Abstract
Propanil is a postemergence herbicide used primarily in rice and wheat production in the United States. The reported toxicities for propanil exposure include methemoglobinemia, immunotoxicity, and nephrotoxicity. A major metabolite of propanil, 3,4-dichloroaniline (3,4-DCA), has been shown to be a nephrotoxicant in vivo and in vitro, but the nephrotoxic potential of propanil has not been examined in detail. The purpose of this study was to determine the nephrotoxic potential of propanil using an in vitro kidney model, determine whether in vitro propanil nephrotoxicity is due to metabolites arising from propanil hydrolysis, and examine mechanistic aspects of propanil nephrotoxicity in vitro. Propanil, 3,4-DCA, propionic acid (0.1–5.0 mM), or vehicle was incubated for 15–120 min with isolated renal cortical cells (IRCC; ~4 million cells/mL) obtained from untreated male Fischer 344 rats. Cytotoxicity was determined by measuring lactate dehydrogenase release from IRCC. In 120-min incubations, propanil induced cytotoxicity at concentrations >0.5 mM. At 1.0 mM, propanil induced cytotoxicity following 60- or 120-min exposure. Cytotoxicity was observed with 3,4-DCA (2.0 mM) at 60 and 120 min, while propionic acid (5.0 mM) induced cytotoxicity at 60 min. In IRCC pretreated with an antioxidant, cytochrome P450(CYP) inhibitor, flavin adenine dinucleotide monooxygenase activity modulator, or cyclooxygenase inhibitor before propanil exposure (1.0 mM; 120 min), only piperonyl butoxide (0.1 mM), a CYP inhibitor, pretreatment decreased propanil cytotoxicity. These results demonstrate that propanil is an in vitro nephrotoxicant in IRCC. Propanil nephrotoxicity is not primarily due to metabolites resulting from hydrolysis of propanil, but a metabolite resulting from propanil oxidation may contribute to propanil cytotoxicity.
Keywords: nephrotoxicity, in vitro, herbicides, halogenated hydrocarbons, Fischer 344 rats, kidney
INTRODUCTION
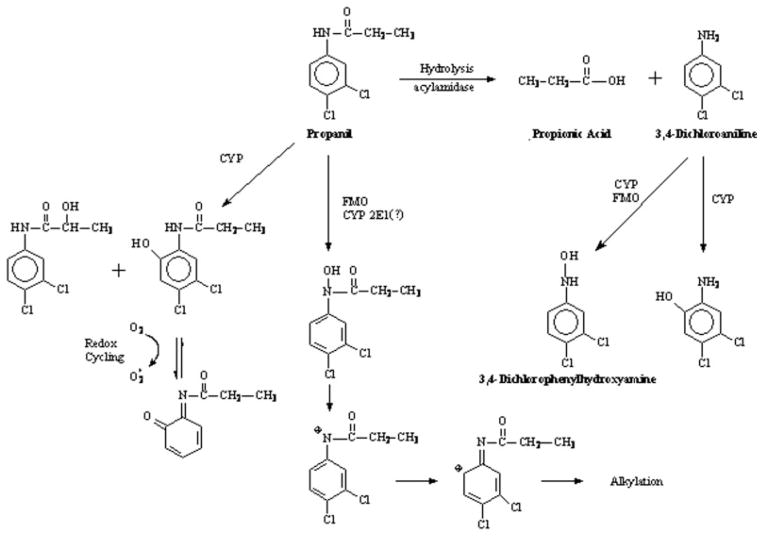
Herbicides represent an important group of agricultural pesticides. One herbicide, propanil (3′,4′-dichloropropionanilide, Fig. 1), is widely used in the United States and other countries as a postemergence contact herbicide, mainly in rice fields, to control weeds (Bartha and Pramer, 1970; Kimbrough, 1980). In plants, propanil inhibits photosynthesis to control broad-leaved and grass weeds.
Fig. 1.
Propanil biotransformation.
Propanil exposure has been associated with toxicity in humans and other mammals. The primary toxicity reported for human poisoning from propanil is methemoglobinemia, which has resulted in fatalities (Kimbrough, 1980; De Silva and Bodinayake, 1997). Methemoglobinemia and hemolytic anemia have also been reported from animal studies with propanil exposure (Ambrose et al., 1972; Singleton and Murphy, 1973; Chow and Murphy, 1975; McMillan et al., 1990b, 1991). Propanil is an immunotoxicant in several models, reducing the production of cytokines as well as the number of CD4+CD8+ thymocytes (Barnett et al., 1992; Blyler et al., 1994; Frost et al., 2001; Malerba et al., 2002). Chloracne observed in industrial workers during propanil manufacture was originally ascribed to propanil, but subsequent studies have demonstrated that this toxicity was due to chemical contaminants (Morse and Baker, 1979; Kimbrough, 1980). Nephrotoxicity and hepatotoxicity have also been reported in cases of human propanil poisoning (Wijekoon et al., 1974; De Silva and Bodinayake, 1997) and animal studies (Stevens and Sumner, 1991; Santillo et al., 1995). Thus, the kidney is one of the targets for propanil-induced toxicity.
Propanil biotransformation includes hydrolysis and oxidation pathways (Fig. 1; McMillan et al., 1990a, 1991). Hydrolysis of propanil yields 3,4-dichloroaniline (3,4-DCA) and propionic acid. Oxidation of propanil most often occurs on the ethyl group of the amide function and at the 6-position on the phenyl ring (McMillan et al., 1990a). The 3,4-DCA produced by propanil hydrolysis can also undergo oxidation at 6-position of the phenyl ring as well as N-oxidation to 3,4-dichlorophenylhydroxylamine (3,4-DCPHA). Although not reported in propanil biotransformation studies, N-hydroxypropanil is a putative metabolite of propanil, since aromatic amides can undergo N-oxidation leading to reactive metabolites (Mulder and Meerman, 1983; Malejka-Giganti and Ritter, 1994).
At least a portion of the toxicity associated with propanil is induced by propanil metabolites. For example, the hematotoxic effects of propanil are due to the hydrolysis of propanil to yield 3,4-DCA (Fig. 1; Chow and Murphy, 1975; McMillan et al., 1990a, 1991; Guilhermino et al., 1998). Subsequent N-oxidation of 3,4-DCA produces 3,4-DCPHA that is responsible for the production of the methemoglobinemia and hemolytic anemia. The propanil metabolite 3,4-DCA can also induce nephrotoxicity in vivo (Lo et al., 1990) and in vitro in rat renal cortical slices (Lo et al., 1990; Valentovic et al., 1995). In addition, 3,4-DCPHA and 2-amino-4,5-dichlorophenol are cytotoxic to renal cortical slices from Fischer 344 rats at concentrations of 0.05 and 0.1mMor higher, respectively (Valentovic et al., 2001, 2002). In some cases, both propanil and its metabolites are directly toxic to target systems. For example, both propanil and 3,4-DCA are toxic to the immune system (Barnett et al., 1992). Studies have indicated that the kidney is a target following propanil exposure (Wijekoon et al., 1974; De Silva and Bodinayake, 1997), but it is unclear whether nephrotoxicity is induced by propanil, its metabolites, or both.
The purpose of this study was to determine the in vitro nephrotoxic potential of propanil using isolated rat renal cortical cells (IRCC). The nephrotoxic potential of the two commercially available propanil hydrolysis metabolites, 3,4-DCA and propionic acid, were also examined. These two metabolites were chosen for investigation because kidney contains the acylamidase enzyme that hydrolyzes propanil (Chow and Murphy, 1975) and will allow for a comparison of the relative nephrotoxic potentials of the parent compound and its two hydrolysis products. The IRCC model was selected for study, because renal cortical cells are the primary target kidney cells for 3,4-DCA in vivo (Lo et al., 1990). By comparing the nephrotoxic potential of propanil, 3,4-DCA and propionic acid in IRCC, the importance of propanil hydrolysis to propanil nephrotoxicity in vitro can be determined. In addition, the effects of several antioxidants, cytochrome P450 (CYP) inhibitors, flavin adenine dinucleotide monooxygenase (FMO) activity modulators, and a cyclooxygenase inhibitor were also examined to gain insights into mechanistic aspects and the role that oxidative metabolites might play in propanil nephrotoxicity.
MATERIALS AND METHODS
Chemicals and Reagents
Propanil (3,4-dichloroproprionanilide), 99% pure, was purchased from ChemService (West Chester, PA). Propionic acid (reagent grade), 3,4-dichloroaniline (3,4-DCA; 98% pure), N-octylamine, and metyrapone were purchased from Aldrich Chemical Co. (Milwaukee, WI). Dimethyl sulfoxide (DMSO; certified grade) was obtained from Fisher Scientific (Fair Lawn, NJ). All other compounds were obtained from Sigma Co. (St. Louis, MO).
Cell Isolation
Male Fischer 344 rats (175–200 g) were obtained from Hilltop Lab Animals (Scottdale, PA) and were placed in standard plastic animal cages prior to use. Animals were allowed at least 1 week to acclimatize to the animal facilities prior to use in experiments. The animal facilities had a controlled temperature (21–23°), humidity (40–55%), and light period (on 0600 h, off 1800 h). All animals were provided humane care in accordance with institutional guidelines and approval.
Following the acclimatization period, rats were anesthetized (pentobarbital sodium, 75 mg/kg, i.p.) and IRCCs were obtained using the collagenase perfusion method of Jones et al. (1979). Initial cell viability was typically 85–95% as judged by the exclusion of trypan blue (2% w/v), and initial lactate dehydrogenase (LDH) release was ~5–10%. The yield of cortical cells for these experiments was typically between 35 and 45 million cells/two kidneys. Cells were resuspended at a concentration of ~4 million cells/mL in Krebs–Henseleit buffer pH 7.37 containing 25 mM Hepes and 2% (w/v) bovine serum albumin. The composition of the IRCC has previously been determined to be ~85% proximal tubular cells, with 15% being distal tubular and collecting duct cells (Jones et al., 1979; Lash and Tokarz, 1989).
Toxicity Studies
Toxicity experiments were conducted by placing 3 mL of the IRCC resuspension in a 25-mL Erlenmeyer flask. The flask was placed in a shaking incubator (37°C water temperature) and sealed with a serum bottle stopper containing an inlet and outlet for gas flow. The atmosphere was equilibrated with 95% O2/5% CO2 with shaking for 5 min. Propanil (0.1, 0.5, 1.0, or 2.0 mM), 3,4-DCA (1.0 or 2.0 mM), propionic acid (1.0 or 5.0 mM), or vehicle (30 μL) was then added. The incubations were then continued between 15 and 120 min. DMSO was the vehicle for propanil and 3,4-DCA, and distilled water was the vehicle for propionic acid. At the end of the incubation period, flasks were removed, and a 0.5-mL aliquot was taken from each flask for determination of LDH release as a measure of cytotoxicity. The aliquots are centrifuged (3000 × g, 5 min), and the supernatant decanted. Cells are resuspended in 1 mL of Triton X-100 (10% solution) to release the remaining cellular LDH activity. LDH activity was then determined in each fraction using Sigma Kit No. LDL-20 or purchased reagents (Sigma) and the same assay procedure. LDH activity quantification is based on a kinetic assay measuring the amount of NADH produced from NAD (as an increased absorbance at 340 nm) when LDH catalyzes the conversion of lactate to pyruvate at 30°C over 60 s. LDH release was expressed as % of total.
In some experiments, cells were pretreated with an antioxidant/ free radical inhibitor, CYP inhibitor, FMO activity modulator, or a cyclooxygenase inhibitor before the addition of propanil (1.0 mM) or propanil vehicle. The pretreatments and pretreatment times are shown in Table I. Following the addition of propanil or propanil vehicle, incubations were continued for 120 min and cytotoxicity determined as described earlier. Pretreatment concentrations were based on previously published studies with these compounds (Lock et al., 1993; Rodriguez and Acosta, 1997; Baliga et al., 1998; Valentovic et al., 1999; Kajita et al., 2002, Duringer et al., 2004; O’Brien and Siraki, 2005).
TABLE I.
Antioxidant and metabolic activity modulator pretreatments
| Pretreatment Compound | Concentration (mM) | Pretreatment Time (min) |
|---|---|---|
| Antioxidants free radical inhibitors | ||
| Ascorbate | 2.0 | 5 |
| Deferoxamine | 0.1 | 5 |
| Glutathione | 1.0 | 30 |
| α-Tocopherol | 1.0 | 5 |
| Cyclooxygenase inhibitor | ||
| Indomethacin | 1.0 | 15 |
| CYP inhibitors | ||
| Isoniazid | 1.0 | 5 |
| Metyrapone | 1.0 | 5 |
| Piperonyl Butoxide | 1.0 | 15 |
| FMO inhibitor/Enhancer | ||
| Methimazole | 1.0 | 5 |
| N-Octylamine | 0.2 | 5 |
Statistics
Values are expressed as the mean ± SE for four experiments per group. Values from each rat represented an N = 1. Data were analyzed using a one-way analysis of variance followed by a Dunnett’s or Newman–Keuls analysis. All statistical tests were run at a 95% confidence interval and significance noted at P<0.05.
RESULTS
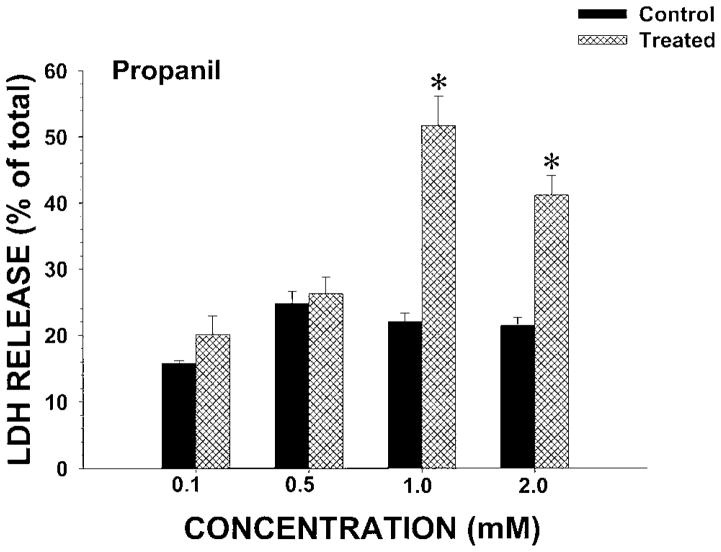
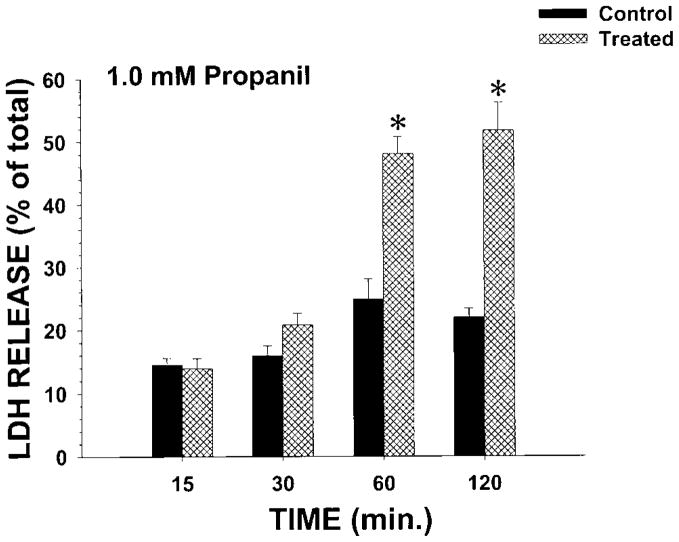
To determine whether propanil was toxic to IRCC, initial studies examined the concentration–cytotoxicity relationship of propanil at 120 min. Propanil concentrations of 0.1 or 0.5 mM did not induce cytotoxicity (↑ LDH release) at 120 min (Fig. 2). However, at propanil bath concentrations of 1.0 mM or greater with 120 min incubations, LDH release was significantly increased (Fig. 2). To determine the temporal aspects of propanil toxicity, a concentration of 1.0 mM propanil was selected for study. Incubations were conducted between 15 and 120 min and LDH release measured (Fig. 3). No cytotoxicity was observed at 15 or 30 min, but cytotoxicity was evident at 60 and 120 min with propanil 1.0 mM. Thus, propanil cytotoxicity was both concentration- and time-dependent.
Fig. 2.
Effect of propanil concentration on LDH release from IRCC. IRCCs (~4 million/mL) were incubated with vehicle (control) or propanil (treated) at various concentrations for 120 min and LDH release (% of total) determined. Values are means ± SE from four separate experiments. An * indicates significantly different from the appropriate control group value, P<0.05.
Fig. 3.
Effect of propanil 1.0 mM on LDH release from IRCC at various times. IRCCs (~4 million/mL) were incubated with vehicle (control) or propanil (treated) for 15 to 120 min and LDH release (% of total) determined. Values are means ± SE from four separate experiments. An * indicates significantly different from the appropriate control group value, P<0.05.
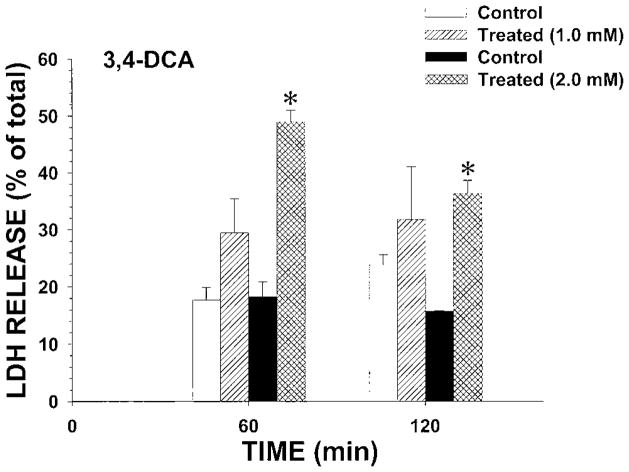
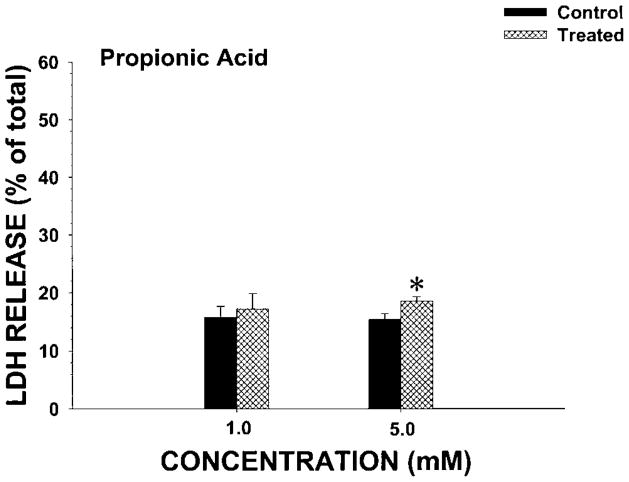
To determine whether the nephrotoxic effects observed with propanil were due to propanil hydrolysis to metabolites, the cytotoxicity of the two commercially available metabolites arising from propanil hydrolysis (3,4-DCA and propionic acid) was also examined for nephrotoxic potential in IRCC. At a bath concentration of 1.0 mM, 3,4-DCA did not induce cytotoxicity at 60 or 120 min (Fig. 4). However, 2.0 mM 3,4-DCA induced cytotoxicity at both 60 and 120 min (Fig. 4). These results were similar to those observed with 1.0 mM propanil (Fig. 3). In contrast, 1.0 mM propionic acid did not induce cytotoxicity at 60 min, and 5.0 mM propionic acid only induced a small increase in LDH release (Fig. 5). Thus, at even a fivefold increase in concentration over the minimal nephrotoxic propanil concentration, propionic acid only weakly induced cytotoxicity. These results suggest that propanil-induced nephrotoxicity was not due to the hydrolysis biotransformation pathway.
Fig. 4.
Effect of 3,4-DCA on LDH release from IRCC. IRCCs (~4 million/mL) were incubated with vehicle (control) or 3,4-DCA (treated) for 60 or 120 min and LDH release (% of total) determined. Values are means ± SE from four separate experiments. An * indicates significantly different from the appropriate control group value, P<0.05.
Fig. 5.
Effect of propionic acid on LDH release from IRCC. IRCCs (~4 million/mL) were incubated with vehicle (control) or propionic acid (treated) for 60 min and LDH release (% of total) determined. Values are means ± SE from four separate experiments. An * indicates significantly different from the appropriate control group value, P<0.05.
To determine whether oxidative metabolites of propanil might contribute to propanil cytotoxicity, three CYP inhibitors were examined: metyrapone, piperonyl butoxide, and isoniazid (Fig. 6). Isoniazid pretreatment had no effect on propanil cytotoxicity, while metyrapone pretreatment slightly, but significantly, increased propanil toxicity. Only piperonyl butoxide pretreatment decreased propanil cytotoxicity. The FMO inhibitor methimazole also had no effect on propanil cytotoxicity (Table II). A similar finding occurred with N-octylamine, a FMO enhancer, and indomethacin, a cyclooxygenase inhibitor (Table II), where propanil cytotoxicity was not altered by these pretreatments. These results suggest that a CYP inhibited by piperonyl butoxide may mediate propanil cytotoxicity, but that FMO or cyclooxygenase oxidation do not contribute to the formation of toxic propanil metabolites.
Fig. 6.
Effect of pretreatment with a CYP inhibitor on propanil-induced LDH release from IRCC. IRCCs (~4 million/ mL) were pretreated with a CYP inhibitor or vehicle prior to addition of propanil vehicle (control) or propanil (1 mM). Incubations were continued for 120 min and LDH release (% of total) determined. Values are means ± SE from four separate experiments. An * indicates significantly different from the appropriate control group value, P < 0.05. A ◆ indicates significantly different from the propanil alone value, P<0.05.
TABLE II.
| Pretreatment | Concentration (mM) | Treatment | %LDH (Mean ± SE) |
|---|---|---|---|
| Indomethacin (Indo) | 1.0 | Control | 25.4 ± 1.9 |
| Propanil | 44.7 ± 1.6 | ||
| Indo | 29.5 ± 0.6 | ||
| Propanil + Indo | 43.4 ± 2.0 | ||
| Methimazole (MTZ) | 1.0 | Control | 21.8 ± 0.4 |
| Propanil | 40.6 ± 1.9 | ||
| MTZ | 22.0 ± 2.0 | ||
| Propanil + MTZ | 41.3 ± 1.1 | ||
| N-Octylamine (N-Oct) | 0.2 | Control | 22.2 ± 2.0 |
| Propanil | 44.8 ± 1.5 | ||
| N-Oct | 25.6 ± 1.0 | ||
| Propanil + N-Oct | 42.3 ± 1.9 |
Incubations were conducted at 37°C for 120 min.
Values are means ± SE for N = 4 experiments.
The effect of several antioxidants on propanil cytotoxicity was also examined (Table III). However, ascorbate, α-tocopherol, or glutathione pretreatments did not alter propanil cytotoxicity. In addition, pretreatment with the iron chelator deferoxamine did not alter propanil nephrotoxicity (Table III). These findings suggest that free radical formation and oxidative stress do not contribute to propanil’s effects on IRCC.
TABLE III.
| Pretreatment | Concentration (mM) | Treatment | %LDH |
|---|---|---|---|
| Ascorbate (Asc) | 2.0 | Control | 22.2 ± 2.1 |
| Propanil | 37.5 ± 4.4 | ||
| Asc | 24.3 ± 2.0 | ||
| Propanil + Asc | 36.7 ± 2.4 | ||
| Glutathione (GSH) | 1.0 | Control | 18.2 ± 1.3 |
| Propanil | 37.0 ± 3.0 | ||
| GSH | 22.2 ± 0.7 | ||
| Propanil + GSH | 32.2 ± 2.7 | ||
| Deferoxamine (Def) | 0.1 | Control | 24.3 ± 1.8 |
| Propanil | 35.4 ± 0.8 | ||
| Def | 23.9 ± 3.0 | ||
| Propanil + Def | 42.1 ± 3.0 | ||
| α-Tocopherol (α-Toc) | 1.0 | Control | 24.8 ± 0.7 |
| Propanil | 36.9 ± 0.8 | ||
| Asc | 24.1 ± 0.9 | ||
| Propanil + α-Toc | 33.7 ± 1.0 |
Incubations were conducted at 37°C for 120 min.
Values are means ± SE for N = 4 experiments.
DISCUSSION
The results of this study demonstrate that propanil has the potential to induce nephrotoxicity in vitro. Acute exposure to a bath concentration of at least 1.0 mM propanil and an exposure time of 60 min are required for cytotoxicity to be observed in this acute exposure kidney model. Although a longer exposure time might have resulted in a lower minimum toxic concentration of propanil, the IRRC model is only viable for ~2 to 3 h. Also, 3,4-DCA was a less potent nephrotoxicant than propanil in this in vitro model, and propionic acid was only a very weak nephrotoxicant at a fivefold higher concentration than the minimum nephrotoxic propanil concentration. These findings suggest that although 3,4-DCA may contribute to propanil nephrotoxicity in vitro, propanil hydrolysis-derived metabolites do not appear to be responsible for inducing most of the toxicity. The finding that both propanil and 3,4-DCA are nephrotoxic in vitro is similar to what is observed for the effects of propanil and 3,4-DCA on the immune system, where both propanil and 3,4-DCA have adverse effects (Barnett et al., 1992). Part of the differential nephrotoxicity observed between propanil and 3,4-DCA may be related to the rates at which these two compounds accumulate in IRCC. However, the relative rates of accumulation of propanil versus 3,4-DCA remains to be determined.
Nonetheless, using rat IRCC, it is clear that propanil can induce nephrotoxicity in vitro. The nephrotoxicity induced by propanil was concentration- and time-dependent, and was not completely due to propanil hydrolysis to 3,4-DCA or propionic acid. Studies with the inhibitors of oxidative biotransformation revealed that propanil cytotoxicity was reduced by piperonyl butoxide, increased by metyrapone, and not altered by isoniazid. These results support one or more CYP as contributing to the formation of a toxic propanil metabolite. Interestingly, piperonyl butoxide is an inhibitor of CYP1A among other CYPs, while metyrapone can enhance CYP1A activity in vitro (Aubrecht et al., 1996; Wassenberg and Di Giulio, 2004). Isoniazid has minimal if any effects on CYP1A. Thus, it might appear that one or more CYP1A family members may contribute to the formation of cytotoxic, propanil-derived metabolites. In addition, 2-amino-4,5-dichlorophenol is a potent in vitro nephrotoxicant, inducing cytotoxicity at bath concentrations of 50 μM or greater (Valentovic et al., 2002). The nephrotoxic potential of 6-hydroxypropanil has not been examined, but it is likely that both phenolic propanil-derived metabolites would be formed via CYP1A-mediated oxidation. However, although CYP1A is inducible in rat proximal tubule cells, CYP1A does not appear to be constitutively expressed in rat kidney (Lock and Reed, 1998) and the CYP activity modulators used in this study can affect several CYPs. Thus, what role renal CYP1A might play in propanil nephrotoxicity remains to be determined. Further studies are needed to more clearly define the role of specific propanil metabolites in propanil nephrotoxicity in vitro and which CYP(s) contribute to the formation of nephrotoxic propanil metabolites.
Studies with the antioxidants suggest that oxidative stress is not an important mechanism for propanil-induced cytotoxicity. In addition, the inability of glutathione to provide any protection from propanil’s effects suggests that reactive propanil metabolites are probably not formed in this model. Thus, redox cycling of phenolic metabolites to create free radicals and oxidative stress is not supported by the results. Likewise, N-oxidation of propanil or other propanil metabolites leading to the formation of a reactive metabolite is not supported by the results. Co-oxidation of propanil metabolites, such as 2-amino-4,5-dichlorophenol, by cyclooxygenase does not appear to be a mechanism for forming cytotoxic metabolites, since indomethacin did not alter propanil cytotoxicity. Possible explanations for these results are that oxidative metabolites of propanil directly inhibit or rapidly alkylate/arylate essential cellular systems (proteins, enzymes, DNA) to lead to cell death. However, which systems/organelles might be the target(s) for propanil is unclear. Thus, additional studies are required to more clearly define the mechanism(s) of propanil cytotoxicity.
The nephrotoxic chemical species following propanil exposure in vivo also remains to be determined. Animal studies that have suggested that the kidney might be a target for propanil did not investigate the role of metabolites (Ambrose et al., 1972). Evidence for propanil-induced nephrotoxicity from human studies comes from case reports where renal function data are not always presented and little evidence of blood levels of propanil or its metabolites are available to calculate organ exposure (Wijekoon et al., 1974; De Silva and Bodinayake, 1997; Yamazaki et al., 2001; Eddleston et al., 2002). Thus, in humans, where propanil nephrotoxicity has been detected, the human studies have not examined the role of propanil metabolites in propanil nephrotoxicity. Previous rat studies have documented that 3,4-DCA, 3,4-DCPHA, and 2-amino-4,5-dichlorophenol are nephrotoxicants in vivo and/or in vitro (Lo et al., 1990; Valentovic et al., 1995, 2001, 2002). Thus, these metabolites may contribute to propanil nephrotoxicity in vivo via direct or indirect mechanisms. However, additional in vivo studies are needed to determine the chemical species responsible for propanil-induced nephrotoxicity.
Acknowledgments
The authors acknowledge the technical assistance of Amanda Casto, Sukanya Baksi, and Tim Crislip.
Contract grant sponsor: NIH (National Center for Research Resources).
Contract grant number: 5 P20 RR016477.
References
- Ambrose AM, Larson PS, Borzelleca JF, Hennigar GR., Jr Toxicological studies on 3′,4′-dichloropropioanilide. Toxicol Appl Pharmacol. 1972;23:650–659. doi: 10.1016/0041-008x(72)90106-8. [DOI] [PubMed] [Google Scholar]
- Aubrecht J, Hirsch-Ernst KI, Foth H, Kahl GF, Hohne MW. Differential induction of mRNA expression of cytochromes P450 (CYP2B1 and CYP1A1/2) by metyrapone in primary rat hepatocytes. Res Commun Mol Pathol Pharmacol. 1996;94:47–61. [PubMed] [Google Scholar]
- Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;54:1562–1569. doi: 10.1046/j.1523-1755.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- Barnett JB, Gandy J, Wilbourn D, Theus SA. Comparison of the immunotoxicity of propanil and its metabolite, 3,4-dichloroaniline, in C57BI/6 mice. Fundam Appl Toxicol. 1992;18:628–631. doi: 10.1016/0272-0590(92)90124-z. [DOI] [PubMed] [Google Scholar]
- Bartha R, Pramer D. Metabolism of acrylanilide herbicides. Adv Appl Microbiol. 1970;13:317–341. [Google Scholar]
- Blyler G, Landreth KS, Lillis T, Schafer R, Theus SA, Gandy J, Barnett JB. Selective myleotoxicity of propanil. Fundam Appl Toxicol. 1994;22:505–510. doi: 10.1006/faat.1994.1057. [DOI] [PubMed] [Google Scholar]
- Chow AYK, Murphy SD. Propanil (3,4-dichloropropionanilide)-induced methemoglobin formation in relation to its metabolism in vitro. Toxicol Appl Pharmacol. 1975;33:14–20. doi: 10.1016/0041-008x(75)90238-0. [DOI] [PubMed] [Google Scholar]
- De Silva WAS, Bodinayake CK. Propanil poisoning. Ceylon Med J. 1997;42:81–84. [PubMed] [Google Scholar]
- Duringer JM, Buhler DR, Craig AM. Comparison of hepatic in vitro metabolism of the pyrrolizidine alkaloid senecionine in sheep and cattle. Am J Vet Res. 2004;65:1563–1572. doi: 10.2460/ajvr.2004.65.1563. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Rajapakshe M, Roberts D, Reginald K, Rezvi Sheriff MH, Dissanayake W, Buckley N. Severe propanil [N-(3, 4-dicholorophenyl)propanamide] pesticide self-poisoning. J Toxicol Clin Toxicol. 2002;40:847–854. doi: 10.1081/clt-120016955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LL, Neeley YX, Schafer R, Gibson LF, Barnett JB. Propanil inhibits tumor necrosis factor-α production by reducing nuclear levels of the transcription factor nuclear factor-κB in the macrophage cell line IC-21. Toxicol Appl Pharmacol. 2001;172:185–193. doi: 10.1006/taap.2001.9153. [DOI] [PubMed] [Google Scholar]
- Guilhermino L, Soares AMVM, Carvalho AP, Lopes MC. Acute effects of 3,4-dichloroaniline on blood of male Wistar mice. Chemosphere. 1998;37:619–632. doi: 10.1016/s0045-6535(98)00087-3. [DOI] [PubMed] [Google Scholar]
- Jones DP, Sundby G-B, Ormsted K, Orrenius S. Use of isolated kidney cells for study of drug metabolism. Biochem Pharmacol. 1979;28:929–935. doi: 10.1016/0006-2952(79)90378-2. [DOI] [PubMed] [Google Scholar]
- Kajita J, Inano K, Fuse E, Kuwabara T, Kobayashi H. Effects of olopatadine, a new antiallergic agent, on human liver microsomal cytochrome P450 activities. Drug Metab Dispos. 2002;30:1504–1511. doi: 10.1124/dmd.30.12.1504. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD. Human health effects of selected pesticides, chloroaniline derivatives. J Environ Sci Health. 1980;15:972–992. doi: 10.1080/03601238009372225. [DOI] [PubMed] [Google Scholar]
- Lash LH, Tokarz JJ. Isolation of two distinct populations of cells from rat kidney cortex and their use in the study of chemical-induced toxicity. Anal Biochem. 1989;182:271–279. doi: 10.1016/0003-2697(89)90593-9. [DOI] [PubMed] [Google Scholar]
- Lo H-H, Brown PI, Rankin GO. Acute nephrotoxicity induced by isomeric dichloroanilines in Fischer 344 rats. Toxicology. 1990;63:215–231. doi: 10.1016/0300-483x(90)90044-h. [DOI] [PubMed] [Google Scholar]
- Lock EA, Reed CJ. Xenobiotic metabolizing enzymes of the kidney. Toxicol Pathol. 1998;26:18–25. doi: 10.1177/019262339802600102. [DOI] [PubMed] [Google Scholar]
- Lock EA, Cross TJ, Schnellmann RG. Studies on the mechanism of 4-aminophenol-induced toxicity to renal proximal tubules. Hum Exp Toxicol. 1993;12:383–388. doi: 10.1177/096032719301200507. [DOI] [PubMed] [Google Scholar]
- Malejka-Giganti D, Ritter CL. Peroxidative metabolism of carcinogenic N-arylhydroxamic acids: Implications for tumorigenesis. Environ Health Perspect. 1994;102 (Suppl 6):75–81. doi: 10.1289/ehp.94102s675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba I, Castoldi AF, Parent-Massin D, Gribaldo L. In vitro myleotoxicity of propanil and 3,4-dichloroaniline on murine and human CFU-E/BFU-E progenitors. Toxicol Sci. 2002;69:433–438. doi: 10.1093/toxsci/69.2.433. [DOI] [PubMed] [Google Scholar]
- McMillan DC, Freeman JP, Hinson JA. Metabolism of the arylamide herbicide propanil. I. Microsomal metabolism and in vitro methemoglobinemia. ToxicolAppl Pharmacol. 1990a;103:90–101. doi: 10.1016/0041-008x(90)90265-v. [DOI] [PubMed] [Google Scholar]
- McMillan DC, McRae TA, Hinson JA. Propanil-induced methemoglobinemia and hemoglobin binding in the rat. Toxicol Appl Pharmacol. 1990b;105:503–507. doi: 10.1016/0041-008x(90)90153-l. [DOI] [PubMed] [Google Scholar]
- McMillan DC, Bradshaw TP, Hinson JA, Jollow DJ. Role of metabolites in propanil-induced hemolytic anemia. Toxicol Appl Pharmacol. 1991;110:70–78. doi: 10.1016/0041-008x(91)90290-u. [DOI] [PubMed] [Google Scholar]
- Morse DL, Baker EL., Jr Propanil–chloracne and methomyl toxicity in workers of a pesticide manufacturing plant. Clin Toxicol. 1979;15:13–21. doi: 10.3109/15563657908992475. [DOI] [PubMed] [Google Scholar]
- Mulder GJ, Meerman JH. Sulfation and glucuronidation as competing pathways in the metabolism of hydroxamic acids: the role of N.O-sulfation in chemical carcinogenesis of aromatic amines. Environ Health Perspect. 1983;49:27–32. doi: 10.1289/ehp.834927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PJ, Siraki AG. Accelerated cytotoxicity mechanism screening using drug metabolizing enzyme modulators. Curr Drug Metab. 2005;6:101–109. doi: 10.2174/1389200053586082. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Acosta D., Jr N-Deacetyl ketoconazole-induced hepatotoxicity in a primary culture system of rat hepatocytes. Toxicology. 1997;117:123–131. doi: 10.1016/s0300-483x(96)03560-3. [DOI] [PubMed] [Google Scholar]
- Santillo M, Rippa C, Della Morte R, Villani GR, Santangelo F, Staiano N, Mondola P. Enhancement of tissue lipoperoxidation in propanil-treated rats. Toxicol Lett. 1995;78:215–218. doi: 10.1016/0378-4274(95)03257-l. [DOI] [PubMed] [Google Scholar]
- Singleton SD, Murphy SD. Propanil (3,4-dichloropropionanilide)-induced methemoglobin formation in mice in relation to acylamidase activity. Toxicol Appl Pharmacol. 1973;25:20–29. doi: 10.1016/0041-008x(73)90158-0. [DOI] [PubMed] [Google Scholar]
- Valentovic MA, Ball JG, Anestis DK, Rankin GO. Comparison of the in vitro toxicity of dichloroaniline structural isomers. Toxicol In Vitro. 1995;9:75–81. doi: 10.1016/0887-2333(94)00188-z. [DOI] [PubMed] [Google Scholar]
- Valentovic M, Meadows MK, Harmon RC, Ball JG, Hong SK, Rankin GO. 2-Amino-5-chlorophenol toxicity in renal cortical slices from Fischer 344 rats: Effects of antioxidants and sulfhydryl agents. Toxicol Appl Pharmacol. 1999;161:1–9. doi: 10.1006/taap.1999.8784. [DOI] [PubMed] [Google Scholar]
- Valentovic M, Ball JG, Stoll S, Rankin GO. 3,4-Dichlorophenylhydroxylamine cytotoxicity in renal cortical slices from Fischer 344 rats. Toxicology. 2001;162:149–156. doi: 10.1016/s0300-483x(01)00356-0. [DOI] [PubMed] [Google Scholar]
- Valentovic MA, Ball JG, Sun H, Rankin GO. Characterization of 2-amino-4,5-dichlorophenol (2A45CP) in vitro toxicity in renal cortical slices from male Fischer 344 rats. Toxicology. 2002;172:113–123. doi: 10.1016/s0300-483x(01)00597-2. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res. 2004;58:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wijekoon PNB, Sivaramakrishna N, Nimalasuriya A. Acute haemolysis and renal failure in chlorinated hydrocarbon poisoning. Ceylon Med J. 1974;19:37–38. [PubMed] [Google Scholar]
- Yamazaki M, Terada M, Kuroki H, Honda K, Matoba R, Mitsukuni Y. Pesticide poisoning initially suspected as a natural death. J Forensic Sci. 2001;46:165–170. [PubMed] [Google Scholar]