Abstract
Background
Cryptococcus neoformans is a ubiquitous environmental fungus that can cause life-threatening meningitis and fungemia, often in the presence of acquired immunodeficiency syndrome (AIDS), liver cirrhosis, diabetes mellitus, or other medical conditions. To distinguish risk factors from comorbidities, we performed a hospital-based, density-sampled, matched case-control study.
Methods
All new-onset cryptococcal meningitis cases and cryptococcemia cases at a university hospital in Taiwan from 2002–2010 were retrospectively identified from the computerized inpatient registry and were included in this study. Controls were selected from those hospitalized patients not experiencing cryptococcal meningitis or cryptococcemia. Controls and cases were matched by admission date, age, and gender. Conditional logistic regression was used to analyze the risk factors.
Results
A total of 101 patients with cryptococcal meningitis (266 controls) and 47 patients with cryptococcemia (188 controls), of whom 32 patients had both cryptococcal meningitis and cryptococcemia, were included in this study. Multivariate regression analysis showed that AIDS (adjusted odds ratio [aOR] = 181.4; p < 0.001), decompensated liver cirrhosis (aOR = 8.5; p = 0.008), and cell-mediated immunity (CMI)-suppressive regimens without calcineurin inhibitors (CAs) (aOR = 15.9; p < 0.001) were independent risk factors for cryptococcal meningitis. Moreover, AIDS (aOR = 216.3, p < 0.001), decompensated liver cirrhosis (aOR = 23.8; p < 0.001), CMI-suppressive regimens without CAs (aOR = 7.3; p = 0.034), and autoimmune diseases (aOR = 9.3; p = 0.038) were independent risk factors for developing cryptococcemia. On the other hand, diabetes mellitus and other medical conditions were not found to be risk factors for cryptococcal meningitis or cryptococcemia.
Conclusions
The findings confirm AIDS, decompensated liver cirrhosis, CMI-suppressive regimens without CAs, and autoimmune diseases are risk factors for invasive C. neoformans diseases.
Introduction
Cryptococcus neoformans is a pathogenic fungus that causes life-threatening meningitis, the first case of which was reported in 1905 [1]. C. neoformans is ubiquitous [2]. The Centers for Disease Control and Prevention (CDC) of the United States estimated that there are approximately one million new cryptococcal meningitis cases every year worldwide, with more than 70% of these cases occurring in sub-Saharan Africa [3]. C. neoformans infection is therefore an important global health concern [4].
Current understanding of the pathogenesis of invasive cryptococcal diseases is primarily based on clinical case series [5–11] and mouse experiments [12–17], which suggest that host conditions that impair cell-mediated immunity (CMI) (e.g., human immunodeficiency virus [HIV] infection with acquired immunodeficiency syndrome [AIDS], immunosuppressive therapy) play a critical role. Liver cirrhosis has also been reported as a condition that may increase the risk of cryptococcosis [18]. Other reported possible risk factors include diabetes mellitus, lymphoproliferative malignancy, hematological malignancy, cancer, autoimmune diseases, and lung diseases [19–21]. However, ascertainment of a condition as a risk factor for a disease requires either a cohort study (to compare the incidence of disease between those with the condition and those without) or a case-control study (to compare the proportion of the condition between those with the disease and those without), in order to distinguish true risk factors from comorbidities. To date, no cohort study or case-control study has been conducted to examine the risk factors of invasive C. neoformans diseases.
The first case of cryptococcal meningitis in Taiwan was diagnosed in 1957 [22]. Large clinical case series on cryptococcal meningitis [23,24], cryptococcemia [25] and cryptococcal diseases [26] reported high rates of HIV/AIDS, immunosuppressive therapy, decompensated liver cirrhosis, malignancy, diabetes mellitus, and kidney diseases among the case patients. To distinguish risk factors from comorbidities, we performed a hospital-based, retrospective, density-sampled, matched case-control study.
Methods
Study Setting
This study was conducted at National Taiwan University Hospital (Taipei, Taiwan), a university-affiliated medical center with a 2,200-bed capacity, which provides both primary and tertiary referral care in northern Taiwan.
Study Design
This was a retrospective, density-sampled, case-control study. Patients with cryptococcal meningitis or cryptococcemia who were diagnosed and hospitalized during 2002–2010 (cases) were compared with those who were hospitalized during the same time period but did not have cryptococcal meningitis or cryptococcemia (controls). The cases and controls were matched according to admission date, age, and gender.
Ethical Statement
Patient medical records were retrospectively reviewed to obtain information on diagnosis, sites of C. neoformans infection, and potential risk factors. All personal information was anonymized. The study procedures were reviewed and approved by National Taiwan University Hospital’s institutional review board (No. 201101083RC). The institutional review board approved the exemption of informed consent.
Recruitment of Cases
All of the patients with the ICD-9 diagnostic code 117.5 (cryptococcal diseases) upon either admission or discharge from January 1, 2002 to December 31, 2010 were identified using a computerized registry of inpatients. The patients’ medical records and microbiological reports were reviewed. All of the new-onset cases of C. neoformans meningitis (cryptococcal meningitis, confirmed by cerebrospinal fluid [CSF] culture) or C. neoformans fungemia (cryptococcemia, confirmed by blood culture) were included in the present study.
Selection of Controls
The controls were selected from inpatients who did not have cryptococcal meningitis or cryptococcemia; they were individually matched to each case by admission date, age (within a 5-year range), and gender at a 2:1 (cryptococcal meningitis) or a 4:1 (cryptococcemia) ratio. We first obtained the list of 600,782 admissions during the period from January 1, 2002 to December 31, 2010 using a computerized registry of inpatients. We then randomly selected controls for each case from the inpatients who met the matching criteria, using a random number generator.
Data Collection
The medical records were systematically reviewed for information regarding age, gender, and the presence of underlying diseases or conditions before the onset of invasive cryptococcal diseases. We assumed an induction time (i.e., the minimum time needed for a causal factor to induce the disease) of one month. Only those conditions that had been present at least one month prior to the onset of the cryptococcal diseases were included in this study.
The data were collected using a standardized recording format, which included items regarding the presence or absence of the following conditions: AIDS, decompensated liver cirrhosis, uremia, cytotoxic chemotherapy, CMI-suppressive therapy, organ transplantation, diabetes mellitus, lymphoma, leukemia/myeloma, solid cancer, other malignancies (e.g., gastrointestinal stromal tumor [GIST], sarcoma, thymoma), autoimmune diseases, cardiovascular disease, chronic obstructive pulmonary disease, asthma, bronchiectasis, pneumoconiosis, and gastrointestinal diseases. All of the data were independently verified by a second researcher.
In addition, the medical records were reviewed for a history of close contact with pigeons before the onset of the invasive cryptococcal diseases, including raising pigeons or visiting pigeon farms, if this information was recorded.
Definitions
AIDS was defined using the CDC’s 1993 revised case definition [27]. Liver cirrhosis was considered to be decompensated if the Child-Pugh score [28] was B or C. CMI-suppressive therapy was defined to include corticosteroids, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus and other immunosuppressive drugs that inhibit T-cell functions. CMI-suppressive therapy was further classified to stipulate whether a regimen was prescribed with or without calcineurin inhibitors (CAs) (cyclosporine or tacrolimus), which have direct in vitro antifungal activity against C. neoformans [29]. Diabetes mellitus was defined as having a fasting plasma glucose level ≥ 126 mg/dL, having a random plasma glucose level ≥ 200 mg/dL, or already receiving antidiabetic agents or insulin to control sugar [30]. Uremia was defined as end-stage renal disease with uremic symptoms or as receiving dialysis for more than three months. Autoimmune diseases included systemic lupus erythematosus, rheumatoid arthritis, dermatomyositis, and pemphigus, among others.
Statistical Analyses
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA). When two proportions were being compared, a chi-square test was used. Fisher’s exact test was used when any value in the cells of the contingency table was smaller than five. Between-group differences for continuous data were compared using Wilcoxon rank sum test. Conditional logistic regression was used to analyze the risk factors. The exact method was used. All variables with p < 0.10 in the univariate analysis were included in the maximum model and underwent stepwise selection during the multivariate analyses. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Cases
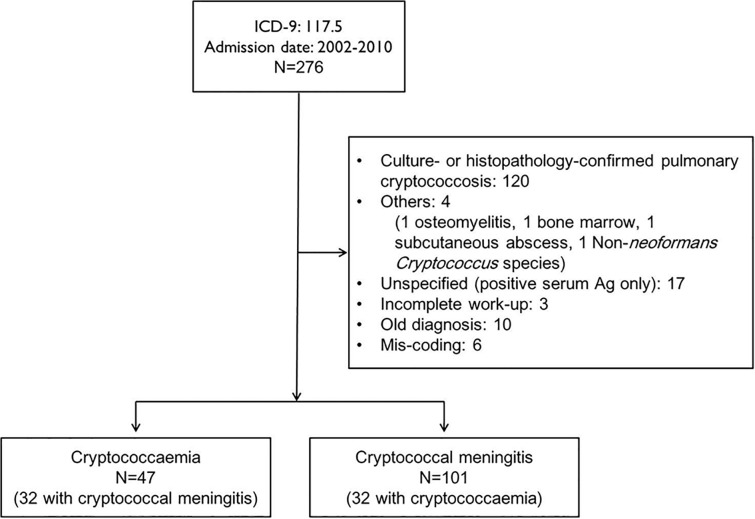
From January 1, 2002 through December 31, 2010, a total of 276 inpatients at National Taiwan University Hospital had the ICD-9 diagnostic code 117.5 upon admission or discharge. Of them, 116 had new-onset cryptococcal meningitis (n = 101) or new-onset cryptococcemia (n = 47), including 32 who had simultaneous cryptococcal meningitis and cryptococcemia. The remaining 160 patients did not meet the inclusion criteria, including those with pulmonary cryptococcosis (n = 120), other local involvement (n = 3), non-Cryptococcus neoformans fungemia (n = 1), an unspecified diagnosis (positive for serum cryptococcal antigen, but without a culture or histopathological confirmation; n = 17), an incomplete work-up (n = 3), an old diagnosis (n = 10), or a mis-coding (n = 6). Fig. 1 shows the flow chart of the recruitment of cases.
Fig 1. Flow chart of the recruitment of cases.
Controls
A total of 326 controls were randomly selected from the 600,782 admissions during the period from January 1, 2002 through December 31, 2010, including 188 controls who were matched to the 47 patients with cryptococcemia (at a 4:1 ratio) and the 138 controls who were matched to the 69 patients who had cryptococcal meningitis but did not have cryptococcemia (at a 2:1 ratio).
Epidemiologic Characteristics
Table 1 shows the characteristics of cryptococcal meningitis and cryptococcemia cases, including demographic features, underlying diseases, history of immunosuppressive therapy, and close contact with pigeons.
Table 1. Epidemiological characteristics of 101 Cryptococcus neoformans meningitis cases and 47 C. neoformans fungemia cases.
| Characteristics | C. neoformans meningitis (N = 101) | C. neoformans fungemia (N = 47) | p-value |
|---|---|---|---|
| Age, range, years | 20–86 | 24–79 | |
| Age, mean ± SD, years | 49.8 ± 17.2 | 51.6 ± 18.2 | |
| Male | 73 (72.3) | 36 (76.6) | 0.58 |
| AIDS | 42 (41.6) | 21 (44.7) | 0.72 |
| Immunosuppressive therapy | 24 (23.8) | 13 (27.7) | 0.68 |
| CMI-suppressive therapy | 23 (22.8) | 13 (27.7) | 0.52 |
| Regimens without CAs | 19 (18.8) | 11 (23.4) | 0.52 |
| Prednisolone a | 11 | 5 | |
| Methylprednisolone a | 1 | 1 | |
| Prednisolone a + azathioprine | 4 | 3 | |
| Prednisolone a + chlorambucil | 1 | 0 | |
| Prednisolone a + cyclophosphamide | 1 | 1 | |
| Prednisolone a + rituximab + etoposide | 1 | 0 | |
| Dexamethasone a + mephalan | 0 | 1 | |
| CA-based regimens | 4 (4.0) | 2 (4.3) | 1.00 |
| Tarcolimus + prednisolone b + mycophenolate mofetil | 3 | 1 | |
| Cyclosporin + prednisolone b | 1 | 0 | |
| Cyclosporin + methylprednisolone b | 0 | 1 | |
| Cytotoxic chemotherapy | 5 (5.0) | 3 (6.4) | 0.71 |
| Cardiovascular disease | 21 (20.8) | 13 (27.7) | 0.36 |
| Autoimmune diseases | 13 (12.9) | 10 (21.3) | 0.19 |
| Decompensated liver cirrhosis | 8 (7.9) | 8 (17.0) | 0.10 |
| Diabetes mellitus | 16 (15.8) | 8 (17.0) | 0.86 |
| Gastrointestinal diseases | 6 (5.9) | 6 (12.8) | 0.16 |
| Lymphoma | 4 (4.0) | 3 (6.4) | 0.68 |
| Leukemia/myeloma | 3 (3.0) | 3 (6.4) | 0.38 |
| Cancer | 14 (13.9) | 6 (12.8) | 1.00 |
| Solid organ transplantation | 4† (4.0) | 1* (2.1) | 1.00 |
| Bone marrow/stem cell transplantation | 0 (0) | 0 (0) | - |
| Uremia | 2 (2.0) | 1 (2.1) | 1.00 |
| COPD | 0 (0) | 0 (0) | - |
| Asthma | 0 (0) | 0 (0) | - |
| Bronchiectasis | 0 (0) | 0 (0) | - |
| Pneumoconiosis | 1 (1) | 0 (0) | 1.00 |
| Close contact with pigeons | 5 (5.0) | 0 (0) | 0.31 |
Data are no. (%) of patients, unless otherwise indicated. AIDS: acquired immunodeficiency syndrome; CAs: calcineurin inhibitors; CMI: cell-mediated immunity; COPD: chronic obstructive pulmonary disease.
†Heart (n = 2), kidney (n = 1), liver (n = 1).
*Heart (n = 1).
a Mean dosage of steroid in CMI-suppressive regimens without CA: equivalent dose of prednisolone 20 mg/day in meningitis cases and 30 mg/day in cryptococcemia cases.
b Mean dosage of steroid in CA-based regimens: equivalent dose of prednisolone 10 mg/day in meningitis cases and 55 mg/day in cryptococcemia cases.
The mean age of cryptococcal meningitis and cryptococcemia cases was approximately 50 years. Most cases (72.3%–76.6%) occurred in men. The age and gender distributions were similar between cases of cryptococcal meningitis and those of cryptococcemia (Table 1). The majority of patients younger than 40 years had AIDS, whereas the older age (≥50 years) group consisted primarily of non-AIDS patients.
AIDS (41.6%–44.7%) was the most common underlying condition in both the cryptococcal meningitis cases and the cryptococcemia cases, followed by immunosuppressive therapy (23.8%–27.7%), cardiovascular diseases (20.8%–27.7%), autoimmune diseases (12.9%–21.3%), decompensated liver cirrhosis (7.9%–17.0%), and diabetes mellitus (15.8%–17.0%). The pattern of underlying conditions was not significantly different between the cryptococcal meningitis cases and the cryptococcemia cases (Table 1).
In-hospital mortality was significantly higher in cryptococcemia cases compared with cryptococcal meningitis cases (23/47 [48.9%] vs. 28/101 [27.7%], p = 0.016). Of the 101 patients with cryptococcal meningitis, 32 (32%) had cryptococcemia, for whom in-hospital mortality occurred at double the rate of those who had cryptococcal meningitis but did not have cryptococcemia (14/32 [43.8%] vs. 14/69 [20.3%], p = 0.027). Cases with decompensated liver cirrhosis had a particularly high mortality, compared with cases without decompensated liver cirrhosis, in both cryptococcal meningitis group (6/8 [75.0%] vs. 22/93 [23.7%], p = 0.005) and cryptococcemia group (6/8 [75.0%] vs. 17/39 [43.6%], p = 0.137).
Table 2 shows the concomitantly involved sites in the 47 patients with cryptococcemia. Those with AIDS (n = 21) were more likely to have culture-proven meningitis than those with decompensated liver cirrhosis (n = 8) (19/21 [90%] vs. 3/8 [44.4%], p = 0.008). However, those patients with decompensated liver cirrhosis were significantly less likely than patients with AIDS to undergo a lumbar puncture for a CSF study (5/8 [62.5%] vs. 21/21 [100%], p = 0.023; Table 2).
Table 2. Characteristics of the 47 patients with cryptococcemia.
| Involved sites† | All cases (N = 47) (%) | Decompensated cirrhosis (N = 8) (%) | AIDS (N = 21) (%) | p-value ξ (Decompensated cirrhosis vs. AIDS) |
|---|---|---|---|---|
| Meningitis | 32 (68.1) | 3 (37.5) | 19 (90) | 0.008 |
| CSF culture (+) | 31 (66.0) | 3 (37.5) | 19 (90) | - |
| CSF Cryptococcal Ag (+) | 32 (68.1) | 3 (37.5) | 19 (90) | - |
| CSF culture (−) | 5 (10.6) | 2 (25.0) | 2 (10) | 0.568 |
| CSF not checked | 8 (17.0) | 3 (37.5) | 0 (0) | 0.023 |
| Peritonitis | 3 (6.4) | 3 (37.5) | 1 (5) | 0.076 |
| Pleurisy | 3 (6.4) | 2 (25.0) | 0 (0) | 0.089 |
| Lung (pathology-proven) | 2 (4.3) | 0 (0) | 0 (0) | 1.000 |
| Urine | 7 (14.9) | 3 (37.5) | 2 (10) | 0.112 |
| Bone and soft tissue | 1 (2.1) | 0 (0) | 0 (0) | 1.000 |
| PTCD | 1 (2.1) | 1 (12.5) | 0 (0) | 0.310 |
PTCD: Percutaneous transhepatic cholangio-drainage.
ξ p-values were computed using Fisher’s exact test to compare cryptococcemia cases with decompensated liver cirrhosis and cases with AIDS.
†Culture-proven, unless otherwise indicated.
Risk Factors for Cryptococcal Meningitis
The significant variables in the univariate analysis included AIDS, decompensated liver cirrhosis, CMI-suppressive regimens without CA, cytotoxic chemotherapy, cardiovascular disease, autoimmune diseases, cancer, and close contact with pigeons (Table 3). Diabetes mellitus was not a risk factor (crude odds ratio [OR] = 1.5; 95% CI: 0.7 to 3.2; p = 0.389). Multivariate regression analysis revealed the following three independent risk factors for cryptococcal meningitis: decompensated liver cirrhosis (adjusted OR = 8.5; 95% CI: 1.6 to 62.3; p = 0.008), AIDS (adjusted OR = 181.4; 95% CI: 28.2 to >999; p < 0.001), and CMI-suppressive regimens without CAs (adjusted OR = 15.9; 95% CI: 4.9 to 68.2; p < 0.001) (Table 4). Close contact with pigeons was a risk factor for cryptococcal meningitis in the univariate analysis (crude OR = 13.5; 95% CI: 1.8 to ∞; p = 0.008); however, this variable did not enter the final model.
Table 3. Univariate analyses of variables associated with cryptococcal meningitis.
| Variables | Cases (N = 101) | Controls (N = 266) | Unadjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Decompensated liver cirrhosis | 8 | 4 | 6.5 (1.5–39.4) | 0.009 |
| AIDS | 42 | 3 | 118.8 (20.0–>999) | <0.001 |
| Immunosuppressive therapy | 24 | 57 | - | - |
| CMI-suppressive therapy | 23 | 15 | - | - |
| Regimens without CAs# | 19# | 10# | 5.5 (2.3–13.9) | <0.001 |
| CA-based regimens | 4 | 5 | 1.7 (0.3–8.1) | 0.630 |
| Cytotoxic chemotherapy | 5 | 47 | 0.2 (0.1–0.7) | 0.002 |
| Solid organ transplantation | 4† | 5* | 1.9 (0.4–8.8) | 0.556 |
| Bone marrow/stem cell transplantation | 0 | 1 | 2.0 (0–78) | 1.000 |
| Diabetes mellitus | 16 | 30 | 1.5 (0.7–3.2) | 0.389 |
| Cardiovascular disease | 21 | 83 | 0.5 (0.2–0.9) | 0.026 |
| Uremia | 2 | 4 | 1.2 (0.1–8.9) | 1.000 |
| Gastrointestinal diseases | 6 | 24 | 0.9 (0.3–2.5) | 1.000 |
| Autoimmune diseases | 13 | 8 | 4.6 (1.7–12.9) | 0.001 |
| COPD | 0 | 5 | 0.6 (0–3.3) | 0.655 |
| Asthma | 0 | 5 | 0.4 (0–2.1) | 0.379 |
| Bronchiectasis | 0 | 2 | 1.7 (0–21.3) | 1.000 |
| Pneumoconiosis | 1 | 0 | 2.0 (0.1–∞) | 0.667 |
| Lymphoma | 4 | 5 | 2.2 (0.4–10.3) | 0.422 |
| Leukemia/myeloma | 3 | 5 | 2.8 (0.5–15.6) | 0.271 |
| Cancer | 14 | 85 | 0.3 (0.1–0.6) | <0.001 |
| Others (GIST/sarcoma/thymoma) | 0 | 5 | 0.5 (0–3.7) | 0.546 |
| Close contact with pigeons | 5 | 0 | 13.5 (1.8–∞) | 0.008 |
CMI: cell-mediated immunity; CAs: calcineurin inhibitors; COPD: chronic obstructive pulmonary disease; GIST: gastrointestinal stromal tumor.
†Heart (n = 2), kidney (n = 1), liver (n = 1).
* Heart (n = 2), kidney (n = 2), liver (n = 1).
# The mean dosage of steroid was significantly higher in cases received CMI-suppressive regimens without CA (n = 19) than that in controls received CMI-suppressive regimens without CA (n = 10) (equivalent dose of prednisolone: 20 vs. 5.4 mg/day, p = 0.041).
Table 4. Independent risk factors for cryptococcal meningitis.
| Variables | Adjusted OR (95% CI) | p-value |
|---|---|---|
| AIDS | 181.4 (28.2–>999) | <0.001 |
| CMI-suppressive regimens without CAs | 15.9 (4.9–68.2) | <0.001 |
| Decompensated liver cirrhosis† | 8.5 (1.6–62.3) | 0.008 |
AIDS: acquired immunodeficiency syndrome. CMI: cell-mediated immunity; CAs: calcineurin inhibitors.
†Decompensated liver cirrhosis: Child-Pugh score of B or C.
Risk Factors for Cryptococcemia
Univariate analyses identified AIDS, decompensated liver cirrhosis, CMI-suppressive regimens without CAs, autoimmune diseases, and cancer as the significant variables (Table 5). Diabetes mellitus was not a risk factor (crude OR = 1.4; 95% CI: 0.5 to 3.5; p = 0.650). Decompensated liver cirrhosis (adjusted OR = 23.8; 95% CI: 3.4 to 340.9; p < 0.001), AIDS (adjusted OR = 216.3; 95% CI: 24.2 to >999; p < 0.001), CMI-suppressive regimens without CAs (adjusted OR = 7.3; 95% CI: 1.1 to 57.5; p = 0.034), and autoimmune diseases (adjusted OR = 9.3; 95% CI: 1.1 to 135.7; p = 0.038) were independent risk factors for cryptococcemia (Table 6).
Table 5. Univariate analyses of variables associated with cryptococcemia.
| Variables | Cases (N = 47) | Controls (N = 188) | Unadjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Decompensated liver cirrhosis | 8 | 4 | 13.7 (2.7–134.6) | <0.001 |
| AIDS | 21 | 2 | 81.5 (13.1–>999) | <0.001 |
| Immunosuppressive therapy | 13 | 40 | - | - |
| CMI-suppressive therapy | 13 | 11 | - | - |
| Regimens without CAs# | 11# | 9# | 5.7 (2.0–17.6) | <0.001 |
| CA-based regimens | 2 | 2 | 4.0 (0.3–55.2) | 0.362 |
| Cytotoxic chemotherapy | 3 | 31 | 0.3 (0.1–1.2) | 0.106 |
| Solid organ transplantation | 1† | 2* | 2.0 (0.03–38.4) | 0.980 |
| Bone marrow/stem cell transplantation | 0 | 0 | - | - |
| Diabetes mellitus | 8 | 25 | 1.4 (0.5–3.5) | 0.650 |
| Cardiovascular disease | 13 | 69 | 0.6 (0.2–1.4) | 0.252 |
| Uremia | 1 | 5 | 0.8 (0.02–8.4) | 1.000 |
| Gastrointestinal diseases | 6 | 21 | 1.2 (0.3–3.6) | 0.935 |
| Autoimmune diseases | 10 | 9 | 5.8 (1.9–19.9) | 0.002 |
| COPD | 0 | 6 | 0.5 (0–2.6) | 0.492 |
| Asthma | 0 | 5 | 0.6 (0–3.3) | 0.655 |
| Bronchiectasis | 0 | 1 | 4.0 (0–76) | 1.000 |
| Pneumoconiosis | 0 | 0 | - | - |
| Lymphoma | 3 | 5 | 2.6 (0.4–15.8) | 0.394 |
| Leukemia/myeloma | 3 | 2 | 6.0 (0.7–71.8) | 0.116 |
| Cancer | 6 | 61 | 0.3 (0.1–0.8) | 0.008 |
| Others (GIST/sarcoma/thymoma) | 0 | 3 | 1.0 (0–6.9) | 1.000 |
CMI: cell-mediated immunity; CAs: calcineurin inhibitors; COPD: chronic obstructive pulmonary disease; GIST: gastrointestinal stromal tumor.
†Heart (n = 1).
*kidney (n = 1), liver (n = 1).
# The mean dosage of steroid was significantly higher in cases CMI-suppressive regimens without CA (n = 11) than that in controls received CMI-suppressive regimens without CA (n = 9) (equivalent dose of prednisolone: 30 mg/day vs. 5 mg/day, p = 0.003).
Table 6. Independent risk factors for cryptococcemia.
| Variables | Adjusted OR (95% CI) | p-value |
|---|---|---|
| AIDS | 216.3 (24.2–>999) | <0.001 |
| CMI-suppressive regimens without CAs | 7.3 (1.1–57.5) | 0.034 |
| Autoimmune diseases | 9.3 (1.1–135.7) | 0.038 |
| Decompensated liver cirrhosis† | 23.8 (3.4–340.9) | <0.001 |
AIDS: acquired immunodeficiency syndrome. CMI: cell-mediated immunity. CAs: calcineurin inhibitors.
†Decompensated liver cirrhosis: Child-Pugh score of B or C.
Discussion
To our knowledge, the present study is the first hospital-based, case-control study to investigate the risk factors for the occurrence of invasive C. neoformans diseases. The results showed that AIDS, decompensated liver cirrhosis, and CMI-suppressive regimens without CAs were three independent risk factors for cryptococcal meningitis and cryptococcemia in Taiwan. Moreover, autoimmune disease was an independent risk factor for cryptococcemia. On the other hand, diabetes mellitus and other medical conditions were not found to be risk factors of cryptococcal meningitis or cryptococcemia.
Both cryptococcal meningitis and cryptococcemia were included in the present study as invasive C. neoformans diseases cases. We had previously reported that cryptococcemia is a fulminant form of cryptococcal disease with a high acute mortality rate, especially for those with liver cirrhosis [25]. The present study further highlights that patients with cryptococcemia have an approximately two-fold higher mortality rate than patients who have had cryptococcal meningitis unaccompanied by cryptococcemia.
AIDS, the end stage of HIV infection [27], is characterized by profound impairment of CMI [5–11], which predisposes people to invasive C. neoformans diseases. Extrapulmonary cryptococcosis is an AIDS-defining illness [27]. Cryptococcal meningitis is one of the most common opportunistic infections in patients who develop AIDS [3,4]. The wide use of highly active antiretroviral therapy (HAART), which can help prevent the development of AIDS if initiated early in the course of HIV infection, led to a decline in the number of C. neoformans cases in North America and Western Europe [20,31]. Our study provides new epidemiologic evidence that confirmed that AIDS is an important risk factor for the occurrence of invasive C. neoformans diseases. Our results support the World Health Organization’s current advice on the prevention of cryptococcal diseases in HIV-infected patients, which emphasizes that the most important and cost-effective strategy is the early initiation of ART through expanded HIV testing and scaled-up access to HAART [4].
CMI has been shown to play a critical role in the host’s defense against C. neoformans in animal models [12–17]. Our study confirmed that CMI-suppressive therapy increases the risk of invasive C. neoformans diseases in humans. Of particular interest is the observation that only the regimen that did not contain a calcineurin inhibitor was found to be a significant risk factor. The virulence of C. neoformans depends on fungal calcineurin [32]. Calcineurin inhibitors, such as cyclosporine and tacrolimus, have in vitro antifungal activity against C. neoformans [29,33] and may mitigate the increased risk of C. neoformans diseases under CMI-suppressive therapy. However, because of the small numbers of subjects receiving cyclosporine- or tacrolimus-based CMI-suppressive regimens in the present study, whether such regimens actually did confer a lower risk of invasive C. neoformans diseases remains statistically inconclusive and needs to be clarified in future studies. It is advisable that all patients receiving CMI-suppressive therapy, regardless of their regimens, be educated to avoid exposure to environmental sources of C. neoformans, such as pigeons and Eucalyptus trees. Physicians should be aware of the risk of invasive C. neoformans diseases in these patients. Early diagnosis and prompt antifungal treatment can be life-saving.
Patients with autoimmune diseases, such as systemic lupus erythematosus (SLE), have been demonstrated to have defects in the functions of phagocytes, humoral immunity, and cellular immunity [34]. In a study involving 150 SLE patients, the functional impairment of T helper cells was found to be correlated with disease activity rather than the medication doses [34], which indicates that autoimmune disease per se is an independent factor that contributes to decreased CMI. In the present study, we confirmed autoimmune disease is an independent risk factor for the occurrence of cryptococcemia.
In the present study, we confirmed that decompensated liver cirrhosis is an independent risk factor for the occurrence of invasive C. neoformans disease. The pathogenesis mechanism could involve multiple aspects. First, although C. neoformans typically enters the human body via inhalation through the respiratory system, people may become infected by ingesting contaminated food [35]. The presence of collateral circulation in decompensated liver cirrhosis allows for ingested C. neoformans to bypass the liver scavenger system and directly enter the circulatory system, thereby causing cryptococcemia and being further disseminated into the central nervous system. In addition, deficiencies in complement [36] and chemotaxis [37] as well as lymphocyte hyporesponsiveness [38] have been reported. The impaired innate and cell-mediated immunity in persons with decompensated liver cirrhosis not only contributes to an increased risk for invasive cryptococcal disease, but also a high risk of mortality once the disease occurs [25]. The high mortality of patients with decompensated liver cirrhosis in the present study (6/8 [75%] in cryptococcal meningitis cases and 6/8 [75%] in cryptococcemia cases) is consistent with that reported by Jean et al. (9/11 [82%]) [25] and Singh et al. (26/32 [81%]) [18]. Whether early recognition and prompt antifungal therapy can reduce this high mortality remains to be determined.
Diabetes mellitus was present in 8.5% to 33% of cryptococcosis cases in reported series [21,23,26,39] and in 15.8% of cryptococcal meningitis cases and 17.0% of cryptococcemia cases in the present study. Nevertheless, diabetes mellitus is a relative common condition in population, and the prevalence increases with age (7.56% among Taiwanese men aged 40–59, 19.97% among Taiwanese men aged 60–79, in Year 2009) [40]. This highlights the necessity of using an age-matched control group in evaluating the role of diabetes. The lack of association between diabetes mellitus and cryptococcosis in the present case-control study implies that diabetes mellitus was a comorbidity in those with two diseases, and that diabetic patients are actually not at increased risk of invasive cryptococcal diseases.
The strengths of the present study include the use of a computerized inpatient registry and ICD-9 diagnostic codes to identify all cases of invasive C. neoformans diseases during the study period and the verification of the diagnoses through review of medical records and microbiologic reports. In addition, a standard data format was used to systematically collect information on potential risk factors from medical records. This method circumvents patient recall bias, which is commonly encountered in case-control studies.
Our study has several limitations. First, because the medical records may not have included comprehensive information on casual exposure to pigeons or Eucalyptus trees, we were unable to confirm that such exposure is a risk factor for invasive C. neoformans disease. Second, close contact with pigeons (including raising pigeons or visiting pigeon farms; n = 5) likely is an important factor in acquiring C. neoformans disease, and it was shown to be a risk factor in the univariate analysis (OR = 13.5, p = 0.008). However, the small number of cases limits the statistical power of the multivariate analysis to implicate close contact with pigeons as an independent risk factor.
In conclusion, AIDS, decompensated liver cirrhosis, CMI-suppressive regimens without CAs, and autoimmune diseases are four independent risk factors for the occurrence of invasive C. neoformans diseases, while diabetes mellitus and other medical conditions are not. The present study confirms previous clinical observations and animal studies on the critical role of impaired cell-mediated immunity in the pathogenesis of invasive C. neoformans diseases, and provides a more precise characterization of high-risk individuals.
Supporting Information
(RAR)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Hansemann DV (1905) Uber eine bisher nicht beobachtete Gehirner Krankung durch Hefen. Verh Dtsch Ges Pathol 9: 21–24. [Google Scholar]
- 2. Mitchell TG, Perfect JR (1995) Cryptococcosis in the era of AIDS―100 years after the discovery of Cryptococcus neoformans . Clin Microbiol Rev 8: 515–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530. 10.1097/QAD.0b013e328322ffac [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (2011) Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva. [PubMed]
- 5. Kovacs JA, Kovacs AA, Polis M, Wright WC, Gill VJ, Tuazon CU, et al. (1985) Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med 103: 533–538. [DOI] [PubMed] [Google Scholar]
- 6. Eng RH, Bishburg E, Smith SM, Kapila R (1986) Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med 81: 19–23. [DOI] [PubMed] [Google Scholar]
- 7. Gal AA, Koss MN, Hawkins J, Evans S, Einstein H (1986) The pathology of pulmonary cryptococcal infections in the acquired immunodeficiency syndrome. Arch Pathol Lab Med 110: 502–507. [PubMed] [Google Scholar]
- 8. Shimizu RY, Howard DH, Clancy MN (1986) The variety of Cryptococcus neoformans in patients with AIDS. J Infect Dis 154: 1042 [DOI] [PubMed] [Google Scholar]
- 9. Zuger A, Louie E, Holzman RS, Simberkoff MS, Rahal JJ (1986) Cryptococcal disease in patients with the acquired immunodeficiency syndrome. diagnostic features and outcome of treatment. Ann Intern Med 104: 234–240. [DOI] [PubMed] [Google Scholar]
- 10. Chuck SL, Sande MA (1989) Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med 321: 794–799. [DOI] [PubMed] [Google Scholar]
- 11. Zoller WG, Kellner H, Goebel FD, Schmiedtke K, Holecek B, Staib F, et al. (1989) Cryptococcus neoformans meningoencephalitis and multiple infections in AIDS. Klin Wochenschr 67: 598–604. [DOI] [PubMed] [Google Scholar]
- 12. Graybill JR, Drutz DJ (1978) Host defense in cryptococcosis. II. Cryptococcosis in the nude mouse. Cell Immunol 40: 263–274. [DOI] [PubMed] [Google Scholar]
- 13. Cauley LK, Murphy JW (1979) Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun 23: 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishimura K, Miyaji M (1979) Histopathological studies on experimental cryptococcosis in nude mice. Mycopathologia 68: 145–153. [DOI] [PubMed] [Google Scholar]
- 15. Salkowski CA, Balish E (1990) Pathogenesis of Cryptococcus neoformans in congenitally immunodeficient beige athymic mice. Infect Immun 58: 3300–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salkowski CA, Balish E (1991) Inflammatory responses to cryptococcosis in congenitally athymic mice. J Leukoc Biol 49: 533–541. [DOI] [PubMed] [Google Scholar]
- 17. Salkowski CA, Balish E (1991) Cutaneous cryptococcosis in athymic and beige-athymic mice. Infect Immun 59: 1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh N, Husain S, De Vera M, Gayowski T, Cacciarelli TV (2004) Cryptococcus neoformans infection in patients with cirrhosis, including liver transplant candidates. Medicine (Baltimore) 83: 188–192. [DOI] [PubMed] [Google Scholar]
- 19. Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36: 1–53. 10.3109/10408410903241444 [DOI] [PubMed] [Google Scholar]
- 20. Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, Brandt ME, et al. (2003) The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis 36: 789–794. [DOI] [PubMed] [Google Scholar]
- 21. Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, Sungkanuparph S (2006) Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis 10: 72–78. [DOI] [PubMed] [Google Scholar]
- 22. Lee TK, Chiu WS, Hsu CJ, Yang SP (1959) Cryptococcosis: a review with two case reports. J Formosan Med Assoc 58: 567–578. [Google Scholar]
- 23. Shih CC, Chen YC, Chang SC, Luh KT, Hsieh WC (2000) Cryptococcal meningitis in non-HIV-infected patients. QJM 93: 245–251. [DOI] [PubMed] [Google Scholar]
- 24. Sun HY, Hung CC, Chang SC (2004) Management of cryptococcal meningitis with extremely high intracranial pressure in HIV-infected patients. Clin Infect Dis 38: 1790–1792. [DOI] [PubMed] [Google Scholar]
- 25. Jean SS, Fang CT, Shau WY, Chen YC, Chang SC, Hsueh PR, et al. (2002) Cryptococcaemia: clinical features and prognostic factors. QJM 95: 511–518. [DOI] [PubMed] [Google Scholar]
- 26. Tseng HK, Liu CP, Ho MW, Lu PL, Lo HJ, Lin YH, et al. (2013) Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PLoS One 8: e61921 10.1371/journal.pone.0061921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Diseases Control and Prevention (1992) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 41: 1–19. [PubMed] [Google Scholar]
- 28. Child CG, Turcotte JG (1964) Surgery and portal hypertension. Major Probl Clin Surg 1: 1–85. [PubMed] [Google Scholar]
- 29. Singh N, Alexander BD, Lortholary O, Dromer F, Gupta KL, John GT, et al. (2007) Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis 195: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Diabetes Association (2006) Diagnosis and classification of diabetes mellitus. Diabetes Care 29 (Suppl 1): S43–48. [PubMed] [Google Scholar]
- 31. Dromer F, Mathoulin-Pelissier S, Fontanet A, Ronin O, Dupont B, Lortholary O, et al. (2004) Epidemiology of HIV-associated cryptococcosis in France (1985–2001): comparison of the pre- and post-HAART eras. AIDS 18: 555–562. [DOI] [PubMed] [Google Scholar]
- 32. Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J, et al. (1997) Calcineurin is required for virulence of Cryptococcus neoformans . EMBO J 16: 2576–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cruz MC, Del Poeta M, Wang P, Wenger R, Zenke G, Quesniaux VF, et al. (2000) Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob Agents Chemother 44: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bermas BL, Petri M, Goldman D, Mittleman B, Miller MW, Stocks NI, et al. (1994) T helper cell dysfunction in systemic lupus erythematosus (SLE): relation to disease activity. J Clin Immunol 14: 169–177. [DOI] [PubMed] [Google Scholar]
- 35. Jean SS, Wang JL, Wang JT, Fang CT, Chen YC, Chang SC. (2005) Cryptococcus neoformans peritonitis in two patients with liver cirrhosis. J Formos Med Assoc 104: 39–42. [PubMed] [Google Scholar]
- 36. Akalin HE, Laleli Y, Telatar H (1985) Serum bactericidal and opsonic activities in patients with non-alcoholic cirrhosis. QJM 56: 431–437. [PubMed] [Google Scholar]
- 37. DeMeo AN, Andersen BR, English DK, Peterson J (1972) Defective chemotaxis associated with a serum inhibitor in cirrhotic patients. N Engl J Med 286: 735–740. [DOI] [PubMed] [Google Scholar]
- 38. Holdstock G, Chastenay BF, Krawitt EL (1982) Studies on lymphocyte hyporesponsiveness in cirrhosis: the role of increased monocyte suppressor cell activity. Gastroenterology 82: 206–212. [PubMed] [Google Scholar]
- 39. Chuang YM, Ho YC, Chang HT, Yu CJ, Yang PC, Hsueh PR (2008) Disseminated cryptococcosis in HIV-uninfected patients. Eur J Clin Microbiol Infect Dis 27: 307–310. [DOI] [PubMed] [Google Scholar]
- 40. Jiang YD, Chang CH, Tai TY, Chen JF, Chuang LM (2012) Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc 111: 599–604. 10.1016/j.jfma.2012.09.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.