Key Points
Rituximab plus recombinant human thrombopoietin is superior to rituximab monotherapy for corticosteroid-resistant or relapsed ITP patients.
Abstract
This study aimed to compare the efficacy and safety of rituximab (RTX) plus recombinant human thrombopoietin (rhTPO) with RTX alone in patients with immune thrombocytopenia (ITP) who had failed to respond to corticosteroids or relapsed. Recruited patients were randomized at a ratio of 2:1 into 2 groups: the combination group (RTX + rhTPO, n = 77) and the monotherapy group (RTX, n = 38). Overall response was achieved in 79.2% of patients in the combination group vs 71.1% in the monotherapy group (P = .36), and the complete response (CR) rate was 45.4% in the combination group compared with 23.7% in the monotherapy group (P = .026). The combination group had significantly shorter time to response (TTR; median and range, 7 and 4-28 days) compared with the monotherapy group (28 and 4-90 days) (P < .01). There was no difference between these 2 groups in terms of the long-term response (P = .12). Our findings demonstrated that the combination of RTX and rhTPO significantly increased the CR rate and shortened TTR compared with RTX monotherapy in the treatment of corticosteroid-resistant or relapsed ITP but failed to show a beneficial effect on the long-lasting response. This study is registered at www.clinicaltrials.gov as #NCT01525836.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder in which both increased platelet destruction and insufficient platelet production are involved.1-5 Corticosteroids and intravenous immunoglobulin are recommended as the first-line treatments of ITP6; however, approximately one-third of ITP patients fail to respond. Besides, a certain amount of ITP patients relapse and require further therapy after one or more treatment strategies (eg, corticosteroids, intravenous immunoglobulin, cyclosporin, danazol, azathioprine, or splenectomy).7 The optimal second-line treatment remains uncertain.8
Rituximab (RTX), a chimeric monoclonal antibody against CD20, has been frequently used in the management of ITP9,10 and recommended as a second-line6 or suggested as a grade 2C7 treatment of ITP. During the past decade, clinical studies on RTX have demonstrated an initial response rate of 50% to 60%, a sustained response rate of 20%, and a median time to response (TTR) of 5.5 weeks in ITP.11-13 Recently, several studies evaluated low-dose RTX at a fixed dose of 100 mg weekly for 4 weeks in ITP patients and found that the response rate was similar to that with standard-dose RTX (375 mg/m2 × 4).14-16
Thrombopoietin (TPO) receptor agonists (romiplostim17 and eltrombopag18,19) have been approved by the US Food and Drug Administration and recommended as second-line options for the management of ITP.6 Platelet response is usually rapid (within 1-3 weeks)17-19 and can be maintained during treatment; however, once the medication is stopped, platelet counts commonly drop to pretreatment levels. Recombinant human thrombopoietin (rhTPO), a full-length and glycosylated TPO developed by 3SBIO (Shenyang, China), was approved by the China State Food and Drug Administration as a second-line option for ITP. A multicenter randomized open-label phase 3 clinical trial assessing the efficacy of rhTPO in corticosteroid-resistant or relapsed ITP patients has demonstrated that rhTPO rapidly increased platelet counts.20
RTX in combination with rhTPO might overcome the long TTR of RTX and drug-dependent response of rhTPO. Furthermore, these 2 drugs could work synergistically based on the mechanism of action targeting both increased platelet destruction and insufficient platelet production. Therefore, our hypothesis is that RTX and rhTPO could complement each other in terms of both the time window and mechanism of action. We evaluated the efficacy and safety of low-dose RTX plus rhTPO in corticosteroid-resistant or relapsed ITP patients in a multicenter randomized open-label clinical trial.
Methods
Study design
The primary objective was to compare the efficacy and safety of RTX plus rhTPO with RTX monotherapy in corticosteroid-resistant or relapsed ITP patients. We conducted an open-label prospective randomized controlled clinical trial in 12 centers in China. Patient enrollment started in January 2012 and ended in December 2013. Approval for the study was obtained from the ethics committees of all participating hospitals. Informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
Patients
The diagnosis of ITP was based on established practice guidelines.5 Inclusion criteria were described as follows: isolated thrombocytopenia (platelet count <30 × 109/L), bone marrow examination showing normal leukocytes and erythrocytes and a normal or increased number of megakaryocytes (all patients underwent a bone marrow examination for the exclusion of myelofibrosis or other thrombocytopenic disorders), normal spleen size, Eastern Cooperative Oncology Group performance status ≤2, and relapse after or no response to at least 1 full course of steroid therapy. Exclusion criteria were secondary ITP; drug-induced thrombocytopenia; virus-induced thrombocytopenia, such as HIV, hepatitis B virus, or hepatitis C virus; severe dysfunction of heart, kidney, liver, or lung; severe immunodeficiency; pregnancy or lactation; and myelofibrosis. Patients who had previously been treated with rituximab were ineligible. Previous treatments for ITP (eg, corticosteroids, splenectomy, and cyclosporin) must have failed and been completed at least 4 weeks prior to enrollment.
Platelet antibody testing was performed using a modified monoclonal antibody-specific immobilization of platelet antigens assay as previously published.21 Briefly, platelets from healthy blood donors (blood group O) were sensitized with tested or control plasma. Diluted sensitized platelet lysate was added in duplicate into the wells of a microtiter plate coated with the monoclonal antibodies SZ2 or P2. Immunoglobulin G bound to the captured glycoproteins was detected. p-Nitrophenyl phosphate was used as substrate and the absorbance was recorded at 405 nm using. Four normal plasmas were analyzed on each plate, and an absorbance above the mean + 3 standard deviations of the controls was considered as a positive reaction.
We performed the assessment of bleeding according to the reported scoring system by the Gruppo Italiano Malattie EMatologiche dell’Adulto ITP Working Party.22 Bleeding severity was graded as follows: (1) grade 0: absence of bleeding, (2) grade 1: petechiae, (3) grade 2: ecchymoses and/or dripping with moderate loss of blood, (4) grade 3: major mucous hemorrhage with copious loss of blood without sequelae, and (5) grade 4: major mucous and/or parenchymal hemorrhage with copious loss of blood with sequelae and/or life-threatening or death.
Treatment strategies
Eligible patients were randomized 2:1 by precoded envelopes to receive either the combination of RTX and rhTPO or RTX alone. RTX was given at a fixed dose of 100 mg weekly for 4 weeks. For the combination group, rhTPO was given concomitantly at a daily dose of 300 U/kg subcutaneously during the first 14 days. The dose frequency of rhTPO was adjusted as follows: (1) rhTPO was given once a day at the beginning to achieve platelet response (platelets ≥30 × 109/L), (2) rhTPO was administered every other day when platelets ascended above 100 × 109/L, and (3) withdrawal of rhTPO could be performed when platelets rose above 300 × 109/L in <14 days.
Study outcomes
The primary end points were complete response (CR), response (R), no response (NR), and relapse during the follow-up period. Secondary end points included TTR, time to relapse (duration of response), and adverse events. The criteria for response were defined as follows: (1) CR: platelet count ≥100 × 109/L and absence of bleeding, (2) R: platelet count ≥30 × 109/L and at least 2-fold increase of the baseline platelet count and absence of bleeding, and (3) NR: platelet count <30 × 109/L or <2-fold increase of the baseline platelet count or bleeding. Loss of CR (or R) was defined as a platelet count <100 × 109/L (or 30 × 109/L) measured on 2 occasions more than 1 day apart and/or the presence of bleeding.7 TTR and time to relapse (duration of response) were defined as the time from baseline to response and the duration from response (CR and R) to relapse, respectively. Response (CR and R) lasting for at least 6 months without any ITP-specific treatments was regarded as sustained response. During the follow-up period, patients were not allowed to receive concomitant medications. Platelet counts were monitored weekly during the first month of treatment, then at biweekly intervals up to the sixth month and monthly thereafter. Adverse events were recorded and graded according to the Common Toxicity Criteria version 2.0.
Statistical analysis
Differences in achieving CR, R, NR, or relapse between the 2 groups and differences in adverse events were analyzed by the Fisher’s exact test. For descriptive statistics, the Mann-Whitney U test, Wilcoxon signed-rank test, and Fisher’s exact test were used. Logistic regression was used to estimate the association of patients’ age, gender, and presence of autoantibodies with response or relapse. P values below .05 were considered statistically significant.
Results
Patient enrollment and follow-up
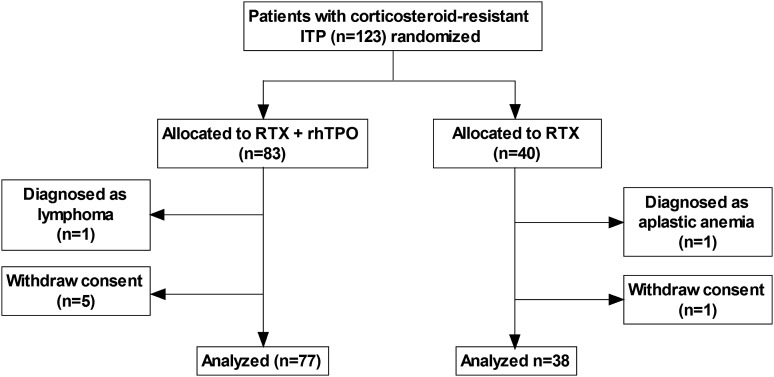
Patients were enrolled into this study from January 2012 to December 2013. A total of 123 patients were recruited and randomized to receive RTX + rhTPO (n = 83) or RTX alone (n = 40). Reasons for exclusion included withdrawn consent (n = 6) and secondary ITP (n = 2; one patient was diagnosed with lymphoma in the second week after enrollment, and the other patient had aplastic anemia but was atypical in the initial bone marrow examination). A total of 115 cases were analyzed, with 77 cases in the RTX + rhTPO group and 38 cases in the RTX group (Figure 1). The median (range) follow-up time in the RTX + rhTPO group and the RTX group was 15 (3-27) months and 13 (6-27) months, respectively. During the follow-up period, responses were evaluated and adverse events were recorded. All patients (except one who died in the third month) were followed for at least 6 months.
Figure 1.
Flowchart indicating the 2 randomized groups.
As shown in Table 1, there were 50 (64.9%) female patients in the RTX + rhTPO group and 25 (65.8%) female patients in the RTX group. The median age (range) of patients in the RTX + rhTPO group and the RTX group was 42 (13-82) years and 42.5 (12-68) years, respectively. The median platelet count at the time of enrollment was 9 × 109/L (0-30 × 109/L) in the RTX + rhTPO group and 12.5 × 109/L (2-30 × 109/L) in the RTX group, respectively. There was no difference in the frequency of platelet glycoprotein autoantibodies between these 2 groups (Table 2).
Table 1.
Baseline characteristics of patients enrolled in the study
| Characteristics | RTX + rhTPO (n = 77) | RTX (n = 38) |
|---|---|---|
| Age (y), median (range) | 42 (13-82) | 42.5 (12-68) |
| Female/male, % (n) | 64.9 (50)/35.1 (27) | 65.8 (25)/34.2 (13) |
| Baseline platelet count (109/L), median (range) | 9 (0-30) | 12.5 (2-30) |
| ITP duration (mo) | ||
| Median | 12.5 | 11 |
| IQR | 7, 22 | 6, 23 |
| Range | 3-72 | 3-65 |
| Previous therapies, % (n) | ||
| Dexamethasone | 55.8 (43) | 50.0 (19) |
| Prednisone | 40.3 (31) | 34.2 (13) |
| Methylprednisolone | 14.3 (11) | 21.0 (8) |
| IVIG | 19.5 (15) | 18.4 (7) |
| Vincristine | 6.5 (5) | 0 |
| rhTPO | 7.8 (6) | 5.3 (2) |
| Cyclosporin | 3.9 (3) | 2.6 (1) |
| Danazol | 6.5 (5) | 10.5 (4) |
| Azathioprine | 1.3 (1) | 0 |
| Herbs | 10.4 (8) | 7.9 (3) |
| Splenectomy* | 11.7 (9) | 7.9 (3) |
| Bleeding score†, % (n) | ||
| 0 | 13.0 (10) | 15.8 (6) |
| 1 | 58.4 (45) | 55.3 (21) |
| 2 | 20.8 (16) | 21.0 (8) |
| 3 | 6.5 (5) | 7.9 (3) |
| 4 | 1.3 (1) | 0 |
IQR, interquartile range; IVIG, intravenous immunoglobulin.
The median time (range) from splenectomy to enrollment of these 12 patients was 7 months (3-22 months).
Bleeding score was at baseline.
Table 2.
Platelet glycoprotein autoantibodies in the 2 groups
| RTX + rhTPO (n = 77) | RTX (n = 38) | |
|---|---|---|
| Anti-GP Ib/IX positive, % (n) | 16.9 (13) | 13.2 (5) |
| Anti-GP IIb/IIIa positive, % (n) | 26.0 (20) | 26.3 (10) |
| Double positive, % (n) | 24.6 (19) | 23.7 (9) |
| Negative, % (n) | 32.5 (25) | 36.8 (14) |
GP, glycoprotein.
The median duration (interquartile range and range) of ITP from the time of diagnosis to enrollment was 12.5 months (7; 22 and 3-72) in the RTX + rhTPO group and 11 months (6; 23 and 3-65) in the RTX group, respectively (Table 1). The median number (range) of previous therapies was 2 (1-5) in the RTX + rhTPO group and 2 (1-4) in the RTX group. More than 60% patients in both groups received 2 or 3 therapies before the enrollment. Among all patients, high-dose dexamethasone was the most commonly used previous therapy, and the second most commonly used treatment was prednisone. There were 9 splenectomized patients in the RTX + rhTPO group and 3 in the RTX group. The median time (range) from splenectomy to enrollment of these 12 patients was 7 months (3-22 months). No patients had previously received RTX administration.
Bleeding assessment was performed. On the day of enrollment, most patients in both groups were graded with a score of 1 with petechiae or a score of 2 with ecchymosis (Table 1). One patient was graded with a score of 4 with cerebral hemorrhage based on the result of magnetic resonance imaging in the RTX + rhTPO group. During the follow-up period, the worst bleeding score of patients was recorded (Table 3). The relief of bleeding symptoms was consistent with the elevated platelet levels.
Table 3.
Worst bleeding scores of patients in this study
| Bleeding score | RTX + rhTPO | RTX | Total | |||
|---|---|---|---|---|---|---|
| Response (n = 61) | Nonresponse (n = 16) | Response (n = 27) | Nonresponse (n = 11) | Response (n = 88) | Nonresponse (n = 27) | |
| 0 | 13.1 (8) | 12.5 (2) | 14.8 (4) | 18.2 (2) | 13.6 (12) | 14.8 (4) |
| 1 | 59.0 (36) | 43.8 (7) | 51.9 (14) | 45.4 (5) | 56.8 (50) | 44.5 (12) |
| 2 | 18.0 (11) | 37.5 (6) | 25.9 (7) | 27.3 (3) | 20.5 (18) | 33.3 (9) |
| 3 | 6.6 (4) | 6.2 (1) | 7.4 (2) | 9.1 (1) | 6.8 (6) | 7.4 (2) |
| 4 | 3.3 (2*) | 0 | 0 | 0 | 2.3 (2*) | 0 |
Data are presented as % (n) of patients.
Two patients had a grade 4 bleeding score: one patient was a prior bleed, and the other (who relapsed in the third month) died of intracranial hemorrhage.
Responses
At the end of the second month of the initial treatment, CR was achieved in 45.4% (35/77) of patients in the RTX + rhTPO group compared with 18.4% (7/38) in the RTX group (P < .01). During the follow-up period, 2 patients in the RTX group initially stabilized at R and subsequently improved to CR at the third month. Therefore, CR was achieved in total 45.4% (35/77) of patients in the RTX + rhTPO group compared with 23.7% (9/38) in the RTX group (P = .026) (Table 4), suggesting that the combination of RTX and rhTPO significantly increased the CR rate. The overall response rate was 79.2% (61/77) in the RTX + rhTPO group and 71.1% (27/38) in the RTX group (P = .36). Age, gender, and the presence of autoantibodies were not associated with response rate or relapse as analyzed by logistic regression.
Table 4.
Responses and outcomes in the RTX + TPO and RTX groups
| RTX + rhTPO | RTX | P | |
|---|---|---|---|
| CR, % (n) | 45.4 (35) | 23.7 (9) | .026* |
| R, % (n) | 33.8 (26) | 47.4 (18) | .22 |
| OR, % (n) | 79.2 (61) | 71.1 (27) | .36 |
| NR, % (n) | 20.8 (16) | 28.9 (11) | .36 |
| For patients with an initial response | |||
| TTR (days), median (range) | 7 (4-28) | 28 (4-90) | .004† |
| Duration of response (≥6 mo), % (n) | 67.2 (41) | 55.6 (15) | .34 |
| Duration of response (≥12 mo), % (n) | 44.3 (27) | 29.6 (8) | .24 |
| Duration of response (≥24 mo), % (n) | 24.6 (15) | 18.5 (5) | .59 |
OR, overall response.
P < .05.
P < .01.
In patients who had achieved CR and R, the median (range) TTR was 7 days (4-28 days) in the RTX + rhTPO group compared with 28 days (4-90 days) in the RTX group (P < .01) (Table 4). Consistent with our hypothesis, the combination of these 2 drugs significantly shortened TTR.
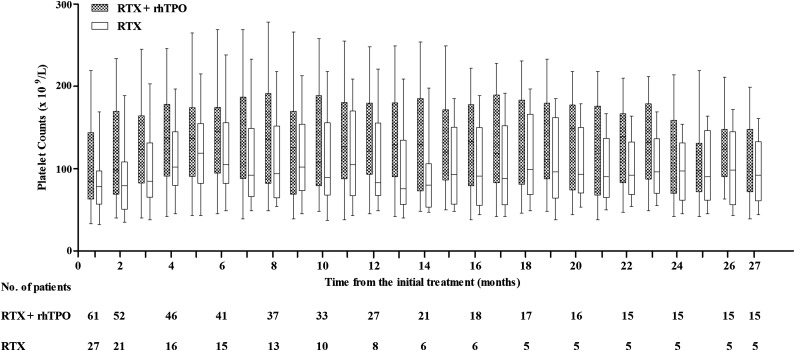
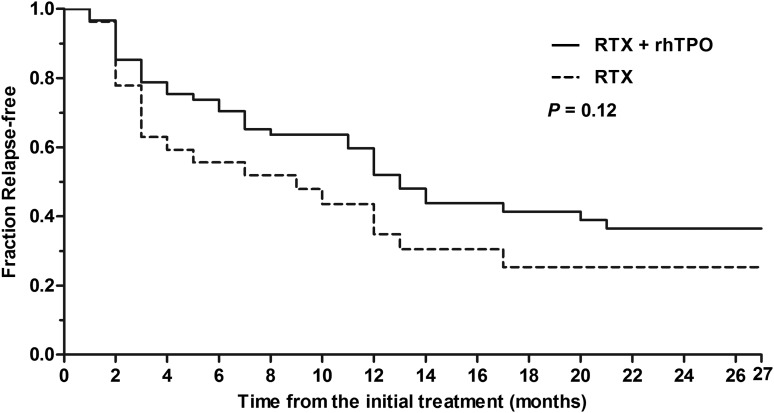
As shown in Table 4 and Figure 2, the response rate at 6 months in the RTX + rhTPO group was 67.2% (41/61) compared with 55.6% (15/27) in the RTX group (P = .34). Sustained response was maintained for 12 months in 44.3% (27/61) of patients in the RTX + rhTPO group and 29.6% (8/27) of patients in the RTX group (P = .24). Furthermore, 24.6% (15/61) of patients in the RTX + rhTPO group and 18.5% (5/27) of patients in the RTX group had a 24-month sustained response (P = .59). There was no difference between these 2 groups in terms of long-term response (P = .12) (Figure 3).
Figure 2.
Box-whisker plots of platelet counts from ITP patients with initial response (including CR and R). The central horizontal line is the median; the lower and upper box limits are the first and third quartiles, respectively; and the whiskers extend to the minimal and maximal data points.
Figure 3.
Kaplan-Meier analysis of patients with an initial response (including CR and R). The Kaplan-Meier curve showed time to relapse with platelet count <30 × 109/L. There was no difference between these 2 groups in terms of proportion of relapse (P = .12).
Adverse events
Adverse events were generally mild and balanced in the grade and incidence between these 2 groups. Over 20% of patients reported adverse events (Table 5). All adverse events observed in this study had been previously reported. The most common adverse event was fever, which was mainly related to the upper respiratory, urinary tract, and gastrointestinal infection. There was an overrepresentation of rash, headache, stomachache, and chest congestion in the RTX + rhTPO group; however, no significant difference was observed between these 2 groups. Among the 77 patients receiving rhTPO therapy, 2 cases were found to have detectable transient anti-TPO antibodies, which disappeared 2 months after the discontinuation of rhTPO.
Table 5.
Adverse events recorded in the 2 groups
| Adverse events, n (%) | RTX + rhTPO (n = 77) | RTX (n = 38) |
|---|---|---|
| Fever | 19 (24.7) | 8 (21.0%) |
| Upper respiratory infection | 12 (15.6) | 5 (13.2) |
| Urinary tract infection | 5 (6.5) | 2 (5.3) |
| Gastrointestinal infections | 3 (3.9) | 1 (2.6) |
| Muscle/joint pain* | 3 (3.9) | 2 (5.3) |
| Insomnia | 4 (5.2) | 2 (5.3) |
| Stomachache | 0 | 1 (2.6) |
| Headache | 0 | 1 (2.6) |
| Chest congestion | 0 | 1 (2.6) |
| Rash* | 0 | 2 (5.3) |
| Malaise | 1 (1.3) | 0 |
| Thrombosis | 1 (1.3) | 0 |
| Encephalorrhagia | 1 (1.3) | 0 |
| Anti-thrombopoietin antibody | 2 (2.6) | 0 |
These 2 adverse events were considered to be serum sickness.
During the follow-up period, 2 deaths occurred in the RTX + rhTPO group. The first patient (72 years old) was a nonresponder and died of myocardial infarction in the eighth month with a platelet count of 26 × 109/L (his pretreatment platelet count was 19 × 109/L). The relationship between his myocardial infarction and the treatment was uncertain, and the patient had underlying risk factors. The second patient (76 years old), who relapsed in the third month, died of intracranial hemorrhage.
Discussion
The ideal treatment option for ITP, especially for corticosteroid-resistant or relapsed patients, is a strategy to achieve a rapid and long-lasting response with a normal treatment-free platelet count in the absence of bleeding. Clinical studies on RTX demonstrated a considerably high response rate in ITP.9,11,23,24 The major limitations of RTX are the relatively long TTR (median TTR 5.5 weeks) and the lack of a high rate of sustained remission.12 The combination of RTX with a rapid onset treatment might be beneficial for ITP patients. TPO receptor agonists have been shown to rapidly increase platelet counts and ameliorate bleeding17-19,25 and therefore can be good candidates to combine with RTX. Unfortunately, romiplostim and eltrombopag are not available in China. In this study, we chose rhTPO instead. A previous study on rhTPO therapy in corticosteroid-resistant or relapsed ITP patients showed similar efficacy to romiplostim and eltrombopag.20
Although a pilot randomized trial to determine the effect of adding RTX to standard treatment in patients with ITP observed little benefit,26 other studies demonstrated that the combination of RTX and dexamethasone induced higher response rates and longer time to relapse than dexamethasone alone.27,28 Later on, the combination of RTX and 3 cycles of dexamethasone showed apparently better efficacy than RTX alone or dexamethasone alone.29 These conflicting findings indicate that the efficacy and safety of the combination of RTX with other drugs in the treatment of ITP needs further study. We hereby performed a multicenter prospective randomized controlled study in corticosteroid-resistant or relapsed ITP patients treated with either RTX + rhTPO or RTX alone, and we found that the combination yielded a significantly higher CR rate and shortened TTR compared with RTX alone but failed to show beneficial effect on the long-lasting response.
Previous studies on RTX treatment in ITP showed that younger age, female gender, and short duration of ITP might be the factors predictive of better outcome.11,24,29-31 In this study, the overall response rate in both groups was fairly high, which might be due to the generally short duration of ITP from diagnosis to enrollment. Age, gender, and the presence of autoantibodies were not associated with response rate or relapse in our study.
A rapid increase in platelet count is beneficial for ITP patients with bleeding symptoms.32,33 The significantly shortened TTR in the combination group was likely to be attributed to the fast onset of rhTPO.20,34 This result suggested that rhTPO could be used as a salvage treatment and combined with RTX in the treatment of ITP. The improvement of CR rate in the RTX + rhTPO group might be related to their synergistic mechanism of action by both inhibiting platelet destruction and promoting platelet production.11,12,20
The mechanism of inhibiting platelet destruction of RTX in ITP was the depletion of B cells and the subsequent effects, such as the decreased production of platelet-specific autoantibodies, decreased secretion of inflammatory factors, and inhibited antigen-presenting cell activity.35 The long-term response was mainly attributed to RTX. Therefore, no statistical significance was observed in sustained response between these 2 groups. Recent studies have demonstrated that TPO mimetic agents could induce sustained remission in some responders.36-38 Both rhTPO and RTX showed effects on the improvement of regulatory T cells (Tregs).39-42 TPO mimetic agents were shown to have profound effects on Tregs function in ITP.42 Similarly, Stasi et al demonstrated that RTX treatment was associated with restored number and regulatory function of Tregs in ITP patients, particularly in those who responded to RTX.41 However, Audia et al reported different results that RTX showed no effect on Tregs in ITP.43 More clinical trials with large sample sizes are required to further investigate the sustained response of the combination of RTX and rhTPO.
This multicenter study indicated that administration of low-dose RTX and rhTPO was well tolerated in a Chinese population. Adverse events were mild and consistent with those previously observed.20,23,28,29 There were no new reported adverse events during the observation period and no adverse event-related study withdrawals. Previous studies reported that 1% to 3% of ITP patients receiving rhTPO therapy developed transient and low-titer anti-TPO antibodies and that no patients had neutralizing anti-TPO antibodies or persistent thrombocytopenia.26,44 In our study, 2 cases receiving rhTPO therapy were found to have detectable transient anti-TPO antibodies that disappeared 2 months after discontinuation of rhTPO. During the follow-up period, 2 deaths (in patients >70 years) occurred in the combination group. The first patient died of myocardial infarction in the eighth month after the discontinuation of rhTPO. The relationship between this death and rhTPO was uncertain, though thrombosis has been previously reported as the adverse event of TPO receptor agonists in ITP patients with normal or subnormal platelet counts.45,46 Patients at risk of thrombosis should be excluded from future clinical trials. The second patient, who initially responded to the treatment but relapsed in the third month, died of intracranial hemorrhage. Intracranial hemorrhage was the most common life-threatening bleeding symptom for ITP patients with low platelet counts.47,48
In this clinical trial, we analyzed 115 patients in 2 randomized groups to investigate efficacy and safety. To the best of our knowledge, this is the largest randomized cohort of corticosteroid-resistant or relapsed ITP patients treated with RTX alone or in combination with rhTPO.
In conclusion, the combination of RTX and rhTPO in ITP yields a significantly higher CR rate and shorter TTR compared with RTX monotherapy, indicating that RTX and rhTPO have an additive effect. The combination shows no beneficial effect on the long-lasting response. Thus, this combination therapy represents an effective treatment option for corticosteroid-resistant or relapsed ITP patients.
Acknowledgments
The authors thank Michael Wang (MD Anderson Cancer Center, Houston, TX) for critical revision of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (81270578, 81370623, 81470284, 81125002, and 91442204), the State Program of National Natural Science Foundation of China for Innovative Research Group (81321061), the National Key Basic Research Program of China (973 Program, 2011CB503906), the State Key Clinical Specialty of China for Blood Disorders, the National Public Health Grand Research Foundation (201202017), the National High Technology Research and Development Program of China (863 Program, 2012AA02A505), and the Shandong Province Science and Technology Development Project (2014GSF118036).
Footnotes
Presented orally at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 7-10, 2013.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.Z., M.X., P.Q., J.P., and M.H. designed and performed research, analyzed data, and wrote the paper; H.-y.Z., C.-l.Y., H.-g.Z., Z.-g.C., Y.-s.M., and L.W. performed research and corrected the paper; F.Z., X.W., D.-q.L., K.-h.B., C.-s.Z., C.-s.G., X.-x.C., Q.-c.W., X.-g.L., X.-y.D., and J.L. performed research and analyzed data; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming Hou, Department of Hematology, Qilu Hospital, Shandong University, 107 Wenhuaxi Rd, Jinan, 250012, China; e-mail: houming@medmail.com.cn; and Jun Peng, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Qilu Hospital, Shandong University, 107 Wenhuaxi Rd, Jinan, 250012, China; e-mail: junpeng88@sina.com.cn.
References
- 1.Berchtold P, Wenger M. Autoantibodies against platelet glycoproteins in autoimmune thrombocytopenic purpura: their clinical significance and response to treatment. Blood. 1993;81(5):1246–1250. [PubMed] [Google Scholar]
- 2.Olsson B, Andersson PO, Jernås M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9(9):1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 3.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103(4):1364–1369. doi: 10.1182/blood-2003-08-2672. [DOI] [PubMed] [Google Scholar]
- 4.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 6.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 7.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 8.Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120(5):960–969. doi: 10.1182/blood-2011-12-309153. [DOI] [PubMed] [Google Scholar]
- 9.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–239. doi: 10.1111/j.1365-2141.2004.04889.x. [DOI] [PubMed] [Google Scholar]
- 10.Stasi R. Rituximab in autoimmune hematologic diseases: not just a matter of B cells. Semin Hematol. 2010;47(2):170–179. doi: 10.1053/j.seminhematol.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Stasi R, Pagano A, Stipa E, Amadori S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001;98(4):952–957. doi: 10.1182/blood.v98.4.952. [DOI] [PubMed] [Google Scholar]
- 12.Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146(1):25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 13.Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provan D, Butler T, Evangelista ML, Amadori S, Newland AC, Stasi R. Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica. 2007;92(12):1695–1698. doi: 10.3324/haematol.11709. [DOI] [PubMed] [Google Scholar]
- 15.Zaja F, Battista ML, Pirrotta MT, et al. Lower dose rituximab is active in adults patients with idiopathic thrombocytopenic purpura. Haematologica. 2008;93(6):930–933. doi: 10.3324/haematol.12206. [DOI] [PubMed] [Google Scholar]
- 16.Zaja F, Vianelli N, Volpetti S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–334. doi: 10.1111/j.1600-0609.2010.01486.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 18.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 19.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641–648. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Yang R, Zou P, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96(2):222–228. doi: 10.1007/s12185-012-1124-8. [DOI] [PubMed] [Google Scholar]
- 21.Hou M, Peng J, Shi Y, et al. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70(6):353–357. doi: 10.1034/j.1600-0609.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 22.Mazzucconi MG, Fazi P, Bernasconi S, et al. Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) Thrombocytopenia Working Party. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood. 2007;109(4):1401–1407. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 23.Braendstrup P, Bjerrum OW, Nielsen OJ, et al. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol. 2005;78(4):275–280. doi: 10.1002/ajh.20276. [DOI] [PubMed] [Google Scholar]
- 24.Godeau B, Porcher R, Fain O, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter phase 2 study. Blood. 2008;112(4):999–1004. doi: 10.1182/blood-2008-01-131029. [DOI] [PubMed] [Google Scholar]
- 25.Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Semin Hematol. 2013;50(suppl 1):S18–S21. doi: 10.1053/j.seminhematol.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356–1362. doi: 10.1182/blood-2011-08-374777. [DOI] [PubMed] [Google Scholar]
- 27.Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115(14):2755–2762. doi: 10.1182/blood-2009-07-229815. [DOI] [PubMed] [Google Scholar]
- 28.Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121(11):1976–1981. doi: 10.1182/blood-2012-09-455691. [DOI] [PubMed] [Google Scholar]
- 29.Bussel JB, Lee CS, Seery C, et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica. 2014;99(7):1264–1271. doi: 10.3324/haematol.2013.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peñalver FJ, Jiménez-Yuste V, Almagro M, et al. Multi-institutional Retrospective Spanish Study Group on the Use of Rituximab in Refractory ITP. Rituximab in the management of chronic immune thrombocytopenic purpura: an effective and safe therapeutic alternative in refractory patients. Ann Hematol. 2006;85(6):400–406. doi: 10.1007/s00277-005-0073-1. [DOI] [PubMed] [Google Scholar]
- 31.Dierickx D, Verhoef G, Van Hoof A, et al. Rituximab in auto-immune haemolytic anaemia and immune thrombocytopenic purpura: a Belgian retrospective multicentric study. J Intern Med. 2009;266(5):484–491. doi: 10.1111/j.1365-2796.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- 32.Stasi R, Murali M, Michel M, et al. Evaluation of bleeding-related episodes in patients with immune thrombocytopenia (ITP) receiving romiplostim or medical standard of care. Int J Hematol. 2012;96(1):26–33. doi: 10.1007/s12185-012-1088-8. [DOI] [PubMed] [Google Scholar]
- 33.Kuter DJ, Mathias SD, Rummel M, et al. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 2012;87(5):558–561. doi: 10.1002/ajh.23163. [DOI] [PubMed] [Google Scholar]
- 34.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 35.Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol. 2008;141(2):149–169. doi: 10.1111/j.1365-2141.2008.07054.x. [DOI] [PubMed] [Google Scholar]
- 36.Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013;53(11):2807–2812. doi: 10.1111/trf.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleh MN, Bussel JB, Cheng G, et al. EXTEND Study Group. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121(3):537–545. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 38.Mahévas M, Fain O, Ebbo M, et al. The temporary use of thrombopoietin-receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Br J Haematol. 2014;165(6):865–869. doi: 10.1111/bjh.12888. [DOI] [PubMed] [Google Scholar]
- 39.Nishimoto T, Kuwana M. CD4+CD25+Foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol. 2013;50(suppl 1):S43–S49. doi: 10.1053/j.seminhematol.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110(8):2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 41.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–1150. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 42.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–4645. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Audia S, Samson M, Guy J, et al. Immunologic effects of rituximab on the human spleen in immune thrombocytopenia. Blood. 2011;118(16):4394–4400. doi: 10.1182/blood-2011-03-344051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao YQ, Wang QY, Zhai M, et al. [A multi-center clinical trial of recombinant human thrombopoietin in chronic refractory idiopathic thrombocytopenic purpura]. Zhonghua Nei Ke Za Zhi. 2004;43(8):608–610. [PubMed] [Google Scholar]
- 45.Lakshmanan S, Cuker A. Contemporary management of primary immune thrombocytopenia in adults. J Thromb Haemost. 2012;10(10):1988–1998. doi: 10.1111/j.1538-7836.2012.04876.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109(11):4607–4616. doi: 10.1182/blood-2006-10-019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MS, Kim WC. Intracranial hemorrhage associated with idiopathic thrombocytopenic purpura: report of seven patients and a meta-analysis. Neurology. 1998;50(4):1160–1163. doi: 10.1212/wnl.50.4.1160. [DOI] [PubMed] [Google Scholar]
- 48.Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood. 2005;106(7):2244–2251. doi: 10.1182/blood-2004-12-4598. [DOI] [PubMed] [Google Scholar]