Abstract
Prenatal sex hormones can induce abnormalities in the reproductive system and adversely impact on genital development. We investigated whether sex hormones in cord blood influenced the ratio of the second to fourth digit lengths (2D/4D) in school-aged children. Of the 514 children who participated in a prospective cohort study on birth in Sapporo between 2002 and 2005, the following sex hormone levels were measured in 294 stored cord blood samples (135 boys and 159 girls); testosterone (T), estradiol (E), progesterone, LH, FSH, inhibin B, and insulin-like factor 3 (INSL3). A total of 350 children, who were of school age and could be contacted for this survey, were then requested via mail to send black-and-white photocopies of the palms of both the left and right hands. 2D/4D was calculated in 190 children (88 boys and 102 girls) using photocopies and derived from participants with the characteristics of older mothers, a higher annual household income, higher educational level, and fewer smokers among family members. 2D/4D was significantly lower in males than in females (p<0.01). In the 294 stored cord blood samples, T, T/E, LH, FSH, Inhibin B, and INSL3 levels were significantly higher in samples collected from males than those from females. A multivariate regression model revealed that 2D/4D negatively correlated with INSL3 in males and was significantly higher in males with <0.32 ng/mL of INSL3 (p<0.01). No correlations were observed between other hormones and 2D/4D. In conclusion, 2D/4D in school-aged children, which was significantly lower in males than in females, was affected by prenatal Leydig cell function.
Introduction
The ratio of the 2nd finger to 4th finger lengths (2D/4D) in humans has been reported to be smaller in males than in females [1]. This sexual difference has been attributed to the prenatal hormonal environment, such as exposure to higher levels of androgens and some other gonad-specific hormones [2] through androgen receptors, which are located in fetal cartilaginous tissue [3]. This hypothesis for the underlying mechanism for this difference is supported by the following findings; lower 2D/4D in girls with congenital adrenal hyperplasia [4], higher 2D/4D in individuals with complete androgen insensitivity syndrome [5], and the existence of a relationship between 2D/4D and polymorphisms in androgen receptors [6].
Prenatal exposure to sex hormones is known to affect human development, including that of the fetal digits, and one of the most important periods for the fetus is from the first to second trimester of pregnancy. Although most organ systems are developing during this period, the endocrine control systems have already been formed. The sexual difference in 2D/4D has already been established during early prenatal development under the influence of sex hormones [7, 8], and 2D/4D is considered to be stable after the early prenatal stages. Therefore, 2D/4D has been used as an easily measurable and stable anthropometric index of prenatal androgen exposure. However, the mechanism responsible for the sexual difference in 2D/4D has not yet been elucidated in detail.
There is currently no established approach for measuring prenatal hormone exposure when investigating the relationship between 2D/4D and the hormonal environment earlier in pregnancy in order to elucidate the mechanism underlying the sexual difference in 2D/4D; measuring prenatal hormone levels is difficult and not feasible for ethical reasons during a normal pregnancy. On the other hand, umbilical cord blood is obtained immediately after delivery, and its hormone levels are broadly considered to reflect the hormonal environment of the fetus at late gestation [9, 10]. Previous studies have been performed using cord blood to investigate the relationship between fetal hormonal exposure and human development [11–13].
In the present study, as a part of the Sapporo Cohort, Hokkaido Study on Environment and Child Health [14, 15], we investigated whether sex hormone levels in cord blood influenced 2D/4D in school-aged children.
Participants and Methods
Participants
This prospective birth cohort study was based on the Sapporo Cohort, Hokkaido Study on Environment and Child Health [14, 15]. Study details regarding the population, data collection, sampling of biological specimens, and contents of the questionnaire have been described previously [14, 15]. Briefly, native Japanese women living in Sapporo City or its surrounding areas were enrolled into the study at 23–35 weeks of gestation at Sapporo Toho Hospital between July 2002 and October 2005. Of the 1796 women approached, 25% were excluded as they decided to enroll in the Japanese cord blood bank or deliver the baby at another hospital; therefore, 514 pregnant women were enrolled in this cohort study (participation rate of 28.6%).
This study was approved by the Institutional Ethical Board for Epidemiological Studies at Hokkaido University Graduate School of Medicine and Hokkaido University Center for Environmental and Health Sciences. All participants provided written informed consent. Informed consent on behalf of the children enrolled was provided by their parents.
Measurement of 2D/4D
Ten out of 514 participants were excluded from the study due to miscarriage, stillbirth, relocation, or voluntary withdrawal from the study before delivery. As 7 sets of twins were born, a total of 511 children (246 males and 265 females) were finally included in the Sapporo Cohort study. Of these, 350 children (68.1%), who are currently school-aged and could be contacted for this survey, were requested via a mail to send black-and-white photocopies of the palms of both the left and right hands. Measurements of digits were made from photocopies of the ventral surface of the right and left hands. The participants were instructed to straighten their fingers and lightly place their hands palm down on the photocopy machine. Measurements were made to the nearest 0.5 mm from the mid-point of the finger crease proximal to the palm to the tip of the finger using steel Vernier calipers. The ratio was calculated by dividing the length of the second digit by that of the fourth digit[1]. All measurements were taken twice by two observers blinded to participants’ information in order to confirm the measurements obtained.
Sex hormone measurements in cord blood samples
At the time of delivery, a blood sample of 10–30mL was collected from the umbilical cord and stored at -80°C for later analysis.
The following hormone levels in 294 stored cord blood samples (135 boys and 159 girls) were measured. Testosterone (T), estradiol (E), and progesterone (P) levels were measured using LC-MS/MS [16, 17]. An immunoradiometric assay was used to measure luteinizing hormone (LH) (Spac-S LH Kit, TFB, Inc., Tokyo Japan) and follicle-stimulating hormone (FSH) levels (Spac-S FSH Kit, TFB, Inc., Tokyo Japan). Inhibin B levels were measured using an enzyme-linked immunosorbent assay (Inhibin B Gen II ELISA, Beckman Coulter, Inc., CA, USA). An enzyme immunoassay (Insulin-like 3 (INSL3) / RLF (Human)—EIA Kit, Phoenix Pharmaceuticals, Inc. CA, USA) was used to measure INSL3 levels. INSL3 was measured in males because it reflects Leydig cell function. It was also measured in 20 randomly selected samples from females. All sex hormone measurements were performed by Aska Pharma Medical Co., Ltd. (Kanagawa, Japan).
Statistical analyses
Data on the characteristics of participants, 2D/4D, and sex hormone levels were presented as a group mean ± standard deviation and were analyzed between groups using a one-way ANOVA. Sex hormones were converted to a log10 scale as these data did not fall into a normal distribution. A half of the detection limit was used when levels were below the detection limit for individual hormones. The relationship between 2D/4D and sex hormone levels in cord blood samples was calculated using a multiple linear regression analysis. The inclusion of covariates was based on biological considerations and adjustments were made for maternal age (continuous), birth weight (continuous), maternal smoking during pregnancy (yes or no), and maternal alcohol consumption during pregnancy (yes or no). All statistical analyses were performed using JMP pro 10 (SAS institute Inc., NC, USA), except for the intra-class correlation coefficient for right and left 2D/4D measurements, which was calculated using SPSS statistics version 19 (IBM, IL, USA). Significance levels were set to 0.05 for all comparisons.
Results
1) Patient characteristics
A total of 190 children from the 189 participants, including 88 males and 102 females, sent back photocopies of their palms. The characteristics of the participants and their children who sent back photocopies for 2D/4D were compared to their children without 2D/4D. 2D/4D was derived from the following participants; older mothers, a higher annual household income, higher educational level, and fewer smokers among family members. No significant differences were observed in gender, birth weight, or gestational age (Table 1).
Table 1. Patient characteristics.
| 2D/4D (+) | 2D/4D (-) | |||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |||
| Maternal characteristics | ||||||
| Age at delivery (years old) | 189 | 31.4 ± 4.2 | 315 | 30.7 ± 5.2 | ** | |
| Pre-pregnancy BMI (m2/kg) | 189 | 21.0 ± 3.1 | 315 | 21.6 ± 3.4 | ||
| Parity | Primiparous | 92 (48.7) | 148 (47.0) | |||
| Multiparous | 97 (51.3) | 167 (53.0) | ||||
| Annual house hold income (million yen per year) | <5 | 108 (57.1) | 237 (75.2) | ** | ||
| ≥5 | 81 (42.9) | 78 (24.8) | ||||
| Educational level (years) | ≤12 | 58 (30.7) | 166 (52.7) | ** | ||
| ≥13 | 131 (69.3) | 149 (47.3) | ||||
| Smoking during pregnancy | Nonsmoker | 174 (92.1) | 232 (73.7) | ** | ||
| Smoker | 12 (7.9) | 83 (26.3) | ||||
| Alcohol consumption during pregnancy | Nondrinker | 120 (63.5) | 235 (74.6) | |||
| Drinker | 69 (36.5) | 80 (25.4) | ||||
| Infant characteristics | ||||||
| Gender | Males | 88 (46.3) | 158 (49.2) | |||
| Females | 102 (53.7) | 163 (50.8) | ||||
| Birth weight (g) | 190 | 3037.6 ± 379.7 | 321 | 3003.9 ± 444.5 | ||
| Gestational age (weeks) | 190 | 38.9 ± 1.5 | 321 | 38.6 ± 1.6 | ||
The values in brackets represent percentages.
**: p<0.01.
2) 2D/4D
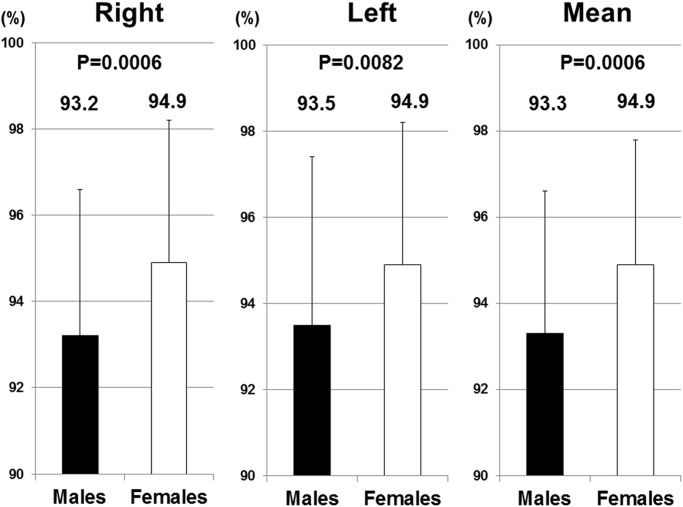
In all right hand, left hand, and mean values, 2D/4D was significantly higher in females than in males (Fig. 1). 2D/4D fell into a normal distribution in all right hand, left hand, and mean values.
Fig 1. 2D/4D in right hands, left hands, and mean values.
2D/4D in right hands, left hands, and mean values were significantly higher in females than in males.
The intra-class correlation coefficient (1, 2) for right and left 2D/4D measurements was 0.720 (95% confidence interval: 0.627–0.789). The mean 2D/4D value in both hands was used to determine its relationship with sex hormones as a representative value of each participant.
3) Sex hormones in cord blood samples
T, E, P, and INSL3 were detected in all samples. INSL3 was only measured in 20 randomly selected samples from females. The detection percentages of LH in males and females were 25.7% and 0.7%, respectively, while those of FSH in males and females were 46.8% and 0%, respectively. Inhibin B was detected in 99.2% of males and 26% of females (Table 2). The mean intra-assay and inter-assay coefficients of variations in terms of sex hormone measurements were as follows; T: 1.4%–5.3%, E: 3.2%–11.3%, P: 2.7%–6.3%, LH: 4.8%–6.5%, FSH: 2.3%–3.7%, Inhibin B: < 3.8%, and INSL3: 1%–5% in the mean intra-assay coefficients of variations, and T: 3.4%–5.1%, E: 4.8%–9.5%, P: 4.7%–6.0%, LH: 7.2%–26.0%, FSH: 5.4%–6.7%, Inhibin B: < 5.6%, and INSL3: 6%–15.0% in the mean inter-assay coefficients of variations.
Table 2. Sex hormone levels in cord blood in males and females.
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DL | n | 50th | 25th-75th | >DL (%) | n | 50th | 25th-75th | >DL (%) | p-value | |
| Testosterone (pg/mL) | 135 | 98.9 | 76.5–126 | 100 | 156 | 69.9 | 51.9–96.3 | 100 | <0.001 | |
| Estradiol (ng/mL) | 135 | 4.86 | 3.33–7.42 | 100 | 159 | 4.67 | 3.15–6.48 | 100 | 0.227 | |
| Progesterone (ng/mL) | 135 | 226 | 184–286 | 100 | 159 | 210 | 167–276 | 100 | 0.184 | |
| T/E | 135 | 18.5 | 13.9–25.7 | 100 | 156 | 15.9 | 11.8–21.8 | 100 | 0.002 | |
| LH (mIU/mL) | 0.5 | 132 | <DL | <DL-0.82 | 25.7 | 155 | <DL | <DL-<DL | 0.7 | <0.001 |
| FSH (mIU/mL) | 0.5 | 132 | <DL | <DL-0.66 | 46.8 | 154 | <DL | <DL-<DL | 0.0 | <0.001 |
| Inhibin B (pg/mL) | 11 | 134 | 44.0 | 33.9–58.3 | 99.2 | 159 | <DL | <DL-11.8 | 26.0 | <0.001 |
| INSL3 (ng/mL) | 0.01 | 132 | 0.29 | 0.25–0.34 | 100 | 20 | 0.18 | 0.17–0.23 | 100 | <0.001 |
DL: detection limit.
The median concentrations of T, LH, FSH, Inhibin B, and INSL3, which indicate androgen activity, were significantly higher in males than in females (Table 2).
4) Relationship between 2D/4D and sex hormones
No significant differences were observed in the hormone levels of children who sent back photocopies for 2D/4D and those who did not (Table 3).
Table 3. Sex hormones in cord blood and 2D/4D.
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2D/4D (+) | 2D/4D (-) | 2D/4D (+) | 2D/4D (-) | |||||||
| n | 50th | n | 50th | p-value | n | 50th | n | 50th | p-value | |
| Min | Min | Min | Min | |||||||
| Max | Max | Max | Max | |||||||
| Testosterone (pg/mL) | 45 | 90.9 | 90 | 101 | 0.240 | 69 | 64.9 | 87 | 71.3 | 0.255 |
| 12.2 | 5.45 | 12.3 | 6.25 | |||||||
| 483 | 620 | 457 | 168 | |||||||
| Estradiol (ng/mL) | 45 | 4.05 | 90 | 5.38 | 0.200 | 72 | 4.86 | 87 | 4.42 | 0.143 |
| 1.91 | 0.01 | 1.66 | 1.44 | |||||||
| 26.6 | 33.5 | 31.2 | 17.4 | |||||||
| Progesterone (ng/mL) | 45 | 183 | 90 | 234 | 0.378 | 72 | 201 | 87 | 216 | 0.457 |
| 13.7 | 0.43 | 6.25 | 8.86 | |||||||
| 455 | 471 | 467 | 514 | |||||||
| T/E | 45 | 21.7 | 90 | 17.5 | 0.477 | 69 | 15.7 | 87 | 15.7 | 0.424 |
| 2.05 | 2.73 | 1.9 | 0.68 | |||||||
| 52.1 | 21839 | 47.6 | 40.3 | |||||||
| LH (mIU/mL) | 45 | <DL | 87 | <DL | 0.986 | 70 | <DL | 85 | <DL | 0.263 |
| <DL | <DL | <DL | <DL | |||||||
| 2.39 | 3.37 | 0.61 | <DL | |||||||
| FSH (mIU/mL) | 45 | <DL | 87 | <DL | 0.765 | 72 | <DL | 82 | <DL | N/A |
| <DL | <DL | <DL | <DL | |||||||
| 1.43 | 1.89 | <DL | <DL | |||||||
| Inhibin B (pg/mL) | 44 | 43.3 | 90 | <DL | 0.957 | 72 | <DL | 87 | <DL | 0.947 |
| <DL | <DL | <DL | <DL | |||||||
| 90.6 | 104 | 76.6 | 65.7 | |||||||
| INSL3 (ng/mL) | 44 | 0.28 | 88 | 0.29 | 0.454 | N/A | N/A | |||
| 0.1 | 0.07 | N/A | N/A | |||||||
| 0.48 | 0.75 | |||||||||
N/A: not applicable.
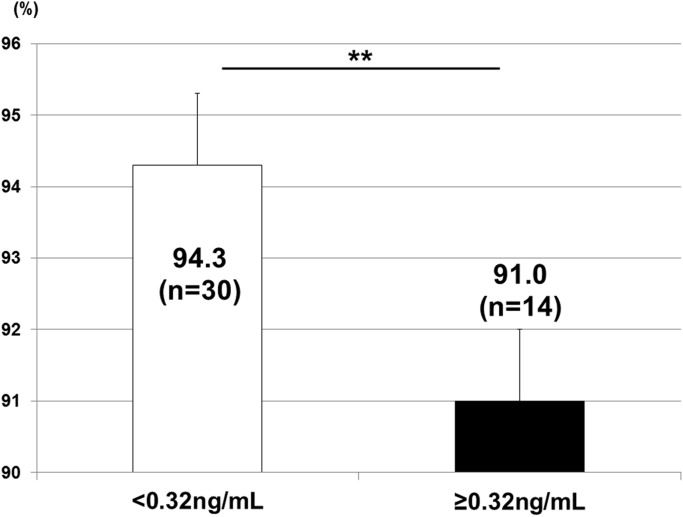
A multivariate regression model showed that 2D/4D negatively correlated with INSL3 only in males. Regarding the other sex hormones in both males and females, no correlations were observed with 2D/4D (Table 4). The application of 0.32 ng/mL of INSL3 from the receiver operating characteristic curve as a cut-off value revealed that 2D/4D was significantly higher in males with <0.32 ng/mL of INSL3 (p<0.01) (Fig. 2). This result indicated that 2D/4D could be affected by prenatal Leydig cell function.
Table 4. Relationship between 2D/4D and sex hormones in cord blood.
| Hormone levels | Total | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | B | R2 | n | B | R2 | n | B | R2 | |
| (95%CI) | (95%CI) | (95%CI) | |||||||
| T (pg/mL) | 114 | -0.021 | 0.113 | 45 | -0.209 | 0.060 | 69 | 0.151 | 0.214 |
| (-2.449, 1.956) | (-8.080, 1.754) | (-0.835, 3.909) | |||||||
| E (ng/mL) | 117 | -0.070 | 0.111 | 45 | -0.051 | 0.022 | 72 | -0.104 | 0.180 |
| (-2.893, 1.257) | (-4.956, 3.625) | (-3.346, 1.219) | |||||||
| P (ng/mL) | 117 | 0.036 | 0.107 | 45 | -0.020 | 0.020 | 72 | 0.078 | 0.175 |
| (-1.323, 1.977) | (-4.461, 3.971) | (-1.114, 3.647) | |||||||
| T/E | 114 | 0.010 | 0.113 | 45 | -0.138 | 0.036 | 69 | 0.200 | 0.228 |
| (-2.259, 2.514) | (-6.331, 2.650) | (-0.440, 5.190) | |||||||
| LH (mIU/mL) | 115 | 0.017 | 0.104 | 45 | 0.207 | 0.055 | 70 | 0.126 | 0.180 |
| (-2.167, 2.610) | (-1.335, 5.346) | (-6.313, 21.64) | |||||||
| FSH (mIU/mL) | 117 | -0.038 | 0.105 | 45 | 0.180 | 0.048 | N/A | ||
| (-3.696, 2.448) | (-2.162, 7.177) | N/A | |||||||
| INSL3 (ng/mL) | N/A | N/A | 44 | -0.377* | 0.145 | N/A | N/A | ||
| N/A | (-30.17, -2.318) | N/A | |||||||
| Inhibin B (pg/mL) | 116 | -0.139 | 0.124 | 44 | -0.068 | 0.024 | 72 | -0.082 | 0.172 |
| (-2.238, 0.331) | (-5.877, 3.891) | (-1.387, 2.732) | |||||||
*: p<0.05,
N/A: not applicable.
Fig 2. 2D/4D and INSL3.
2D/4D was significantly higher in males with <0.32 ng/mL of INSL3 in cord blood (p<0.01). **: p<0.01.
Discussion
In the present study, the ratio of the digit length of the 2nd finger to that of the 4th finger, which has been used as an easily measurable and stable anthropometric index of prenatal exposure to androgens, was calculated in school-aged children, and sex hormone levels in cord blood samples were then measured. The levels of sex hormones indicating androgen activity in cord blood were significantly higher in males than in females. 2D/4D was significantly higher in females than in males, and negatively correlated with INSL3 only in males.
The biosynthesis of testosterone hypothetically occurs at a gestational age of 9 weeks, whereas 2D/4D dimorphism appears as early as at 14 weeks of gestation [7, 8], which indicated that early levels of sex hormones can influence 2D/4D. A previous study reported that 2D/4D reflected a genetic background subjected to a given level of exposure to prenatal androgens [1]. A gestational peak in testosterone production due to the development of Leydig cells occurred between 14 and 18 weeks. Thus, compelling evidence currently shows that 2D/4D is affected by prenatal exposure to androgens in humans.
In the present study, we used the mean 2D/4D value in both hands as a representative value of each participant, as previously reported, because the influence of the stronger side of the hands in 2D/4D on correlations with any factors has not yet been established and the intra-class correlation coefficient (1, 2) for right and left 2D/4D measurements was 0.720 (95% confidence interval: 0.627–0.789). 2D/4D in the left hand negatively correlated with INSL3 (β = -0.414, p = 0.0125), whereas 2D/4D in the right hand was not correlated with INSL3 (β = -0.268, p = 0.1093). We attributed these differences in 2D/4D between the right and left hands to various factors including measurement errors, the relatively small sample size, and the limitations associated with physical measurements. Thus, we considered it reasonable to use the mean value of 2D/4D as a representative value of each participant.
In the present study, no correlation was observed between the level of testosterone in cord blood and 2D/4D. This result was compatible with previous findings, which demonstrated that the concentration of testosterone in cord blood could not predict 2D/4D[18]. Furthermore, a previous study suggested that amniotic fluid, but not cord blood, was the best candidate for investigating the effects of early fetal exposure to androgens [19]. These findings taken together with our results indicated that testosterone in cord blood did not influence 2D:4D or reflect fetal exposure during the critical period of digit development at approximately 14 weeks of gestation. The measurement of sex hormones in cord blood may be affected by obstetric and maternal factors, such as prematurity, labor onset, placental weight, intrauterine infection, and preeclampsia, which have not yet been established in detail [9].
INSL3 levels in cord blood samples correlated with 2D/4D in males. INSL3 is constitutively produced by Leydig cells in the fetal testis, not by other organs, after sex determination [20], and is a gender-specific fetal hormone. The fetal testis is established at approximately 7 weeks of pregnancy and the INSL3 gene in fetal Leydig cells is detectable by 8–10 weeks of pregnancy in humans [21]. This period of transition from the first to the second trimester is important for development, and is very vulnerable to a range of endocrine-disrupting insults to male reproductive development. Thus, the detection of INSL3 in fetal blood during mid-gestation reliably indicates a male fetal gender [21]. INSL3 in cord blood reflects prenatal Leydig cell function, which serves in the production of testosterone, and may also reflect androgen exposure during the important developmental window of earlier pregnancy for the digits as well as male reproductive development. In the present study, a correlation was observed between INSL3, but not testosterone, in cord blood and 2D/4D, and a previous study also demonstrated that 2D/4D was significantly related to adult testosterone levels and the presence of testosterone deficiency syndrome [22].
No correlation was noted between other hormones with androgen activity, such as LH, FSH, and Inhibin B, and 2D/4D. This may have been due to more than 50% of the stored cord blood samples being below the detection limit for LH and FSH. Therefore, more sensitive kits are needed to measure LH and FSH. Since Inhibin B reflects Sertoli cell function, its levels may not directly indicate androgen exposure in utero for digit development. Furthermore, a previous study using mice showed that receptors for androgen and estrogen were particularly located in the 4th digit and the growth of this digit was stimulated by androgen, but arrested by estrogen[23]. Although it has already been reported that 2D/4D cannot be determined by prenatal testosterone alone and the balance between prenatal testosterone and prenatal estrogen is another important factor in fetal digit development [24], our results showed that T/E in cord blood did not correlate with 2D/4D. Thus, the present study revealed that only prenatal Leydig cell function, indicating early exposure during gestation to androgens, could be implicated in 2D/4D.
As one of factors that affects sex hormones during gestation, endocrine-disrupting chemicals, e.g. phthalates, dioxins, polychlorinated biphenyls (PCBs), and perfluorinated alkyl acids (PFAAs), have been shown to induce a broad spectrum of toxic effects on the reproductive system and genital development in the prenatal period in humans. Our cohort study already demonstrated that maternal exposure to phthalates reduced the levels of T/E, P, inhibin B, and INSL3 in cord blood, suggesting that exposure to DEHP in utero may have adverse effects on both Sertoli and Leydig cell development in males [25]. Previous studies also revealed that other endocrine-disrupting chemicals affected the hormonal environment during the prenatal period in humans. Cao et al. demonstrated that maternal exposure to dioxins decreased T and E in cord blood [26]. Furthermore, Hsu et al. showed that maternal exposure to PCBs decreased T/E in boys at puberty [27]. Regarding PFAAs, Vested et al. reported that maternal exposure to perfluorooctane sulfonate (PFOS) during gestation decreased the concentration and counts of sperm and increased LH and FSH levels in males after puberty, suggesting that maternal exposure to PFOS may affect semen quality and reproductive hormone levels in adult human males. Thus, maternal exposure to endocrine-disrupting chemicals influences sex hormones during gestation, as demonstrated by anti-androgen activity in males. These findings indicate that maternal exposure to endocrine-disrupting chemicals affects sex hormone levels during gestation and induces physical changes to the digits of children. An animal study has already showed that prenatal exposure to low doses of endocrine-disrupting chemicals induced feminized digit ratios in male rats[28]. Further studies are warranted to confirm this in humans.
Polymorphisms in androgen receptors (AR) may also affect sensitivity to androgen exposure in 2D/4D. AR are produced by the AR gene, which is located on the X-chromosome and repeats the nucleotide sequence CAG on exon 1. Furthermore, the number of CAG repeats varies in length among individuals and code for the length of a polyglutamine stretch on the N-terminal domain of AR. Although previous studies revealed that there was no evidence for a clear association between CAG repeats and 2D/4D [29, 30], the synergic effects of polymorphisms in AR and sex hormones in cord blood on 2D/4D remain unclear. Therefore, further investigations are needed in our cohort study.
The first limitation of this study was that we performed multiple analyses, which are associated with the risk of false positives in the main result of a correlation between 2D:4D and INSL3. The second limitation of this study was the relatively small cohort of school-aged children for whom we had data on both 2D:4D and sex hormones because only 190 (54.3%) of 350 children sent photocopies of their palms for the measurement of 2D/4D. Larger studies are needed to reveal the effects of sex hormone levels in utero on physical changes to children.
Conclusions
The levels of sex hormones indicating androgen activity in cord blood were significantly higher in males than in females. 2D/4D in school-aged children, which was significantly lower in boys than in girls, was affected by prenatal Leydig cell function in males.
Acknowledgments
We thank all the mothers and their children who participated in this study, and all the staff at Sapporo Toho Hospital.
Data Availability
Due to ethical restrictions, data are available to qualified researchers on request from the corresponding author.
Funding Statement
The study was supported by the Ministry of Health, Labour and Welfare, Health and Labour Sciences Research Grants, Grants-in Aid for Scientific Research from the Japan Society for the Promotion of Science, and Environment Research and Technology Development Fund (5C-1252) from the Ministry of the Environment, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998, 13;11:3000–3004. [DOI] [PubMed] [Google Scholar]
- 2. Breedlove SM. Minireview: Organizational hypothesis: instances of the fingerpost. Endocrinology. 2010, 151;9:4116–4122. 10.1210/en.2010-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben-Hur H, Thole HH, Mashiah A, Insler V, Berman V, Shezen E et al. Estrogen, progesterone and testosterone receptors in human fetal cartilaginous tissue: immunohistochemical studies. Calcif Tissue Int. 1997, 60;6:520–526. [DOI] [PubMed] [Google Scholar]
- 4. Okten A, Kalyoncu M, Yaris N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Hum Dev. 2002, 70;1–2:47–54. [DOI] [PubMed] [Google Scholar]
- 5. Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009, 150;11:5119–5124. 10.1210/en.2009-0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manning JT, Henzi P, Venkatramana P, Martin S, Singh D. Second to fourth digit ratio: ethnic differences and family size in English, Indian and South African populations. Ann Hum Biol. 2003, 30;5:579–588. [DOI] [PubMed] [Google Scholar]
- 7. Galis F, Ten Broek CM, Van Dongen S, Wijnaendts LC. Sexual dimorphism in the prenatal digit ratio (2D:4D). Arch Sex Behav. 2010, 39;1:57–62. 10.1007/s10508-009-9485-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malas MA, Dogan S, Evcil EH, Desdicioglu K. Fetal development of the hand, digits and digit ratio (2D:4D). Early Hum Dev. 2006, 82;7:469–475. [DOI] [PubMed] [Google Scholar]
- 9. Hollier LP, Keelan JA, Hickey M, Maybery MT, Whitehouse AJ. Measurement of Androgen and Estrogen Concentrations in Cord Blood: Accuracy, Biological Interpretation, and Applications to Understanding Human Behavioral Development. Front Endocrinol (Lausanne). 2014, 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keelan JA, Mattes E, Tan H, Dinan A, Newnham JP, Whitehouse AJ et al. Androgen concentrations in umbilical cord blood and their association with maternal, fetal and obstetric factors. PLoS One. 2012, 7;8:e42827 10.1371/journal.pone.0042827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hollier LP, Mattes E, Maybery MT, Keelan JA, Hickey M, Whitehouse AJ. The association between perinatal testosterone concentration and early vocabulary development: a prospective cohort study. Biol Psychol. 2013, 92;2:212–215. 10.1016/j.biopsycho.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 12. Whitehouse AJ, Mattes E, Maybery MT, Sawyer MG, Jacoby P, Keelan JA et al. Sex-specific associations between umbilical cord blood testosterone levels and language delay in early childhood. J Child Psychol Psychiatry. 2012, 53;7:726–734. 10.1111/j.1469-7610.2011.02523.x [DOI] [PubMed] [Google Scholar]
- 13. Robinson M, Whitehouse AJ, Jacoby P, Mattes E, Sawyer MG, Keelan JA et al. Umbilical cord blood testosterone and childhood internalizing and externalizing behavior: a prospective study. PLoS One. 2013, 8;4:e59991 10.1371/journal.pone.0059991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kishi R, Kobayashi S, Ikeno T, Araki A, Miyashita C, Itoh S et al. Ten years of progress in the Hokkaido birth cohort study on environment and children’s health: cohort profile—updated 2013. Environ Health Prev Med. 2013, 18;6:429–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kishi R, Sasaki S, Yoshioka E, Yuasa M, Sata F, Saijo Y et al. Cohort profile: the Hokkaido study on environment and children’s health in Japan. Int J Epidemiol. 2011, 40;3:611–618. 10.1093/ije/dyq071 [DOI] [PubMed] [Google Scholar]
- 16. Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography—electrospray ionization tandem mass spectrometry. Steroids. 2007, 72;11–12:819–827. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita K, Takahashi M, Tsukamoto S, Numazawa M, Okuyama M, Honma S. Use of novel picolinoyl derivatization for simultaneous quantification of six corticosteroids by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A. 2007, 1173;1–2:120–128. [DOI] [PubMed] [Google Scholar]
- 18. Hickey M, Doherty DA, Hart R, Norman RJ, Mattes E, Atkinson HC et al. Maternal and umbilical cord androgen concentrations do not predict digit ratio (2D:4D) in girls: a prospective cohort study. Psychoneuroendocrinology. 2010, 35;8:1235–1244. 10.1016/j.psyneuen.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 19. van de Beek C, Thijssen JH, Cohen-Kettenis PT, van Goozen SH, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav. 2004, 46;5:663–669. [DOI] [PubMed] [Google Scholar]
- 20. Anand-Ivell R, Ivell R, Driscoll D, Manson J. Insulin-like factor 3 levels in amniotic fluid of human male fetuses. Hum Reprod. 2008, 23;5:1180–1186. 10.1093/humrep/den038 [DOI] [PubMed] [Google Scholar]
- 21. Anand-Ivell R, Ivell R. Insulin-like factor 3 as a monitor of endocrine disruption. Reproduction. 2014, 147;4:R87–95. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Cruz E, Huguet J, Piqueras M, Ribal MJ, Alcaraz A. Second to fourth digit ratio, adult testosterone level and testosterone deficiency. BJU Int. 2012, 109;2:266–271. 10.1111/j.1464-410X.2011.10249.x [DOI] [PubMed] [Google Scholar]
- 23. Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci U S A. 2011, 108;39:16289–16294. 10.1073/pnas.1108312108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manning JT. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc Natl Acad Sci U S A. 2011, 108;39:16143–16144. 10.1073/pnas.1113312108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Araki A, Mitsui T, Miyashita C, Nakajima T, Naito H, Ito S et al. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: The Hokkaido Study on Environment and Children’s Health. PLoS One. 2014, 9;10:e109039 10.1371/journal.pone.0109039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao Y, Winneke G, Wilhelm M, Wittsiepe J, Lemm F, Furst P et al. Environmental exposure to dioxins and polychlorinated biphenyls reduce levels of gonadal hormones in newborns: results from the Duisburg cohort study. Int J Hyg Environ Health. 2008, 211;1–2:30–39. [DOI] [PubMed] [Google Scholar]
- 27. Hsu PC, Lai TJ, Guo NW, Lambert GH, Guo YL. Serum hormones in boys prenatally exposed to polychlorinated biphenyls and dibenzofurans. J Toxicol Environ Health A. 2005, 68;17–18:1447–1456. [DOI] [PubMed] [Google Scholar]
- 28. Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC et al. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proc Biol Sci. 2013, 280;1768:20131532 10.1098/rspb.2013.1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Folland JP, Mc Cauley TM, Phypers C, Hanson B, Mastana SS. Relationship of 2D:4D finger ratio with muscle strength, testosterone, and androgen receptor CAG repeat genotype. Am J Phys Anthropol. 2012, 148;1:81–87. 10.1002/ajpa.22044 [DOI] [PubMed] [Google Scholar]
- 30. Honekopp J. No Evidence that 2D:4D is Related to the Number of CAG Repeats in the Androgen Receptor Gene. Front Endocrinol (Lausanne). 2013, 4:185 10.3389/fendo.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions, data are available to qualified researchers on request from the corresponding author.