Abstract
Tumour and organ microenvironments are crucial for cancer progression and metastasis. Crosstalk between multiple non-malignant cell types in the microenvironments and cancer cells promotes tumour growth and metastasis. Blood and lymphatic endothelial cells (BEC and LEC) are two of the components in the microenvironments. Tumour blood vessels (BV), comprising BEC, serve as conduits for blood supply into the tumour, and are important for tumour growth as well as haematogenous tumour dissemination. Lymphatic vessels (LV), comprising LEC, which are relatively leaky compared with BV, are essential for lymphogenous tumour dissemination. In addition to describing the conventional roles of the BV and LV, we also discuss newly emerging roles of these endothelial cells: their crosstalk with cancer cells via molecules secreted by the BEC and LEC (also called angiocrine and lymphangiocrine factors). This review suggests that BEC and LEC in various microenvironments can be orchestrators of tumour progression and proposes new mechanism-based strategies to discover new therapies to supplement conventional anti-angiogenic and anti-lymphangiogenic therapies.
Introduction
Hallmarks of cancer have been proposed by Hanahan and Weinberg: the hallmarks include proliferative signalling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis (Ref. 1). Recently, tumour and organ microenvironments have been emerging as targets to effectively treat tumour growth and metastasis (Refs 2, 3). Non-cancer stromal and parenchymal cells residing in these microenvironments largely contribute to cancer progression through their crosstalk with cancer cells, extracellular matrix (ECM) and other non-cancer cells (Ref. 4). This crosstalk is achieved by numerous secreted factors from diverse cell types, and their corresponding receptor signalling pathways (Ref. 5). These cell-to-cell cross-communications promote tumour growth (Ref. 6), angiogenesis (Ref. 7) and invasion (Ref. 8); provide cancer cells with stem cell-like properties (Ref. 9) and epithelial-to-mesenchymal transition (EMT) phenotypes (Ref. 10); and cause tumour drug resistance (Ref. 11) and modify host immunity to protect cancer cells from anti-tumour immune reaction. Importantly, these non-cancer cells are genetically stable, thus more targetable, compared with cancer cells that undergo frequent genetic mutations, epigenetic alterations and exhibit heterogeneity (Ref. 12). Therefore, targeting these non-cancer cell types and their secreted factors and signals in the tumour and organ microenvironments can serve as an effective strategy to defeat cancer.
Among the crucial cell types in the tumour and organ microenvironments, blood and lymphatic endothelial cells (BEC and LEC) are the components of blood vessels (BV) and lymphatic vessels (LV), respectively (Refs 13, 14). Tumour BV play a role as conduits for blood supply into the tumour, which is pivotal for tumour growth. These BV also contribute to haematogenous tumour cell spreading. Tumour LV are particularly important for metastasis, as the LV are only sparsely covered by pericytes and smooth muscle cells, and thus more permeable compared with BV (Ref. 15). These are among the reasons that in certain cancers, such as breast cancer, tumour dissemination occurs preferentially via stromal and peritumoural LV. The conventional roles of BV and LV are limited to their functions as conduits for the delivery of oxygen, nutrients, lymph fluid and for metastatic tumour cells. Roles of the factors secreted by BV and LV and the signals mediated by them in the promotion of cancer and metastasis in particular are relatively less well understood. Recently, it has been reported that the cells lining the blood (BEC) and lymphatic (LEC) vessels exhibit distinct gene expression profiles (Ref. 16), suggesting that BV and LV and the diverse set of proteins they secrete may play more inductive roles in cancer progression. The subsets of proteins present in the conditioned media from cultured cells are referred to as ‘secretomes’ (Ref. 17). Specifically, BEC- and LEC-secreted factors are referred to as ‘angiocrine’ (Ref. 18) and ‘lymphangiocrine’ factors, respectively (Ref. 19). These endothelium-derived factors are actively involved in tumour progression. Therefore, the understanding of the angiocrine and lymphangiocrine factors adds BEC and LEC to cancer-promoting orchestrators in microenvironments beyond their conventional roles as components of the passive conduits and suggests more improved, mechanism-based strategies upon current anti-angiogenic or anti-lymphangiogenic therapies.
In this review, we first discuss tumour and organ microenvironments, with a focus on angiogenesis and lymphangiogenesis in these microenvironments. We next discuss BEC- and LEC-secreted factors and their roles in cancer. Lastly, we address clinical implications and applications and outstanding research questions.
Microenvironment in cancer
Directly targeting tumour cells, which are genetically unstable and prone to mutations, often leads to resistance to therapy and a risk of tumour recurrence. However, because the non-cancer cell types in the tumour and organ microenvironments are genetically stable, targeting them and the microenvironmental regulation of tumour progression is an attractive alternative. Here we discuss two distinct microenvironments in cancer: the tumour microenvironment and the organ microenvironment.
Tumour microenvironment
The tumour microenvironment is the cellular environment in which the tumour exists and it consists of ECM and diverse types of non-malignant cells, including cancer-associated fibroblasts (CAF), pericytes, macrophages, dendritic cells (DC), mast cells, lymphocytes, endothelial cells and their precursors in tumours (Ref. 4). Among them, immune cells and mesenchymal cell types have been well studied.
Immunecells (e.g. macrophages, DC, lymphocytes and mast cells) are recruited to the TME where they express diverse tumour-promoting signals. Tumour-associated macrophages (TAM) are well-studied immune cell types and are generally known to be immunosuppressive and pro-angiogenic. TAM are one of the myeloid-derived suppressor cells (MDSC) which are a heterogeneous population of myeloid cells with a potential to repress T cell responses (Ref. 20). Specifically, the M2-type TAM overexpress and secrete pro-angiogenic factors such as vascular endothelial growth factor (VEGF), tumour necrosis factor alpha (TNFα), fibroblast growth factor basic (bFGF), matrix metalloproteinase (MMP)-2, −7, −9 and −12, as well as epidermal growth factor (EGF) to facilitate tumour growth, invasion and metastasis (Ref. 21). TAM also induce lymphangiogenesis (Ref. 22). Subpopulations of TAM expressing VEGFC/D and vascular endothelial growth factor receptor 3 (VEGFR3) have been identified. It is thought that the TAM-expressed VEGFC/D could induce peritumoural lymphangiogenesis (Ref. 22). TAM express immunosuppressive factors, including prostaglandin E2, interleukin 10 (IL10) and transforming growth factor beta (TGFβ) to facilitate escape of the tumour from attack by the immune system (Ref. 23). DC are the most potent antigen-presenting cells (APC) in general, thus they can activate T lymphocytes and trigger antigen-specific anti-tumour immune responses (Ref. 24). However, the DC recruited to the TME are impaired in their immunity and start to produce pro-angiogenic factors such as TNFα, TGFβ and granulocyte macrophage colony-stimulating factor (GM-CSF; Ref. 25). Mechanistically, tumour-secreted factors such as IL6 and TNF suppress DC maturation by activating the phospho-signal transducer and activator of transcription 3 (Stat3) pathways or inhibiting the toll-like receptor (TLR) pathways (Ref. 26). Tumour infiltrating lymphocytes (TIL) and their modifications are also crucial for tumour cell survival in TME (Refs 27, 28). Although the TIL are supposed to be anti-tumourigenic, TME induces apoptosis of the TIL by secreting tumour exosomes that contain apoptosis ligands (Ref. 29). T lymphocytes activity in TME can also be edited by LV activity. CCL21 expressed by the LV recruits CCR7-positive naive T cells into the lymphatic systems in the tumour stroma where they can be conditioned to be less immune reactive by tumour-secreted cytokines (Ref. 30). Tumour-secreted TGFβ promotes tumour-associated regulatory T cell (Treg) and MDSC activities. These cells impair cytotoxic T lymphocytes (CTL) and helper T1 cell functions (Ref. 30).
Mesenchymal cell types, such as fibroblasts are also pivotal for tumour progression (Refs 31, 32). Physiological fibroblasts or their precursors are recruited to the TME where several paracrine factors from cancer cells or stromal cells reprogram the fibroblasts to be pro-tumourigenic: these pathological fibroblasts are referred to as CAF (Ref. 33). CAF can also be derived from pericytes, smooth muscle cells and mesenchymal stem cells in the TME. CAF generally feature rapid self-proliferation and up-regulation of tumour-promoting genes [CXC chemokine ligand 12 (CXCL12); TGFβ; hepatocyte growth factor (HGF)], pro-inflammatory genes (IL6, IL8) and pro-angiogenic genes [MMPs; platelet-derived growth factors (PDGFs)] (Ref. 31). CAF also confer cancer cells with resistance to RAF inhibitors by HGF production (Ref. 34); with resistance to anti-VEGF therapy by activating the PDGFC signal (Ref. 35). Moreover, CAF modify host immunity (Refs 36, 37) and induce cancer cells to undergo EMT (Ref. 38).
Organ microenvironment
Metastasis requires multiple steps; tumour cell intravasation into the vasculature at the primary tumour site, recruitment and survival of the tumour cells in the blood during circulation, extravasation into the metastatic organ, and seeding, proliferation, angiogenesis and colonisation in the organ microenvironment (Refs 39, 40). The organ microenvironment is the cellular environment in which organ-specific parenchymal and stromal cells exist and tumour cells can metastasise (Ref. 3). Specific organ microenvironment serves as metastatic niche and determines the extent of tumour cell proliferation, invasion and survival (Ref. 41).
According to the ‘seed and soil hypothesis’ by Stephen Paget (Ref. 42), metastatic cancer cells function as ‘seeds’ and a particular organ microenvironment serves as the ‘soil’. The ‘soil’ must be prepared and pre-conditioned to properly function as a metastatic niche. A number of studies have shown evidence supporting this hypothesis. For example, orthotopic versus ectopic murine tumour models revealed that cancer cells implanted in ectopic sites grow slowly and metastasise rarely compared with those in orthotopic sites (Ref. 43). Organs with low incidence of neoplasm metastasis have been reported and it has been found that these organs are also associated with poor metastatic niche formation (Ref. 44). Some organs that are vulnerable to metastasis such as lymph nodes (LN), bone marrow and lungs are found to promote tumour cell recruitment, immune modification, vascular remodelling and enhanced angiogenesis (Refs 45, 46, 47). It has also been reported that tumour metastasis is organ-specific (Refs 48, 49, 50, 51). Breast cancer mostly metastasise to the bones, lungs, brain and liver (Ref. 50). Leukaemias metastasise preferentially to certain parts of the nervous system; they metastasise to the leptomeninges, which are the two innermost layers of the membrane that envelops the brain and spinal cord, but rarely to the brain parenchyma (Ref. 52). Most head and neck cancers metastasise to the regional LN (e.g. cervical LN) and salivary glands, while distal metastases are uncommon (Ref. 53). For example, autopsies of over 4000 patients who died of head and neck squamous cell carcinoma (HNSCC) showed that the fraction of distant metastasis was <1% of local spread (Ref. 54). These data suggest that the nature of the organ microenvironment has a significant influence on tumour metastasis. Therefore, a better understanding of the molecular mechanisms that link the organ microenvironment to metastasis should provide ideas for more effective anti-metastatic therapies.
Several mechanistic studies have demonstrated how the organ microenvironments are primed to facilitate cancer metastasis. TGFβ promotes breast tumour cell seeding in the lungs by activating angiopoietin-like four induced tumour cell extravasation (Ref. 55). Other mediators (Angiopoietin 2, MMP3, MMP10, TGFβ and TNFα) of vascular remodelling that are important in metastasis to the lungs have also been discovered (Refs 56, 57). CXCL12 and insulin-like growth factor 1 (IGF1) are two key mesenchymal signals that select seeds from breast tumours to metastasise and colonise the bone (Ref. 58). Tumour-conditioned media (TCM) from triple negative breast cancer cells induced lymphangiogenesis and angiogenesis in regional LN (Ref. 59). Increased angiogenesis in tumour-draining LN (TDLN) were observed and correlated with LN metastasis and lower survival in breast cancer patients (Refs 60, 61, 62). Exosomes are microvesicles derived from tumours and their role in tumour–organ communication for metastatic progression is emerging. Exosomes secreted from melanoma-induced LN angiogenesis and BV remodelling (Ref. 63). Melanoma exosome-mediated cMet and its effects on bone marrow-derived cells and organ niche formation have been reported (Ref. 64). These examples suggest that tumour-draining secretions, organ-residing cells, and their resulting signals and phenotypes can be targeted to improve upon current therapeutic strategies.
Angiogenesis and lymphangiogenesis in cancer
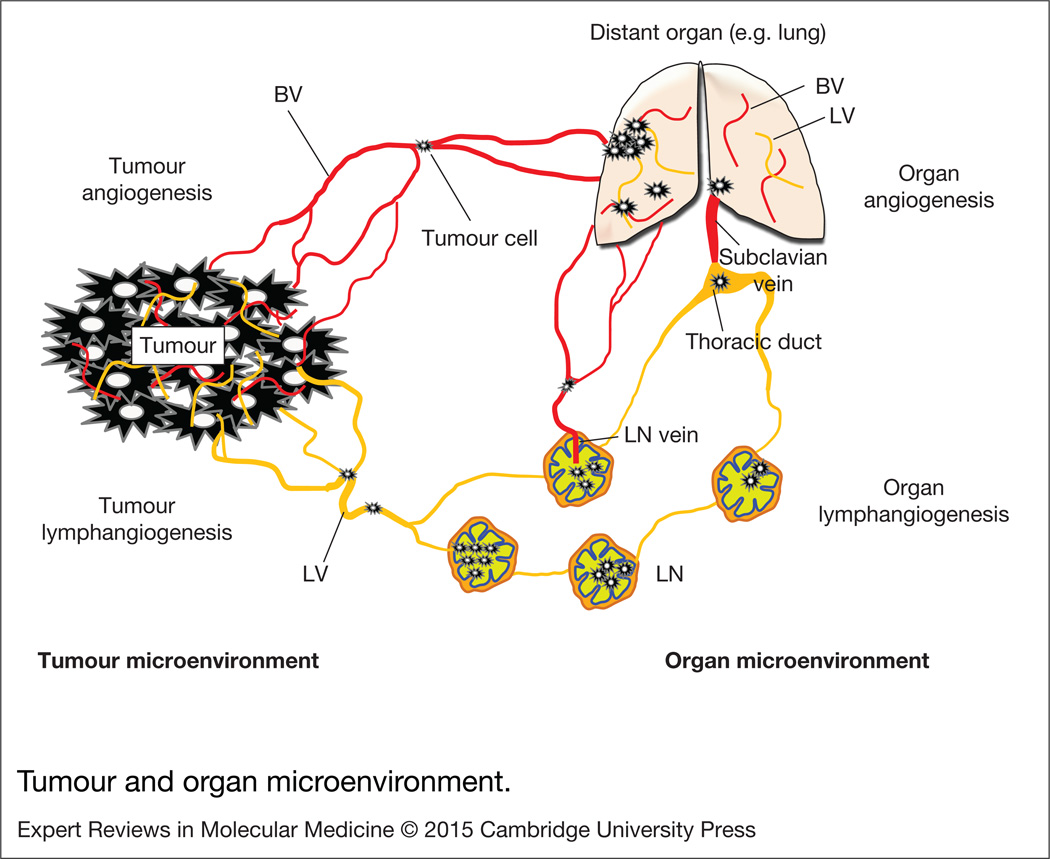
BV and LV are important components of both the tumour and the organ microenvironments. Once tumours or metastases are formed either at primary sites or distal organs, adjacent BEC and LEC, lining the pre-existing BV and LV are activated, migrate and proliferate under the influence of angiogenic and lymphangiogenic signals initiated by tumour cells or microenvironments to support continuous tumour growth and metastasis. These processes are referred to as tumour /organ angiogenesis and lymphangiogenesis (Refs 18, 19). We briefly discuss the state of understanding of these phenomena in cancer (Fig. 1).
Figure 1. Tumour and organ microenvironment.
Tumour cells express angiogenic growth factors and lymphangiogenic growth factors, promoting tumour angiogenesis and lymphangiogenesis. Tumour blood vessels primarily serve as conduits for blood supply and a route for haematogenous tumour spreading. Tumour lymphatic vessels particularly play a role in tumour cell escape from the primary tumour by expressing tumour cell recruiting chemokine factors. Tumour secretions from primary tumours or metastases can promote angiogenesis and lymphangiogenesis in pre-metastatic organs such as lymph nodes and distant organs. Sentinel lymph nodes are initially invaded by tumour cells (also called tumour-draining lymph nodes, TDLN). The TDLN serve as a reservoir for tumour cells before distal metastasis. Angiogenesis in the TDLN is a way to facilitate metastatic colonisation and further dissemination; lymphangiogenesis in TDLN is crucial for initial tumour invasion and tumour immune tolerance by modifying host immunity. Tumour cells in the lymphatic system can be transported into the blood vessels through the LN vein and thoracic duct and subclavian vein where the blood and lymphatic systems are temporarily fused each other. Vascular permeability in the lung is critical for tumour cell extravasation. BV, blood vessels; LN, lymph node; LV, lymphatic vessels.
Angiogenesis
Angiogenesis, the formation of new BV from preexisting BV, has been recognised as a target to inhibit primary tumour growth and haematogenous tumour cell dissemination (Ref. 65). In general, cells have to be located within 150–200 µm of blood capillaries to survive, although the distance can vary depending on the cell type (Ref. 66). For this reason, tumours are not able to grow to more than 1–2 mm3 without angiogenesis within the stroma (Ref. 67). Tumour cells under hypoxia express and secrete VEGFA, which is up-regulated by the transcription factor hypoxia inducible factor 1 alpha (HIF1α), to trigger tumour angiogenesis and make the tumour resistant to hypoxia (Ref. 68). Though other growth factors are also involved in angiogenesis, the signals mediated by VEGFR and neuropilin (NRP) are relatively well understood (Ref. 69). There are five growth factors in the VEGF family: VEGFA/B/C/D, and placental growth factor (PlGF); and three VEGF receptors: VEGFR1/2/3. Two neuropilins (NRP1/2) are also involved in VEGFR signalling. Among them, angiogenesis is primarily regulated by VEGFA, VEGFR1, VEGFR2 and NRP1. VEGFC/D, VEGFR3 and NRP2 regulate lymphangiogenesis. NRP1 makes a co-receptor complex with VEGFR2, promoting VEGFA-induced VEGFR2 signalling in BECs. The current understanding of how anti-angiogenic therapies targeting VEGFA or VEGFR2 signalling work is that they inhibit BV formation in tumours, thus limiting the blood supply, depriving the tumour of oxygen and nutrients, and hence induce severe hypoxia and necrosis of the tumours leading to an inhibition of tumour growth (Ref. 70).
The stromal BV also serve as routes of haematogenous spread of cancer cells from the primary tumour (Ref. 71). Haematogenous metastasis has been studied using the tail vein or intracardiac tumour injection models (Refs 72, 73). In addition to the stromal BV, organ-residing BV are also important for metastasis. They facilitate tumour cell extravasation, seeding, and colonisation. Particularly, permeability of the organ BV (e.g. lung vascular permeability and blood–brain barrier integrity) is crucial for extravasation of tumour cells from the circulating blood (Ref. 55). Several studies have shown mechanisms of how the tight junctions between BEC in different organ microenvironments are opened to facilitate extravasation of tumour cells (Refs 74, 75). Moreover, the enhancement of organ angiogenesis is essential for tumour cell survival and colonisation. It is well established that up-regulation of angiogenesis in the organ microenvironment is synchronised with the increase in oncogenic activation and the mutation of tumour suppressor genes (Ref. 76). For example, activation of the Ras oncogene promotes VEGF expression in tissues and p53 mutation results in reduced expression of the anti-angiogenic factor, thrombospondin 1. However, it is still not clear whether the angiogenic switch is turned on before the actual metastasis to prepare and condition the pre-metastatic niches, or initiated only after tumour cell invasion into the niches (Refs 63, 77). In addition to enhanced angiogenesis, remodelling of the blood vasculature in the organ microenvironment also contributes to metastasis. For example, high endothelial venules (HEV) in LN experience morphological changes during LN metastasis. The HEV are modified so that they are more dilated and cancer cells and red blood cells are detected within the lumen rather than the expected immune cells in tongue cancer compared with normal, physiological LN (Ref. 78).
Lymphangiogenesis
Tumour cells express and secrete lymphangiogenic factors, including VEGFC/D, angiopoietins, PDGF-BB/AA and bFGF to promote tumour lymphangiogenesis, the formation of new LV in the tumour stroma (Refs 15, 79, 80). LV are distinct from BV in their anatomy, growth signals, gene expression and functions (Refs 16, 81). The LV lack a well-defined basement and are scarcely covered by pericytes or smooth muscle cells thus are much more permeable than BV (Ref. 15). Due to their leaky nature, LV play a role as a reservoir for proteins and cells that have leaked from the BV and function to transport them back from the tissues to the blood circulation (Ref. 19). Tumour lymphangiogenesis has not been studied as long as tumour angiogenesis primarily because of the relatively recent discoveries of lymphatic markers such as VEGFR3 (Ref. 82), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1; Ref. 83), prospero homeobox protein 1 (Prox1; Ref. 84), NRP2 (Ref. 85) and podoplanin (Ref. 86). It is, however, well understood that the tumour lymphatic vasculature serves as the initial route of tumour lymphatic dissemination (Ref. 80). Lymphatic metastasis occurs at least as frequently as BV-mediated metastasis (Ref. 15), and in certain kinds of cancer, such as breast cancer, LV are the primary routes of tumour dissemination (Refs 87, 88, 89). Slow lymphatic flow rates and tumour cell survival factors, such as hyaluronic acids in the lymph fluid also support lymphogenous metastasis (Refs 90, 91). Tumour LV are connected to the regional LN (Refs 92, 93) and the tumour cell positive or tumour secretion influenced LN are referred to as TDLN (Ref. 30). These TDLN are considered to be transient reservoirs of metastatic tumour cells, and as a support system for helping the tumour cells make colonies for further metastasis to distant organs (Refs 94, 95). From the lymphatic system, cancer cells can also be transported back to the circulating blood from where they extravasate and invade distant organs (Ref. 96). Thus blocking tumour lymphangiogenesis can inhibit metastasis by limiting the likelihood of initial tumour dissemination via intra- and peritumoural LV (Refs 97, 98). Lymphangiogenesis in the pre-metastatic organs is also central for successful metastasis (Refs 99, 100, 101). For example, LV in the TDLN are connected with the tumour LV so that they can serve as direct routes for invasion of the tumour into the LN (Ref. 102). Also, LV in the pre-metastatic organs can modify host immunity, so that cancer cells can escape immune attack (Ref. 103). This pro-tumour immuno-editing activity is regulated by LEC-secreted factors. We discuss this in more detail in the next section. Recently, we developed a new type of metastasis model in which we inject TCM subcutaneously into animals for 2 weeks followed by orthotopic breast cancer cell inoculation (Refs 59, 104). We saw very rapid formation of metastases in the LN and lungs in this model. At the same time the TCM treatment resulted in enhanced lymphangiogenesis in the pre-metastatic organs (Ref. 105). These results suggest that both organ lymphangiogenesis and tumour lymphangiogenesis may be therapeutic targets to efficiently treat metastasis.
BEC- and LEC-secreted factors and signals
Now we discuss BV- and LV-mediated signals and their inductive roles. First, we discuss BEC- and LEC-mediated signals in physiology. Second, we focus on BEC-derived signals in cancer. Lastly, LEC-induced signals in cancer are discussed.
BEC- and LEC-mediated signals in physiology
BEC secrete a large number of molecules, including proteins, lipids and metabolites. These molecules are called angiocrine factors (Ref. 106). These factors allow BEC to communicate with other cells and they play a role in maintaining physiological homeostasis, regulating cell fate and host immunity, and promoting tissue regeneration (Ref. 18). For example, angiocrine factors balance self-renewal and differentiation of haematopoietic stem cells (HSC) to maintain haematopoietic homeostasis (Ref. 107). The blood endothelium also maintains blood fluidity and immunity through crosstalk with platelets (Ref. 108). There have been several studies claiming that angiocrine factors are central for tissue regeneration; e.g. angiocrine signals from liver sinusoidal endothelial cells (LSEC) are required for liver regeneration (Ref. 109); angiocrine signals induce and maintain the regeneration of lung alveoli (Ref. 110).
LV are distinct from BV in that they do not have a well-defined basement membrane, do not carry erythrocytes, and are composed of LEC that are phenotypically different from BEC. Generally LV function as reservoirs for immune cells, antigens, lipids and macromolecules that have leaked from the vascular system and transport lymph fluid back to the circulatory system (Refs 93, 111). If the LV are abnormal, the lymph fluid cannot be efficiently drained and results in lymphedema. LV also function in fat absorption and lipid transport and the consumption of fatty foods increases lymph flow in humans (Ref. 112). The lymphatic endothelium also expresses several adhesion molecules and chemokine ligands that interact with immune cells to facilitate maintenance of the host immunity. LEC express CC chemokine ligand 21 (CCL21) (Ref. 113). CCL21 or CCL19 can attract CC chemokine receptor 7 (CCR7) positive DC and APC (Refs 114, 115, 116). These DC and APC are transported into LN where they meet T lymphocytes in the T cell zone: the T lymphocytes and DC are activated and matured in the LN and depart from the LN through the efferent LV. In addition, resident T and B lymphocytes are important in the regulation of lymphangiogenesis in the LN. T lymphocytes negatively regulate lymphangiogenesis via interferon gamma-mediated mechanisms (Ref. 117). On the other hand, B lymphocytes promote lymphangiogenesis in the LN (Ref. 118).
BEC-mediated signals in cancer
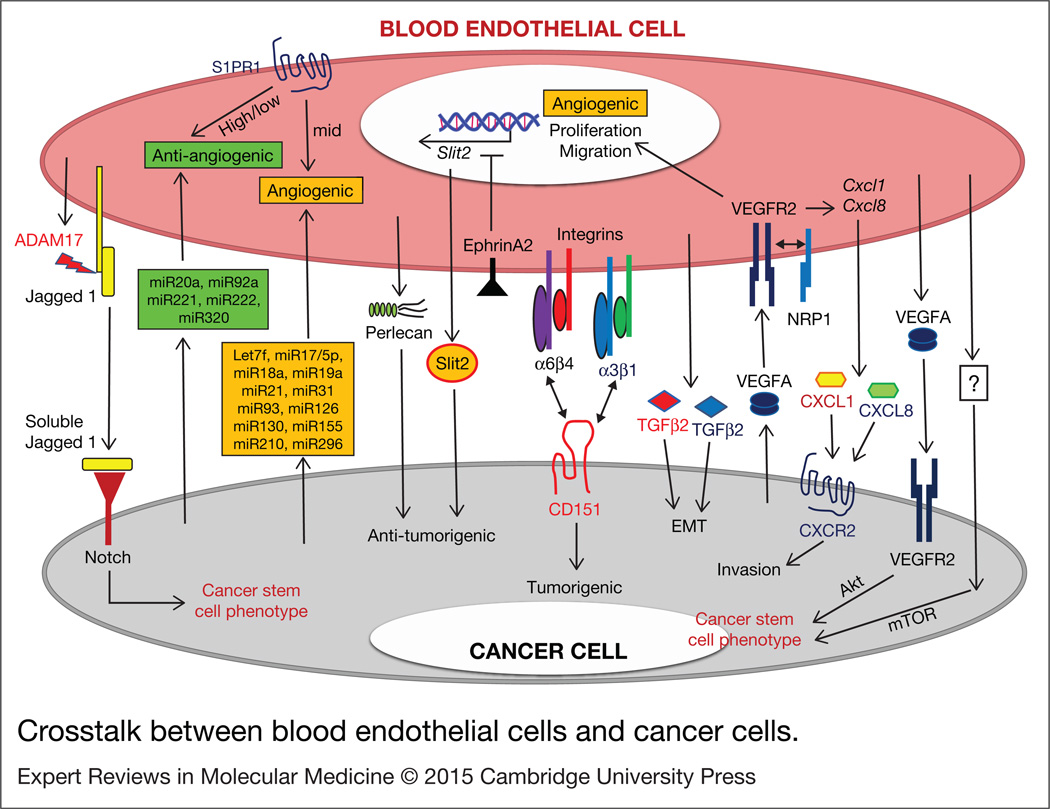
As discussed above, tumour angiogenesis is a target for inhibiting tumour growth by blocking the supply of oxygen to the growing tumour. Recently tumour-associated BEC have been revisited with a focus on BEC-secreted factors and their crosstalk with cancer cells and other stromal cells (Ref. 18). BEC-mediated signals in cancer are summarised in Figure 2.
Figure 2. Crosstalk between blood endothelial cells and cancer cells.
Tumour cell and blood endothelial cell -secreted factors, ECM components, microRNAs, and membrane bound or soluble receptors can mediate tumour/blood endothelial crosstalk signals to promote tumour cell proliferation, migration, invasion, EMT, and cancer stem cell phenotypes. Question marks represent unknown mechanisms. ADAM17, ADAM metallopeptidase domain 17; EMT, epithelial-to-mesenchymal transition; miRxx, micro RNA xx; mTOR, mammalian target of rapamycin; NRP1, neuropilin1; S1PR1, Sphingosine-1-phosphate receptor 1; Slit2, Slit homolog 2 protein; TGFβ, transforming growth factor beta; VEGFA, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2.
There have been a number of studies that show that BEC-cancer cell crosstalk regulates cancer cell proliferation and migration. Slit homologue protein 2 (Slit2), which is negatively regulated by Ephrin A2 receptor (EphA2) in BEC, is a breast tumour suppressive angiocrine factor (Ref. 119). Tumour BEC express high levels of EphA2. Enhanced EphA2 expression in BEC is associated with low Slit2 expression in the tumour blood endothelium and this predicted poor prognosis in human breast cancer patients (Ref. 119). Large-cell lymphoma cell growth and migration were stimulated by conditioned media obtained from liver-, lung-, brain- microvascular endothelial cells (MEC), suggesting that tumour angiocrine factors induce tumour cell growth and migration (Ref. 120). Tetraspanin CD151, which is expressed in breast cancer cells, regulates breast tumourigenesis by interacting with integrins α3β1 and α6β4 on BEC (Ref. 121). Although CD151 did not influence the inherent proliferative potential of breast cancer cells, it positively affected tumour cell proliferation in a cancer cell-BEC co-culture system (Ref. 121). BEC also promoted invasion of oral squamous cell carcinoma and Kaposi’s sarcoma through CXC chemokine signalling (Ref. 122). CXCL1 and CXCL8 were up-regulated in VEGF-treated EC, and these CXC chemokines initiated the invasion of CXCR2-positive tumour cells (Ref. 122). Surprisingly, perlecan-expressing BEC suppressed breast and lung carcinoma tumourigenesis by reducing pro-tumourigenic and pro-inflammatory signalling in the cancer cells. Perlecan silencing in BEC eliminated this tumour suppressive activity of the endothelial cells via increasing IL6 secretion by the cancer cells (Ref. 123).
BEC-cancer cell crosstalk regulates tumour angiogenesis as well. Tumour blood endothelium-derived microRNAs influence tumour progression by regulating tumour angiogenesis (Ref. 124). A number of studies have explored diverse microRNAs, reporting that Let7f, miR17/5p, miR18a, miR19a, miR21, miR31, miR93, miR126, miR130, miR155, miR210 and miR296 are BEC-expressed pro-angiogenic miRNAs and that miR20a, miR92a, miR221, miR222 and miR320 are anti-angiogenic miRNAs (Ref. 124). Crosstalk between BEC and breast cancer cells also results in reciprocal regulation of some angiogenic factors, including VEGF and angiopoietin 1/2. Co-culture of BEC with breast cancer cells resulted in enhanced VEGF and angiopoietin 2 expression but reduced angiopoietin 1 expression in the breast cancer cells compared with a monoculture of the breast cancer cells. These changes increased vascular sprouting and destabilisation (Ref. 125). In a recent study, sphingosine-1-phosphate receptor (S1PR1), one of the G-protein-coupled receptors (GPCR) expressed in BEC, regulated tumour vascular permeability and modulated tumour growth (Ref. 126). When S1PR1 signalling was very low or very high, tumour growth was delayed while, when S1PR1 signalling was intermediate, tumour growth was enhanced. The intermediate levels of S1PR1 appear to be important for stabilising tumour BV and promoting tumour growth (Ref. 126).
BEC-cancer cell crosstalk also influences the stem cell-like properties of cancer cells. BEC-derived VEGF inhibited anoikis, a form of programmed cell death, of head and neck cancer stem cells (CSC) by activating protein kinase B (PKB, also known as Akt) signalling pathways (Ref. 127). Conditioned media obtained from brain BEC maintained glioblastoma stem-like cell expansion through the mammalian target of rapamycin (mTOR) pathway (Ref. 128). BEC-derived signalling promoted survival and self-renewal of CSC in HNSCC (Ref. 129). Selective inhibition of tumour-associated BEC by transducing a caspase-based artificial death switch (iCaspase-9) reduced CSC in the HNSCC tumour xenografts (Ref. 129). The EC-derived soluble form of Jagged 1 generated by the proteolytic activity of ADAM metallopeptidase domain 17 (ADAM17), led to Notch activation in human colorectal cancer (CRC) cells, promoting CSC phenotypes in CRC (Ref. 130).
BEC-cancer cell crosstalk also induces EMT in cancer cells. Conditioned media from bovine aortic endothelial cells (BAEC) induced EMT in A549 and PANC-1 tumour cell lines. In that study, neutralising antibody against either TGFβ1 or TGFβ2 did not reverse endothelial-dependent EMT, but simultaneous inhibition of both TGFβ1 and TGFβ2 abolished the EMT (Ref. 131). Three-dimensional (3D) culture of BEC and breast epithelial cells induced EMT in the breast epithelial cells, and basal-like breast tumours contained cells undergoing EMT around the vascular-rich areas of the tumours (Ref. 132).
LEC-mediated signals in cancer
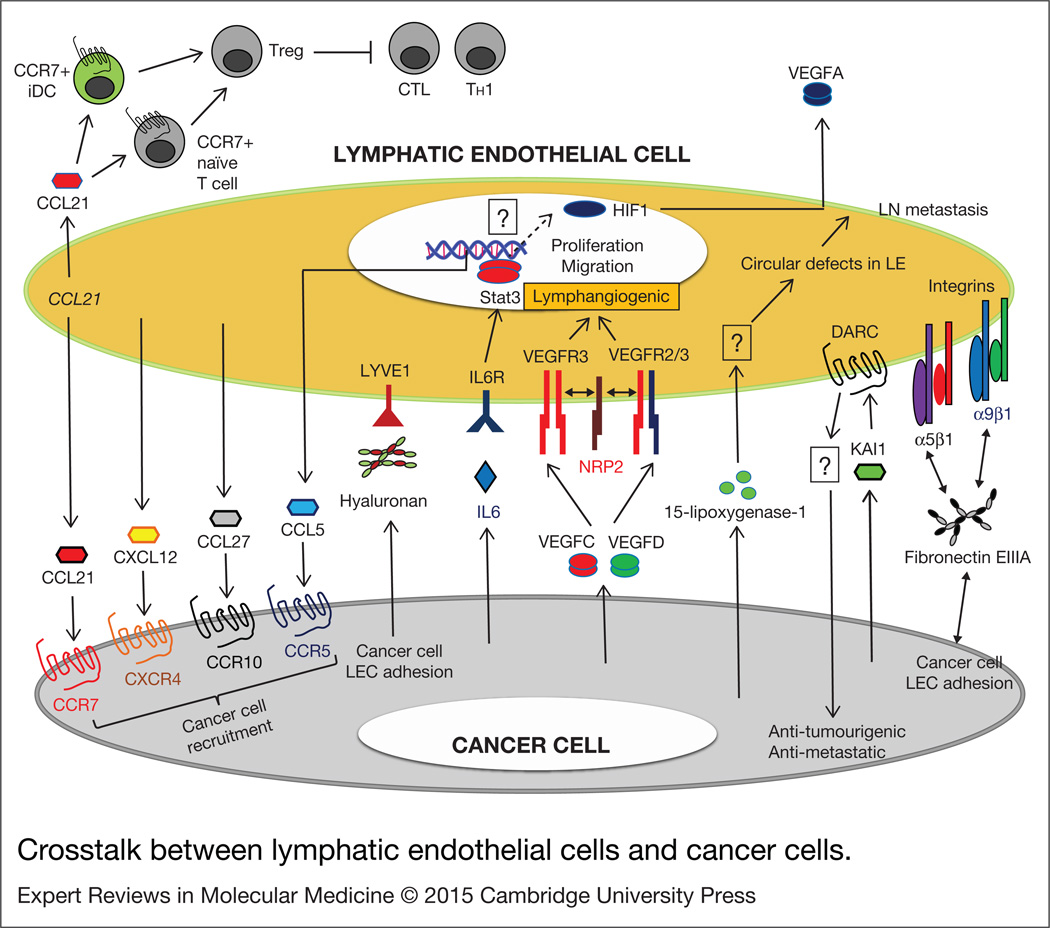
Tumour lymphangiogenesis, initiated by lymphangiogenic signals from tumour cells, is a therapeutic target to inhibit tumour dissemination through the lymphatic system. Tumour-associated LEC, the LEC-secreted factors (also called ‘lymphangiocrine factors’) and their crosstalk with cancer cells and stromal cells have been studied with this possibility in mind. LEC-mediated signals in cancer are summarised in Figure 3.
Figure 3. Crosstalk between lymphatic endothelial cells and cancer cells.
Tumour cell and lymphatic endothelial cell-secreted factors, ECM components, and membrane bound receptors can mediate tumour/ lymphatic endothelial crosstalk signals to promote tumour cell migration, recruitment and adhesion. Lymphatic endothelial cell-secreted factors also induce angiogenesis and enhance vascular permeability in pre-metastatic organs. They can also modify host immunity to mediate tumour immune escape by recruiting immature dendritic cells (iDC) and naïve T cells. Question marks represent unknown mechanisms. CCRxx, CC chemokine receptor xx; CCLxx, CC chemokine ligand xx; DARC, duffy antigen receptor for chemokines; HIF1, hypoxia inducible factor 1; iDC, immature dendritic cells; IL6, interleukin 6; IL6R, interleukin 6 receptor; KAl1, Kallmann syndrome 1; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; LN, lymph node; LE, lymphatic endothelium; NRP1, neuropilin1; Stat3, signal transducer and activator of transcription 3; Treg, regulatory T cells; CTL, cytotoxic T lymphocytes; TH1, helper T 1; VEGFC/D, vascular endothelial growth factorC/D; VEGFR3, vascular endothelial growth factor receptor 3 (homodimer); VEGFR 2/3, vascular endothelial growth factor receptor 2/3 (heterodimer).
Crosstalk between LEC and cancer cells can facilitate the recruitment of cancer cells from the primary tumours to the lymphatic system. The lymphatic endothelium expresses CXCL12 and CCL21 chemokines and these chemokines recruit CXCR4- or CCR7-expressing cancer cells by chemoattraction (Refs 133, 134, 135). CXCL12 and CCL21 expression was observed in physiological LEC as well where they function to recruit immune cells to maintain host immunity. The chemokines secreted by LEC in highly metastatic oral cancer cell conditioned media was reported to be highly altered compared with that by normal LEC (Ref. 136), suggesting that there can be tumour-specific LEC-mediated chemokine ligand/receptor mechanisms for tumour cell invasion into the LV. Recently, Lee et al. showed that triple-negative breast tumour-induced LEC secrete CCL5 and recruit CCR5-expressing cancer cells into the lymphatic system, supporting LN metastasis (Ref. 104). This recruitment was inhibited by maraviroc, the CCR5 inhibitor with anti-retroviral activity. In that study, tumour secreted IL6-induced Stat3 phosphorylation in LEC resulting in enhanced CCL5 expression and secretion by the LEC. Inhibition of IL6 or Stat3 signalling resulted in reduced CCL5 secretion by the LEC and significant reduction of lymphogenous metastasis (Ref. 104). Hyaluronan (HA), which is expressed in cancer cells, also mediates cancer cell adhesion to the lymphatic endothelium by binding to LYVE1 (Refs 83, 137) and induces tumour cell invasion into the LV. Other adhesion molecules such as CLEVER-1/ Stabilin-1, mannose receptor and Thy1 (CD90) have also been reported to contribute to tumour cell adhesion to LV (Ref. 138). A morphological study of the interaction between M21 melanoma cells and LEC showed that tumour cells adhere via pseudopodia to LEC at the site near the intercellular junction. Upon tumour cell adhesion, the LEC junctions between LEC appear to dissociate and allow invasion by the tumour cells (Ref. 139). A recent discovery showed that tumour cell derived 15-lipoxygenase-1 mediates LN metastasis by allowing the tumour cells to make circular holes in the lymphatic endothelium. Selective inhibition of 15-lipoxygenase-1 in tumour cells repressed formation of the circular defects in the LV and resulted in reduced LN metastasis (Ref. 140).
LEC-cancer cell crosstalk can also regulate cancer cell proliferation. Interaction of the duffy antigen receptor for chemokines (DARC) in LEC with the Kallmann syndrome 1 (KAI1) ligand from cancer cells reduces cancer cell proliferation and metastatic progression. This model suggests that tumour-expressed KAI1 can have a tumour suppressing activity by interacting with the host LV (Ref. 141). LEC conditioned by MDA-MB-231 breast TCM express EGF and PDGF-BB to promote tumour cell proliferation, angiogenesis and pericyte infiltration into the stroma (Ref. 142).
Recently LEC-induced immune modification and tumour immune tolerance has been reported (Refs 30, 143). Tumour cell spreading is mediated by sentinel, TDLN. Hence, the presence of tumour cells in the TDLN is used as an important prognostic indicator of metastasis in several cancers, such as breast cancer and skin cancer. Generally, LN are the primary organs that regulate host immunity, as diverse immune cells are travelling through the LV and the LN; lymphocytes are abundant in the T–B cell regions of the LN, and antigen-specific immune reactions normally occur in the LN. Thus, the LN can establish and maintain immunity against pathogens and possibly tumour cells. However, interestingly, many studies have shown that TDLN do not function as normal LN do against normal inflammation or infection. They instead appear to play a role as transient reservoirs of tumour cells for distal metastasis (Ref. 144). These results suggest that tumour cells or tumour secretions can modify the LN or lymphatic systems in the LN, thus impairing their original functions. Mechanistically, CCL21 expressed by the lymphatic endothelium can recruit CCR7-positive naive T cells into the lymphatic systems in the TDLN and tumour stroma where they are educated to be less immune reactive; similarly, CCR7-positive DC are maintained in their immature state [immature dendritic cells (iDC)] in the tumour and the TDLN. The iDC promotes tumour-associated regulatory T cell (Treg) activity to suppress CTL (Ref. 30). Cytokines around the lymphatic endothelium in the various microenvironments also contribute to immune tolerance. High amounts of TGFβ are secreted by the tumour ECM, as the tumour matrix tension caused by interstitial flow influences TGFβ expression. TGFβ inhibits natural killer cell functions. TGFβ can also promote tumour-associated regulatory T cell (Treg) and MDSC activities: these immune suppressive cell activities impair CTL and helper T1 cell (TH1) functions, causing tumour cell immune tolerance (Ref. 30).
Clinical implications and applications
Therapeutic strategies to target blood and LV
Multiple strategies to target the tumour and organ microenvironments, particularly the BV and LV are in the clinic and in development. To target the BV in the microenvironment, several drugs, including the anti-VEGF monoclonal antibody bevacizumab, the receptor tyrosine kinase inhibitor sunitinib and the decoy receptor aflibercept (VEGF-trap) are clinically used. Anti-angiogenic monotherapy, such as anti-VEGF treatment, has shown limitation in clinic. This can be partially explained by that even after inhibiting single factor or pathway, other tumour-secreted factors or signals continue to support angiogenesis persistently (Refs 35, 145, 146, 147). New data on the combination of anti-angiogenic therapies and other pathway blockages showed improved outcomes. For example, anti-VEGF therapy with COX-2 inhibition more potently blocked the angiogenesis, compared with just anti-VEGF treatment by blocking COX-2 derived prostaglandin E2 (PGE2) production. PGE2 is a VEGF-independent pro-angiogenic factor secreted by COX-2 expressing tumour cells (Ref. 148). No drugs are clinically used to target the LV but some are in preclinical development. They mostly target VEGFC/D, VEGFR3 and NRP2. Although anti-angiogenic therapies have shown promising outcomes in reducing tumour size, a large number of patients suffer from tumour metastasis facilitated by tumour LV which current anti-angiogenic agents are not able to inhibit. Specifically in breast cancer, tumour LV are the predominant routes of tumour dissemination. Thus current interest in the application of anti-angiogenic agents as cancer therapeutics has led to strategies combining inhibitors of angiogenesis and lymphangiogenesis with the goal of developing more effective anti-cancer therapies (Refs 62, 105, 149, 150).
Therapeutic and diagnostic values of the tumour–endothelial crosstalk signals
As described in this review, BEC and LEC secretomes play important roles in tumour progression, suggesting that we can target tumour–endothelial crosstalk signals in addition to targeting conventional tumour angiogenesis and lymphangiogenesis. In our previous study, we found that breast tumour cell secreted IL6 educated LEC in the pre-metastatic organs to express CCL5 and facilitate metastatic breast tumour recruitment into those organs (Ref. 104). In that study, we blocked IL6 and CCR5 resulting in dramatic reduction in lung and LN metastasis. These signalling molecules governing tumour–endothelial crosstalk have a diagnostic value as well. Human sample-based bioinformatics analysis of TCGA (The Cancer Genome Atlas) showed higher levels of IL6 and CCL5 mRNAs in highly metastatic human triple-negative breast cancer (TNBC) tumour samples compared with those in less metastatic human oestrogen receptor positive (ER+) tumour samples. Furthermore, TNBC tumours obtained from LN positive patients showed better correlation of IL6 and CCL5 gene expression compared with those from LN negative patients. These results suggest that repurposing the anti-retroviral CCR5 inhibitor (maraviroc) or the anti-rheumatoid arthritis IL6 receptor antibody (tocilizumab) for advanced and metastatic breast cancer may have substantial clinical benefit (Ref. 104). Also these repurposed drugs can be combined with conventional anti-angiogenic drugs (e.g. anti-VEGF antibodies) or chemotherapies. For example, we showed that an anti-VEGF antibody exhibited additive anti-metastatic effects when it was combined with maraviroc.
Research in progress and outstanding research questions
Over the past 40 years, much progress has been made in understanding the role of angiogenesis in tumour growth and metastasis. This understanding has facilitated anti-angiogenic drug development to efficiently treat primary tumour growth in patients. It is very important that the current interest in tumour lymphangiogenesis also results in the development of drugs to target tumour LV formation and lymphogenous metastasis. Possibly these anti-angiogenic and anti-lymphangiogenic therapies can be combined with each other for improved outcomes for patients. Other recent studies of tumour and organ microenvironments have also contributed to our understanding of diverse non-cancer cell types that can promote tumour growth, metastasis, immune tolerance, drug resistance, etc. However, the perspective that BEC and LEC can be important orchestrators in the microenvironments are still under-appreciated compared with the role of other cell types such as immune cells, bone marrow-derived cells and mesenchymal like cells. Analyses of secretome of BEC and LEC from different kinds of tumours (breast, gastric, brain tumours, etc.), from metastatic versus non-metastatic tumours, from different sites of metastases (LN, lung, bone metastases, etc.) need to be further investigated to generate more informative secretome libraries. Tumour secretomes can cause dysregulation of endothelial secretomes, thus we also need to understand which factors (cytokines, nucleic acids, small molecules, etc.) in tumour secretomes are governing these tumour-promoting scenarios.
Acknowledgements and funding
This work was supported by the National Institutes of Health grants R01 CA138264, R21 CA152473 and the Safeway Foundation.
Footnotes
Conflicts of Interest
None.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clinical & Experimental Metastasis. 2012;29:381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 3.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocrine Reviews. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 4.Catalano V, et al. Tumor and its microenvironment: a synergistic interplay. Seminars in Cancer Biology. 2013;23(6 Pt B):522–532. doi: 10.1016/j.semcancer.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Chen ST, et al. Breast tumor microenvironment: proteomics highlights the treatments targeting secretome. Journal of Proteome Research. 2008;7:1379–1387. doi: 10.1021/pr700745n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watnick RS. The role of the tumor microenvironment in regulating angiogenesis. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006676. doi: 10.1101/cshperspect.a006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funasaka T, Raz A. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer and Metastasis Review. 2007;26:725–735. doi: 10.1007/s10555-007-9086-7. [DOI] [PubMed] [Google Scholar]
- 9.Lin Q, Yun Z. Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics. Cancer Biology & Therapy. 2010;9:949–956. doi: 10.4161/cbt.9.12.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao D, Mittal V. Tumor microenvironment regulates epithelial-mesenchymal transitions in metastasis. Expert Review of Anticancer Therapy. 2012;12:857–859. doi: 10.1586/era.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tredan O, et al. Drug resistance and the solid tumor microenvironment. Journal of the National Cancer Institute. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 12.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends in Genetics. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Samples J, Willis M, Klauber-Demore N. Targeting angiogenesis and the tumor microenvironment. Surgical Oncology Clinics of North America. 2013;22:629–639. doi: 10.1016/j.soc.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, et al. Molecular regulation of lymphangiogenesis in development and tumor microenvironment. Cancer Microenvironment. 2012;5:249–260. doi: 10.1007/s12307-012-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nature Reviews Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 16.Hirakawa S, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. American Journal of Pathology. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volmer MW, et al. Tumor suppressor Smad4 mediates downregulation of the anti-adhesive invasion-promoting matricellular protein SPARC: landscaping activity of Smad4 as revealed by a ‘secretome’ analysis. Proteomics. 2004;4:1324–1334. doi: 10.1002/pmic.200300703. [DOI] [PubMed] [Google Scholar]
- 18.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nature Reviews Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alitalo K. The lymphatic vasculature in disease. Nature Medicine. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 20.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. European Journal of Immunology. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 21.Stearman RS, et al. A macrophage gene expression signature defines a field effect in the lung tumor microenvironment. Cancer Research. 2008;68:34–43. doi: 10.1158/0008-5472.CAN-07-0988. [DOI] [PubMed] [Google Scholar]
- 22.Schoppmann SF, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. American Journal of Pathology. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard JW. Trophic macrophages in development and disease. Nature Reviews Immunology. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banchereau J, et al. Immunobiology of dendritic cells. Annual Review of Immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 25.Sozzani S, et al. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends in Immunology. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Fainaru O, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB Journal. 2010;24:1411–1418. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pages F, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 28.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Research. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 29.Valenti R, et al. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Research. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 30.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nature Reviews Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 31.Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’–more than meets the eye. Trends in Molecular Medicine. 2013;19:447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, Chen J. Mesenchymal stem cells in the tumor microenvironment. Biomedical Reports. 2013;1:517–521. doi: 10.3892/br.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol. 2014 Apr;25:3–9. doi: 10.1016/j.semcancer.2013.12.010. Epub 2014 Jan 7. [DOI] [PubMed] [Google Scholar]
- 34.Straussman R, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford Y, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Feig C, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraman M, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 38.Yu B, et al. Stromal fibroblasts in the microenvironment of gastric carcinomas promote tumor metastasis via upregulating TAGLN expression. BMC Cell Biology. 2013;14:17. doi: 10.1186/1471-2121-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stracke ML, Liotta LA. Multi-step cascade of tumor cell metastasis. In Vivo. 1992;6:309–316. [PubMed] [Google Scholar]
- 40.Sahai E. Illuminating the metastatic process. Nature Reviews Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 41.Fidler IJ, Kim SJ, Langley RR. The role of the organ microenvironment in the biology and therapy of cancer metastasis. Journal of Cellular Biochemistry. 2007;101:927–936. doi: 10.1002/jcb.21148. [DOI] [PubMed] [Google Scholar]
- 42.Paget S. The distribution of secondary growths in cancer of the breast. Cancer and Metastasis Review. 1989;8:98–101. [PubMed] [Google Scholar]
- 43.Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer and Metastasis Review. 1991;10:229–243. doi: 10.1007/BF00050794. [DOI] [PubMed] [Google Scholar]
- 44.Araki C. Organs with low incidence of neoplasm metastasis through blood circulation. Nihon Rinsho. 1968;26:3217–3221. [PubMed] [Google Scholar]
- 45.Nishida Y, Tsukushi S, Urakawa H, Sugiura H, Nakashima H, Yamada Y, Ishiguro N. High incidence of regional and in-transit lymph node metastasis in patients with alveolar rhabdomyosarcoma. Int J Clin Oncol. 2014 Jun;19(3):536–543. doi: 10.1007/s10147-013-0571-4. Epub 2013 Jun 4. [DOI] [PubMed] [Google Scholar]
- 46.Dos Santos LA, et al. Incidence of lymph node and adnexal metastasis in endometrial stromal sarcoma. Gynecologic Oncology. 2011;121:319–322. doi: 10.1016/j.ygyno.2010.12.363. [DOI] [PubMed] [Google Scholar]
- 47.Hirasawa T, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 48.Lu X, et al. In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Cancer Research. 2010;70:3905–3914. doi: 10.1158/0008-5472.CAN-09-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigamonti N, De Palma M. A role for angiopoietin-2 in organ-specific metastasis. Cell Reports. 2013;4:621–623. doi: 10.1016/j.celrep.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 50.Lorusso G, Ruegg C. New insights into the mechanisms of organ-specific breast cancer metastasis. Seminars in Cancer Biology. 2012;22:226–233. doi: 10.1016/j.semcancer.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature Reviews Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 52.Nolan CP, Abrey LE. Leptomeningeal metastases from leukemias and lymphomas. Cancer Treatment and Research. 2005;125:53–69. doi: 10.1007/0-387-24199-x_4. [DOI] [PubMed] [Google Scholar]
- 53.Patel V, et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Research. 2011;71:7103–7112. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen CT, et al. Emerging insights into head and neck cancer metastasis. Head Neck. 2013;35:1669–1678. doi: 10.1002/hed.23202. [DOI] [PubMed] [Google Scholar]
- 55.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta GP, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 57.Goncharova EA. mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects. FASEB Journal. 2013;27:1796–1807. doi: 10.1096/fj.12-222224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang XH, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee E, Pandey NB, Popel AS. Pre-treatment of mice with tumor-conditioned media accelerates metastasis to lymph nodes and lungs: a new spontaneous breast cancer metastasis model. Clinical & Experimental Metastasis. 2014;31:67–79. doi: 10.1007/s10585-013-9610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guidi AJ, et al. Association of angiogenesis in lymph node metastases with outcome of breast cancer. Journal of the National Cancer Institute. 2000;92:486–492. doi: 10.1093/jnci/92.6.486. [DOI] [PubMed] [Google Scholar]
- 61.Edel MJ, Harvey JM, Papadimitriou JM. Comparison of vascularity and angiogenesis in primary invasive mammary carcinomas and in their respective axillary lymph node metastases. Clinical & Experimental Metastasis. 2000;18:695–702. doi: 10.1023/a:1013139022051. [DOI] [PubMed] [Google Scholar]
- 62.Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey NB, Popel AS. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Sci Rep. 2014 Nov 20;4:7139. doi: 10.1038/srep07139. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Research. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 64.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET. Nature Medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annual Review of Biomedical Engineering. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 67.Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nature Reviews Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 69.Mac Gabhann F, et al. Systems biology of pro-angiogenic therapies targeting the VEGF system. Wiley Interdisciplinary Reviews: System Biology and Medicine. 2010;2:694–707. doi: 10.1002/wsbm.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarmiento R, et al. Antiangiogenic therapies in breast cancer. Current Opinion in Investigational Drugs. 2009;10:1334–1345. [PubMed] [Google Scholar]
- 71.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discovery. 2012;2:1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rashid OM, et al. Is tail vein injection a relevant breast cancer lung metastasis model? Journal of Thoracic Disease. 2013;5:385–392. doi: 10.3978/j.issn.2072-1439.2013.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zadnik P, et al. A novel animal model of human breast cancer metastasis to the spine: a pilot study using intracardiac injection and luciferase-expressing cells. Journal of Neurosurgery: Spine. 2013;18:217–225. doi: 10.3171/2012.11.SPINE12325. [DOI] [PubMed] [Google Scholar]
- 74.Arshad F, et al. Blood-brain barrier integrity and breast cancer metastasis to the brain. Pathology Research International. 2010;2011:920509. doi: 10.4061/2011/920509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irmisch A, Huelsken J. Metastasis: new insights into organ-specific extravasation and metastatic niches. Exp Cell Res. 2013 Jul 1;319(11):1604–1610. doi: 10.1016/j.yexcr.2013.02.012. Epub 2013 Feb 21. [DOI] [PubMed] [Google Scholar]
- 76.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Reviews Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 77.Farnsworth RH, et al. A role for bone morphogenetic protein-4 in lymph node vascular remodeling and primary tumor growth. Cancer Research. 2011;71:6547–6557. doi: 10.1158/0008-5472.CAN-11-0200. [DOI] [PubMed] [Google Scholar]
- 78.Lee SY, et al. Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. Journal of Translational Medicine. 2012;10:206. doi: 10.1186/1479-5876-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Von Marschall Z, et al. Vascular endothelial growth factor-D induces lymphangiogenesis and lymphatic metastasis in models of ductal pancreatic cancer. International Journal of Oncology. 2005;27:669–679. [PubMed] [Google Scholar]
- 80.Kesler CT, et al. Lymphatic vessels in health and disease. Wiley Interdisciplinary Reviews: System Biology and Medicine. 2013;5:111–124. doi: 10.1002/wsbm.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohammed RA, et al. Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Modern Pathology. 2011;24:774–785. doi: 10.1038/modpathol.2011.4. [DOI] [PubMed] [Google Scholar]
- 82.Kaipainen A, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banerji S, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. Journal of Cell Biology. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 85.Yuan L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 86.Schacht V, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO Journal. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Porter GJ, et al. Patterns of metastatic breast carcinoma: influence of tumour histological grade. Clinical Radiology. 2004;59:1094–1098. doi: 10.1016/j.crad.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 89.Moskowitz M, et al. Breastcancer screening. Preliminary report of 207 biopsies performed in 4, 128 volunteer screenees. Cancer. 1975;36:2245–2250. doi: 10.1002/cncr.2820360943. [DOI] [PubMed] [Google Scholar]
- 90.Matsuo S. Studies on the factors inducing metastasis of breast cancer to lymph node. I. Lymph flow in the thoracic wall. Acta Medica Okayama. 1974;28:259–270. [PubMed] [Google Scholar]
- 91.Lipponen P, et al. High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. European Journal of Cancer. 2001;37:849–856. doi: 10.1016/s0959-8049(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 92.Skobe M, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Medicine. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 93.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 94.Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014 May 1;32(13):1365–1383. doi: 10.1200/JCO.2013.54.1177. Epub 2014 Mar 24. [DOI] [PubMed] [Google Scholar]
- 95.Boughey JC, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Journal of American Medical Association. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sleeman JP, Cady B, Pantel K. The connectivity of lymphogenous and hematogenous tumor cell dissemination: biological insights and clinical implications. Clinical & Experimental Metastasis. 2012;29:737–746. doi: 10.1007/s10585-012-9489-x. [DOI] [PubMed] [Google Scholar]
- 97.Jain RK, Padera TP. Prevention and treatment of lymphatic metastasis by antilymphangiogenic therapy. Journal of the National Cancer Institute. 2002;94:785–787. doi: 10.1093/jnci/94.11.785. [DOI] [PubMed] [Google Scholar]
- 98.Achen MG, Mann GB, Stacker SA. Targeting lymphangiogenesis to prevent tumour metastasis. British Journals of Cancer. 2006;94:1355–1360. doi: 10.1038/sj.bjc.6603120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirakawa S, et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. American Journal of Pathology. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012 Oct 18;31(42):4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 102.Quagliata L, et al. Inhibition of VEGFR-3 activation in tumor-draining lymph nodes suppresses the outgrowth of lymph node metastases in the MT-450 syngeneic rat breast cancer model. Clinical & Experimental Metastasis. 2014;31:351–365. doi: 10.1007/s10585-013-9633-2. [DOI] [PubMed] [Google Scholar]
- 103.Thomas SN, et al. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Lee E, et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nature Communications. 2014;5:4715. doi: 10.1038/ncomms5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee E, et al. Inhibition of lymphangiogenesis and angiogenesis in breast tumor xenografts and lymph nodes by a peptide derived from transmembrane protein 45A. Neoplasia. 2013;15:112–124. doi: 10.1593/neo.121638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nolan DJ, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Developmental Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature Cell Biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. Journal of Thrombosis and Haemostasis. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 109.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weech AA, Goettsch E, Reeves EB. The flow and composition of lymph in relation to the formation of edema. Journal of Experimental Medicine. 1934;60:63–84. doi: 10.1084/jem.60.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turner SG, Barrowman JA. Intestinal lymph flow and lymphatic transport of protein during fat absorption. Quaterly Journal of Experimental Physiology and Cognate Medical Sciences. 1977;62:175–180. doi: 10.1113/expphysiol.1977.sp002387. [DOI] [PubMed] [Google Scholar]
- 113.Nykanen AI, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- 114.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nature Reviews Immunology. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 115.Scandella E, et al. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–1601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 116.Riedl K, et al. Overexpression of CCL-21/ secondary lymphoid tissue chemokine in human dendritic cells augments chemotactic activities for lymphocytes and antigen presenting cells. Molecular Cancer. 2003;2:35. doi: 10.1186/1476-4598-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kataru RP, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 118.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Brantley-Sieders DM, et al. Angiocrine factors modulate tumor proliferation and motility through EphA2 repression of Slit2 tumor suppressor function in endothelium. Cancer Research. 2011;71:976–987. doi: 10.1158/0008-5472.CAN-10-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamada J, et al. Separable growth and migration factors for large-cell lymphoma cells secreted by microvascular endothelial cells derived from target organs for metastasis. British Journal of Cancer. 1992;66:349–354. doi: 10.1038/bjc.1992.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sadej R, et al. CD151 regulates tumorigenesis by modulating the communication between tumor cells and endothelium. Molecular Cancer Research. 2009;7:787–798. doi: 10.1158/1541-7786.MCR-08-0574. [DOI] [PubMed] [Google Scholar]
- 122.Warner KA, et al. Endothelial cells enhance tumorcel linvasion through a crosstalk mediated by CXC chemokine signaling. Neoplasia. 2008;10:131–139. doi: 10.1593/neo.07815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011 Jan 19;3(66) doi: 10.1126/scitranslmed.3001542. 66ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heusschen R, et al. MicroRNAs in the tumor endothelium: novel controls on the angioregulatory switchboard. Biochimica et Biophysica Acta. 2010;1805:87–96. doi: 10.1016/j.bbcan.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Buchanan CF, et al. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. Journal of Cellular Biochemistry. 2012;113:1142–1151. doi: 10.1002/jcb.23447. [DOI] [PubMed] [Google Scholar]
- 126.Host endothelial S1PR1 regulation of vascular permeability modulates tumor growth. Am J Physiol Cell Physiol. 2014 Jul 1;307(1):C14–C24. doi: 10.1152/ajpcell.00043.2014. Epub 2014 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Campos MS, et al. Endothelial derived factors inhibit anoikis of head and neck cancer stem cells. Oral Oncology. 2012;48:26–32. doi: 10.1016/j.oraloncology.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galan-Moya EM, et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Reports. 2011;12:470–476. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krishnamurthy S, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Research. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu J, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kimura C, et al. Endothelium-dependent epithelial-mesenchymal transition of tumor cells: exclusive roles of transforming growth factor beta1 and beta2. Biochimica et Biophysica Acta. 2013;1830:4470–4481. doi: 10.1016/j.bbagen.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Sigurdsson V, et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PLoS ONE. 2011;6:e23833. doi: 10.1371/journal.pone.0023833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim M, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Research. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- 134.Wiley HE, et al. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. Journal of the National Cancer Institute. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 135.Hwang TL, et al. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World Journal of Gastroenterology. 2012;18:1249–1256. doi: 10.3748/wjg.v18.i11.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhuang Z, et al. Altered phenotype of lymphatic endothelial cells induced by highly metastatic OTSCC cells contributed to the lymphatic metastasis of OTSCC cells. Cancer Science. 2010;101:686–692. doi: 10.1111/j.1349-7006.2009.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Du Y, et al. The interaction between LYVE-1 with hyaluronan on the cell surface may playa role in the diversity of adhesion to cancer cells. PLoS ONE. 2013;8:e63463. doi: 10.1371/journal.pone.0063463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paupert J, Sounni NE, Noel A. Lymphangiogenesis in post-natal tissue remodeling: lymphatic endothelial cell connection with its environment. Molecular Aspects of Medicine. 2011;32:146–158. doi: 10.1016/j.mam.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Ding Z, et al. Morphological study of the interaction between M21 melanoma and lymphatic endothelium. Lymphology. 2005;38:87–91. [PubMed] [Google Scholar]
- 140.Kerjaschki D, et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. Journal of Clinical Investigation. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Iiizumi M, Bandyopadhyay S, Watabe K. Interaction of Duffy antigen receptor for chemokines and KAI1: a critical step in metastasis suppression. Cancer Research. 2007;67:1411–1414. doi: 10.1158/0008-5472.CAN-06-3801. [DOI] [PubMed] [Google Scholar]
- 142.Lee E, Pandey NB, Popel AS. Lymphatic endothelial cells support tumor growth in breast cancer. Scientific Reports. 2014;4:5853. doi: 10.1038/srep05853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Swartz MA, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Research. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zheng R, et al. Significance of regional draining lymph nodes in the development of tumor immunity: implications for cancer immunotherapy. Cancer Treatment and Research. 2007;135:223–237. doi: 10.1007/978-0-387-69219-7_17. [DOI] [PubMed] [Google Scholar]
- 145.Cascone T, et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. Journal of Clinical Investigation. 2011;121:1313–1328. doi: 10.1172/JCI42405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nature Reviews Clinical Oncology. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kerbel RS. Reappraising antiangiogenic therapy for breast cancer. Breast. 2011;20(Suppl 3):S56–S60. doi: 10.1016/S0960-9776(11)70295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu L, et al. Sci Transl Med. 2014 Jun 25;6(242) doi: 10.1126/scitranslmed.3008455. (2014) 242ra84. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Science Translational Medicine 6, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee E, et al. Small peptides derived from somatotropin domain-containing proteins inhibit blood and lymphatic endothelial cell proliferation, migration, adhesion and tube formation. International Journal of Biochemistry & Cell Biology. 2011;43:1812–1821. doi: 10.1016/j.biocel.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koskimaki JE, et al. Synergy between a collagen IV mimetic peptide and a somatotropin-domain derived peptide as angiogenesis and lymphangiogenesis inhibitors. Angiogenesis. 2013;16:159–170. doi: 10.1007/s10456-012-9308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]