgroup, body mass increased with latitude can also be understood in terms of increased capacity for body fat stores in larger animals. However, this effect may also be explained in terms of Bergmann’s rule (Bergmann, 1848), i.e., the concept that colder climates select for increased body sizes because the associated decrease of relative body surface reduces heat loss. For a more detailed discussion of the effects of environmental conditions on body mass in hibernators see Gür (2010) and Ozgul et al. (2010).
The absolute latitude of the species’ geographic range was also a significant predictor of TBDmax, and even more so of TBDmean, among hibernators (Figs 5, 6). To our knowledge this is also a novel finding, as the effects of latitude on hibernation characteristics have not been investigated before (but see Boyles et al., 2013 for effects of latitude on the general degree of heterothermy). TBD was not associated with latitude, however, among daily heterotherms (Figs 5, 6). The absence of this effect among daily heterotherms, as outlined above, is most likely due to the fact that TBDmax is constrained to <24 h in order to maintain entrainment with the light-dark cycle. Mean and maximum TBD in hibernators increased towards higher latitudes, which indicates that most hibernating mammals prolong torpor (even if it does increase energy savings) only if environmental conditions dictate such a behaviour. In some cases, species that hibernate for several months at higher latitudes may remain euthermic and even reproduce during winter in warmer parts of their geographical distribution range (e.g., the Garden dormouse, Gil-Delgado et al., 2006). Striking differences in the use of torpor and hibernation, likely due to local climatic conditions, may even occur on a small geographical scale (Lehmer et al., 2006). Incidentally, this degree of flexibility in the use of prolonged torpor also questions the validity of the terms “obligate” versus “facultative” hibernator.
It is evident that the coldest winter habitats at extreme latitudes require the most profound energy savings. This would explain why hibernators lengthen TBD with decreasing Ta and Tb in torpor (e.g., French, 1982; Hut et al., 2002a; Bieber & Ruf, 2009). This seems the most straightforward proximate physiological mechanism that links low hibernaculum temperatures – via Tb min – to decreased energy expenditure. On the other hand, high-latitude geographical ranges are also characterized by long periods of low food availability, which also should facilitate the use of energy stores and hibernation, independent of cold load. It has long been recognized that factors other than low Ta can be a major selective force favouring hibernation, such as seasonal droughts, which often occur even in subtropical and tropical environments (Darwin, 1845).
Even when adjusted for the effect of latitude, there was still a large residual variation in both maximum and mean TBD in our analyses, likely caused by species-specific factors we could not account for here. It appears that TBDmax and other characteristics of hibernation (or torpor in general) result from both the benefits and costs of hypometabolic states (Humphries et al., 2003a; Humphries et al., 2003b). The costs of prolonged torpor are evident from the fact that within a species, animals overwintering in mild winter apparently avoid its use (e.g., Gil-Delgado et al., 2006; Lehmer et al., 2006). Further, supplemental feeding of food-hoarding hibernators can significantly reduce their use of torpor (Humphries et al., 2003a) and individuals with high body energy reserves among fat-storing hibernators reduce torpor use and increase euthermic episodes during winter (Zervanos et al., 2013; Bieber et al., 2014). While these observations indicate that torpor use is minimized whenever possible, the physiological mechanisms generating costs are not as easy to pinpoint. One physiological function that may be impaired is memory (Millesi et al., 2001), but this effect may be species specific (Clemens et al., 2009). It has also been hypothesized that torpor may represent a state of sleep deprivation, but there was little experimental support for this hypothesis (review in Kräuchi & Deboer, 2011). Further, torpor at low Tb is associated with extreme immune-suppression that is reversed during periodic arousals (Burton & Reichman, 1999; Prendergast et al., 2002; Bouma et al., 2010). Generally, immune-suppression during torpor is probably beneficial as it saves energy, protects from inflammatory processes, and typically has little risks since most microbes proliferate very slowly at low temperatures (Ma et al., 2005; Bouma et al., 2010). However, impaired immune function during hibernation may increase the risk of contracting certain viral or fungal diseases that can be lethal (Prendergast et al., 2002; Bouma et al., 2010). Torpor also seems associated with increased oxidative stress and the potentially costly up-regulation of antioxidant defences (Buzadžić et al., 1997; Carey et al., 2000; Ni & Storey, 2010). Recent evidence from edible dormice suggests that despite up-regulated antioxidant defences, arousals from deep hibernation are associated with cellular damage in terms of shortening of telomeres, i.e., the repeated sections of DNA that ensure the integrity of the ends of chromosomes (Turbill et al., 2013). This effect may be directly related to rewarming from low Tb as the use of daily torpor in Djungarian hamsters (with much higher Tb min), was found to have a positive effect on relative telomere-lengths (Turbill et al., 2012).
The benefits of torpor are easier to characterize, because they were the focus of research in this field in the past. In particular, it has been known for some time that hibernation and daily torpor result in significant energy savings (Hall, 1832; Darwin, 1839; Wyss, 1932; Kayser, 1939). However, recent data identify several other beneficial functions of torpor. Torpor facilitates migration in certain birds, it is an integral part of reproductive strategies that involve sperm storage in certain bats and other mammals, it can primarily serve as a water conservation mechanism, and it was found to lower the risk of extinction (review in Geiser & Brigham, 2012). The latter finding is due to the fact that hibernation, which typically is associated with retreat into underground burrows and other secluded areas, decreases predation risk and, although often assumed otherwise, leads to much higher survival rates than during the active season in the same species (review in Turbill et al., 2011). The fact that torpor indirectly facilitates predator avoidance may help to explain why both hibernators and daily heterotherms have relatively low body masses, compared to terrestrial mammals on average: Large mammals have fewer predators and extrinsic mortality decreases as body mass increases (Owen-Smith & Mills, 2008; Ricklefs, 2008). Further, large animals seem unable to dig underground burrows, which prohibits this avenue of predator avoidance, but maintain high alertness and fast flight, which are incompatible with deep torpor. Obviously, further important energetic reasons, related to Tb and TMRmin (see below) may account for the finding that hibernation and daily torpor become exceedingly rare as body size increases.
(3) Minimum body temperature in torpor
The Tb min of a typical 30-g mammal was 13°C lower in hibernators than in mammalian daily heterotherms (and 18°C lower than in birds showing daily torpor). However, there was also considerable overlap between these subgroups (Figs 3, 7) confirming the earlier conclusion that Tb min alone is not well suited to discriminate between hibernation and daily torpor (Geiser & Ruf, 1995).
Tb min increased significantly with body mass in all groups, whether apparent outliers (i.e., large carnivores with Tb min >28°C) were included or not. This differs from euthermic mammals, in which resting Tb shows no allometric relationship to body mass (Lovegrove, 2012b). Our present result also differs from previous analyses of smaller samples that had not shown any body mass effect on Tb min among hibernators (Geiser & Ruf, 1995). One might be tempted to attribute this increase of Tb min to effects of thermal conductance decreasing as body mass increases. However, based on the allometric equation for conductance in mammals (Bradley & Deavers, 1980) the increase of Tb min (in animals at steady state with identical TMRmin) would only be of 2.5°C, for an increase in body mass from 10 g to 10 kg. The observed effect was much larger (+6°C) over the same body mass range, among mammalian hibernators even after removal of outliers, and even larger (+ 11 to 17°C) in daily heterotherms (Fig. 7). Moreover, whereas large body size and the ensuing large heat capacity may create time-constraints for entrance into and rewarming from daily torpor, this factor should be negligible in hibernators that may stay torpid for several weeks. Therefore, we suggest that small animals, with higher mass-specific metabolic rates, select a low Tb min in order to reach TMRmin similar to those reached by larger animals. Lower Tb min will always be associated with lower TMRmin irrespective of whether metabolic reduction results from active metabolic depression (with decreasing Tb min as a side effect) or from temperature effects (cf. Carey et al., 2003). The finding that many large animals maintain relatively high Tb min provides further support for the view that the torpid state appears to be also associated with risks or physiologically adverse effects, making the regulated depth of torpor the evolved result of a trade-off between its costs and benefits. Since the use of energy reserves, namely body fat stores, to cover energy expenditure will be inevitably lower in small animals (Calder, 1996), it is not surprising that they are apparently forced to undergo extremely deep torpor bouts, and to risk adverse consequences such as cellular damage during arousals (Turbill et al., 2013).
Another factor associated with very low Tb min during hibernation are very low ambient temperatures, down to ~ −20°C in hibernacula of the Arctic ground squirrel (Barnes, 1989). Whereas several species may reach Tb min below 0°C at low Ta, supercooling seems limited to Tb min above −3°C (Table 1). This is probably because in the supercooled state, animals are vulnerable to ice nucleation and freezing (Boyer & Barnes, 1999), and ice-formation is much slower at high subzero temperatures (Storey & Storey, 2013), which also may allow hibernators to escape freezing by arousal.
The highest Tb min during torpor, on the other hand, are maintained by large carnivores (Meles meles, Ursus americanus, Ursus arctos). This trait differentiates them from other hibernators more than the TMR (although data on TMRmin are currently available only for Ursus americanus). This suggests that adverse effects of torpor may be due to, in particular, low Tb min. Black bears, and other large carnivores, seem to avoid adverse effects of low Tb, by maintaining body temperatures >28°C (Fowler & Racey, 1988; Tøien et al., 2011). Notably, in Ursus americanus, even at this high Tb, TMRmin reached a level (0.042 mLO2 g−1 h−1) that was within the 95% confidence interval of a typical 30-g hibernator (0.036-0.043 mLO2 g−1 h−1). Arguably then, the most efficient type of torpor that combines metabolic reduction typical for hibernation with an almost euthermic Tb, has evolved in these large carnivores. It remains to be clarified, however, whether torpor in these “denning” species is a separate type of heterothermy or simply represents the extreme in a continuum of hypometabolic states, and resultant body mass related Tb reductions among hibernators. In any case, the fact that high Tb apparently does not constrain the TMRmin argues against the usefulness of the term “deep hibernator” sometimes used in the literature to characterize species. Neither our analysis of Tb min nor that of TMRmin or TMRrel point to the existence of distinct subgroups within hibernators.
Adverse effects of a reduction in Tb, even if decreases in Tb are small, as in bears, may also explain the conspicuous absence of torpor and hibernation, in its typical form, in certain taxonomical groups such as the ungulates. Even under extremely harsh environmental conditions, as experiences for instance by the Svalbard reindeer, these animals not use torpor but remain euthermic throughout winter (Blix, 1989). In the past, drastic reductions of food intake during winter were thought to be primarily due to a marked reduction in locomotor activity, a behaviour called “Arctic resignation” (Blix, 1989). There is, however, increasing evidence that both Cetartiodactyla and Perissodactyla are also capable of profound hypometabolism in winter (Arnold et al., 2004; Kuntz et al., 2006; Signer et al., 2011). Importantly however, such reductions in winter metabolic rate in red deer, horses, or ibex were associated with reduced peripheral tissue temperatures, rather than core temperatures (e.g., Arnold et al., 2011). It remains to be seen how many endothermic species (perhaps including marine mammals) use this type of hypometabolism, which is much less conspicuous than hibernation or daily torpor, as an alternative over-wintering strategy. As suggested by Lovegrove (2012b) one reason for the maintenance of high euthermic Tb and the avoidance of torpor may be a cursorial lifestyle, e.g., in several Lagomorpha, Artiodactyla, Perrisodactyla and Carnivora, which requires high Tb for maximum muscle function and high running speed (Clarke & Pörtner, 2010; Rojas et al., 2012). An interesting open question in this context is however, whether even cursors when using peripheral cooling may sacrifice some flight or hunting capability for energy savings under harsh winter conditions.
(4) Metabolic reduction
TMR, both in terms of absolute and relative MR, was another trait that clearly separated daily heterotherms from hibernators. For a 30-g mammal, TMRmin in hibernators was only 16.7% of that in daily heterotherms, and there was little overlap in TMRmin between these subgroups (Fig. 8). The degree of reduction of TMRmin below BMR was similar in avian and mammalian daily heterotherms (Fig. 8), resulting in statistically equivalent relative TMRrel in these groups (Fig. 9).
TMRmin decreased with increasing body mass in both daily heterotherms and hibernators (Fig. 8). Among hibernators, the slope of this relationship was only −0.116, but significantly different from 0. In a smaller subsample of hibernators TMRmin previously appeared to be body mass independent (Geiser & Ruf, 1995), but a slope (−0.128) very similar to our present findings has been reported before (Geiser, 2004), suggesting that TMRmin in hibernators does indeed decrease slightly with body mass.
This observation raises the question why small mammalian hibernators do not reduce TMRmin further? We hypothesize there may be at least two reasons: 1) High thermal conductance forces small mammals to spend more energy to maintain a certain Tb-Ta gradient in torpor than larger animals. 2) Small species, which have to reduce MR when entering torpor from a high mass-specific BMR, may be limited by the extent of possible TMR reductions. For instance, one component of the “metabolic machinery” that generates higher BMR in small animals is mitochondrial basal proton leak, which is associated with increased membrane polyunsaturated fatty acid content (reviews in Hulbert & Else, 2005; Polymeropoulos et al., 2012). Although proton leak decreases with Tb (Polymeropoulos et al., 2012), and seems to be actively suppressed during hibernation (Barger et al., 2003), it is most likely never entirely absent. Hence, differences in membrane composition and proton leak that affect BMR may well still be present in the torpid state and could at least partly explain the mass-dependency of TMRmin.
The relative reduction of metabolic rate, i.e., TMRrel as % of BMR was highly variable among both avian and mammalian daily heterotherms (Fig. 9). Partly, this can be attributed to measurement error in both BMR and TMRmin, used to compute TMRrel. In very small mammals and birds, it may be almost impossible to obtain “true” values of BMR, because these animals are rarely in a genuinely post-absorptive, euthermic state at rest. Among mammalian hibernators, however, there was little variation in TMRrel, and after the removal of two apparent outliers it was best described by a constant, i.e., 4% of BMR. Some of the larger variability in species displaying daily torpor may also be due to the maintenance of large Tb-Ta gradients in some daily heterotherms, while, at least in most hibernators, Tb typically is very close to Ta (e.g., Heldmaier & Ruf, 1992; Arnold, 1993; Bieber & Ruf, 2009).
Interestingly, the degree of metabolic reduction during mammalian hibernation is similar to the extent of metabolic depression in many other animals (e.g., molluscs, crustaceans, or reptiles; Guppy & Withers, 1999). However, much lower metabolic rates and degrees of depression can be reached by invertebrates under conditions such as desiccation, freezing, supercooling, and oxygen deficiency, down to a virtually complete absence of metabolism in diapaused eggs of brine-shrimps (Clegg, 1997). In mammals, the lowest MRmin reported was 0.01 mL O2 g−1 h−1 in the hedgehog (Thäti, 1978). Conceivably, this may be close to the absolute minimum metabolic rate attainable by mammals, or even endothermic vertebrates in general. If this is the case, it would have interesting consequences, because this minimum metabolic rate will be identical to BMR once an animal reaches a body mass of 115 tonnes, which is intriguingly close to the mass of the largest known animal, the blue whale (Balaenoptera musculus; weighing up to 170 tonnes; Fig. 11). Even if mammals could further decrease their cellular metabolism, as predicted by the regression through TMRmin, minimum metabolism would be equal to BMR at a body mass of 750 tonnes, which is still within the same order of magnitude (Fig. 11). Thus, an absolute lower limit of MR around or slightly below 0.01 mL O2 g−1 h−1 may well constrain the upper limit of body mass that can be reached by a mammal. This is because BMR generates just sufficient heat at thermoneutrality to keep Tb at euthermic levels. It seems that blue whales indeed always live at temperatures within or above their thermoneutral zone (Lavigne et al., 1990), and arguably will face additional thermoregulatory problems when heat production is elevated due to locomotion or lactation (Hokkanen, 1990). Of course, specialized heat-dissipation mechanisms, such as increased blood flow to body appendages may have co-evolved with larger body sizes. Still, even with such adaptations, there may be a maximum body mass at which endotherms are unable to dissipate the excess heat generated by their BMR plus other processes, and overheat with detrimental consequences (Speakman & Król, 2010).
Fig. 11.
Basal and hibernation metabolic rates among mammals intersect at a body mass close to that of the largest animal known to have ever existed, the blue whale. Body mass of endotherms may in fact reach an upper limit due to excess heat production if BMR cannot be reduced below minimum MR as reached during hibernation.
(5) Phylogeny, torpor use, and its apparent absence in certain taxa
Many of the variables investigated here contained a significant phylogenetic signal. This underlines the need for phylogenetically informed statistics in this context, although it seems that using these methods has only confirmed the classical view that daily torpor and hibernation are distinct adaptations (e.g., Geiser & Ruf, 1995). Differences in the strength of a phylogenetic signal between traits and subgroups can be partly explained by differences in the variation of torpor variables. For instance, coefficients of variation (i.e., SD/mean) were very low for TBDmax in avian (0.27) and mammalian (0.47) daily heterotherms, in which the timing of bouts is controlled by the circadian system. Not surprisingly then, there was no significant phylogenetic signal in this variable. In mammalian hibernators, on the other hand, TBDmax varied considerably (CV=1.58), which allowed for a strong phylogenetic signal. Overall there was a significant correlation between the coefficient of variation of a variable and Pagel’s λ (Spearman’s rho=0.68, P<0.002, n=18), indicating that phylogenetically close species had similar characteristics whenever variation was not constrained. Notably, several variables, such as Tb min in mammals, contained a strong phylogenetic signal even when the effect of body mass on these variables was statistically eliminated. Thus, phylogenetic signals in torpor variables were not just due to the fact that closely related species typically have similar body weights.
The finding that closely-related species showed similar torpor characteristics is fully compatible with a plesiomorphic origin of heterothermy. The view that torpor is an ancestral trait dates back at least to the 19th century (reviewed in Johnson, 1931) and was subsequently reinforced by several authors, based on different lines of arguments (e.g., Eisentraut, 1956; Kayser, 1961; Grigg et al., 1990; Augee & Gooden, 1992; Malan, 1996; Grigg et al., 2004; Lovegrove, 2012a). There also seems to be a prevailing view that daily torpor, i.e., “circadian heterothermy” (Malan, 1996), represents the ancient trait, whereas prolonged hibernation, especially in cold environments, appears to be viewed as an advanced, secondary adaptation (Malan, 1996; Grigg et al., 2004; Lovegrove, 2012a). However, our present phylogenetic analysis does not rule out, of course, that heterothermy may have evolved independently in birds and mammals (c.f., Geiser, 1998). Also, significant phylogenetic signals in torpor characteristics could also be detectable if heterothermy evolved more than once in separate mammalian clades, e.g., in marsupials and placentals (discussed in Geiser, 2008). It seems entirely unlikely, however, that heterothermy involved independently in at least 11 mammalian orders (Fig. 1). Thus, a single origin of heterothermy, with subsequent adaptive adjustments depending on the biology of each species, seems the most parsimonious view, and this explanation has recently gained some evidence from a phylogenetic statistical analysis (Lovegrove, 2012c).
If heterothermy indeed is a plesiomorphic trait, this raises the question why it is absent in many extant endotherms. As pointed out before, there may well be physiological specializations and ecological conditions of certain taxa that preclude modes of energy conservation involving large drops in core Tb (c.f., Lovegrove, 2012a; Ruf et al., 2012). Adverse effects of torpor, such as memory loss (Millesi et al., 2001) or impaired muscle function (Clarke & Pörtner, 2010; Rojas et al., 2012) may have selected against torpor whenever environmental conditions in terms of climate and food availability permit continued euthermia. This view is supported by the observation that certain species may make extensive use of heterothermy under harsh, but to a much lesser extent or not at all under mild winter conditions (e.g., Lehmer & Biggins, 2005; Gil-Delgado et al., 2006; Dunbar & Brigham, 2010). Even in cold climates, costs associated with torpor may have favoured the evolution of alternatives such as increased body size (e.g., Secord et al., 2012), which lowers heat-loss due to a reduced relative body surface and additionally facilitates the development of a long, well-insulating fur (Scholander et al., 1950). Surprisingly then, at least at first glance, many small mammals alternatively save energy by a reduction of body mass towards winter. Because such a reduction of body size is typically accompanied by moult to winter fur with improved insulation, the net effect is a decrease of total energy expenditure (review in Heldmaier, 1989). Small endotherms also show various other adaptations that allow overwintering under harsh conditions without torpor. In several species of shrews, tree squirrels or moles, for instance, these adaptations include the use of elaborate nests, thermally buffered burrow systems, reliance on abundant, energy-rich prey, and food hoarding (e.g., Thompson & Thompson, 1980; Genoud, 1985; Merritt, 1986; Larsen et al., 1997). In tree squirrels, the hoarding strategy is further augmented by specialized cognitive functions that allow for very high rates of recovery of cached food (Jacobs & Liman, 1991). Another adaptation of both small and large endotherms that significantly decreases energy expenditure is reduced activity during the coldest winter periods (e.g., Merritt, 1986; Blix, 1989). There are of course further avenues by which endotherms, depending on their ecology, may avoid torpor even under severe climatic conditions.
However, even considering adverse effects of torpor and alternative adaptations, the number of heterotherms (171 mammalian, 43 avian species) examined here appears to represent only a stunningly small minority of all mammals (>5,000 species) and birds (>10,000 species). As already noted by McKechnie and Lovegrove (2002) for birds, the limited number of orders and families with species known to use heterothermy is surprising. There are of course a number of species for which some indication for heterothermy was observed (for examples of such cases, see Lovegrove, 2012a), but not enough quantitative information was provided to be included in our present analysis. Even so, the number of known heterotherms remains very limited. One might be tempted to conclude that this is simply related to the fact that the vast majority of species live in the tropics. Indeed, the mean geographical distribution range of all terrestrial mammals, for instance, is located at an absolute latitude of approximately 17.5°, which is considerably lower than that of daily heterotherms (25°) or hibernators (35°) alone. The same holds for birds for which species richness peaks in the tropical band (0-5°, Kissling et al., 2012), while heterothermic birds had geographical ranges at a mean latitude of 23°. However, heterothermic species – at least in certain taxa – commonly occur even in the tropics (e.g., McKechnie & Lovegrove, 2002; Dausmann et al., 2009; Geiser & Stawski, 2011). Relatively low numbers of known tropical heterotherms – as in the dataset examined here – are, to a certain degree, likely caused by a bias in the distribution of researchers and their preferred study species (Lovegrove, 2000). To some degree, this may have also biased our results on the latitudinal distribution of heterotherms. Another reason for the limited overall number of known heterothermic species may be the fact that only relative small fraction of species and families have been systematically investigated in regard to torpor use (Geiser & Körtner, 2010; Lovegrove, 2012a).
An additional cause for the apparent absence of heterothermy in some species can be the study of captive individuals, which does not necessarily reflect the biology of free-ranging individuals. Animals in captivity are often stressed and may not behave like their free-ranging con-specifics in regard to torpor use. This seems to be especially pronounced in birds. For instance, in captivity, frogmouths (Podargus strigoides) and kookaburras (Dacelo novaeguinea) maintain stable euthermic Tb even at low Ta (McNab & Bonaccorso, 1995; Bech & Nicol, 1999; Buttemer et al., 2003), whereas free-ranging individuals regularly reduce Tb by up to 10°C on cold winter nights (Körtner et al., 2000; Cooper et al., 2008). Moreover, under laboratory conditions, substantial decreases in Tb have been recorded only in small birds and often only after severe starvation (Marshall, 1955; Lasiewski & Dawson, 1964; Peiponen, 1965; Dawson & Fisher, 1969). In the field, some of the same species frequently enter torpor even under apparently favourable environmental conditions (Brigham, 1992; McKechnie & Lovegrove, 2002; Doucette et al., 2012). Therefore, we expect that even among birds, which typically can avoid periods of unfavourable conditions by migration, future studies will uncover many more heterothermic species that express not only daily torpor but perhaps also hibernation.
Mammals also may resist entering torpor in captivity. Free-ranging echidnas (Tachyglossus aculeatus) hibernate in many areas of Australia, including warm habitats (Grigg et al., 1989; Nicol & Andersen, 1996). In contrast, they are reluctant to do so in captivity. In sugar gliders (Petaurus breviceps), daily torpor is shallow and rare in captivity, whereas deep and frequent torpor bouts occur in the wild under similar environmental conditions (Geiser et al., 2007). Moreover, edible dormice (Glis glis) are extremely reluctant to hibernate in captivity unless they are allowed to dig their own hibernacula or are provided with elaborate artificial burrows (Wilz & Heldmaier, 2000; Bieber & Ruf, 2009). Finally, species or entire groups may be misclassified as homoeothermic based on non-systematic, short-term investigations of torpor use. This was the case, for example, for shrews and pteropodid bats, which were regularly described as being entirely homeothermic (Stoddart, 1979; Ransome, 1990), although experimental evidence clearly shows otherwise (Table 1). Especially among bats, there are probably a much larger number of heterothermic species than currently known (Geiser & Stawski, 2011; Lovegrove, 2012c; Lovegrove, 2012a). Similar incorrect conclusions were drawn from early evolutionary examinations on murid rodents including the house mouse (Mus musculus; Cade (1964)) or studies on the rock elephant shrew (Elephantulus edwardii; Leon et al. (1983)). Thus, it is likely that with an increasing number of studies on free-living animals and more systematic work on thermal biology of mammals and birds, the number of known heterotherms will increase enormoususly.
V. CONCLUSIONS
Daily torpor and hibernation are distinct physiological adaptations, and species employing these two types of metabolic reduction differ particularly in their maximum (and mean) torpor bout duration, as well as their capacity for absolute and relative reduction of metabolic rate. Moreover, hibernators, on average, reach lower Tb min than daily heterotherms, have higher body masses, and live at geographical ranges closer to the poles.
Arguably, a fundamental difference between daily heterotherms and hibernators is the temporal structure of torpor patterns. Daily heterotherms employ the circadian system to control torpor timing in order to stay entrained with the light-dark cycle, which facilitates continued foraging. In contrast, hibernators appear to have uncoupled their temporal control of torpor from the circadian system to allow prolonged bouts of hypometabolism and reliance on energy stores.
Within each functional group, most torpor traits (i.e., Tb min, TMRmin, TMRrel) are significantly affected by body mass, suggesting a dependence on metabolic processes. Even though torpor bout duration is independent of body mass, it decreases with an increase in mass specific TMR among hibernators. Moreover, the duration of euthermic intervals between hibernation torpor bouts decreases as euthermic metabolic rate (BMR) increases. These observations support the classical hypothesis that torpor-arousal cycles in hibernators (but not in daily heterotherms) are driven by a metabolism-dependent imbalance that accumulates during torpor and is eliminated during inter-bout euthermic phases.
The degree of metabolic reduction during mammalian hibernation is similar to the extent of metabolic depression in many other animal groups (e.g., molluscs, crustaceans, or reptiles). We suggest that the absolute minimum MR observed in hibernators (~0.01 mL O2 g−1 h−1) may constrain the maximum body mass of mammals – or even of endotherms in general – because this MR will generate excessive heat load under thermoneutrality at body masses > ~100 tonnes.
Most torpor traits (if they are not constrained for ecological (i.e., TBDmax in daily heterotherms) or physiological reasons (i.e., TMRmin in hibernators) contain a significant phylogenetic signal, that is, closely-related species often show similar characteristics. This supports the view of a plesiomorphic origin of torpor, with adaptive adjustments to the environmental conditions and ecology of each species.
Species-specific adaptations to certain habitats or resources may, on the other hand, also lead to the avoidance of torpid states that are associated with low core Tb. This is because decreased Tb also has adverse effects, such as an impairment of muscle function and maximum running speed. These costs of torpor may explain why many endotherms even in harsh environments employ alternative avenues of overwintering, such as food hoarding, building of elaborate nests, reducing activity or, particularly in large endotherms, regional heterothermy.
Possible adverse effects of torpor alone do not sufficiently explain however, why the occurrence of torpid states has been demonstrated only in several hundred out of >15,000 bird and mammal species. We largely attribute this fact to a scarcity of studies on undisturbed, free-ranging animals. Given the availability of new devices to measure Tb and/or MR in the field, we expect that the number of species known to exhibit torpor will increase substantially.
Supplementary Material
ACKNOWLEDGEMENTS
This study was financially supported by the Austrian Science Fund (FWF grant no. P25023), the DVCR of UNE and the Australian Research Council. We thank Renate Hengsberger for her help with the formatting of the manuscript.
Footnotes
For a definition of key terms used see Appendix 1
REFERENCES
- Anderson MD. Aardwolf adaptations: a review. Transactions of the Royal Society of South Africa. 2004;59:99–104. DOI: 10.1080/00359190409519168. [Google Scholar]
- Arlettaz R, Ruchet C, Aeschimann J, Brun E, Genoud M, Vogel P. Physiological traits affecting the distribution and wintering strategy of the bat Tadarida teniotis. Ecology. 2000;81:1004–1014. DOI: 10.1890/0012-9658(2000)081[1004:PTATDA]2.0.CO;2. [Google Scholar]
- Armitage KB, Woods BC, Salsbury CM. In: Heldmaier G, Klingenspor M, editors. Energetics of hibernation in woodchucks (Marmota monax); Life in the Cold. 11th International Hibernation Symposium; Springer, Berlin Heidelberg New York. 2000.pp. 73–80. [Google Scholar]
- Arnold JM. Dasyurus geoffroii. University of Western Australia; 1976. Growth and bioenergetics of the Chuditch. [Google Scholar]
- Arnold W. Energetics of social hibernation. In: Carey C, Florant GL, Wunder BA, Horwitz B, editors. Life in the Cold: Ecological, Physiological, and Molecular Mechanisms. Westview Press; Boulder: 1993. pp. 65–80. [Google Scholar]
- Arnold W, Ruf T, Frey-Roos F, Bruns U. Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a hibernator. PLoS ONE. 2011;6:e18641. doi: 10.1371/journal.pone.0018641. DOI: 10.1371/journal.pone.0018641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus) American Journal of Physiology - Regulatory and Integrative Comparative Physiology. 2004;286:R174–R181. doi: 10.1152/ajpregu.00593.2002. DOI: 10.1152/ajpregu.00593.2002. [DOI] [PubMed] [Google Scholar]
- Audet D, Thomas DW. Facultative hypothermia as a thermoregulatory strategy in the phyllostomid bats, Carollia perspicillata and Sturnira lilium. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 1997;167:146–152. doi: 10.1007/s003600050058. DOI: 10.1007/s003600050058. [DOI] [PubMed] [Google Scholar]
- Augee ML, Ealey EHM. Torpor in the Echidna, Tachyglossus aculeatus. Journal of Mammalogy. 1968;49:446–454. DOI: 10.2307/1378202. [PubMed] [Google Scholar]
- Augee ML, Gooden BA. Monotreme hibernation-some afterthoughts. In: Augee ML, editor. Platypus and Echidnas. Royal Zoological Society of New South Wales; Sydney: 1992. pp. 174–176. [Google Scholar]
- Bakko EB, Nahornia J. Torpor patterns in captive white-tailed prairie dogs (Cynomys leucurus) Journal of Mammalogy. 1986;67:576–578. http://www.jstor.org/stable/1381290 [Google Scholar]
- Barger JL, Brand MD, Barnes BM, Boyer BB. Tissue-specific depression of mitochondrial proton leak and substrate oxidation in hibernating arctic ground squirrels. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;284:R1306–R1313. doi: 10.1152/ajpregu.00579.2002. DOI: 10.1152/ajpregu.00579.2002. [DOI] [PubMed] [Google Scholar]
- Barnes BM. Freeze avoidance in a mammal: body temperatures below 0° Celsius in an arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. DOI: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- Barnes BM, Ritter D. Patterns of body temperature change in hibernating arctic ground squirrels. In: Carey C, Florant GL, Wunder BA, Horwitz B, editors. Life in the Cold: Ecological, Physiological, and Molecular Mechanisms. Westview Press; Boulder: 1993. pp. 119–130. [Google Scholar]
- Bartels W, Law BS, Geiser F. Daily torpor and energetics in a tropical mammal, the northern blossom-bat Macroglossus minimus (Meagchiroptera) Journal of Comparative Physiology B. 1998;168:233–239. doi: 10.1007/s003600050141. DOI: 10.1007/s003600050141. [DOI] [PubMed] [Google Scholar]
- Bartholomew GA, Cade TJ. Temperature Regulation, Hibernation, and Aestivation in the Little Pocket Mouse, Perognathus longimembris. Journal of Mammalogy. 1957;38:60–72. DOI: 10.2307/1376476. [Google Scholar]
- Bartholomew GA, Dawson WR, Lasiewski RC. Thermoregulation and Heterothermy in Some of the Smaller Flying Foxes (Megachiroptera) of New Guinea. Zeitschrift für vergleichende Physiologie. 1970;70:196–209. DOI: 10.1007/BF00297716. [Google Scholar]
- Bartholomew GA, Howell TR, Cade TJ. Torpidity in the White-Throated Swift, Anna Hummingbird, and Poor-Will. The Condor. 1957;59:145–155. DOI: 10.2307/1364720. [Google Scholar]
- Bartholomew GA, MacMillen RE. Oxygen Consumption, Estivation, and Hibernation in the Kangaroo Mouse, Microdipodops pallidus. Physiological Zoology. 1961;34:177–183. DOI: 10.2307/30152696. [Google Scholar]
- Bartholomew GA, Vleck CM, Bucher TL. Energy Metabolism and Nocturnal Hypothermia in Two Tropical Passerine Frugivores, Manacus vitellinus and Pipra mentalis. Physiological Zoology. 1983;56:370–379. DOI: 10.2307/30152601. [Google Scholar]
- Baxter RM. Evidence for spontaneous torpor in Crocidura flavescens. Acta Theriologica. 1996;41:327–330. [Google Scholar]
- Bech C, Abe AS, Steffensen JF, Berger M, Bicudo JEPW. Torpor in Three Species of Brazilian Hummingbirds under Semi-Natural Conditions. The Condor. 1997;99:780–788. DOI: 10.2307/1370489. [Google Scholar]
- Bech C, Nicol SC. Thermoregulation and ventilation in the tawny frogmouth, Podargus strigoides: a low-metabolic avian species. Australian Journal of Zoology. 1999;47:143–153. DOI: 10.1071/Zo98058. [Google Scholar]
- Bech C, Steffensen JF, Berger M, Abe AS, Bicudo JEPW. Metabolic aspects of torpor in hummingbirds. Acta Zoologica Sinica. 2006;52(Suppl.):397–400. [Google Scholar]
- Bergmann CGLC. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Vandenhoeck und Ruprecht; Göttingen: 1848. [Google Scholar]
- Bickler PE. CO2 balance of a heterothermic rodent: comparison of sleep, torpor, and awake states. American Journal of Physiology - Regulatory, Integrative Comparative Physiology. 1984;246:R49–R55. doi: 10.1152/ajpregu.1984.246.1.R49. [DOI] [PubMed] [Google Scholar]
- Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. Body mass dependent use of hibernation: why not prolong the active season, if they can? Functional Ecology. 2014;28:167–177. DOI: 10.1111/1365-2435.12173. [Google Scholar]
- Bieber C, Ruf T. Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften. 2009;96:165–171. doi: 10.1007/s00114-008-0471-z. DOI: 10.1007/s00114-008-0471-z. [DOI] [PubMed] [Google Scholar]
- Bininda-Edmonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. DOI: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- Blanco MB, Rahalinarivo V. First direct evidence of hibernation in an eastern dwarf lemur species (Cheirogaleus crossleyi) from the high-altitude forest of Tsinjoarivo, central-eastern Madagascar. Naturwissenschaften. 2010;97:945–950. doi: 10.1007/s00114-010-0707-6. DOI: 10.1007/s00114-010-0707-6. [DOI] [PubMed] [Google Scholar]
- Blix AS. Arctic resignation: winter dormancy without hypothermia. In: Malan A, Canguilhem B, Institut national de la santé et de la recherche médicale, editors. Living in the Cold: 2nd International Symposium; John Libbey Eurotext Ltd., France. 1989; pp. 117–119. Colloque Inserm. [Google Scholar]
- Bouma HR, Carey HV, Kroese FGM. Hibernation: the immune system at rest? Journal of Leukocyte Biology. 2010;88:619–624. doi: 10.1189/jlb.0310174. DOI: 10.1189/Jlb.0310174. [DOI] [PubMed] [Google Scholar]
- Boyer BB, Barnes BM. Molecular and metabolic aspects of mammalian hibernation. Bioscience. 1999;49:713–724. DOI: 10.2307/1313595. [Google Scholar]
- Boyles JG, Thompson AB, McKechnie AE, Malan E, Humphries MM, Careau V. A global heterothermic continuum in mammals. Global Ecology and Biogeography. 2013;22:1029–1039. DOI: 10.1111/Geb.12077. [Google Scholar]
- Bozinovic F, Marquet PA. Energetics and Torpor in the Atacama Desert-Dwelling Rodent Phyllotis darwini rupestris. Journal of Mammalogy. 1991;72:734–738. DOI: 10.2307/1381835. [Google Scholar]
- Bozinovic F, Rosenmann M. Daily Torpor in Calomys musculinus, a South-American Rodent. Journal of Mammalogy. 1988;69:150–152. DOI: 10.2307/1381762. [Google Scholar]
- Bozinovic F, Ruiz G, Rosenmann M. Energetics and torpor of a South American “living fossil”, the microbiotheriid Dromiciops gliroides. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2004;174:293–297. doi: 10.1007/s00360-004-0414-8. DOI: 10.1007/s00360-004-0414-8. [DOI] [PubMed] [Google Scholar]
- Brack V, Twente JW. The duration of the period of hibernation of 3 species of vespertilionid bats. 1. Field studies. Canadian Journal of Zoology. 1985;63:2952–2954. DOI: 10.1139/z85-442. [Google Scholar]
- Bradley SR, Deavers DR. A re-examination of the relationship between thermal conductance and body weight in mammals. Comparative Biochemistry and Physiology Part A: Physiology. 1980;65:465–476. DOI: 10.1016/0300-9629(80)90060-2. [Google Scholar]
- Brigham RM. Daily Torpor in a Free-ranging Goatsucker, the Common Poorwill (Phalaenoptilus nuttallii) Physiological Zoology. 1992;65:457–472. DOI: 10.2307/30158263. [Google Scholar]
- Brigham RM, Körtner G, Maddocks TA, Geiser F. Seasonal use of torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus) Physiological and Biochemical Zoology. 2000;73:613–620. doi: 10.1086/317755. DOI: 10.1086/317755. [DOI] [PubMed] [Google Scholar]
- Brown CR, Bernard RT. Thermal preference of Schreiber’s long-fingered (Miniopterus schreiberisii) and Cape horseshoe (Rhinolophus capensis) bats. Comparative Biochemistry and Physiology Part A: Physiology. 1994;107:439–449. doi: 10.1016/0300-9629(94)90023-x. DOI: 10.1016/0300-9629(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Brown JH, Bartholomew GA. Periodicity and Energetics of Torpor in the Kangaroo Mouse, Microdipodops pallidus. Ecology. 1969;50:705–709. http://www.jstor.org/stable/1936263 [Google Scholar]
- Bucher TL, Chappell MA. Ventilatory and Metabolic Dynamics during Entry into and Arousal from Torpor in Selasphorus Hummingbirds. Physiological Zoology. 1992;65:978–993. DOI: 10.2307/30158553. [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;279:R255–R262. doi: 10.1152/ajpregu.2000.279.1.R255. http://ajpregu.physiology.org/content/279/1/R255.full.html [DOI] [PubMed] [Google Scholar]
- Buffenstein R. The effect of starvation, food restriction, and water deprivation on thermoregulation and average daily metabolic rates in Gerbillus pusillus. Physiological Zoology. 1985;58:320–328. http://www.jstor.org/stable/30156003 [Google Scholar]
- Burton RS, Reichman OJ. Does immune challenge affect torpor duration? Functional Ecology. 1999;13:232–237. DOI: 10.2307/2656337. [Google Scholar]
- Buttemer WA, Nicol SC, Sharman A. Thermoenergetics of pre-moulting and moulting kookaburras (Dacelo novaeguineae): they’re laughing. Journal of Comparative Physiology B. 2003;173:223–230. doi: 10.1007/s00360-003-0326-z. DOI: 10.1007/s00360-003-0326-z. [DOI] [PubMed] [Google Scholar]
- Buzadžić B, Blagojević D, Korać B, Saičic ZS, Spasić MB, Petrović VM. Seasonal variation in the antioxidant defense system of the brain of the ground squirrel (Citellus citellus) and response to low temperature compared with rat. Comparative Biochemistry and Physiology C: Pharmacology, Toxicology and Endocrinology. 1997;117:141–149. doi: 10.1016/s0742-8413(97)00061-3. DOI: 10.1016/S0742-8413(97)00061-3. [DOI] [PubMed] [Google Scholar]
- Cade TJ. Annales Academiae Scientiarum Fennicae Ser. A 4 Biologica. Vol. 71. Helsinki: 1964. The evolution of torpidity in rodents; pp. 77–111. [Google Scholar]
- Calder WA. Size, function, and life history. 2nd edition Dover Publications; Mineola, N.Y: 1996. [Google Scholar]
- Calder WA, Booser J. Hypothermia of broad-tailed hummingbirds during incubation in nature with ecological correlations. Science. 1973;180:751–753. doi: 10.1126/science.180.4087.751. DOI: 10.1126/science.180.4087.751. [DOI] [PubMed] [Google Scholar]
- Canale CI, Levesque DL, Lovegrove BG. Tropical heterothermy: does the exception prove the rule? In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a seasonal world. Thermoregulatory and Metabolic Adaptations. Springer Berlin; Heidelberg, New York: 2012. pp. 29–40. DOI: 10.1007/978-3-642-28678-0_3. [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiological Reviews. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. DOI: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Carey HV, Frank CL, Seifert JP. Hibernation induces oxidative stress and activation of NF-κB in ground squirrel intestine. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2000;170:551–559. doi: 10.1007/s003600000135. DOI: 10.1007/s003600000135. [DOI] [PubMed] [Google Scholar]
- Carpenter FL. Torpor in an Andean hummingbird: its ecological significance. Science. 1974;183:545–547. doi: 10.1126/science.183.4124.545. DOI: 10.1126/science.183.4124.545. [DOI] [PubMed] [Google Scholar]
- Caviedes-Vidal E, Codelia EC, Roig V, Doña R. Facultative Torpor in the South American Rodent Calomys venustus (Rodentia: Cricetidae) Journal of Mammalogy. 1990;71:72–75. DOI: 10.2307/1381319. [Google Scholar]
- Clarke A, Pörtner H-O. Temperature, metabolic power and the evolution of endothermy. Biological Reviews. 2010;85:703–727. doi: 10.1111/j.1469-185X.2010.00122.x. DOI: 10.1111/j.1469-185X.2010.00122.x. [DOI] [PubMed] [Google Scholar]
- Clegg J. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. The Journal of Experimental Biology. 1997;200:467–75. doi: 10.1242/jeb.200.3.467. http://jeb.biologists.org/content/200/3/467.abstract [DOI] [PubMed] [Google Scholar]
- Clemens LE, Heldmaier G, Exner C. Keep cool: Memory is retained during hibernation in Alpine marmots. Physiology & Behavior. 2009;98:78–84. doi: 10.1016/j.physbeh.2009.04.013. DOI: 10.1016/j.physbeh.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Coburn DK, Geiser F. Seasonal Changes in Energetics and Torpor Patterns in the Subtropical Blossom-Bat Syconycteris australis (Megachiroptera) Oecologia. 1998;113:467–473. doi: 10.1007/s004420050399. DOI: 10.2307/4221876. [DOI] [PubMed] [Google Scholar]
- Çolak E, Yiğit N. Ecology and biology of Allactaga elater, Allactaga euphratica and Allactaga williamsi (Rodentia: Dipodidae) in Turkey. Turkish Journal of Zoology. 1998;22:105–117. [Google Scholar]
- Collins BG, Wooller RD, Richardson KC. Torpor by the honey possum, Tarsipes rostratus (Marsupialia: Tarsipedidae), in response to food intake and low environmental temperature. Australian Mammalogy. 1987;11:51–57. [Google Scholar]
- Cooper CE, Körtner G, Brigham M, Geiser F. Body temperature and activity patterns of free-living laughing Kookaburras: The largest kingfisher is heterothermic. The Condor. 2008;110:110–115. DOI: 10.1525/cond.2008.110.1.110. [Google Scholar]
- Cooper CE, Withers PC. Patterns of body temperature variation and torpor in the numbat, Myrmecobius fasciatus (Marsupialia: Myrmecobiidae) Journal of Thermal Biology. 2004;29:277–284. DOI: 10.1016/j.jtherbio.2004.05.003. [Google Scholar]
- Cooper CE, Withers PC. Comparative physiology of Australian quolls (Dasyurus; Marsupialia) Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2010;180:857–868. doi: 10.1007/s00360-010-0452-3. DOI: 10.1007/s00360-010-0452-3. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Withers PC, Cruz-Neto AP. Metabolic, Ventilatory, and Hygric Physiology of the Gracile Mouse Opossum (Gracilinanus agilis) Physiological and Biochemical Zoology. 2009;82:153–162. doi: 10.1086/595967. DOI: 10.1086/595967. [DOI] [PubMed] [Google Scholar]
- Cory Toussaint D, McKechnie AE. Interspecific variation in thermoregulation among three sympatric bats inhabiting a hot, semi-arid environment. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2012;182:1129–40. doi: 10.1007/s00360-012-0683-6. DOI: 10.1007/s00360-012-0683-6. [DOI] [PubMed] [Google Scholar]
- Cory Toussaint D, McKechnie AE, van der Merwe M. Heterothermy in free-ranging male Egyptian Free-tailed bats (Tadarida aegyptiaca) in a subtropical climate. Mammalian Biology. 2010;75:466–470. DOI: 10.1016/j.mambio.2009.06.001. [Google Scholar]
- Cranford JA. Body temperature, heart rate and oxygen consumption of normothermic and heterothermic western jumping mice (Zapus princeps) Biochemical Physiology. 1983;74A:595–599. doi: 10.1016/0300-9629(83)90553-4. DOI: 10.1016/0300-9629(83)90553-4. [DOI] [PubMed] [Google Scholar]
- Cranford JA. Field and laboratory annual cycles of activity and hibernation in the Uinta Basin ground squirrel (Spermophilus armatus) In: Heller HC, Musacchia XJ, Wang LCH, editors. Living in the Cold: Physiological and Biochemical Adaptations. Elsevier; New York: 1986. pp. 411–418. [Google Scholar]
- Cryan PM, Wolf BO. Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. The Journal of Experimental Biology. 2003;206:3381–3390. doi: 10.1242/jeb.00574. DOI: 10.1242/jeb.00574. [DOI] [PubMed] [Google Scholar]
- Daan S. Periodicity of heterothermy in the garden doormouse, Eliomys quercinus (L.) Netherlands Journal of Zoology. 1973;23:237–265. DOI: 10.1163/002829673X00067. [Google Scholar]
- Daan S, Beersma DGM, Borbély AA. Timing of human sleep: Recovery process gated by a circadian pacemaker. American Journal of Physiology. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Darwin C. Journal and remarks: 1832-1836. H. Colburn; London: 1839. [Google Scholar]
- Darwin C. Journal of researches into the natural history and geology of the countries visited during the voyage of H.M.S. Beagle round the world: under the command of Capt. Fitz Roy, R.N. 2nd edition. J. Murray; London: 1845. [Google Scholar]
- Dausmann KH, Ganzhorn JU, Heldmaier G. Body temperature and metabolic rate of a hibernating primate in Madagascar: preliminary results from a field study. In: Heldmaier G, Klingenspor M, editors. Life in the Cold IV. Springer; Berlin, Heidelberg, New York: 2000. pp. 41–47. [Google Scholar]
- Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. Hibernation in a tropical primate. Nature. 2004;429:825–826. doi: 10.1038/429825a. DOI: 10.1038/429825a. [DOI] [PubMed] [Google Scholar]
- Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. Hibernation in the tropics: lessons from a primate. Journal of Comparative Physiology B. 2005;175:147–155. doi: 10.1007/s00360-004-0470-0. DOI: 10.1007/s00360-004-0470-0. [DOI] [PubMed] [Google Scholar]
- Dausmann KH, Glos J, Heldmaier G. Energetics of tropical hibernation. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2009;179:345–357. doi: 10.1007/s00360-008-0318-0. DOI: 10.1007/s00360-008-0318-0. [DOI] [PubMed] [Google Scholar]
- Dausmann KH, Nowack J, Kobbe S, Mzilikazi N. Afrotropical heterothermy: a continuum of possibilities. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a seasonal world. Thermoregulatory and Metabolic Adaptations. Springer Berlin, Heidelberg; New York: 2012. pp. 13–27. DOI: 10.1007/978-3-642-28678-0_2. [Google Scholar]
- Dawson TJ, Wolfers JM. Metabolism, thermoregulation and torpor in shrew sized marsupials of the genus planigale. Comparative Biochemistry and Physiology Part A: Physiology. 1978;59:305–309. DOI: 10.1016/0300-9629(78)90167-6. [Google Scholar]
- Dawson WR, Fisher CD. Responses to Temperature by the Spotted Nightjar (Eurostopodus guttatus) The Condor. 1969;71:49–53. DOI: 10.2307/1366047. [Google Scholar]
- Deavers DR, Hudson JW. Temperature regulation in two rodents (Clethrionomys gapperi and Peromyscus leucopus) and a shrew (Blarina brevicaudata) inhabiting the same environment. Physiological Zoology. 1981;54:94–108. http://www.jstor.org/stable/30155808 [Google Scholar]
- Doucette LI, Brigham RM, Pavey CR, Geiser F. Prey availability affects daily torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus) Oecologia. 2012;169:361–372. doi: 10.1007/s00442-011-2214-7. DOI: 10.1007/s00442-011-2214-7. [DOI] [PubMed] [Google Scholar]
- Downs CT, Brown M. Nocturnal Heterothermy and Torpor in the Malachite Sunbird (Nectarinia famosa) The Auk. 2002;119:251–260. DOI: 10.2307/4090032. [Google Scholar]
- Dryden GL, Gȩbczyński M, Douglas EL. Oxygen consumption by nursling and adult musk shrews. Acta Theriologica. 1974;19:453–461. [Google Scholar]
- Dunbar MB, Brigham RM. Thermoregulatory variation among populations of bats along a latitudinal gradient. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2010;180:885–893. doi: 10.1007/s00360-010-0457-y. DOI: 10.1007/s00360-010-0457-y. [DOI] [PubMed] [Google Scholar]
- Dunbar MB, Tomasi TE. Arousal Patterns, Metabolic Rate, and an Energy Budget of Eastern Red Bats (Lasiurus borealis) in Winter. Journal of Mammalogy. 2006;87:1096–1102. DOI: 10.1644/05-MAMM-A-254R3.1. [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall; New York, NY: 1993. [Google Scholar]
- Ehrhardt N, Heldmaier G, Exner C. Adaptive mechanisms during food restriction in Acomys russatus: the use of torpor for desert survival. Journal of Comparative Physiology B. 2005;175:193–200. doi: 10.1007/s00360-005-0475-3. DOI: 10.1007/s00360-005-0475-3. [DOI] [PubMed] [Google Scholar]
- Eisentraut M. Winterstarre, Winterschlaf und Winterruhe. Eine kurze biologischphysiologische Studie. Mitteilungen aus dem Zoologischen Museum in Berlin. 1933;19:48–63. [Google Scholar]
- Eisentraut M. Der Winterschlaf mit seinen ökologischen und physiologischen Begleiterscheinungen. VEB G Fischer; Jena: 1956. [Google Scholar]
- El Ouezzani S, Janati IA, Magoul R, Pevet P, Saboureau M. Overwinter body temperature patterns in captive jerboas (Jaculus orientalis): influence of sex and group. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2011;181:299–309. doi: 10.1007/s00360-010-0519-1. DOI: 10.1007/s00360-010-0519-1. [DOI] [PubMed] [Google Scholar]
- Ellison GTH. Thermoregulatory Responses of Cold-Acclimated Fat Mice (Steatomys pratensis) Journal of Mammalogy. 1995;76:240–247. DOI: 10.2307/1382332. [Google Scholar]
- Ericson PGP, Zuccon D, Ohlson JI, Johansson US, Alvarenga H, Prum RO. Higher-level phylogeny and morphological evolution of tyrant flycatchers, cotingas, manakins, and their allies (Aves: Tyrannida) Molecular Phylogenetics and Evolution. 2006;40:471–483. doi: 10.1016/j.ympev.2006.03.031. DOI: 10.1016/j.ympev.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Fisher KC. On the mechanism of periodic arousal in the hibernating ground squirrel. Annales Academiae Scientiarum Fennicae Ser. A. 1964;71:143–156. [Google Scholar]
- Fleming MR. Thermoregulation and Torpor in the Sugar Glider, Petaurus breviceps (Marsupialia, Petauridae) Australian Journal of Zoology. 1980;28:521–534. DOI: 10.1071/Zo9800521. [Google Scholar]
- Fleming MR. The Thermal Physiology of the Feathertail Glider, Acrobates pygmaeus (Marsupialia, Burramyidae) Australian Journal of Zoology. 1985;33:667–681. DOI: 10.1071/Zo9850667. [Google Scholar]
- Florant GL, Heller HC. CNS regulation of body temperature in euthermic and hibernating marmots (Marmota flaviventris) American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1977;232:R203–R208. doi: 10.1152/ajpregu.1977.232.5.R203. [DOI] [PubMed] [Google Scholar]
- Florant GL, Hill V, Ogilvie MD. Circadian rhythms of body temperature in laboratory and field marmots (Marmota flaviventris) In: Heldmaier G, Klingenspor M, editors. Life in the Cold IV. Springer; Berlin Heidelberg New York: 2000. pp. 223–231. [Google Scholar]
- Fowler PA, Racey PA. Overwintering strategies of the badger, Meles meles, at 57 °N. Journal of Zoology (London) 1988;214:635–651. DOI: 10.1111/j.1469-7998.1988.tb03763.x. [Google Scholar]
- Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. Journal of the American Statistical Association. 2002;97:611–631. DOI: 10.1198/016214502760047131. [Google Scholar]
- Franco M, Contreras C, Cortes P, Chappell MA, Soto-Gamboa M, Nespolo RF. Aerobic power, huddling and the efficiency of torpor in the South American marsupial, Dromiciops gliroides. Biology Open. 2012;1:1178–84. doi: 10.1242/bio.20122790. DOI: 10.1242/bio.20122790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckelton RP, Harvey PH, Pagel MD. Phylogenetic analysis and comparative data: A test and review of evidence. The American Naturalist. 2002;160:712–726. doi: 10.1086/343873. http://www.jstor.org/stable/10.1086/343873 [DOI] [PubMed] [Google Scholar]
- French AR. Periodicity of recurrent hypothermia during hibernation in the pocket mouse, Peromyscus longimembris. Journal of Comparative Physiology A. 1977;115:86–100. DOI: 10.1007/BF00667787. [Google Scholar]
- French AR. Effects of temperature on the duration of arousal episodes during hibernation. Journal of Applied Physiology - Respiratory Environmental and Exercise Physiology. 1982;52:216–220. doi: 10.1152/jappl.1982.52.1.216. [DOI] [PubMed] [Google Scholar]
- French AR. Allometries of the duration of torpid and euthermic intervals during mammalian hibernation: A test of the theory of metabolic control of the timing of changes in body temperature. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1985;156:13–19. doi: 10.1007/BF00692921. DOI: 10.1007/BF00692921. [DOI] [PubMed] [Google Scholar]
- Frey H. La température corporelle de Suncus etruscus (Soricidae, Insectivora) au cours de l’activité, du repos normothermique et de la torpeur. Revue Suisse de Zoologie. 1979;86:653–662. http://www.biodiversitylibrary.org/part/82328 [Google Scholar]
- Frey H. Le métabolisme énergétique de Suncus etruscus (Soricidae, Insectivora) en torpeur. Revue Suisse de Zoologie. 1980;87:739–748. http://www.biodiversitylibrary.org/part/85543 [Google Scholar]
- Fritz SA, Bininda-Emonds ORP, Purvis A. Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecology Letters. 2009;12:538–549. doi: 10.1111/j.1461-0248.2009.01307.x. DOI: 10.1111/j.1461-0248.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- Geiser F. Thermoregulation and torpor in the Kultarr, Antechinomys laniger (Marsupialia: Dasyuridae) Journal of Comparative Physiology B. 1986;156:751–757. DOI: 10.1007/BF00692755. [Google Scholar]
- Geiser F. Hibernation and daily torpor in two pygmy possums (Cercartetus spp., Marsupialia) Physiological Zoology. 1987;60:93–102. http://www.jstor.org/stable/30158631 [Google Scholar]
- Geiser F. Daily torpor and thermoregulation in Antechinus (Marsupialia): influence of body mass, season, development, reproduction, and sex. Oecologia. 1988;77:395–399. doi: 10.1007/BF00378050. DOI: 10.1007/BF00378050. [DOI] [PubMed] [Google Scholar]
- Geiser F. The effect of unsaturated and saturated dietary lipids on the pattern of daily torpor and the fatty-acid composition of tissues and membranes of the deer mouse Peromyscus maniculatus. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 1991;161:590–597. doi: 10.1007/BF00260749. DOI: 10.1007/BF00260749. [DOI] [PubMed] [Google Scholar]
- Geiser F. Hibernation in the Eastern Pygmy Possum, Cercartetus nanus (Marsupialia, Burramyidae) Australian Journal of Zoology. 1993;41:67–75. DOI: 10.1071/Zo9930067. [Google Scholar]
- Geiser F. Evolution of daily torpor and hibernation in birds and mammals: importance of body size. Clinical and Experimental Pharmacology and Physiology. 1998;25:736–739. doi: 10.1111/j.1440-1681.1998.tb02287.x. DOI: 10.1111/j.1440-1681.1998.tb02287.x. [DOI] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annual Review of Physiology. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. DOI: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Geiser F. Yearlong hibernation in a marsupial mammal. Naturwissenschaften. 2007;94:941–944. doi: 10.1007/s00114-007-0274-7. DOI: 10.1007/s00114-007-0274-7. [DOI] [PubMed] [Google Scholar]
- Geiser F. Ontogeny and phylogeny of endothermy and torpor in mammals and birds. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2008;150:176–80. doi: 10.1016/j.cbpa.2007.02.041. DOI: 10.1016/j.cbpa.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Geiser F, Augee ML, McCarron HCK, Raison JK. Correlates of torpor in the insectivorous dasyurid marsupial Sminthopsis murina. Australian Mammalogy. 1984;7:185–191. [Google Scholar]
- Geiser F, Baudinette RV. Seasonality of torpor and thermoregulation in three dasyurid marsupials. Journal of Comparative Physiology B. 1987;157:335–344. DOI: 10.1007/BF00693360. [Google Scholar]
- Geiser F, Baudinette RV. Daily Torpor and Thermoregulation in the Small Dasyurid Marsupials Planigale gilesi and Ningaui yvonneae. Australian Journal of Zoology. 1988;36:473–481. DOI: 10.1071/Zo9880473. [Google Scholar]
- Geiser F, Brigham RM. Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus) Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2000;170:153–162. doi: 10.1007/s003600050270. DOI: 10.1007/s003600050270. [DOI] [PubMed] [Google Scholar]
- Geiser F, Brigham RM. The other functions of torpor. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a seasonal world. Thermoregulatory and Metabolic Adaptations. Springer Berlin; Heidelberg, New York: 2012. pp. 109–121. DOI: 10.1007/978-3-642-28678-0_10. [Google Scholar]
- Geiser F, Broome LS. Hibernation in the mountain pygmy possum Burramys parvus (Marsupialia) Journal of Zoology (London) 1991;223:593–602. DOI: 10.1111/j.1469-7998.1991.tb04390.x. [Google Scholar]
- Geiser F, Ferguson C. Intraspecific differences in behaviour and physiology: effects of captive breeding on patterns of torpor in feathertail gliders. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2001;171:569–576. doi: 10.1007/s003600100207. DOI: 10.1007/s003600100207. [DOI] [PubMed] [Google Scholar]
- Geiser F, Hiebert SM, Kenagy GJ. Torpor bout duration during the hibernation season of two sciurid rodents: interrelations with temperature and metabolism. Physiological Zoology. 1990;63:489–503. http://www.jstor.org/stable/30156224 [Google Scholar]
- Geiser F, Holloway JC, Körtner G. Thermal biology, torpor and behaviour in sugar gliders: a laboratory-field comparison. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2007;177:495–501. doi: 10.1007/s00360-007-0147-6. DOI: 10.1007/s00360-007-0147-6. [DOI] [PubMed] [Google Scholar]
- Geiser F, Kenagy GJ. Torpor duration in relation to temperature and metabolism in hibernating ground squirrels. Physiological Zoology. 1988;61:442–449. http://www.jstor.org/stable/30161266 [Google Scholar]
- Geiser F, Körtner G. Hibernation and daily torpor in Australian mammals. Australian Zoologist. 2010;35:204–215. [Google Scholar]
- Geiser F, Masters P. Torpor in Relation to Reproduction in the Mulgara, Dasycercus cristicauda (Dasyuridae, Marsupialia) Journal of Thermal Biology. 1994;19:33–40. DOI: 10.1016/0306-4565(94)90007-8. [Google Scholar]
- Geiser F, Mzilikazi N. Does torpor of elephant shrews differ from that of other heterothermic mammals? Journal of Mammalogy. 2011;92:452–459. DOI: 10.1644/10-MAMM-A-097.1. [Google Scholar]
- Geiser F, Pavey CR. Basking and torpor in a rock-dwelling desert marsupial: survival strategies in a resource-poor environment. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2007;177:885–892. doi: 10.1007/s00360-007-0186-z. DOI: 10.1007/s00360-007-0186-z. [DOI] [PubMed] [Google Scholar]
- Geiser F, Ruf T. Hibernation versus Daily Torpor in Mammals and Birds: Physiological Variables and Classification of Torpor Patterns. Physiological Zoology. 1995;68:935–966. http://www.jstor.org/stable/30163788 [Google Scholar]
- Geiser F, Stawski C. Hibernation and Torpor in Tropical and Subtropical Bats in Relation to Energetics, Extinctions, and the Evolution of Endothermy. Integrative and Comparative Biology. 2011;51:337–348. doi: 10.1093/icb/icr042. DOI: 10.1093/icb/icr042. [DOI] [PubMed] [Google Scholar]
- Genoud M. Ecological energetics of two European shrews: Crocidura russula and Sorex coronatus (Soricidae: Mammalia) Journal of Zoology. 1985;207:63–85. DOI: 10.1111/j.1469-7998.1985.tb04916.x. [Google Scholar]
- Gil-Delgado JA, Cabaret P, Declercq S, Gomez J, Sánchez I. Winter reproduction of Eliomys quercinus (Rodentia) in the orange groves of Sagunto (Valencia, Spain) / La reproduction en hiver d’Eliomys quercinus (Rodentia) dans les orangeraies de Sagunto (Valence, Espagne) Mammalia. 2006;70:76–79. DOI: 10.1515/Mamm.2006.017. [Google Scholar]
- Giroud S, Frare C, Strijkstra A, Boerema A, Arnold W, Ruf T. Membrane Phospholipid Fatty Acid Composition Regulates Cardiac SERCA Activity in a Hibernator, the Syrian Hamster (Mesocricetus auratus) PLoS ONE. 2013;8:e63111. doi: 10.1371/journal.pone.0063111. DOI: 10.1371/journal.pone.0063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BG. Effect of photoperiod on the hibernation cycle of the Turkish hamster. In: Malan A, Canguilhem B, editors. Living in the Cold: 2nd International Symposium. Colloque INSERM/John Libbey Eurotext; 1989. pp. 5–15. [Google Scholar]
- Grahn DA, Miller JD, Houng VS, Heller HC. Persistence of circadian rhythmicity in hibernating ground squirrels. American Journal of Physiology - Regulatory Integrative Comparative Physiology. 1994;266:R1251–R1258. doi: 10.1152/ajpregu.1994.266.4.R1251. [DOI] [PubMed] [Google Scholar]
- Grant TR, Temple-Smith PD. Observations on torpor the small marsupial Dromiciops australis (Marsupialia: Microbiotheriidae) from southern Chile. In: Archer M, editor. Possums and Opossums: studies in evolution. Surrey Beatty and The Royal Zoological Society of NSW, New South Wales; Australia: 1987. pp. 273–277. [Google Scholar]
- Grigg GC, Beard L, Augee M. Echidnas In The High Country. Australian Natural History. 1990;23:528–537. [Google Scholar]
- Grigg GC, Beard LA, Augee ML. Hibernation in a Monotreme, the Echidna (Tachyglossus aculeatus) Comparative Biochemistry and Physiology Part A: Physiology. 1989;92:609–612. doi: 10.1016/0300-9629(89)90375-7. DOI: 10.1016/0300-9629(89)90375-7. [DOI] [PubMed] [Google Scholar]
- Grigg GC, Beard LA, Augee ML. The Evolution of Endothermy and Its Diversity in Mammals and Birds. Physiological and Biochemical Zoology. 2004;77:982–997. doi: 10.1086/425188. DOI: 10.1086/425188. [DOI] [PubMed] [Google Scholar]
- Guppy M, Withers P. Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biological Reviews of the Cambridge Philosophical Society. 1999;74:1–40. doi: 10.1017/s0006323198005258. DOI: 10.1111/j.1469-185X.1999.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Gür H. Why do Anatolian ground squirrels exhibit a Bergmannian size pattern? A phylogenetic comparative analysis of geographic variation in body size. Biological Journal of the Linnean Society. 2010;100:695–710. DOI: 10.1111/j.1095-8312.2010.01447.x. [Google Scholar]
- Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han K-L, Harshman J, Huddelston C, Marks BD, Miglia KJ, Moore WS, Sheldon FH, Steadman DW, Witt CC, Yuri T. A Phylogenomic Study of Birds Reveals Their Evolutionary History. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. DOI: 10.1126/science.1157704. [DOI] [PubMed] [Google Scholar]
- Hainsworth FR, Collins BG, Wolf LL. The function of torpor in hummingbirds. Physiological Zoology. 1977;50:215–222. http://www.jstor.org/stable/30155724 [Google Scholar]
- Hainsworth FR, Wolf LL. Regulation of oxygen consumption and body temperature during torpor in a hummingbird, Eulampis jugularis. Science. 1970;168:368–369. doi: 10.1126/science.168.3929.368. DOI: 10.1126/science.168.3929.368. [DOI] [PubMed] [Google Scholar]
- Hall LS. The Effect of Cave Microclimate on Winter Roosting Behavior in the Bat, Miniopterus schreibersii blepotis. Australian Journal of Ecology. 1982;7:129–136. DOI: 10.1111/j.1442-9993.1982.tb01586.x. [Google Scholar]
- Hall M. On Hybernation. Philosophical Transactions of the Royal Society of London. 1832;122:335–360. DOI: 10.1098/rstl.1832.0017. [Google Scholar]
- Hallam SL, Mzilikazi N. Heterothermy in the southern African hedgehog, Atelerix frontalis. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2011;181:437–445. doi: 10.1007/s00360-010-0531-5. DOI: 10.1007/s00360-010-0531-5. [DOI] [PubMed] [Google Scholar]
- Hammel HT, Dawson TJ, Abrams RM, Anderson HJ. Total calorimetric measurements on Citellus lateralis in hibernation. Physiological Zoology. 1968;41:341–357. http://www.jstor.org/stable/30155466 [Google Scholar]
- Harlow HJ. Torpor and other physiological adaptations of the badger (Taxidea taxus) to cold environment. Physiological Zoology. 1981;54:267–275. http://www.jstor.org/stable/30159941 [Google Scholar]
- Harlow HJ, Menkens GE. A comparison of hibernation in the black-tailed prairie dog, white-tailed prairie dog, and Wyoming ground squirrel. Canadian Journal of Zoology. 1986;64:793–796. DOI: 10.1139/z86-118. [Google Scholar]
- Harmata W. The frequency of winter sleep interruptions in two species of bats hibernating in limestone tunnels. Acta Theriologica. 1987;32:331–332. [Google Scholar]
- Hayes JP. Mass-specific and whole-animal metabolism are not the same concept. Physiological and Biochemical Zoology. 2001;74:147–150. doi: 10.1086/319310. DOI: 10.1086/319310. [DOI] [PubMed] [Google Scholar]
- Healy JE, Burdett KA, Buck CL, Florant GL. Sex differences in torpor patterns during natural hibernation in golden-mantled ground squirrels (Callospermophilus lateralis) Journal of Mammalogy. 2012;93:751–758. DOI: 10.1644/11-Mamm-a-120.1. [Google Scholar]
- Heldmaier G. Seasonal acclimatization of energy requirements in mammals: Functional significance of body weight control, hypothermia, torpor and hibernation. In: Wieser W, Gnaiger E, editors. Energy Transformations in Cells and Organisms. Georg Thieme; Stuttgart: 1989. pp. 130–139. [Google Scholar]
- Heldmaier G, Ruf T. Body temperature and metabolic rate during natural hypothermia in endotherms. Journal of Comparative Physiology B. 1992;162:696–706. doi: 10.1007/BF00301619. DOI: 10.1007/BF00301619. [DOI] [PubMed] [Google Scholar]
- Henshaw RE. Thermoregulation in bats. In: Slaughter BH, Wilson DW, editors. About bats. Southern Methodist University Press; Dallas: 1970. pp. 188–233. [Google Scholar]
- Herreid CF. Metabolism of the Mexican free-tailed bat. Journal of Cellular and Comparative Physiology. 1963;61:201–207. doi: 10.1002/jcp.1030610210. DOI: 10.1002/jcp.1030610210. [DOI] [PubMed] [Google Scholar]
- Herreid CF, Schmidt-Nielsen K. Oxygen Consumption Temperature and Water Loss in Bats from Different Environments. American Journal of Physiology. 1966;211:1108–1112. doi: 10.1152/ajplegacy.1966.211.5.1108. [DOI] [PubMed] [Google Scholar]
- Hiebert SM. Energy costs and temporal organization of torpor in the rufous hummingbird (Selasphorus rufus) Physiological Zoology. 1990;63:1082–1097. http://www.jstor.org/stable/30152634 [Google Scholar]
- Hiebert SM. Seasonality of daily torpor in a migratory hummingbird. In: Carey C, Florant GL, Wunder BA, Horwitz B, editors. Life in the Cold: Ecological, Physiological and Molecular Mechanisms. Westview; Boulder: 1993. pp. 25–32. [Google Scholar]
- Hildwein G. Capacités thermorégulatrices d’un mammifère insectivore primitif, le tenrec: leurs variations saisonnières. Archives des Sciences Physiologiques. 1970;24:55–71. [PubMed] [Google Scholar]
- Hill RW. Daily torpor in Peromyscus leucopus on an adequate diet. Comparative Biochemistry and Physiology Part A: Physioloy. 1975;51:413–423. doi: 10.1016/0300-9629(75)90389-8. DOI: 10.1016/0300-9629(75)90389-8. [DOI] [PubMed] [Google Scholar]
- Hissa R. Physiology of the European brown bear (Ursus arctos arctos) Annales Zoologici Fennici. 1997;34:267–287. http://elektra.helsinki.fi/se/a/0003-455x/34/4/physiolo.pdf [Google Scholar]
- Hock RJ. The Metabolic Rates and Body Temperatures of Bats. The Biological Bulletin. 1951;101:289–299. http://www.biolbull.org/content/101/3/289.abstract [Google Scholar]
- Hock RJ. Seasonal variations in physiologic functions of arctic ground squirrels and black bears. Bulletin of the Museum of Comparative Zoology. 1960;124:155–171. [Google Scholar]
- Hoffmann R, Prinzinger R. Torpor und Nahrungsausnutzung bei 4 Mausvogelarten (Coliiformes) Journal für Ornithologie. 1984;125:225–237. DOI: 10.1007/BF01640590. [Google Scholar]
- Hokkanen JEI. Temperature regulation of marine mammals. Journal of Theoretical Biology. 1990;145:465–485. doi: 10.1016/s0022-5193(05)80482-5. DOI: 10.1016/S0022-5193(05)80482-5. [DOI] [PubMed] [Google Scholar]
- Hope PR, Jones G. Warming up for dinner: torpor and arousal in hibernating Natterer’s bats (Myotis nattereri) studied by radio telemetry. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2012;182:569–578. doi: 10.1007/s00360-011-0631-x. DOI: 10.1007/s00360-011-0631-x. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, Withers PC. Temperature regulation and metabolism of an Australian bat, Chalinolobus gouldii (Chiroptera: Vespertilionidae) when euthermic and torpid. Journal of Comparative Physiology B. 1997;167:71–80. doi: 10.1007/s003600050049. DOI: 10.1007/s003600050049. [DOI] [PubMed] [Google Scholar]
- Hudson JW. Temperature Regulation and Torpidity in the Pygmy Mouse, Baiomys taylori. Physiological Zoology. 1965;38:243–254. DOI: 10.2307/30152836. [Google Scholar]
- Hudson JW, Deavers DR. Thermoregulation at High Ambient Temperatures of Six Species of Ground Squirrels (Spermophilus spp.) from Different Habitats. Physiological Zoology. 1973;46:95–109. DOI: 10.2307/30155591. [Google Scholar]
- Hudson JW, Scott IM. Daily torpor in the laboratory mouse Mus musculus var. albino. Physiological Zoology. 1979;52:205–218. http://www.jstor.org/stable/30152564 [Google Scholar]
- Hulbert AJ, Else PL. Membranes and the setting of energy demand. The Journal of Experimental Biology. 2005;208:1593–1599. doi: 10.1242/jeb.01482. DOI: 10.1242/Jeb.01482. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Kramer DL, Thomas DW. The Role of Energy Availability in Mammalian Hibernation: An Experimental Test in Free-Ranging Eastern Chipmunks. Physiological and Biochemical Zoology. 2003a;76:180–186. doi: 10.1086/367949. DOI: 10.1086/367949. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Thomas DW, Kramer DL. The Role of Energy Availability in Mammalian Hibernation: A Cost-Benefit Approach. Physiological and Biochemical Zoology. 2003b;76:165–179. doi: 10.1086/367950. DOI: 10.1086/367950. [DOI] [PubMed] [Google Scholar]
- Hut RA, Barnes BM, Daan S. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2002a;172:47–58. doi: 10.1007/s003600100226. DOI: 10.1007/s003600100226. [DOI] [PubMed] [Google Scholar]
- Hut RA, Van der Zee EA, Jansen K, Gerkema MP, Daan S. Gradual reappearance of post-hibernation circadian rhythmicity correlates with numbers of vasopressin-containing neurons in the suprachiasmatic nuclei of European ground squirrels. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2002b;172:59–70. doi: 10.1007/s003600100227. DOI: 10.1007/s003600100227. [DOI] [PubMed] [Google Scholar]
- Hwang YT, Larivière S, Messier F. Energetic Consequences and Ecological Significance of Heterothermy and Social Thermoregulation in Striped Skunks (Mephitis mephitis) Physiological and Biochemical Zoology. 2007;80:138–145. doi: 10.1086/509211. DOI: 10.1086/509211. [DOI] [PubMed] [Google Scholar]
- Ives AR, Midford PE, Garland T., Jr. Within-species variation and measurement error in phylogenetic comparative methods. Systematic Biology. 2007;56:252–270. doi: 10.1080/10635150701313830. DOI: 10.1080/10635150701313830. [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Kelly EJ, Mason M, Stoffberg S. Thermoregulation in two free-ranging subtropical insectivorous bat species: Scotophilus species (Vespertilionidae) Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2007;85:883–890. DOI: 10.1139/Z07-067. [Google Scholar]
- Jacobs LF, Liman ER. Grey squirrels remember the locations of buried nuts. Animal Behaviour. 1991;41:103–110. DOI: 10.1016/S0003-3472(05)80506-8. [Google Scholar]
- Johnson GE. Hibernation in Mammals. The Quarterly Review of Biology. 1931;6:439–461. DOI: 10.2307/2808210. [Google Scholar]
- Johnson JS. Doctoral Dissertation. University of Kentucky; 2012. Foraging and roosting behaviors of Rafinesque’s big-eared bat (Corynorhinus rafinesquii) as the northern edge of the species’ range. [Google Scholar]
- Jonasson KA, Willis CKR. Hibernation energetics of free-ranging little brown bats. The Journal of Experimental Biology. 2012;215:2141–2149. doi: 10.1242/jeb.066514. DOI: 10.1242/Jeb.066514. [DOI] [PubMed] [Google Scholar]
- Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orme CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Connolly C, Cutts MJ, Foster JK, Grenyer R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J, Teacher A, Bininda-Emonds ORP, Gittleman JL, Mace GM, Purvis A, Michener WK. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology. 2009;90:2648–2648. DOI: 10.1890/08-1494.1. [Google Scholar]
- Karpovich S, Tøien Ø, Buck C, Barnes B. Energetics of arousal episodes in hibernating arctic ground squirrels. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2009;179:691–700. doi: 10.1007/s00360-009-0350-8. DOI: 10.1007/s00360-009-0350-8. [DOI] [PubMed] [Google Scholar]
- Kart Gür M, Refinetti R, Gür H. Daily rhythmicity and hibernation in the Anatolian ground squirrel under natural and laboratory conditions. Journal of Comparative Physiology B. 2009;179:155–164. doi: 10.1007/s00360-008-0298-0. DOI: 10.1007/s00360-008-0298-0. [DOI] [PubMed] [Google Scholar]
- Kayser C. Exchanges respiratoires des hibernants réveillés. Annales de physiologie et de physicochimie biologique. 1939;15:1087–1219. [Google Scholar]
- Kayser C. The physiology of natural hibernation. Pergamon Press; Oxford: 1961. [Google Scholar]
- Kayser C. La dépense d’énergie des mammiferes en hibernation. Archives des Sciences Physiologiques. 1964;18:137–150. [PubMed] [Google Scholar]
- Kelm DH, von Helversen O. How to budget metabolic energy: torpor in a small Neotropical mammal. Journal of Comparative Physiology B. 2007;177:667–677. doi: 10.1007/s00360-007-0164-5. DOI: 10.1007/s00360-007-0164-5. [DOI] [PubMed] [Google Scholar]
- Kenagy GJ, Vleck D. Daily temporal organisation of metabolism in small mammals: adaptation and diversity. In: Aschoff J, Daan S, Groos GA, editors. Vertebrate Circadian Systems. Springer; Berlin, Heidelberg, New York: 1982. [Google Scholar]
- Kirsch R, Ouarour A, Pevet P. Daily torpor in the djungarian hamster (Phodopus sungorus) - photoperiodic regulation, characteristics and circadian organization. Journal of Comparative Physiology A. 1991;168:121–128. doi: 10.1007/BF00217110. DOI: 10.1007/BF00217110. [DOI] [PubMed] [Google Scholar]
- Kisser B, Goodwin HT. Hibernation and Overwinter Body Temperatures in Free-Ranging Thirteen-Lined Ground Squirrels, Ictidomys tridecemlineatus. The American Midland Naturalist. 2012;167:396–409. DOI: 10.1674/0003-0031-167.2.396. [Google Scholar]
- Kissling WD, Sekercioglu CH, Jetz W. Bird dietary guild richness across latitudes, environments and biogeographic regions. Global Ecology and Biogeography. 2012;21:328–340. DOI: 10.1111/j.1466-8238.2011.00679.x. [Google Scholar]
- Kobbe S, Ganzhorn JU, Dausmann KH. Extreme individual flexibility of heterothermy in free-ranging Malagasy mouse lemurs (Microcebus griseorufus) Journal of Comparative Physiology B. 2011;181:165–173. doi: 10.1007/s00360-010-0507-5. DOI: 10.1007/s00360-010-0507-5. [DOI] [PubMed] [Google Scholar]
- Körtner G, Brigham RM, Geiser F. Metabolism: Winter torpor in a large bird. Nature. 2000;407:318. doi: 10.1038/35030297. DOI: 10.1038/35030297. [DOI] [PubMed] [Google Scholar]
- Körtner G, Brigham RM, Geiser F. Torpor in free-ranging tawny frogmouths (Podargus strigoides) Physiological and Biochemical Zoology. 2001;74:789–97. doi: 10.1086/324097. DOI: 10.1086/324097. [DOI] [PubMed] [Google Scholar]
- Körtner G, Geiser F. The temporal organization of daily torpor and hibernation: circadian and circannual rhythms. Chronobiology International. 2000a;17:103–128. doi: 10.1081/cbi-100101036. DOI: 10.1081/CBI-100101036. [DOI] [PubMed] [Google Scholar]
- Körtner G, Geiser F. Torpor and Activity Patterns in Free-Ranging Sugar Gliders Petaurus breviceps (Marsupialia) Oecologia. 2000b;123:350–357. doi: 10.1007/s004420051021. DOI: 10.2307/4222627. [DOI] [PubMed] [Google Scholar]
- Körtner G, Geiser F. The key to winter survival: daily torpor in a small arid-zone marsupial. Naturwissenschaften. 2009;96:525–530. doi: 10.1007/s00114-008-0492-7. DOI: 10.1007/s00114-008-0492-7. [DOI] [PubMed] [Google Scholar]
- Körtner G, Pavey CR, Geiser F. Thermal biology, torpor, and activity in free-living mulgaras in arid zone Australia during the winter reproductive season. Physiological and Biochemical Zoology. 2008;81:442–451. doi: 10.1086/589545. DOI: 10.1086/589545. [DOI] [PubMed] [Google Scholar]
- Körtner G, Rojas AD, Geiser F. Thermal biology, torpor use and activity patterns of a small diurnal marsupial from a tropical desert: sexual differences. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2010;180:869–876. doi: 10.1007/s00360-010-0459-9. DOI: 10.1007/s00360-010-0459-9. [DOI] [PubMed] [Google Scholar]
- Koskimies J. On temperature regulation and metabolism in the swift, Micropus a. apus L., during fasting. Experientia. 1948;4:274–276. doi: 10.1007/BF02164408. DOI: 10.1007/BF02164408. [DOI] [PubMed] [Google Scholar]
- Koteja P, Jurczyszyn M, Woloszyn B. Energy balance of hibernating mouse-eared bat Myotis myotis: a study with a TOBEC instrument. Acta Theriologica. 2001;46:1–12. DOI: 10.1007/BF03192411. [Google Scholar]
- Kräuchi K, Deboer T. Body Temperatures, Sleep, and Hibernation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier Health Sciences; 2011. pp. 323–334. [Google Scholar]
- Kristoffersson R, Soivio A. Hibernation of the hedgehog (Erinaceus europaeus L.): the periodicity of hibernation of undisturbed animals during the winter in a constant ambient temperature. Annales Academiae Scientiarum Fennicae A IV Biologica. 1964;80:3–22. [Google Scholar]
- Krüger K, Prinzinger R, Schuchmann KL. Torpor and metabolism in hummingbirds. Comparative Biochemistry and Physiology Part A: Physiology. 1982;73:679–689. DOI: 10.1016/0300-9629(82)90275-4. [Google Scholar]
- Kulzer E. Temperaturregulation bei Fledermäusen (Chiroptera) aus verschiedenen Klimazonen. Zeitschrift für vergleichende Physiologie. 1965;50:1–34. DOI: 10.1007/BF00388050. [Google Scholar]
- Kulzer E, Nelson JE, McKean JL, Möhres FP. Untersuchungen über die Temperaturregulation australischer Fledermäuse (Microchiroptera) Zeitschrift für vergleichende Physiologie. 1970;69:426–451. DOI: 10.1007/BF00333769. [Google Scholar]
- Kulzer E, Storf R. Schlaf-Lethargie bei dem afrikanischen Langzungenflughund Megaloglossus woermanni Pagenstecher, 1885. Zeitschrift für Säugetierkunde. 1980;45:23–29. [Google Scholar]
- Kuntz R, Kubalek C, Ruf T, Tataruch F, Arnold W. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake. The Journal of Experimental Biology. 2006;209:4557–4565. doi: 10.1242/jeb.02535. DOI: 10.1242/jeb.02535. [DOI] [PubMed] [Google Scholar]
- Lane JE, Brigham RM, Swanson DL. Daily torpor in free-ranging whip-poor-wills (Caprimulgus vociferus) Physiological and Biochemical Zoology. 2004;77:297–304. doi: 10.1086/380210. DOI: 10.1086/380210. [DOI] [PubMed] [Google Scholar]
- Larsen KW, Becker CD, Boutin S, Blower M. Effects of hoard manipulations on life history and reproductive success of female red squirrels (Tamiasciurus hudsonicus) Journal of Mammalogy. 1997;78:192–203. http://www.jstor.org/stable/1382652 [Google Scholar]
- Lasiewski RC. Oxygen consumption of torpid, resting, active and flying hummingbirds. Physiological Zoology. 1963;36:122–140. http://www.jstor.org/stable/30155436 [Google Scholar]
- Lasiewski RC. Body Temperatures, Heart and Breathing Rate, and Evaporative Water Loss in Hummingbirds. Physiological Zoology. 1964;37:212–223. DOI: 10.2307/30152332. [Google Scholar]
- Lasiewski RC, Dawson WR. Physiological Responses to Temperature in the Common Nighthawk. The Condor. 1964;66:477–490. DOI: 10.2307/1365224. [Google Scholar]
- Lavigne DM, Innes S, Worthy GAJ, Edwards EF. Lower critical temperatures of blue whales, Balaenoptera musculus. Journal of Theoretical Biology. 1990;144:249–257. DOI: 10.1016/S0022-5193(05)80323-6. [Google Scholar]
- Lee TN, Barnes BM, Buck CL. Body temperature patterns during hibernation in a free-living Alaska marmot (Marmota broweri) Ethology Ecology & Evolution. 2009;21:403–413. DOI: 10.1080/08927014.2009.9522495. [Google Scholar]
- Lehmer EM, Biggins DE. Variation in torpor patterns of free-ranging black-tailed and Utah prairie dogs across gradients of elevation. Journal of Mammalogy. 2005;86:15–21. DOI: 10.1644/1545-1542(2005)086<0015:VITPOF>2.0.CO;2. [Google Scholar]
- Lehmer EM, Savage LT, Antolin MF, Biggins DE. Extreme plasticity in thermoregulatory behaviors of free-ranging black-tailed prairie dogs. Physiological and Biochemical Zoology. 2006;79:454–467. doi: 10.1086/502816. DOI: 10.1086/502816. [DOI] [PubMed] [Google Scholar]
- Lehmer EM, Van Horne B, Kulbartz B, Florant GL. Facultative torpor in free-ranging black-tailed prairie dogs (Cynomys ludovicianus) Journal of Mammalogy. 2001;82:551–557. DOI: 10.1644/1545-1542(2001)082<0551:FTIFRB>2.0.CO;2. [Google Scholar]
- Leon B, Shkolnik A, Shkolnik T. Temperature Regulation and Water Metabolism in the Elephant Shrew Elephantulus edwardi. Comparative Biochemistry and Physiology Part A: Physiology. 1983;74:399–407. doi: 10.1016/0300-9629(83)90623-0. DOI: 10.1016/0300-9629(83)90623-0. [DOI] [PubMed] [Google Scholar]
- Levesque DL, Tattersall GJ. Seasonal torpor and normothermic energy metabolism in the Eastern chipmunk (Tamias striatus) Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2010;180:279–292. doi: 10.1007/s00360-009-0405-x. DOI: 10.1007/s00360-009-0405-x. [DOI] [PubMed] [Google Scholar]
- Levin E, Ar A, Hefetz A, Yom-Tov Y, Kronfeld-Schor N. Some like it hot: Hibernation patterns of the greater mouse-tailed bat (Rhinopoma microphyllum); 27th Annual Meeting, Australian and New Zealand Society for Comparative Physiology and Biochemistry; Australian National University, Canberra. 2010.p. 26. [Google Scholar]
- Levy O, Dayan T, Kronfeld-Schor N. Adaptive Thermoregulation in Golden Spiny Mice: The Influence of Season and Food Availability on Body Temperature. Physiological and Biochemical Zoology. 2011;84:175–184. doi: 10.1086/658171. DOI: 10.1086/658171. [DOI] [PubMed] [Google Scholar]
- Lindstedt SL. Regulated Hypothermia in the Desert Shrew. Journal of Comparative Physiology. 1980;137:173–176. DOI: 10.1007/BF00689217. [Google Scholar]
- Lindstedt SL, Boyce MS. Seasonality, Fasting Endurance, and Body Size in Mammals. The American Naturalist. 1985;125:873–878. DOI: 10.1086/284385. [Google Scholar]
- Liu JN, Karasov W. Hibernation in warm hibernacula by free-ranging Formosan leaf-nosed bats, Hipposideros terasensis, in subtropical Taiwan. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2011;181:125–135. doi: 10.1007/s00360-010-0509-3. DOI: 10.1007/s00360-010-0509-3. [DOI] [PubMed] [Google Scholar]
- Liu JN, Karasov WH. Metabolism during winter in a subtropical hibernating bat, the Formosan leaf-nosed bat (Hipposideros terasensis) Journal of Mammalogy. 2012;93:220–228. DOI: 10.1644/11-Mamm-a-144.1. [Google Scholar]
- Lobban KD, Lovegrove BG. Heterothermy and the evolution of endothermy: lessons from Tenrec ecaudatus; 14th International Hibernation Symposium (IHS); Semmering, Austria. 2012.p. 51. [Google Scholar]
- Lovegrove BG. The zoogeography of mammalian basal metabolic rate. The American Naturalist. 2000;156:210–219. doi: 10.1086/303383. DOI: 10.1086/303383. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG. The evolution of endothermy in Cenozoic mammals: a plesiomorphic-apomorphic continuum. Biological Reviews. 2012a;87:128–162. doi: 10.1111/j.1469-185X.2011.00188.x. DOI: 10.1111/j.1469-185X.2011.00188.x. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG. The evolution of mammalian body temperature: the Cenozoic supraendothermic pulses. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2012b;182:579–589. doi: 10.1007/s00360-011-0642-7. DOI: 10.1007/s00360-011-0642-7. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG. A Single Origin of Heterothermy in Mammals. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a Seasonal World. Thermoregulatory and Metabolic Adaptations. Springer; Berlin, Heidelberg, New York: 2012c. pp. 3–11. DOI: 10.1007/978-3-642-28678-0_1. [Google Scholar]
- Lovegrove BG, Canale C, Levesque D, Fluch G, Řeháková P, Ruf T. Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiological and Biochemical Zoology. 2013 doi: 10.1086/673313. In press. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG, Lawes MJ, Roxburgh L. Confirmation of pleisiomorphic daily torpor in mammals: the round-eared elephant shrew Macroscelides proboscideus (Macroscelidea) Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1999;169:453–460. doi: 10.1007/s003600050242. DOI: 10.1007/s003600050242. [DOI] [PubMed] [Google Scholar]
- Lovegrove BG, Raman J, Perrin MR. Heterothermy in elephant shrews, Elephantulus spp. (Macroscelidea): Daily torpor or hibernation? Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2001;171:1–10. doi: 10.1007/s003600000139. DOI: 10.1007/s003600000139. [DOI] [PubMed] [Google Scholar]
- Lyman CP. The oxygen consumption and temperature regulation of hibernating hamsters. Journal of Experimental Zoology. 1948;109:55–78. doi: 10.1002/jez.1401090105. DOI: 10.1002/jez.1401090105. [DOI] [PubMed] [Google Scholar]
- Lyman CP. Oxygen consumption, body temperature and heart rate of woodchucks entering hibernation. American Journal of Physiology. 1958;194:83–91. doi: 10.1152/ajplegacy.1958.194.1.83. [DOI] [PubMed] [Google Scholar]
- Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and torpor in mammals and birds. Academic Press; New York, San Diego: 1982. [Google Scholar]
- Lynch GR, Bunin J, Schneider JE. The effect of constant light and dark on the circadian nature of daily torpor in Peromyscus leucopus. Journal of Interdisciplinary Cycle Research. 1980;11:85–93. DOI: 10.1080/09291018009359691. [Google Scholar]
- Lynch GR, White SE, Grundel R, Berger MS. Effects of photoperiod, melatonin administration and thyroid block on spontaneous daily torpor and temperature regulation in the white-footed mouse, Peromyscus leucopus. Journal of Comparative Physiology B. 1978;125:157–163. DOI: 10.1007/BF00686752. [Google Scholar]
- Ma YL, Zhu XW, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. American Journal of Physiology- Regulatory Integrative and Comparative Physiology. 2005;289:R1297–R1306. doi: 10.1152/ajpregu.00260.2005. DOI: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- Macmillen RE. Aestivation in the cactus mouse, Peromyscus eremicus. Comparative Biochemistry and Physiology. 1965;16:227–248. doi: 10.1016/0010-406x(65)90062-9. DOI: 10.1016/0010-406X(65)90062-9. [DOI] [PubMed] [Google Scholar]
- MacMillen RE. Adaptive physiology of heteromyid rodents. Great Basin Naturalist Memoirs. 1983;7:65–76. https://ojs.lib.byu.edu/ojs/index.php/gbnmem/article/viewArticle/3017 [Google Scholar]
- MacMillen RE, Nelson JE. Bioenergetics and body size in dasyruid marsupials. American Journal of Physiology. 1969;217:1246–1251. doi: 10.1152/ajplegacy.1969.217.4.1246. http://ajplegacy.physiology.org/content/217/4/1246.short [DOI] [PubMed] [Google Scholar]
- MacMillen RE, Trost CH. Nocturnal hypothermia in the Inca dove, Scardafella inca. Comparative Biochemistry and Physiology. 1967;23:243–253. doi: 10.1016/0010-406x(67)90492-6. DOI: 10.1016/0010-406X(67)90492-6. [DOI] [PubMed] [Google Scholar]
- Maddocks TA, Geiser F. Heterothermy in an Australian passerine, the Dusky Woodswallow (Artamus cyanopterus) Journal of Ornithology. 2007;148:571–577. DOI: 10.1007/s10336-007-0205-6. [Google Scholar]
- Malan A. In: Geiser F, Hulbert AJ, Nicol SC, editors. The origins of hibernation: a reappraisal; Adaptations to the Cold. Tenth International Hibernation Symposium; University of New England Press,, Armidale, New South Wales. 1996.pp. 1–6. [Google Scholar]
- Malan A. Is the torpor-arousal cycle of hibernation controlled by a non-temperature-compensated circadian clock? Journal of Biological Rhythms. 2010;25:166–175. doi: 10.1177/0748730410368621. DOI: 10.1177/0748730410368621. [DOI] [PubMed] [Google Scholar]
- Marom S, Korine C, Wojciechowski MS, Tracy CR, Pinshow B. Energy Metabolism and Evaporative Water Loss in the European Free-Tailed Bat and Hemprich’s Long-Eared Bat (Microchiroptera): Species Sympatric in the Negev Desert. Physiological and Biochemical Zoology. 2006;79:944–956. doi: 10.1086/505999. DOI: 10.1086/505999. [DOI] [PubMed] [Google Scholar]
- Marshall JT., Jr. Hibernation in Captive Goatsuckers. The Condor. 1955;57:129–134. DOI: 10.2307/1364860. [Google Scholar]
- Masaki M, Koshimoto C, Tsuchiya K, Nishiwaki A, Morita T. Body temperature profiles of the Korean field mouse Apodemus peninsulae during winter aggregation. Mammal Study. 2005;30:33–40. DOI: 10.3106/1348-6160(2005)30[33:BTPOTK]2.0.CO;2. [Google Scholar]
- McKechnie AE, Lovegrove BG. Heterothermic responses in the speckled mousebird (Colius striatus) Journal of Comparative Physiology. 2001a;171:507–518. doi: 10.1007/s003600100201. DOI: 10.1007/s003600100201. [DOI] [PubMed] [Google Scholar]
- McKechnie AE, Lovegrove BG. Thermoregulation and the energetic significance of clustering behavior in the white-backed mousebird (Colius colius) Physiological and Biochemical Zoology. 2001b;74:238–49. doi: 10.1086/319669. DOI: 10.1086/319669. [DOI] [PubMed] [Google Scholar]
- McKechnie AE, Lovegrove BG. Avian facultative hypothermic responses: A review. The Condor. 2002;104:705–724. DOI: 10.1650/0010) 5422(2002-104[0705:AFHRAR]2.0.CO;2. [Google Scholar]
- McKechnie AE, Mzilikazi N. Heterothermy in Afrotropical Mammals and Birds: A Review. Integrative and Comparative Biology. 2011;51:349–363. doi: 10.1093/icb/icr035. DOI: 10.1093/Icb/Icr035. [DOI] [PubMed] [Google Scholar]
- McNab BK, Bonaccorso FJ. The Energetics of Australasian Swifts, Frogmouths, and Nightjars. Physiological Zoology. 1995;68:245–261. DOI: 10.2307/30166502. [Google Scholar]
- McNab BK, Morrison P. Body Temperature and Metabolism in Subspecies of Peromyscus from Arid and Mesic Environments. Ecological Monographs. 1963;33:63–82. DOI: 10.2307/1948477. [Google Scholar]
- Merola-Zwartjes M, Ligon JD. Ecological Energetics of the Puerto Rican Tody: Heterothermy, Torpor, and Intra-Island Variation. Ecology. 2000;81:990–1003. DOI: 10.2307/177173. [Google Scholar]
- Merritt JF. Winter survival adaptations of the short-tailed shrew (Blarina brevicauda) in Appalachian montane forest. Journal of Mammalogy. 1986;67:450–464. DOI: 10.2307/1381276. [Google Scholar]
- Millesi E, Prossinger H, Dittami JP, Fieder M. Hibernation effects on memory in European ground squirrels (Spermophilus citellus) Journal of Biological Rhythms. 2001;16:264–271. doi: 10.1177/074873040101600309. DOI: 10.1177/074873040101600309. [DOI] [PubMed] [Google Scholar]
- Morhardt JE. Body temperatures of white-footed mice (Peromyscus sp.) during daily torpor. Comparative Biochemistry and Physiology. 1970;33:423–439. doi: 10.1016/0010-406x(70)90359-2. DOI: 10.1016/0010-406X(70)90359-2. [DOI] [PubMed] [Google Scholar]
- Morris P. Winter Nests of the Hedgehog (Erinaceus europaeus L.) Oecologia. 1973;11:299–313. doi: 10.1007/BF00345702. DOI: 10.2307/4214832. [DOI] [PubMed] [Google Scholar]
- Morrison P, McNab BK. Daily torpor in a brazilian murine opossum (Marmosa) Comparative Biochemistry and Physiology. 1962;6:57–68. DOI: 10.1016/0010-406X(62)90043-9. [Google Scholar]
- Morton SR, Lee AK. Thermoregulation and Metabolism in Planigale maculata (Marsupialia Dasyuridae) Journal of Thermal Biology. 1978;3:117–120. DOI: 10.1016/0306-4565(78)90003-7. [Google Scholar]
- Mouhoub-Sayah C, Robin J-P, Malan A, Pevet P, Saboureau M. Patterns of body temperature change in the Algerian hedgehog (Atelerix algirus) In: Lovegrove BG, McKechnie AE, editors. Hypometabolism in animals: torpor, hibernation and cryobiology. 13th International Hibernation Symposium. University of KwaZulu-Natal; Pietermaritzburg: 2008. pp. 307–316. [Google Scholar]
- Muchlinski AE, Rybak EN. Energy-Consumption of Resting and Hibernating Meadow Jumping Mice. Journal of Mammalogy. 1978;59:435–437. DOI: 10.2307/1379934. [PubMed] [Google Scholar]
- Muller J. Honours Thesis. LaTrobe University; 1996. Torpor in Sminthopsis douglasi. [Google Scholar]
- Muñoz-Garcia A, Ben-Hamo M, Korine C, Pinshow B, Williams JB. A new thermoregulatory index for heterothermy. Methods in Ecology and Evolution. 2013;5:141–145. DOI: 10.1111/2041-210X.12131. [Google Scholar]
- Mzilikazi N, Lovegrove BG. Reproductive activity influences thermoregulation and torpor in pouched mice, Saccostomus campestris. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2002;172:7–16. doi: 10.1007/s003600100221. DOI: 10.1007/s003600100221. [DOI] [PubMed] [Google Scholar]
- Mzilikazi N, Lovegrove BG. Daily torpor in free-ranging rock elephant shrews, Elephantulus myurus: A year-long study. Physiological and Biochemical Zoology. 2004;77:285–296. doi: 10.1086/381470. DOI: 10.1086/381470. [DOI] [PubMed] [Google Scholar]
- Mzilikazi N, Madikiza Z, Oelkrug R, Baxter RM. Hibernation in free-ranging African woodland dormice, Graphiurus murinus. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a Seasonal World. Thermoregulatory and Metabolic Adaptations. Springer Verlag; Heidelberg, New York, Dordrecht, London: 2012. pp. 41–50. DOI: 10.1007/978-3-642-28678-0_4. [Google Scholar]
- Nagel A. Torpor in the European white-toothed shrews. Experientia. 1977;33:1455–1456. DOI: 10.1007/BF01918804. [Google Scholar]
- Nagel A. Sauerstoffverbrauch, Temperaturregulation und Herzfrequenz bei europäischen Spitzmäusen (Soricidae) Zeitschrift für Säugetierkunde. 1985;50:249–266. [Google Scholar]
- Németh I, Nyitrai V, Altbäcker V. Ambient temperature and annual timing affect torpor bouts and euthermic phases of hibernating European ground squirrels (Spermophilus citellus) Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2009;87:204–210. DOI: 10.1139/Z08-150. [Google Scholar]
- Neumann RL, Cade TJ. Torpidity in the Mexican ground squirrel Citellus mexicanus parvidens (Mearns) Canadian Journal of Zoology. 1965;43:133–40. doi: 10.1139/z65-011. DOI: 10.1139/z65-011. [DOI] [PubMed] [Google Scholar]
- Newman JR, Rudd RL. Observations of torpor-like behavior in the shrew, Sorex sinuosus. Acta Theriologica. 1978;23:446–448. [Google Scholar]
- Ni ZL, Storey KB. Heme oxygenase expression and Nrf2 signaling during hibernation in ground squirrels. Canadian Journal of Physiology and Pharmacology. 2010;88:379–387. doi: 10.1139/Y10-017. DOI: 10.1139/Y10-017. [DOI] [PubMed] [Google Scholar]
- Nicol S, Andersen NA. The timing of hibernation in Tasmanian echidnas: why do they do it when they do? Comparative Biochemistry and Physiology Part B: Biochemistry & Molecular Biology. 2002;131:603–611. doi: 10.1016/s1096-4959(02)00018-0. DOI: 10.1016/S1096-4959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Nicol SC, Andersen NA. In: Geiser F, Hulbert AJ, Nicol SC, editors. Hibernation in the echidna: not an adaptation to cold?; Adaptations to the Cold. Tenth International Hibernation Symposium; University of New England Press, Armidale. 1996.pp. 7–12. [Google Scholar]
- Norman JA, Ericson PGP, Jønsson KA, Fjeldså J, Christidis L. A multi-gene phylogeny reveals novel relationships for aberrant genera of Australo-Papuan core Corvoidea and polyphyly of the Pachycephalidae and Psophodidae (Aves: Passeriformes) Molecular Phylogenetics and Evolution. 2009;52:488–497. doi: 10.1016/j.ympev.2009.03.019. DOI: 10.1016/j.ympev.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Nowack J, Mzilikazi N, Dausmann KH. Torpor on Demand: Heterothermy in the Non-Lemur Primate Galago moholi. PLoS ONE. 2010;5:e10797. doi: 10.1371/journal.pone.0010797. DOI: 10.1371/journal.pone.0010797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkrug R, Meyer CW, Heldmaier G, Mzilikazi N. Seasonal changes in thermogenesis of a free-ranging afrotherian small mammal, the Western rock elephant shrew (Elephantulus rupestris) Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2012;182:715–727. doi: 10.1007/s00360-012-0647-x. DOI: 10.1007/s00360-012-0647-x. [DOI] [PubMed] [Google Scholar]
- Opazo JC, Nespolo RF, Bozinovic F. Arousal from torpor in the chilean mouse-opposum (Thylamys elegans): does non-shivering thermogenesis play a role? Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1999;123:393–397. doi: 10.1016/s1095-6433(99)00081-1. DOI: 10.1016/S1095-6433(99)00081-1. [DOI] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5.2. 2013. [Google Scholar]
- Ortmann S, Heldmaier G. Regulation of body temperatures and energy requirements of hibernating Alpine marmots (Marmota marmota) American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278:698–704. doi: 10.1152/ajpregu.2000.278.3.R698. [DOI] [PubMed] [Google Scholar]
- Otsu R, Kimura T. Effects of food availability and ambient temperature on hibernation in the Japanese dormouse, Glirulus japonicus. Journal of Ethology. 1993;11:37–42. DOI: 10.1007/BF02350004. [Google Scholar]
- Owen-Smith N, Mills MGL. Predator-prey size relationships in an African large-mammal food web. Journal of Animal Ecology. 2008;77:173–183. doi: 10.1111/j.1365-2656.2007.01314.x. DOI: 10.1111/j.1365-2656.2007.01314.x. [DOI] [PubMed] [Google Scholar]
- Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. Coupled dynamics of body mass and population growth in response to environmental change. Nature. 2010;466:482–487. doi: 10.1038/nature09210. DOI: 10.1038/nature09210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard GC, Boardman TJ. The Misuse of Ratios, Indexes, and Percentages in Ecophysiological Research. Physiological Zoology. 1988;61:1–9. http://www.jstor.org/stable/30163730 [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. DOI: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Pajunen I. Ambient temperature dependence of the periodic respiratory pattern during longterm hibernation in the garden dormouse, Eliomys quercinus L. Annales Zoologici Fennici. 1984;21:143–148. [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. DOI: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Park KJ, Jones G, Ransome RD. Torpor, arousal and activity of hibernating Greater Horseshoe Bats (Rhinolophus ferrumequinum) Functional Ecology. 2000;14:580–588. DOI: 10.1046/j.1365-2435.2000.t01-1-00460.x. [Google Scholar]
- Peiponen VA. On Hypothermia and Torpidity in the Nightjar (Caprimulgus europaeus L.) Annales Academiae Scientiarum Fennicae A IV. Biologica. 1965;87:1–15. http://books.google.at/books?id=k3bFGwAACAAJ [Google Scholar]
- Pengelley ET. Responses of a New Hibernator (Citellus variegatus) to Controlled Environments. Nature. 1964;203:892–892. doi: 10.1038/203892a0. DOI: 10.1038/203892a0. [DOI] [PubMed] [Google Scholar]
- Pengelley ET, Asmundson SM. Free-running periods of endogenous circannual rhythms in the golden mantled ground squirrel, Citellus lateralis. Comparative Biochemistry and Physiology. 1969;30:177–183. doi: 10.1016/0010-406x(69)91312-7. DOI: 10.1016/0010-406X(69)91312-7. [DOI] [PubMed] [Google Scholar]
- Pengelley ET, Fisher KC. Rhythmical arousal from Hibernation in the Golden-Mantled Ground Squirrel, Citellus lateralis tescorum. Canadian Journal of Zoology. 1961;39:105–120. DOI: 10.1139/z61-013. [Google Scholar]
- Pengelley ET, Fisher KC. The effect of temperature and photoperiod on the yearly hibernating behavior of captive golden-mantled ground squirrels (Citellus lateralis tescorum) Canadian Journal of Zoology. 1963;41:1103–1120. DOI: 10.1139/z63-087. [Google Scholar]
- Pengelley ET, Kelley KH. A “circannian” rhythm in hibernating species of the genus Citellus with observation on their physiological evolution. Comparative Biochemistry and Physiology. 1966;19:603–617. doi: 10.1016/0010-406x(66)90043-0. DOI: 10.1016/0010-406X(66)90043-0. [DOI] [PubMed] [Google Scholar]
- Perret M. Energetic Advantage of Nest-Sharing in a Solitary Primate, the Lesser Mouse Lemur (Microcebus murinus) Journal of Mammalogy. 1998;79:1093–1102. DOI: 10.2307/1383001. [Google Scholar]
- Perrin MR, Ridgard BW. Thermoregulation and patterns of torpor in the spectacled dormouse, Graphiurus ocularis (A. Smith 1829) (Gliridae) Tropical Zoology. 1999;12:253–266. DOI: 10.1080/03946975.1999.10539392. [Google Scholar]
- Pettigrew JD, Wilson P. Nocturnal hypothermia in the White-throated Needletail, Hirundapus caudacutus. Emu. 1985;85:200–201. DOI: 10.1071/MU9850200. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core team . nlme: Linear and Nonlinear Mixed Effects Models (R Package Version 3.0.2) 2013. [Google Scholar]
- Pivorun EB. A biotelemetry study of the thermoregulatory patterns of Tamias striatus and Eutamias minimus during hibernation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1976;53:265–271. doi: 10.1016/s0300-9629(76)80034-5. DOI: 10.1016/S0300-9629(76)80034-5. [DOI] [PubMed] [Google Scholar]
- Pohl H. Temperaturregulation und Tagesperiodik des Stoffwechsels bei Winterschläfern. Zeitschrift für vergleichende Physiologie. 1961;45:109–153. DOI: 10.1007/BF00297762. [Google Scholar]
- Pohl H. Circadian pacemaker does not arrest in deep hibernation - evidence for desynchronization from the light cycle. Experientia (Basel) 1987;43:293–294. doi: 10.1007/BF01945554. DOI: 10.1007/BF01945554. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos ET, Heldmaier G, Frappell PB, McAllan BM, Withers KW, Klingenspor M, White CR, Jastroch M. Phylogenetic differences of mammalian basal metabolic rate are not explained by mitochondrial basal proton leak. Proceedings of the Royal Society B-Biological Sciences. 2012;279:185–193. doi: 10.1098/rspb.2011.0881. DOI: 10.1098/rspb.2011.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2002;282:R1054–R1082. doi: 10.1152/ajpregu.00562.2001. DOI: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- Prezlaff I, Dausmann KH. Impact of climatic variation on the hibernation physiology of Muscardinus avellanarius. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a Seasonal World. Thermoregulatory and Metabolic Adaptations. Springer Verlag; Heidelberg, New York, Dordrecht, London: 2012. pp. 85–97. [Google Scholar]
- Prinzinger R, Göppel R, Lorenz A, Kulzer E. Body temperature and metabolism in the Red-backed mousebird (Colius castanotus) during fasting and torpor. Comparative Biochemistry and Physiology Part A: Physiology. 1981;69:689–692. DOI: 10.1016/0300-9629(81)90157-2. [Google Scholar]
- Prinzinger R, Siedle K. Experimenteller Nachweis von Torpor bei jungen Mehlschwalben, Delichon urbica. Journal für Ornithologie. 1986;127:95–96. DOI: 10.1007/BF01641448. [Google Scholar]
- Prinzinger R, Siedle K. Ontogeny of Metabolism, Thermoregulation and Torpor in the House Martin Delichon u. urbica (L.) and its Ecological Significance. Oecologia. 1988;76:307–312. doi: 10.1007/BF00379969. DOI: 10.2307/4218674. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. Version 3.0.2. [Google Scholar]
- Ransome R. The natural history of hibernating bats. C. Helm; London: 1990. [Google Scholar]
- Reardon M. Quolls on the run. Australian Geographic. 1999;54:89–105. [Google Scholar]
- Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pevet M, Simonneaux V, Saboureau M. The circadian clock stops ticking during deep hibernation in the European hamster. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13816–13820. doi: 10.1073/pnas.0704699104. DOI: 10.1073 pnas.0704699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. The evolution of senescence from a comparative perspective. Functional Ecology. 2008;22:379–392. DOI: 10.1111/j.1365-2435.2008.01420.x. [Google Scholar]
- Riedesel ML, Williams BA. Continuous 24-hour oxygen consumption studies of Myotis velifer. Comparative Biochemistry and Physiology Part A: Physiology. 1976;54:95–99. doi: 10.1016/s0300-9629(76)80076-x. DOI: 10.1016/S0300-9629(76)80076-X. [DOI] [PubMed] [Google Scholar]
- Rojas AD, Körtner G, Geiser F. Cool running: locomotor performance at low body temperature in mammals. Biology Letters. 2012;8:868–870. doi: 10.1098/rsbl.2012.0269. DOI: 10.1098/rsbl.2012.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF. Hibernation: when good clocks go cold. Journal of Biological Rhythms. 2003;18:275–86. doi: 10.1177/0748730403254971. DOI: 10.1177/0748730403254971. [DOI] [PubMed] [Google Scholar]
- Ruf T, Arnold W. Mechanisms of social thermoregulation in hibernating alpine marmots (Marmota marmota) In: Heldmaier G, Klingenspor M, editors. Life in the Cold IV. Springer; Berlin, Heidelberg, New York: 2000. pp. 81–94. [Google Scholar]
- Ruf T, Arnold W. Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. American Journal of Physiology - Regulatory and Integrative Comparative Physiology. 2008;294:R1044–R1052. doi: 10.1152/ajpregu.00688.2007. DOI: 10.1152/ajpregu.00688.2007. [DOI] [PubMed] [Google Scholar]
- Ruf T, Bieber C, Arnold W, Millesi E. Living in a Seasonal World. Thermoregulatory and Metabolic Adaptations. Springer Verlag; Heidelberg, New York, Dordrecht, London: 2012. pp. I–XIV.pp. 1–563. Index. [Google Scholar]
- Ruf T, Steinlechner S, Heldmaier G. In: Malan A, Canguilhem B, editors. Rhythmicity of body temperature and torpor in the Djungarian hamster, Phodopus sungorus; Living in the Cold: 2nd International Symposium; John Libbey & Comp, London. 1989.pp. 53–62. [Google Scholar]
- Ruf T, Stieglitz A, Steinlechner S, Blank JL, Heldmaier G. Cold exposure and food restriction facilitate physiological responses to short photoperiod in djungarian hamsters (Phodopus sungorus) Journal of Experimental Zoology. 1993;267:104–112. doi: 10.1002/jez.1402670203. DOI: 10.1002/jez.1402670203. [DOI] [PubMed] [Google Scholar]
- Scantlebury M, Lovegrove B, Jackson C, Bennett N, Lutermann H. Hibernation and non-shivering thermogenesis in the Hottentot golden mole (Amblysomus hottentottus longiceps) Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2008;178:887–897. doi: 10.1007/s00360-008-0277-5. DOI: 10.1007/s00360-008-0277-5. [DOI] [PubMed] [Google Scholar]
- Schaub R, Prinzinger R, Schleucher E. Energy metabolism and body temperature in the Blue-naped Mousebird (Urocolius macrourus) during torpor. Ornis Fennica. 1999;76:211–219. [Google Scholar]
- Schlegel R. Der Ziegenmelker (Caprimulgus europaeus L.) A. Ziemsen; Wittenberg Lutherstadt: 1969. [Google Scholar]
- Schleucher E. Heterothermia in pigeons and doves reduces energetic costs. Journal of Thermal Biology. 2001;26:287–293. DOI: 10.1016/S0306-4565(01)00032-8. [Google Scholar]
- Schmid J. Daily torpor in the gray mouse lemur (Microcebus murinus) in Madagascar: energetic consequences and biological significance. Oecologia. 2000;123:175–183. doi: 10.1007/s004420051003. DOI: 10.1007/s004420051003. [DOI] [PubMed] [Google Scholar]
- Schmid J, Ruf T, Heldmaier G. Metabolism and temperature regulation during daily torpor in the smallest primate, the pygmy mouse lemour (Microcebus myoxinus) in Madagascar. Journal of Comparative Physiology B. 2000;170:59–68. doi: 10.1007/s003600050008. DOI: 10.1007/s003600050008. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. Cambridge University Press; New York: 1979. [Google Scholar]
- Scholander PF, Hock R, Walters V, Johnson F, Irving L. Body insulation of some arctic and tropical mammals and birds. Biological Bulletin. 1950;99:225–236. doi: 10.2307/1538740. [DOI] [PubMed] [Google Scholar]
- Scholl P. Temperaturregulation beim madegassischen Igeltanrek Echinops telfairi (Martin, 1838) Journal of Comparative Physiology A. 1974;89:175–195. DOI: 10.1007/BF00694790. [Google Scholar]
- Secord R, Bloch JI, Chester SGB, Boyer DM, Wood AR, Wing SL, Kraus MJ, McInerney FA, Krigbaum J. Evolution of the Earliest Horses Driven by Climate Change in the Paleocene-Eocene Thermal Maximum. Science. 2012;335:959–962. doi: 10.1126/science.1213859. DOI: 10.1126/science.1213859. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM. Thermoregulatory changes anticipate hibernation onset by 45 days: data from free) living arctic ground squirrels. Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2012;182:841–847. doi: 10.1007/s00360-012-0661-z. DOI: 10.1007/s00360-012-0661-z. [DOI] [PubMed] [Google Scholar]
- Sibley CG, Ahlquist JE. Phylogeny and classification of birds. A study in molecular evolution. Yale University Press; New Haven: 1990. [Google Scholar]
- Signer C, Ruf T, Arnold W. Hypometabolism and basking: The strategies of Alpine ibex to endure harsh over-wintering conditions. Functional Ecology. 2011;25:537–547. DOI: 10.1111/j.1365-2435.2010.01806.x. [Google Scholar]
- Silva-Duran IP, Bozinovic F. Food availability regulates energy expenditure and torpor in the Chilean mouse-opossum Thylamys elegans. Revista Chilena De Historia Natural. 1999;72:371–375. [Google Scholar]
- Siutz C, Pluch M, Ruf T, Millesi E. Sex Differences in Foraging Behaviour, Body Fat and Hibernation Patterns of Free-Ranging Common Hamsters. In: Ruf T, Bieber C, Arnold W, Millesi E, editors. Living in a Seasonal World. Thermoregulatory and Metabolic Adaptations. Springer Verlag; Heidelberg, New York, Dordrecht, London: 2012. pp. 155–165. DOI: 10.1007/978-3-642-28678-0_14. [Google Scholar]
- Smit B, Boyles JG, Brigham RM, McKechnie AE. Torpor in Dark Times: Patterns of Heterothermy Are Associated with the Lunar Cycle in a Nocturnal Bird. Journal of Biological Rhythms. 2011;26:241–248. doi: 10.1177/0748730411402632. DOI: 10.1177/0748730411402632. [DOI] [PubMed] [Google Scholar]
- Smit B, McKechnie AE. Do owls use torpor? Winter thermoregulation in free-ranging pearl-spotted owlets and African scops-owls. Physiological and Biochemical Zoology. 2010;83:149–56. doi: 10.1086/605457. DOI: 10.1086/605457. [DOI] [PubMed] [Google Scholar]
- Song X, Körtner G, Geiser F. Thermal relations of metabolic rate reduction in a hibernating marsupial. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1997;273:R2097–R2104. doi: 10.1152/ajpregu.1997.273.6.R2097. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Król E. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. Journal of Animal Ecology. 2010;79:726–746. doi: 10.1111/j.1365-2656.2010.01689.x. DOI: 10.1111/j.1365-2656.2010.01689.x. [DOI] [PubMed] [Google Scholar]
- Stawski C, Geiser F. Seasonality of torpor patterns and physiological variables of a free-ranging subtropical bat. The Journal of Experimental Biology. 2010;213:393–399. doi: 10.1242/jeb.038224. DOI: 10.1242/Jeb.038224. [DOI] [PubMed] [Google Scholar]
- Stawski C, Geiser F. Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301:R542–R547. doi: 10.1152/ajpregu.00792.2010. DOI: 10.1152/ajpregu.00792.2010. [DOI] [PubMed] [Google Scholar]
- Stawski C, Turbill C, Geiser F. Hibernation by a free-ranging subtropical bat (Nyctophilus bifax) Journal of Comparative Physiology B: Biochemical Systemic and Environmental Physiology. 2009;179:433–441. doi: 10.1007/s00360-008-0328-y. DOI: 10.1007/s00360-008-0328-y. [DOI] [PubMed] [Google Scholar]
- Stephenson PJ, Racey PA. Reproductive Energetics of the Tenrecidae (Mammalia: Insectivora). I. The Large-Eared Tenrec, Geogale aurita. Physiological Zoology. 1993a;66:643–663. DOI: 10.2307/30163816. [Google Scholar]
- Stephenson PJ, Racey PA. Reproductive Energetics of the Tenrecidae (Mammalia: Insectivora). II. The Shrew-Tenrecs, Microgale spp. Physiological Zoology. 1993b;66:664–685. DOI: 10.2307/30163817. [Google Scholar]
- Stoddart DM. Ecology of small mammals. Chapman and Hall; Wiley, London; New York: 1979. p. xiii.p. 386. [Google Scholar]
- Storey KB, Storey JM. Molecular Biology of Freezing Tolerance. Comprehensive Physiology. 2013;3:1283–1308. doi: 10.1002/cphy.c130007. DOI: 10.1002/Cphy.C130007. [DOI] [PubMed] [Google Scholar]
- Streicher S. MSc Thesis. University of Pretoria; 2010. The effect of environmental variables on patterns of body temperature in the Damaraland mole-rat, Fukomys damarensis (Ogilby 1838) [Google Scholar]
- Strumwasser F. Some physiological principles governing hibernation in Citellus beecheyi. Bulletin of the Museum of Comparative Zoology. 1960;124:282–320. [Google Scholar]
- Strumwasser F, Schlechte FR, Streeter J. The internal rhythms of hibernators. In: Fisher KC, Dawe AR, Lyman CP, Schönbaum E, South FE, editors. Mammalian Hibernation III. Oliver and Boyd; London: 1967. pp. 110–139. [Google Scholar]
- Superina M, Boily P. Hibernation and daily torpor in an armadillo, the pichi (Zaedyus pichiy) Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2007;148:893–898. doi: 10.1016/j.cbpa.2007.09.005. DOI: 10.1016/j.cbpa.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Tannenbaum MG, Pivorun EB. Differences in daily torpor patterns among three southeastern species of Peromyscus. Journal of Comparative Physiology B. 1984;154:233–236. DOI: 10.1007/BF02464401. [Google Scholar]
- Tannenbaum MG, Pivorun EB. Seasonal study of daily torpor in southeastern Peromyscus maniculatus and Peromyscus leucopus from mountains and foothills. Physiological Zoology. 1988;61:10–16. http://www.jstor.org/stable/30163731 [Google Scholar]
- Thäti H. Seasonal differences in O2 consumption and respiratory quotient in a hibernator (Erinaceus europaeus L.) Annales Zoologici Fennici. 1978;15:69–75. [Google Scholar]
- Thompson DC, Thompson PS. Food habits and caching behavior of urban grey squirrels. Canadian Journal of Zoology. 1980;58:701–710. DOI: 10.1139/z80-101. [Google Scholar]
- Thompson SD. Subspecific Differences in Metabolism, Thermoregulation, and Torpor in the Western Harvest Mouse Reithrodontomys megalotis. Physiological Zoology. 1985;58:430–444. DOI: 10.2307/30156018. [Google Scholar]
- Tinkle DW, Patterson IG. A Study of Hibernating Populations of Myotis velifer in Northwestern Texas. Journal of Mammalogy. 1965;46:612–633. DOI: 10.2307/1377932. [PubMed] [Google Scholar]
- Tøien Ø, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–909. doi: 10.1126/science.1199435. DOI: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Withers PC, Cooper C. Hypothermia versus torpor in response to cold stress in the native Australian mouse Pseudomys hermannsburgensis and the introduced house mouse Mus musculus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2007;148:645–650. doi: 10.1016/j.cbpa.2007.08.013. DOI: 10.1016/j.cbpa.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Withers PC, Maloney SK. Flexibility in thermoregulatory physiology of two dunnarts, Sminthopsis macroura and Sminthopsis ooldea (Marsupialia; Dasyuridae) The Journal of Experimental Biology. 2012;215:2236–2246. doi: 10.1242/jeb.065516. DOI: 10.1242/jeb.065516. [DOI] [PubMed] [Google Scholar]
- Tucker VA. Diurnal torpidity in the California pocket mouse. Science. 1962;136:380–381. doi: 10.1126/science.136.3514.380. DOI: 10.1126/science.136.3514.380. [DOI] [PubMed] [Google Scholar]
- Tucker VA. Oxygen consumption, thermal conductance, and torpor in the California pocket mouse, Perognathus californicus. Journal of Cellular and Comparative Physiology. 1965;65:393–403. doi: 10.1002/jcp.1030650313. DOI: 10.1002/jcp.1030650313. [DOI] [PubMed] [Google Scholar]
- Turbill C, Bieber C, Ruf T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proceedings of the Royal Society B. 2011;278:3355–3363. doi: 10.1098/rspb.2011.0190. DOI: 10.1098/rspb.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbill C, Geiser F. Hibernation by tree-roosting bats. Journal of Comparative Physiology B. 2008;178:597–605. doi: 10.1007/s00360-007-0249-1. DOI: 10.1007/s00360-007-0249-1. [DOI] [PubMed] [Google Scholar]
- Turbill C, Ruf T, Smith S, Bieber C. Seasonal variation in telomere length of a hibernating rodent. Biology Letters. 2013;9:20121095. doi: 10.1098/rsbl.2012.1095. DOI: 10.1098/rsbl.2012.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbill C, Smith S, Deimel C, Ruf T. Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biology Letters. 2012;8:304–307. doi: 10.1098/rsbl.2011.0758. DOI: 10.1098/rsbl.2011.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Körtner G, Warnecke L, Geiser F. Summer and winter torpor use by a free-ranging marsupial. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2012;162:274–280. doi: 10.1016/j.cbpa.2012.03.017. DOI: 10.1016/j.cbpa.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Twente JW, Twente JA. Regulation of Hibernating Periods by Temperature. Proceedings of the National Academy of Sciences of the United States of America. 1965;54:1058–1061. doi: 10.1073/pnas.54.4.1058. http://www.jstor.org/stable/73050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier L, van der Merwe M. The incidence of torpor in winter and summer in the Angolan free-tailed bat, Mops condylurus (Microchiroptera: Molossidae), in a subtropical environment, Mpumulanga, South Africa. African Zoology. 2011:42. http://africanzoology.journals.ac.za/pub/article/view/379 [Google Scholar]
- Vogel P. Kälteresistenz und reversible Hypothermie der Etruskerspitzmaus (Suncus etruscus, Soricidae, Insectivora) Zeitschrift für Säugetierkunde. 1974;39:78–88. [Google Scholar]
- Wang LCH, Hudson JW. Energetics and field aspects of mammalian torpor: the Richardson’s ground squirrel. In: Wang LCH, editor. Strategies in Cold: Natural Torpidity and Thermogenesis. Academic Press; New York: 1978. pp. 109–145. [Google Scholar]
- Wang LCH, Hudson JW. Some physiological aspects of temperature regulation in the normothermic and torpid hispid pocket mouse, Perognathus hispidus. Comparative Biochemistry and Physiology. 1970;32:275–293. doi: 10.1016/0010-406x(70)90941-2. DOI: 10.1016/0010-406X(70)90941-2. [DOI] [PubMed] [Google Scholar]
- Wang LCH, Hudson JW. Temperature regulation in normothermic and hibernating eastern chipmunk, Tamias striatus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1971;38:59–90. doi: 10.1016/0300-9629(71)90098-3. DOI: 10.1016/0300-9629(71)90098-3. [DOI] [PubMed] [Google Scholar]
- Warnecke L, Turner JM, Geiser F. Torpor and basking in a small arid zone marsupial. Naturwissenschaften. 2008;95:73–78. doi: 10.1007/s00114-007-0293-4. DOI: 10.1007/s00114-007-0293-4. [DOI] [PubMed] [Google Scholar]
- Waßmer T, Wollnik F. Timing of torpor bouts during hibernation in European hamsters (Cricetus cricetus L.) Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1997;167:270–279. doi: 10.1007/s003600050074. DOI: 10.1007/s003600050074. [DOI] [PubMed] [Google Scholar]
- Watts PD, Øritsland NA, Jonkel C, Ronald K. Mammalian hibernation and the oxygen consumption of a denning black bear (Ursus americanas) Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1981;69:121–123. DOI: 10.1016/0300-9629(81)90645-9. [Google Scholar]
- Willis CKR, Brigham RM, Geiser F. Deep, prolonged torpor by pregnant, free-ranging bats. Naturwissenschaften. 2006;93:80–83. doi: 10.1007/s00114-005-0063-0. DOI: 10.1007/s00114-005-0063-0. [DOI] [PubMed] [Google Scholar]
- Willis CKR, Lane JE, Liknes ET, Swanson DL, Brigham RM. Thermal energetics of female big brown bats (Eptesicus fuscus) Canadian Journal of Zoology. 2005a;83:871–879. DOI: 10.1139/z05-074. [Google Scholar]
- Willis CKR, Turbill C, Geiser F. Torpor and thermal energetics in a tiny Australian vespertilionid, the little forest bat (Vespadelus vulturnus) Journal of Comparative Physiology B. 2005b;175:479–486. doi: 10.1007/s00360-005-0008-0. DOI: 10.1007/s00360-005-0008-0. [DOI] [PubMed] [Google Scholar]
- Wilz M, Heldmaier G. Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2000;170:511–521. doi: 10.1007/s003600000129. DOI: 10.1007/s003600000129. [DOI] [PubMed] [Google Scholar]
- Withers PC. Respiration, Metabolism, and Heat Exchange of Euthermic and Torpid Poorwills and Hummingbirds. Physiological Zoology. 1977;50:43–52. DOI: 10.2307/30155714. [Google Scholar]
- Withers PC, Louw GN, Henschel J. Energetics and water relations of Namib desert rodents. South African Journal of Zoology. 1980;15:131–137. [Google Scholar]
- Withers PC, Richardson KC, Wooller RD. Metabolic physiology of euthermic and torpid honey possums, Tarsipes rostratus. Australian Journal of Zoology. 1990;37:685–693. DOI: 10.1071/ZO9890685. [Google Scholar]
- Wolf LL, Hainsworth FR. Environmental influence on regulated body temperature in torpid hummingbirds. Comparative Biochemistry and Physiology Part A: Physiology. 1972;41:167–173. doi: 10.1016/0300-9629(72)90044-8. DOI: 10.1016/0300-9629(72)90044-8. [DOI] [PubMed] [Google Scholar]
- Woods CP, Brigham RM. The Avian Enigma: “Hibernation” by Common Poorwills (Phalaenoptilus nuttallii) In: Barnes BM, Carey HV, editors. Life in the Cold V: Evolution, Mechanism, Adaptation, and Application. Twelfth International Hibernation Symposium. Biological Papers of the University of Alaska, number 27. Institute of Arctic Biology, University of Alaska; Fairbanks, Alaska, USA: 2004. pp. 231–240. [Google Scholar]
- Wyss OAM. Winterschlaf und Wärmehaushalt, untersucht am Siebenschläfer (Myoxus glis) Pflügers Archiv European Journal of Physiology. 1932;229:599–635. DOI: 10.1007/BF01754494. [Google Scholar]
- Yang M, Xing X, Guan S, Zhao Y, Wang Z, Wang D-H. Hibernation patterns and changes of body temperature in Daurian ground squirrels (Spermophilus dauricus) during hibernation. Acta Theriologica Sinica. 2011;31:387–395. [Google Scholar]
- Young PJ. Hibernating patterns of free-ranging Columbian Ground Squirrels. Oecologia. 1990;83:504–511. doi: 10.1007/BF00317201. DOI: 10.1007/BF00317201. [DOI] [PubMed] [Google Scholar]
- Zervanos SM, Maher CR, Florant GL. Effect of Body Mass on Hibernation Strategies of Woodchucks (Marmota monax) Integrative and Comparative Biology. 2013:1–9. doi: 10.1093/icb/ict100. DOI: 10.1093/icb/ict100. [DOI] [PubMed] [Google Scholar]
- Zervanos SM, Maher CR, Waldvogel JA, Florant GL. Latitudinal differences in the hibernation characteristics of woodchucks (Marmota monax) Physiological and Biochemical Zoology. 2010;83:135–41. doi: 10.1086/648736. DOI: 10.1086/648736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.