Abstract
Basic fibroblast growth factor (FGF) mRNA is increased 4 h after cortical brain injury. In situ hybridization reveals that the increased mRNA persists for at least 2 weeks and that, in areas adjacent and ipsilateral to the lesion, the expression of basic FGF mRNA is also modified. As an example, at three days distal from the lesion, mRNA can be detected in ependymal cells of the lateral ventricle and in selected cells of the hippocampus and cortex. Endothelial cells also synthesize basic FGF mRNA. The increase in basic FGF mRNA is paralleled by similar changes in the localization of the basic FGF protein. Both the intensity and number of cells which stain for basic FGF are increased when they are compared to staining in either the contralateral side or to comparable areas of unlesioned brains. The pattern of mRNA expression is similar from 4 hours to 14 days. Early in the response (4 h to 3 days) on the border of the lesion, the presence of basic FGF is most obvious within the MAC-1-immunopositive population (macrophages and/or microglia). From 7 days to 2 weeks, there has been extensive hypertrophy of the reactive astrocytes which stain intensely for anti-basic FGF(1–24). We conclude that there is increased basic FGF as a function of injury to the CNS. In view of the observation that it is an early and persistent response, the possibility that it plays multiple functions in the regenerative capacity of the CNS is discussed.
Keywords: Fibroblast growth factor, Brain, Injury, Immunohistochemistry, In situ hybridization
INTRODUCTION
Although the cellular events that accompany brain injury have been well characterized, the factors that mediate growth and repair in the CNS are only beginning to be identified. One such factor is basic fibroblast growth factor (FGF). Originally isolated from pituitary and brain extracts, the molecule has been recently shown to be almost ubiquitous in its distribution in tissues5,13. It has also been shown to be particularly pleiotropic4. As an example although it was first recognized for its ability to stimulate the proliferation of fibroblasts, it is an equally powerful (ED50 ~30 pg/ml) growth factor for endothelial cells, chondrocytes, smooth muscle cells, adrenocortical cells, Schwann cells, melanocytes, granulosa cells, astrocytes and oligodendrocytes4,20,26. Furthermore, it is a potent neurotropic factor capable of supporting survival of various neurons in cell culture27,40. Of even greater importance, the in vitro activities of basic FGF have invariably been shown to correlate with important in vivo functions when administered to experimental animals2,29. It is a potent angiogenic factor in models of neovascularization, stimulates cartilage repair, enhances peripheral and optic nerve regeneration and can prevent experimentally induced neuronal death in the CNS.
We have attempted to determine whether basic FGF is associated with the plasticity and regenerative capacity of the CNS by studying the effects of injury on basic FGF. In most models of injury, polymorphonuclear leukocytes attract monocytes to the site of injury which then differentiate into macrophages; the macrophages are one of the most predominant and persistent cells associated with injury6,8,31. Because activated macrophages contain basic FGF3, they can presumably deliver this growth factor to the site of cell injury. Microglia of macrophage lineage and CNS origin migrate to the injured area and proliferate17. Although, it is not known whether they produce basic FGF in vivo, they could potentially be providing the first stimulus for oligodendrocyte proliferation during the early phase of repair11,26 and for astrocyte and endothelial cell proliferation during the later stages10,26,28.
A few recent studies have suggested that a common response to injury is its ability to increase basic FGF. In peripheral tissues, the levels of growth factor are increased in wound fluid22. Similarly, the cell proliferation that accompanies vascular injury also increases basic FGF9. In the CNS, Logan25 has reported that injury increases the levels of immunoreactive and biologically active basic FGF and Finkelstein et al. have localized the increase to cells at the site of injury14. We have extended these studies and examined the effect of CNS injury on basic FGF gene expression.
MATERIALS AND METHODS
Animals and surgery
Female Sprague–Dawley rats (200 g) were anesthetized (i.m.) with a mixture of acepromazine (1.875 mg/kg), ketamine (37.5 mg/kg) and xylazine (1.9 mg/kg). A 1.8 × 2.5 mm region of the right cingulate and frontal cortex and corpus callosum was aspirated (0 to −1.8 AP, 0 to 2.5 ML to bregma). At 4 h, 1, 3, 7, or 14 days after surgery, animals (n = 3/group) were put under deep anesthesia then perfused transcardially with phosphate buffered saline containing 4% paraformaldehyde (PFA) and 0.05% glutaraldehyde using the pH shift method38. Brains were excised and further fixed in 10% sucrose and 4% PFA for 24 h. They were then snap frozen in OCT and stored at −80 °C. Sections (25 µm) were prepared from regions within and adjacent to the lesion, collected in cryoprotectant (20% glycerol and 30% ethylene glycol) and stored at −20 °C until used in either in situ hybridization studies for basic FGF mRNA or for the immunolocalization of basic FGF. The brains of three unlesioned rats and the contralateral side of the brain of each lesioned rat were processed and examined for comparative purposes.
Immunohistochemistry
The immunoperoxidase technique was used to stain and identify FGF-containing cells using the ABC Vectastain Elite kit (Vector Lab.). The primary antiserum raised against basic FGF(1–24) has been previously described19. The IgG fraction was partially purified by protein-A Sepharose chromatography and used at 2.5 µg/ml. Control sections were stained with the eluate of antibody that was not retained on basic FGF-Affi-gel columns.
In situ hybridization
The methods used for the in situ hybridization of basic FGF mRNA in the CNS have previously been described12. The XhoI–XhoI fragment derived from rat basic FGF cDNA36 was subcloned into a riboprobe and transcription of the antisense strand of the coding sequence performed using T7 polymerase in the presence of [35S]UTP. A [35S]UTP-labeled RNA probe encoding the sense strand of the 5′ non-coding sequence was used for controls. Autoradiograms of the mounted sections were processed using Kodak XAR-5 film in order to obtain a macroscopic analysis of any changes in the distribution of basic FGF mRNA. For microscopic analysis, slides were exposed to a Kodak NTB-2 liquid autoradiograph emulsion for 3 weeks, processed with Kodak D19 developer and rapid fixer, counterstained with haemotoxylin and examined by dark-field microscopy.
RESULTS
Detection of basic FGF mRNA after a lesion to the CNS
The distribution of basic FGF mRNA was examined in sections at or near the lesions at 4 h, 3,7 and 14 days after injury. The pattern of distribution surrounding the injury is similar at all stages and a representative example at day 3 is shown in Fig. 1. In all animals, basic FGF mRNA is detectable at 4 h, peaks at day 3 and persists up to 14 days in the region surrounding the lesion. Corresponding sections from control animals (not shown) or an examination of the contralateral side (Fig. 1g) of the lesioned animal reveals the expected4 distribution of basic FGF mRNA in the indusium griseum and CA2 region of the hippocampus.
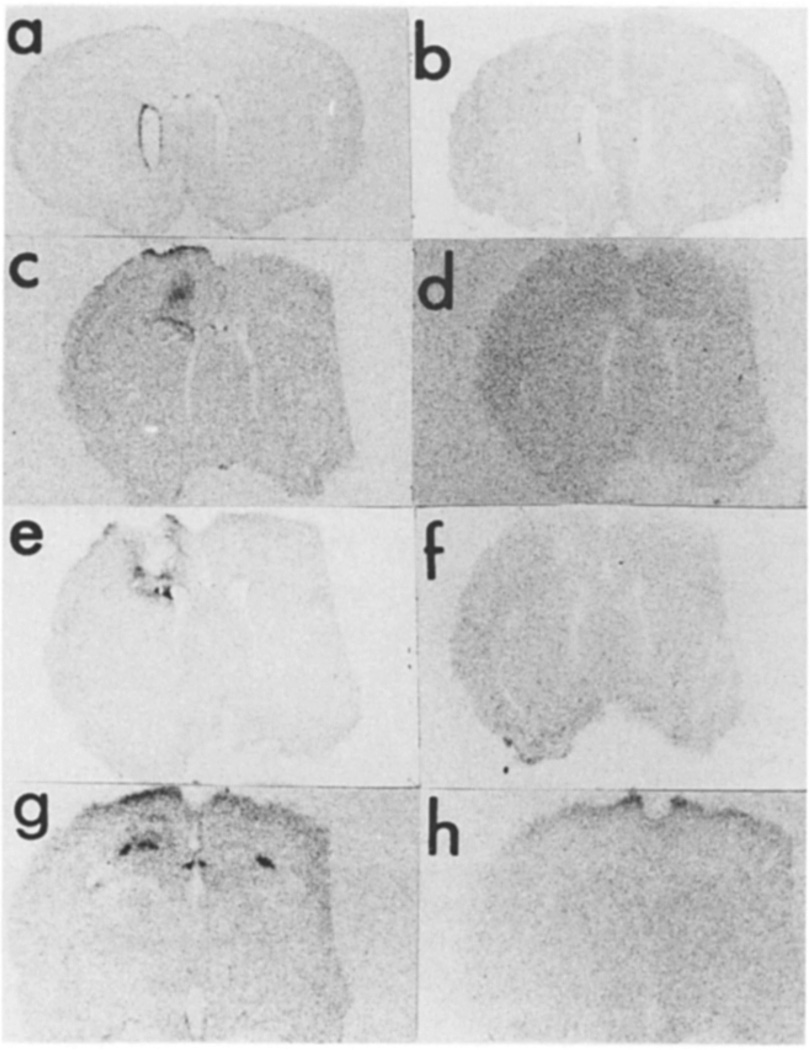
Fig. 1.
Basic FGF mRNA 3 days after CNS lesion. In situ hybridization of basic FGF mRNA was performed using sections of rat brain obtained from an animal perfused 3 days after lesion. The antisense (a,c,e,g) and sense (b,d,f,h) strands were used on sections that represent rostral (a–d), central (e,f), and caudal (g,h) to the lesion site.
The basic FGF mRNA signal is most intense on the third day after injury and is detectable in cells lining the ipsilateral, but not in the contralateral ventricle (Fig. 1a). This induction of mRNA is also detectable in sections anterior to the lesion and increased mRNA is only detected on the ipsilateral side (Fig. 1c). In both instances, the signal is absent in the corresponding region of control rats (not shown) or when sense strands are used as probes (Fig. 1b,d). When sections taken through the center of the lesion are examined (Fig. 1e), there is a strong signal for basic FGF mRNA surrounding the lesion. No signal is present in adjacent sections that are hybridized with the sense probe (Fig. 1f). When more caudal sections are examined, basic FGF mRNA is readily detected in the hippocampus on both the ipsilateral and contralateral sides (Fig. 1g). The ipsilateral hybridization however appears considerably more robust and different from the normal distribution of basic FGF mRNA we have previously reported12. Adjacent sections, hybridized with the sense probe are negative (Fig. 1h).
Identification of loci of FGF mRNA expression
Microscopic examination of the brains 3 days after lesion confirmed that the detection of injury-associated basic FGF mRNA was confined to a select group of cells surrounding the lesion, in the hippocampus and in the lateral ventricle (Figs. 2–Fig. 4).
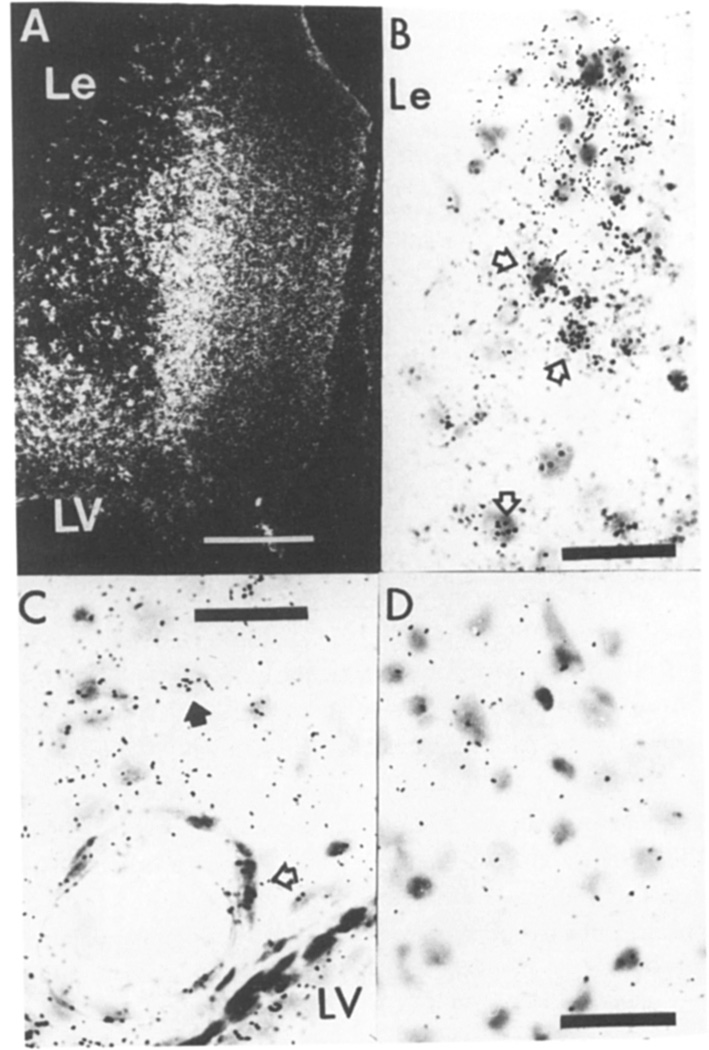
Fig. 2.
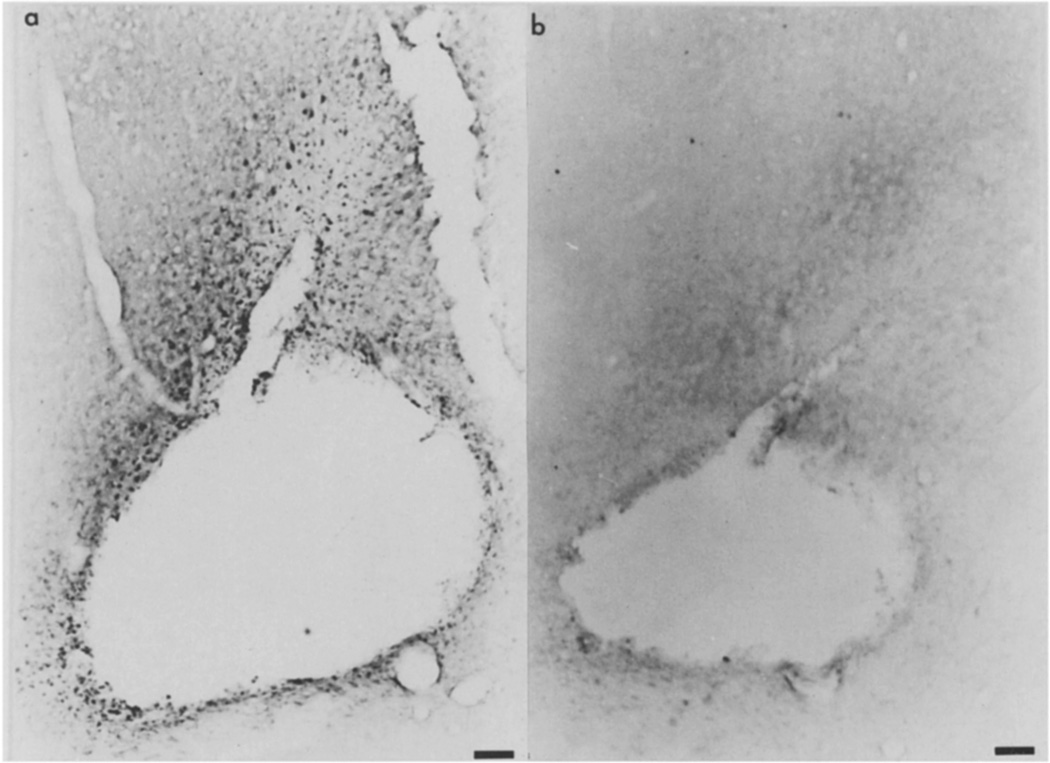
Basic FGF mRNA at the site of injury 3 days after CNS lesion. A: dark-field micrograph of the cortical lesion (Le) dorsal to the lateral ventricle (LV). Bar = 50 µm. B: bright-field micrograph of selective cells in the lesion margin actively expressing mRNA (open arrows). C: distal to the lesion in the cortex, cells surround a large vessel (open arrow) and a cell with a pale ovoid nucleus (closed arrow) express basic FGF mRNA. D: an adjacent section incubated with the control sense strand in a region corresponding to the section shown in (B). In B–D the bar = 50 µm.
Fig 4.
Basic FGF mRNA in the hippocampus 3 days after CNS lesion. A bright-field micrograph of basic FGF mRNA in (a) cells associated with large vessels but not in (b) the adjacent section labeled with the sense transcript. Bar = 50 µm.
Loci surrounding the lesion
As shown in Fig. 2, the cells that surround the lesion express basic FGF mRNA more strongly than those distal to the site of injury. Within the margin of the lesion, diverse but selective cells appear to express basic FGF mRNA (Fig. 2B,C). The hybridization is specific since none is observed with the sense transcript. There was also no evidence for basic FGF mRNA expression in the contralateral cortex or in age and sex-matched controls.
Loci in the hippocampus
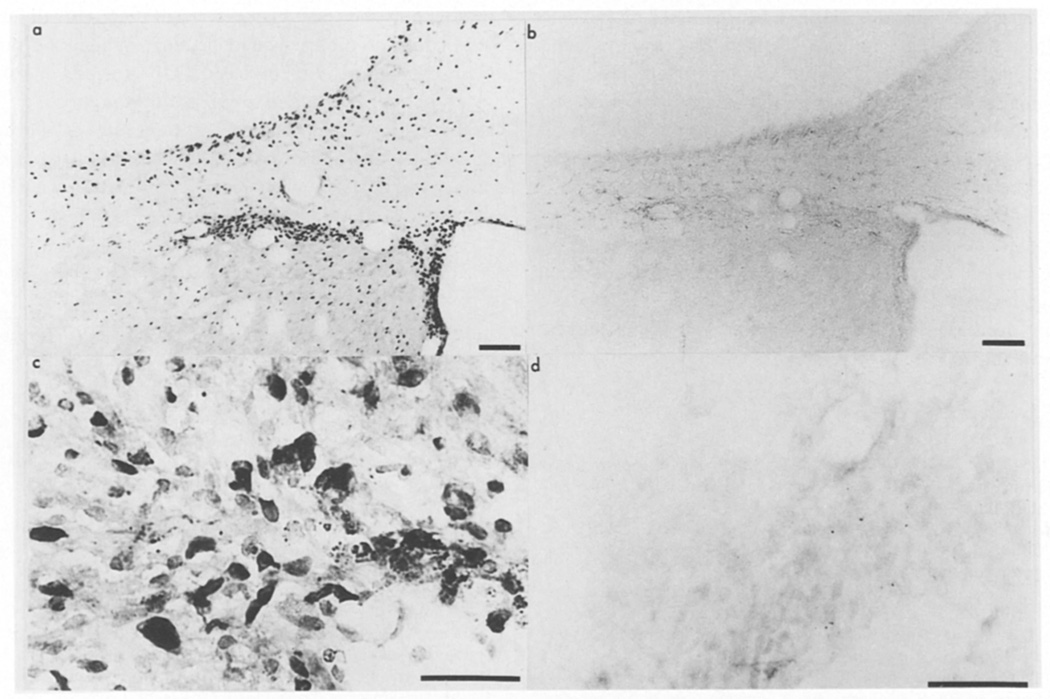
Unlike in the control hippocampus where basic FGF mRNA is restricted to the CA2 layer of cells12, in the lesioned animals basic FGF mRNA is detected in all hippocampal layers throughout a 0.4 mm region caudal to the lesion. The strongest signal is observed in cells surrounding the hippocampal fissure (Fig. 3a). There appears to be a specific induction of basic FGF mRNA in this area. First, no signal is detected with the sense probe (Fig. 3b) and second, an examination of and comparison with the contralateral side (see Fig. 1g) shows that mRNA is distributed throughout the hippocampal region of the ipsilateral side and not only in the CA2 pyramidal neurons. Higher magnification of these FGF mRNA expressing cells reveals that, in bright field, grains are associated with large vessels rather than neurons and overlie cells that have morphological resemblance to macrophages and ameboid microglia (Fig. 4). Although the injury induced the expression of basic FGF mRNA in regions which normally do not express detectable levels by in situ, the normal expression of basic FGF mRNA in the CA2 region was not affected by injury.
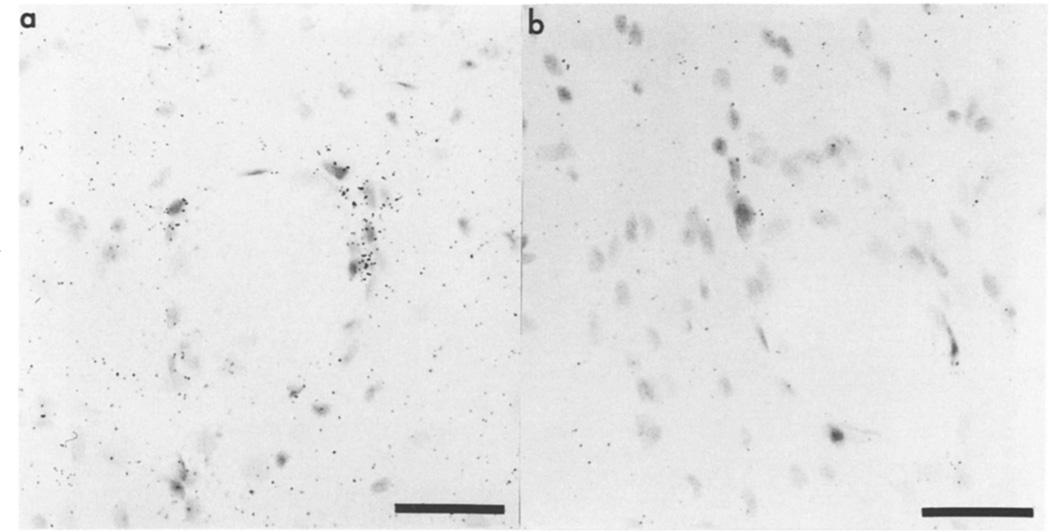
Fig. 3.
Basic FGF mRNA in the hippocampus 3 days after CNS lesion. In situ hybridization for basic FGF mRNA was performed on sections of lesioned brains and dark-field micrographs, showing basic FGF mRNA in the hippocampus ipsilateral to the lesion; (a) antisense or (b) sense strand. Bar = 100 µm.
Loci in the lateral ventricle
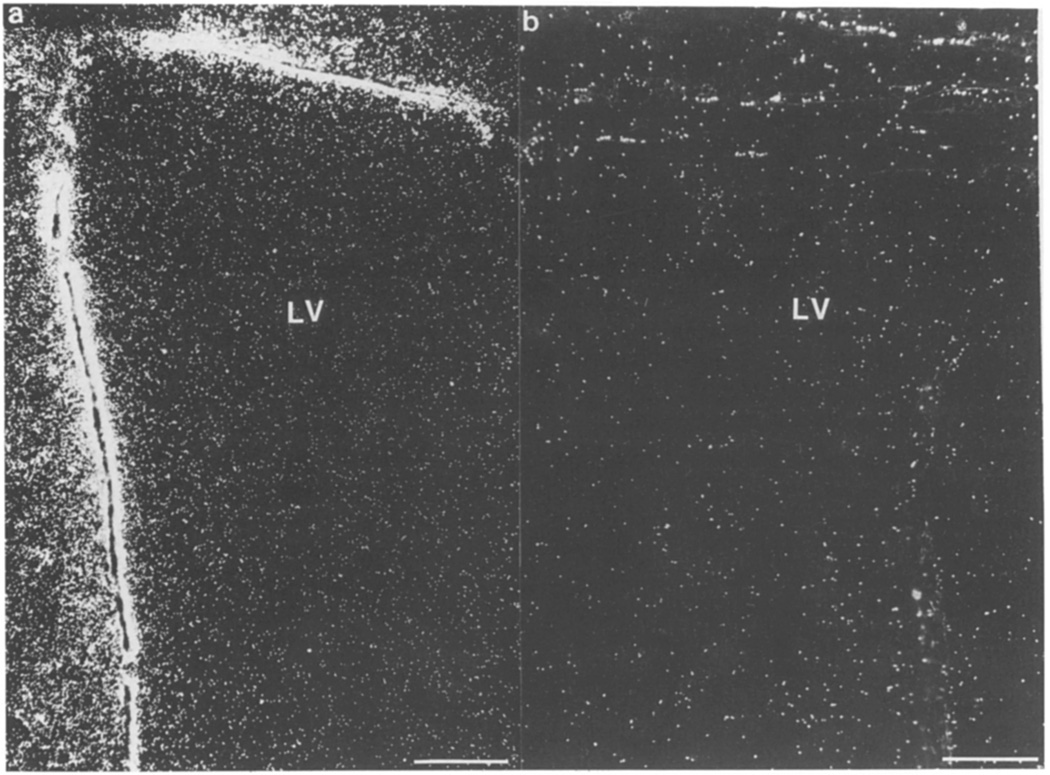
Ependymal cells lining the lateral ventricle show a strong signal for basic FGF mRNA when examined anterior and ipsilateral to the lesion (Fig. 5a). This signal was absent in the cells in the contralateral ventricle (Fig. 5b) and in sections labeled with the sense probe (not shown).
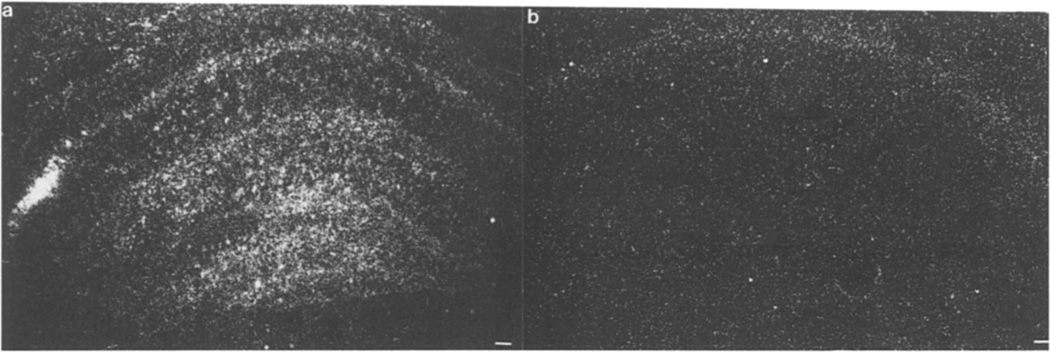
Fig 5.
Basic FGF mRNA in ependymal cells of the lateral ventricle 3 days after CNS lesion. Dark-field micrographs demonstrate the basic FGF mRNA in the ependymal layers lining the lateral ventricle (LV) (a) ipsilateral but not (b) contralateral to the lesion. Bar = 100 µm.
Basic FGF immunoreactivity after lesions
Immunocytochemical analysis of the distribution of basic FGF demonstrated that, throughout the time period examined (4 h to 2 weeks), the increase in basic FGF mRNA is accompanied by an increase in the number and staining intensity of immunopositive cells for basic FGF (Figs. 6–Fig. 8).
Fig. 6.
Basic FGF immunoreactivity 3 days after CNS lesion. Photomicrographs of (a) anti-basic FGF(1–24) and (b) preabsorbed anti-FGF staining in the anterior region of the lesion was examined in an animal perfused 3 days after surgery. Bar = 100 µm.
Fig. 8.
Basic FGF in the ependymal cells of the lateral ventricle. Photomicrographs of the lateral ventricle ipsilateral and rostral to the lesion were taken from an animal perfused 3 days after surgery. Sections were incubated with (a) anti-FGF(1–24) or (b) preabsorbed antiserum. Bar = 100 µm. Sections of the border of the cortical aspiration lesion were incubated with (c) anti-FGF(1–24) or (d) preabsorbed antiserum from an animal perfused 3 days after surgery illustrate the pattern of staining at the border of the cortical lesion. Bar = 50 µm.
Immunoreactivity surrounding the lesion
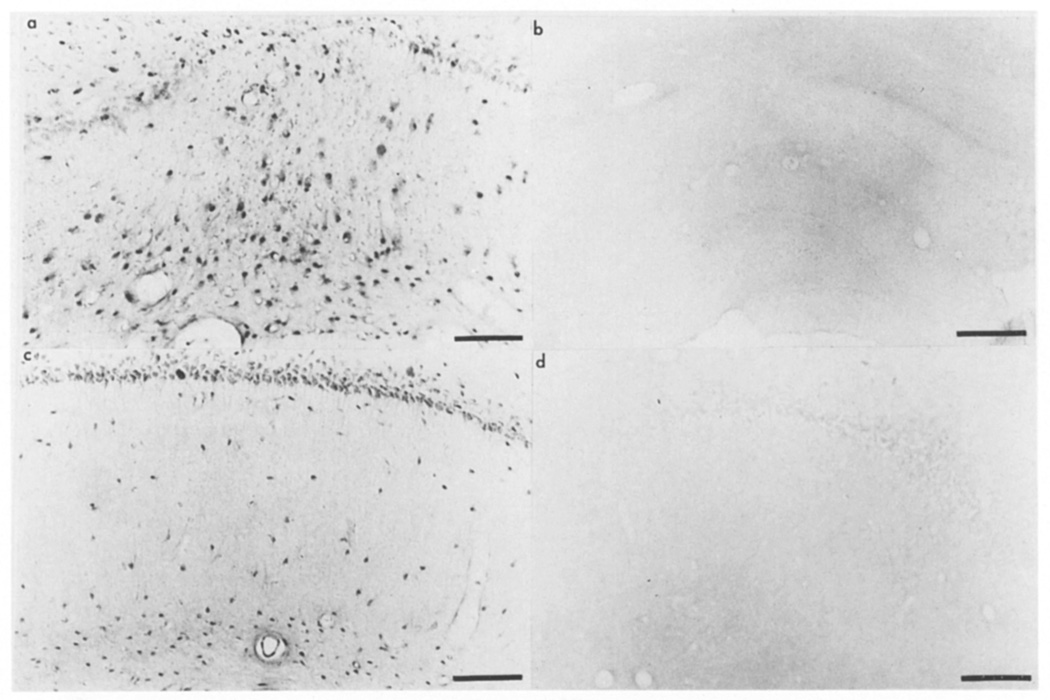
At 3 days (Fig. 6a), cells are extensively stained on the margin of the lesion when compared to the contralateral side or to comparative sections of other lesion time points. The staining is specific as illustrated by the inability of the flow through IgG to stain cells (Fig. 6b). At this time point, some of the immunopositive cells have the features of macrophage- or ameboid microglia-like cells (see Fig. 8c as an example) but like in uninjured rat cingulate cortex and in the contralateral side of lesioned rats (not shown), neurons surrounding the margin of the lesion are immunopositive for basic FGF (Fig. 6a). By 7 and 14 days after lesion, the cells that contain an immunoreactive basic FGF have more of the appearance of astrocytes and stain for the marker GFAP (not shown). Fewer neurons are immunoreactive for basic FGF after day 7 when the cystic glial scar forms.
Immunoreactivity in the hippocampus
The immunohistochemical localization of basic FGF in the hippocampus confirmed the effect of the injury on FGF mRNA. As little as 4 h after surgery, at sites caudal to the cortical lesion and surrounding the hippocampal fissure, there is an appearance of basic FGF immunopositive cells in the anterior hippocampus (Fig. 7a). This is in stark contrast to the unlesioned side where only neurons and a few scattered glial cells stained for basic FGF (Fig. 7c). Sections incubated with preabsorbed antisera did not stain for basic FGF (Fig. 7b,d) and at other time points, the staining pattern was similar except that many of the basic FGF immunopositive cells were now hypertrophied (not shown).
Fig. 7.
Basic FGF in the hippocampus after CNS lesion. Photomicrographs of the hippocampus in an animal perfused 4 h after surgery on the side ipsilateral (a,b) and contralateral (c,d) to the lesion. Sections were incubated with (a,c) anti-FGF(1–4) or (b,d) preabsorbed antiserum. Bar = 100 µm.
Immunoreactivity lateral ventricle
Three days after the lesion, basic FGF immunoreactivity is also detected in ependymal cells (Fig. 8a). At a higher magnification, the increased immunostaining surrounding the lesion appears to be largely attributable to immunopositive macrophage-like cells (Fig. 8c). These cells also stain for the macrophage differentiation antigen MAC-1 (not shown) which is also present on ameboid microglia17.
DISCUSSION
The results presented here establish that the injury associated with a cortical aspiration lesion increases basic FGF and its mRNA. They confirm earlier findings that this lesion increases immunoreactive basic FGF14 and suggest that it is due to increased mRNA. Basic FGF mRNA is increased in the ependymal cells lining the lateral ventricle, in selected cells on the border of the lesion and in cells associated with the vessels of the hippocampal fissure. Whether the injury induces basic FGF gene transcription or enhanced mRNA stability has not been investigated. Clearly however, multiple cell types in the CNS have the capacity to express basic FGF mRNA in response to injury. Sections from control animals or from the contralateral side were consistently negative.
The results presented here are certainly compatible with the suggestion that basic FGF may have a multifunctional role in promoting glial hypertrophy and proliferation33 and in stimulating angiogenesis21. At 3 days, many of the basic FGF-immunopositive cells are immunopositive for an antigen (MAC-1) common to cells of the monocyte-macrophage lineage, including ameboid microglia. This suggests that one of the cell-types synthesizing basic FGF is probably macrophage-derived. In addition to synthesizing basic FGF, these cells may acquire basic FGF from neuronal debris collected by phagocytosis. The detection of basic FGF immunoreactivity in these cells persisted over the course of the experiments and are consistent with the fact that there is an early extensive infiltration of macrophages and monocytes and proliferation of microglia in response to CNS injury24. In lesioned rats, monocytes have previously been detected within hours after injury17. These cells which acquire characteristics of microglia and macro-phages1 may thus contribute basic FGF-like activities. First and foremost, they stimulate angiogenesis6 and glial proliferation33,34. Because activated macrophages contain basic FGF3, it is thus possible that, as part of the early phase of the regenerative process, basic FGF is available locally from monocytes and/or macrophages and helps initiate the response to CNS injury. Similar paradigms have been proposed for interleukin-1 (IL-1), a growth factor with 19–25% structural homology with basic FGF that is synthesized by macrophages16,41.
Of particular interest was the observation that the distribution of basic FGF mRNA and protein is affected in areas distal to the lesion. One mechanism that might account for these changes is if the lesions interfered with specific neuronal pathways. Deafferentation leads to aggregation and activation of microglia surrounding degenerating axons17,30 which in turn, stimulates the proliferation of astrocytes18. While this process might explain changes in basic FGF expression in the hippocampus, it is more difficult to reconcile this mechanism with the increased basic FGF mRNA detected in ependymal cells of the lateral ventricle. Accordingly, it will be important to determine if the basic FGF that is localized in the ependyma is released by the injury into the third ventricle to accelerate the response to trauma.
The observation that basic FGF increases after CNS injury raises the question of how its synthesis and biological activities are eventually turned off when the healing process ends. This is particularly important for basic FGF in view of its ability to affect the function of diverse cell types in the CNS. Perhaps the failure of neurons to regenerate when cystic glial scars form16 provides a clue to the regulation of FGF activity. The appearance of the scar coincides with the phagocytosis of the cellular debris derived from degenerating neurons, oligodendrocytes and other cells17,30. Basic FGF expression decreases at this time and it is interesting to speculate that the protein and glycosaminoglycan matrix in the scars acts much like the basement membrane of peripheral tissues, to sequester the remaining basic FGF. If this is the case, then these scars could be potentially deleterious by removing basic FGF from the normal neurotrophic milieu. This model is compatible with our recent observation that basic FGF is associated with the senile plaques that characterize Alzheimer’s disease39 and might explain the increased plasticity and regenerative capacity of the injured fetal and neonatal brain which fail to scar7.
In the experiments described here, prior to day 7 there were only occasional astrocytes in the vicinity of the lesion that were basic FGF immunoreactive. In contrast, basic FGF-positive astrocytes became a major component of the lesion after day 7, a time that is coincident with extensive glial hypertrophy and development of the glial scar10. While in the normal brain, basic FGF is primarily present in neurons32, in culture systems the reverse has been observed; only astrocytes produce detectable levels of basic FGF mRNA12. In this sense, the proliferating astrocytes in the area of the lesion resemble astrocytes in culture. Because reactive astrocytes have been shown to synthesize platelet-derived growth factor35 nerve growth factor1516, β-amyloid precursor protein37, tumor necrosis factor, IL-1 and IL-623 presumably the collective delivery of these growth factors ultimately promotes wound healing. While, the potent neurotrophic activity of basic FGF suggests a role in neuronal sprouting and survival after injury2,27,29,40, it will remain of paramount importance to establish the functional significance of elevated basic FGF after CNS injury and during neuronal recovery to determine if it can be used as an adjunct to therapy.
Acknowledgements
We thank Ali Vazirizand and Cassandra Harrison for technical assistance. This work was supported by NIH DK-18811, NS 28121 and a grant from Erbamont. Preliminary results of this investigation were presented at the UCLA Symposium on Neurotrophic Factors at San Padre Id., TX, April, 1990.
REFERENCES
- 1.Akiyama H, McGeer PL. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Research. 1989;489:247–253. doi: 10.1016/0006-8993(89)90857-3. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KJ, Dam D, Lee S, Cotman CW. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature. 1988;332:360–361. doi: 10.1038/332360a0. [DOI] [PubMed] [Google Scholar]
- 3.Baird A, Mormède P, Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem. Biophys. Res. Commun. 1985;126:358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- 4.Baird A, Böhlen P. Fibroblast growth factors. In: Sporn MB, Roberts AB, editors. Peptide Growth Factors and Their Receptors. Vol. 95. Berlin: Springer; 1990. pp. 369–417. [Google Scholar]
- 5.Baird A, Ueno N, Esch F, Ling N. Distribution of fibroblast growth factors (FGFs) in tissues and structure-function studies with synthetic fragments of basic FGF. J. Cell Physiol. 1987;5:101–106. doi: 10.1002/jcp.1041330419. [DOI] [PubMed] [Google Scholar]
- 6.Beck DW, Hart MN, Cancilla PA. The role of the macrophage in microvascular regeneration following brain injury. J. Neuropathol. Exp. Neurol. 1983;42:601–614. doi: 10.1097/00005072-198311000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Berry M, Maxwell WL, Logan A, Mathewson A, Mc-Connell P, Ashhurst DE, Thomas GH. Deposition of scar tissue in the central nervous system. Acta Neurochir. 1983;32(Suppl):31–35. doi: 10.1007/978-3-7091-4147-2_3. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh JB. The proliferation of astrocytes around a needle wound in the rat brain. J. Anat. 1970;106:471–487. [PMC free article] [PubMed] [Google Scholar]
- 9.Cuevas P, Baird A, Guillemin R. Angiogenic response to fibroblast growth factor in the rat brain in vivo. In: Gagliardi R, Benvenuti L, editors. 8th International Symposium on Microsurgical Anastomoses for Cerebral Ischemia. Florence, Italy: Monduzzi Editore; 1986. pp. 731–737. [Google Scholar]
- 10.du Bois M, Bowman PD, Goldstein GW. Cell proliferation after ischemic injury in gerbil brain: an immunocytochemical and autoradiographic study. Cell Tissue Res. 1985;242:17–23. doi: 10.1007/BF00225558. [DOI] [PubMed] [Google Scholar]
- 11.Eccleston PA, Silverberg DH. Fibroblast growth factor is a mitogen for oligodendrocytes in vitro. Dev. Brain Res. 1985;21:315–318. doi: 10.1016/0165-3806(85)90221-4. [DOI] [PubMed] [Google Scholar]
- 12.Emoto N, Gonzalez AM, Walicke PA, Wada E, Simmons DM, Shimasaki S, Baird A. Identification of specific loci of basic fibroblast growth factor synthesis in the rat brain. Growth Factors. 1989;2:21–29. doi: 10.3109/08977198909069078. [DOI] [PubMed] [Google Scholar]
- 13.Esch E, Baird A, Ling N, Ueno N, Hill E, Denoroy L, Klepper R, Gospodarowicz D, Böhlen P, Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine acidic FGF. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein SP, Apostolides PJ, Caday CG, Prosser J, Philips MF, Klagsbrun M. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Research. 1988;460:253–259. doi: 10.1016/0006-8993(88)90370-8. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa Y, Tomioka N, Sato W, Satoyoshi E, Hayashi K, Furukawa S. Catecholamines increase nerve growth factor mRNA content in both mouse astroglial cells and fibroblast cells. FEBS Lett. 1989;247:463–467. doi: 10.1016/0014-5793(89)81391-2. [DOI] [PubMed] [Google Scholar]
- 16.Gage FH, Olejnizak P, Armstrong DM. Astrocytes are important for sprouting in the septohippocampal circuit. Exp. Neurol. 1988;102:2–13. doi: 10.1016/0014-4886(88)90073-8. [DOI] [PubMed] [Google Scholar]
- 17.Giulian D, Tapscott MJ. Immunoregulation of cells within the central nervous system. Brain Behav. Immunol. 1988;2:352–358. doi: 10.1016/0889-1591(88)90040-2. [DOI] [PubMed] [Google Scholar]
- 18.Giulian D, Young DJ, Woodward J, Brown DC, Lachman BL. Interleukin-1 is an astroglial growth factor in the developing brain. J. Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez AM, Buscaglia M, Ong M, Baird A. Immunohistochemical localization of basic FGF in tissues of the 18 d-fetal rat. J. Cell Biol. 1990;110:347–358. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gospodarowicz D, III CR, Hornsby PJ, Gill G. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor: lack of effect of epidermal growth factor. Endocrinology. 1977;100:1080–1089. doi: 10.1210/endo-100-4-1080. [DOI] [PubMed] [Google Scholar]
- 21.Gospodarowicz D, Massoglia S, Cheng J, Fujii DK. Effect of fibroblast growth factor and lipoproteins on the proliferation of endothelial cells derived from bovine adrenal cortex, brain cortex, and corpus luteum capillaries. J. Cell Physiol. 1986;127:121–136. doi: 10.1002/jcp.1041270116. [DOI] [PubMed] [Google Scholar]
- 22.Hamerman D, Taylor S, Kirschenbaum I, Klagsbrun M, Raines EW, Ross R, Thomas KA. Growth factors with heparin binding affinity in human synovial fluid. Proc. Soc. Exp. Biol. Med. 1987;186:384–389. doi: 10.3181/00379727-186-3-rc2. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccaride or a neurotropic virus. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay RM. In: Astrocytes — cell biology and pathology of astrocytes. Federoff S, Vernadakis A, editors. New York: Academic Press; 1986. pp. 231–262. [Google Scholar]
- 25.Logan A. CNS growth factors. Br. J. Hosp. Med. 1990;43:428–437. [PubMed] [Google Scholar]
- 26.Ludwin SK. Reaction of oligodendrocytes and astrocytes to trauma and implantation. Lab. Invest. 1985;52:20–30. [PubMed] [Google Scholar]
- 27.Morrison RS, Sharma A, De Vellis J, Bradshaw RA. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc. Natl. Acad. Sci. U.S.A. 1986;83:7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murabe Y, Ibata Y, Sano Y. Morphological studies on neuroglia: response of glial cells to kainic acid-induced lesions. Cell Tissue Res. 1981;216:581–589. doi: 10.1007/BF00238652. [DOI] [PubMed] [Google Scholar]
- 29.Otto D, Frotscher M, Unsicker K. Basic fibroblast growth factor and nerve growth factor administered in gel foam rescue medial septal neurons after fimbria fornix transection. J. Neurosci. Res. 1989;22:83–91. doi: 10.1002/jnr.490220111. [DOI] [PubMed] [Google Scholar]
- 30.Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11:273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 31.Persson L. Cellular reactions to small cerebral stab wounds in the rat frontal lobe. Virchows Arch. Cell. Pathol. 1976;22:21–37. doi: 10.1007/BF02889204. [DOI] [PubMed] [Google Scholar]
- 32.Pettmann B, Labourdette G, Weibel M, Sensenbrenner M. The brain fibroblast growth factor (FGF) is localized in neurons. Neurosci. Lett. 1986;68:175–180. doi: 10.1016/0304-3940(86)90137-0. [DOI] [PubMed] [Google Scholar]
- 33.Pettmann B, Weibel M, Sensenbrenner M, Labourdette G. Purification of two astroglial growth factors from bovine brain. FEBS Lett. 1985;189:102–108. doi: 10.1016/0014-5793(85)80851-6. [DOI] [PubMed] [Google Scholar]
- 34.Pruss RM, Bartlett PF, Gavrilovic J, Lisak RP, Rattray S. Mitogens for glial cells: a comparison of the response of cultured astrocytes, oligodendrocytes and Schwann cells. Dev. Brain Res. 1982;2:19–35. doi: 10.1016/0165-3806(81)90056-0. [DOI] [PubMed] [Google Scholar]
- 35.Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- 36.Shimasaki S, Emoto N, Koba A, Mercado M, Shibata F, Cooksey K, Baird A, Ling N. Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem. Biophys. Res. Commun. 1988;157:256–263. doi: 10.1016/s0006-291x(88)80041-x. [DOI] [PubMed] [Google Scholar]
- 37.Siman R, Card JP, Nelson RB, Davis LG. Expression of β-amyloid presursor protein in reactive astrocytes following neuronal damage. Neuron. 1989;3:275–285. doi: 10.1016/0896-6273(89)90252-3. [DOI] [PubMed] [Google Scholar]
- 38.Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAS in brain and other tissues with radiolabeled single-stranded RNA probes. J. Histotechnol. in press. [Google Scholar]
- 39.Stopa EG, Gonzalez AM, Chorsky R, Corona RJ, Alvarez J, Bird ED, Baird A. Basic fibroblast growth factor in Alzheimer's disease. Biochem. Biophys. Res. Commun. doi: 10.1016/0006-291x(90)91201-3. in press. [DOI] [PubMed] [Google Scholar]
- 40.Walicke P, Cowan WM, Ueno N, Baird A, Guillemin R. Fibroblast growth factor promotes survival of dissociated hippocampal neurons and enhances neurite extension. Proc. Natl. Acad. Sci. U.S.A. 1986;83:3012–3016. doi: 10.1073/pnas.83.9.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimecki M, Wieczorek Z, Kapp JA, Pierce CW. Secretion of interleukin 1 (IL-1) by peritoneal macrophages upon contact with syngeneic T cells is la-restricted and antigen-independent process. Arch. Immunol. Ther. Exp. 1988;36:661–671. [PubMed] [Google Scholar]