Abstract
Objectives:
The objectives of this study were to develop a conceptual model of quality of life (QOL) in muscular dystrophies (MDs) and review existing QOL measures for use in the MD population.
Methods:
Our model for QOL among individuals with MD was developed based on a modified Delphi process, literature review, and input from patients and patient advocacy organizations. Scales that have been used to measure QOL among patients with MD were identified through a literature review and evaluated using the COSMIN (Consensus-Based Standards for the Selection of Health Measurement Instruments) checklist.
Results:
The Comprehensive Model of QOL in MD (CMQM) captures 3 broad domains of QOL (physical, psychological, and social), includes factors influencing self-reported QOL (disease-related factors, support/resources, and expectations/aspirations), and places these concepts within the context of the life course. The literature review identified 15 QOL scales (9 adult and 6 pediatric) that have been applied to patients with MD. Very few studies reported reliability data, and none included data on responsiveness of the measures to change in disease progression, a necessary psychometric property for measures included in treatment and intervention studies. No scales captured all QOL domains identified in the CMQM model.
Conclusions:
Additional scale development research is needed to enhance assessment of QOL for individuals with MD. Item banking and computerized adaptive assessment would be particularly beneficial by allowing the scale to be tailored to each individual, thereby minimizing respondent burden.
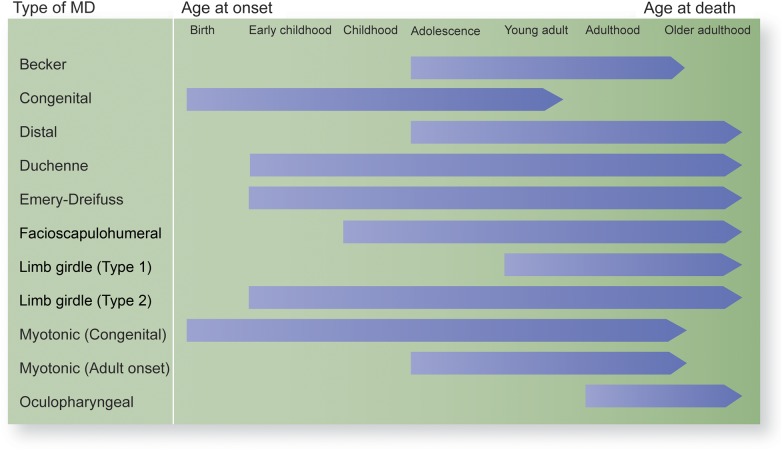
Muscular dystrophies (MDs) are a clinically diverse group of debilitating diseases characterized by progressive muscle weakness that can affect not only mobility but respiration and cardiac function as well. These diseases have varying ages of onset and severity of disease progression (figure 1; see reference 1 for a review).
Figure 1. Progression from disease onset to death throughout the life course for 9 types of muscular dystrophies (MDs).
Older age, longer illness duration, and greater disease severity are associated with lower perceived health-related quality of life (HRQOL) among individuals with MD.2–6,e1,e2 However, treatments such as corticosteroids have been linked with improved physical functioning, greater life expectancy, and better HRQOL in some forms of MD.2,5,6,e3,e4
Reliable and valid quality of life (QOL) measures are needed to assess the impact of MD on QOL and to accurately measure changes in QOL due to interventions and treatments. However, existing QOL measures and models do not capture all components of QOL that are meaningful to individuals with MD. For example, stakeholders (patients/families, clinicians, researchers, and advocates) participating in the 2011 Centers for Disease Control and Prevention's meeting on priorities for MD research identified significant gaps in existing QOL models, noting that they do not adequately capture emotional effects of disease, personal sense of meaningfulness, participation in society, access to care, and QOL issues at transitional points in a progressive disease.
The current study introduces a new conceptual model of QOL in MD based on stakeholder input and a literature review and outlines the domains and psychometric properties of existing QOL measures applicable to adults and children with MD.
METHODS
To facilitate measurement of QOL that is responsive to the unique experiences of individuals living with MD, we undertook the multistep process outlined in figure 2. First, we developed an expanded conceptual model of QOL in MD, using a modified Delphi process. We reviewed existing QOL measures applied to neuromuscular disorders, including MD, and compiled a list of 104 possible QOL domains for inclusion in the model (table e-1 on the Neurology® Web site at Neurology.org). A diverse group of 12 stakeholders (patient/family members, clinicians, advocates, and researchers) then independently ranked these domains on a 4-point scale (critical, important, somewhat important, or not essential) regarding importance to measuring QOL in MD. The group reviewed the rankings during a 2-day, in-person meeting in July 2013 and reached a consensus on which domains should be included in the model, which was then refined through a series of additional group discussions.
Figure 2. Study process.
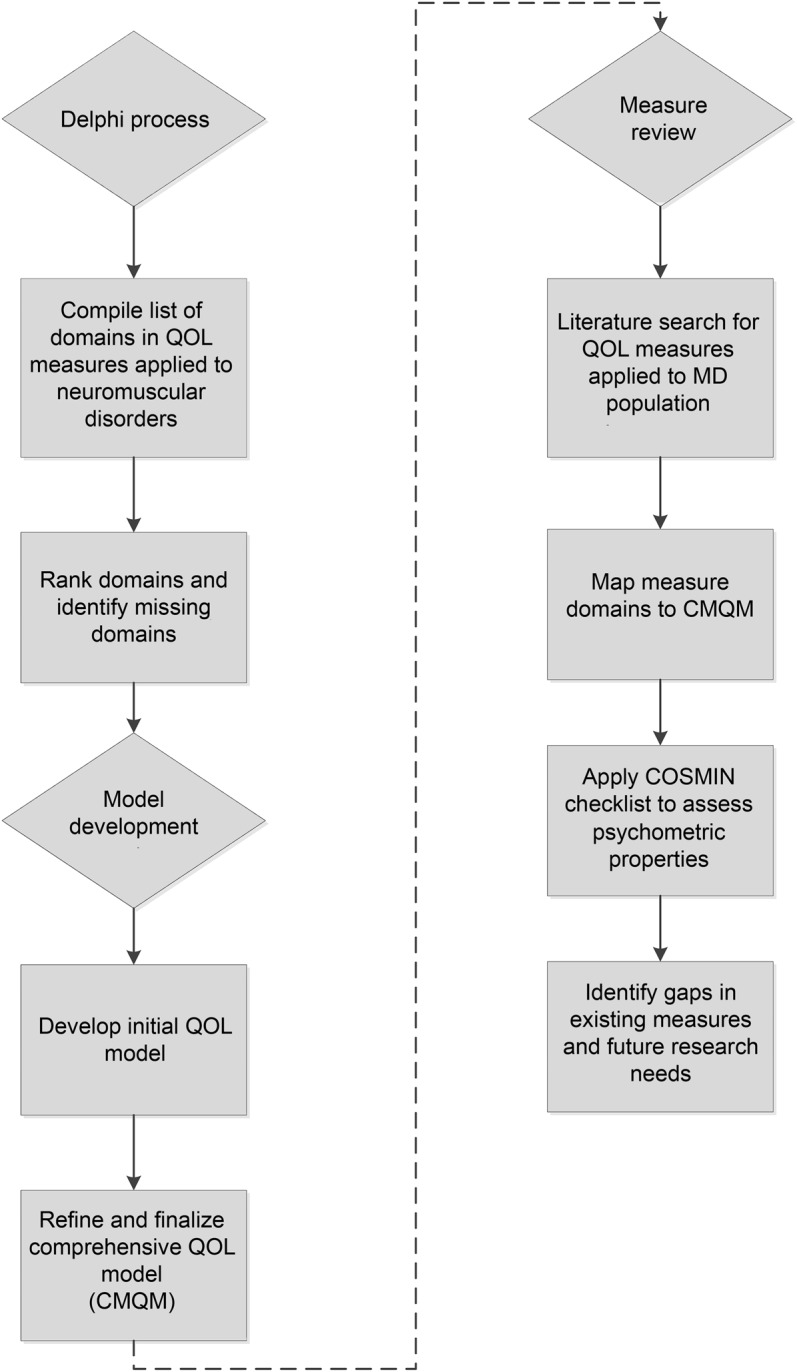
The flowchart outlines the steps undertaken in the study, including the Delphi process, model development, and review of existing quality-of-life measures. CMQM = Comprehensive Model of Quality of Life in Muscular Dystrophy; COSMIN = Consensus-Based Standards for the Selection of Health Measurement Instruments; MD = muscular dystrophy; QOL = quality of life.
After developing the conceptual model and identifying important components of QOL in MD, we examined the properties of existing QOL measures in individuals with MD. To identify studies that have used these measures, we conducted a search of PubMed, PsycINFO, and Embase for English language articles published between January 1, 1990, and November 22, 2013, with the following search terms: Muscular Dystrophy AND quality of life AND (measure OR scale OR assessment OR survey OR questionnaire OR instrument OR inventory). Additional articles and measures were identified through a review of the bibliographies of the articles resulting from the database search.
We reviewed the identified measures using the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) checklist, a rigorous, standardized tool for assessing psychometric properties of patient-reported outcomes.7 The checklist includes 10 broad categories of psychometric properties: internal consistency, reliability, measurement error, content validity, structural validity, hypotheses testing, cross-cultural validity, criterion validity, responsiveness, and interpretability. We briefly summarize here the psychometric properties of each measure among individuals with MD as reported in the articles. Because of limited information on some COSMIN categories reported in the current studies, we have condensed the information into 2 categories: reliability/internal consistency and validity. When providing mean scores, if available, we have used individuals' self-reports rather than proxies' reports.
RESULTS
QOL model.
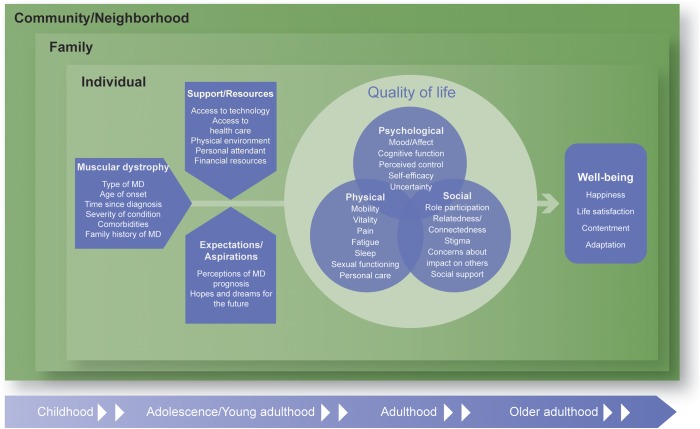
Based on the Delphi process, literature review, and input from individuals with MD and advocacy organizations, we developed the Comprehensive Model of QOL in Muscular Dystrophy (CMQM) shown in figure 3. The CMQM portrays the impact of MD on an individual's QOL and the related construct of well-being. Overall, the CMQM is consistent with the World Health Organization (WHO) definition of QOL as “individuals' perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns. It is a broad ranging concept affected in a complex way by the person's physical health, psychological state, level of independence, social relationships, personal beliefs and their relationship to salient features of their environment.”8(p1)
Figure 3. Comprehensive Model of Quality of Life in Muscular Dystrophy.
The diagram outlines the domains comprising 3 dimensions of quality of life in MDs (physical, psychological, and social) and the pathways connecting disease-related factors, support and resources, and expectations to quality of life and well-being. MD = muscular dystrophy.
The model development process was informed by examining existing QOL conceptual models and frameworks applied to MD and/or non-MD populations. For example, in a systematic review, Bakas et al.9 found that the 3 most frequently used HRQOL models were (1) the Wilson and Cleary model,10 (2) revised Wilson and Cleary model,11 and (3) WHO International Classification of Functioning, Disability and Health (ICF).12 The revised Wilson and Cleary HRQOL model illustrates a progression from biological function to HRQOL through symptoms, functional status, and general health perceptions; individual and environmental characteristics are shown as having an influence throughout this entire process. This model is useful for identifying physical determinants of HRQOL; however, it does not provide a breakdown of the specific components comprising QOL and does not capture other types of determinants of QOL.
The ICF model operationalizes HRQOL in terms of functioning divided into (1) body functions, (2) activities, and (3) participation. For example, participation would include an individual's ability to engage in social interactions. Similar to the Wilson and Cleary models, in the ICF model, an individual's health condition, personal factors, and environmental factors can have an impact on HRQOL. With its focus on functioning, the ICF model omits other components of QOL that may be applicable to individuals with MD, such as mood/affect, perceived control, stigma, and social support.
After reviewing these and other existing QOL models, we chose to adapt the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) HRQOL framework by adding domains identified as essential for a comprehensive evaluation of QOL among individuals with MD. The PROMIS framework includes 3 broad domains of QOL—physical, mental, and social13—and has been applied to many different patient populations. These domains are consistent with the WHO definition of health as a “state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.”14 In addition, these broad domains encompass many of the domains included in other QOL models; for example, the social domain captures much of the participation domain in the ICF model.
The CMQM expands the physical domain in the PROMIS model, which captures fatigue, pain, and sexual functioning, to include the concepts of mobility, vitality, and personal care (figure 3). These issues may be of particular concern to individuals with MD who, by nature of the disease, may lose their mobility and require assistance with activities of daily living. The Psychological domain overlaps with the PROMIS Mental domain and includes mood and affect, broadly defined to include concepts such as depression, anxiety, and anger, as well as cognitive functioning, which may be affected by MD. The domain was also expanded to include feelings of control and self-efficacy, as well as uncertainty about the future. Finally, the Social domain includes components similar to those in the PROMIS model (i.e., role participation, relatedness/connectedness, social support); however, it also captures concerns reported by individuals with MD regarding stigma related to their disease and concerns about the impact of their disease on others, especially caregivers.
In addition to characterizing domains of QOL, the CMQM includes potential determinants and moderators of QOL. These factors include disease-related factors, such as the type of MD, age at onset, time since diagnosis, severity of condition, comorbidities, and family history of MD. Potential moderators of self-appraisals include support/resources and expectations/aspirations. Greater access to support and resources, such as adaptive technology, may help ameliorate the impact of MD on QOL. Individuals experiencing greater misalignment between their expectations/aspirations and their actual abilities as the disease progresses may experience a greater impact on their QOL.
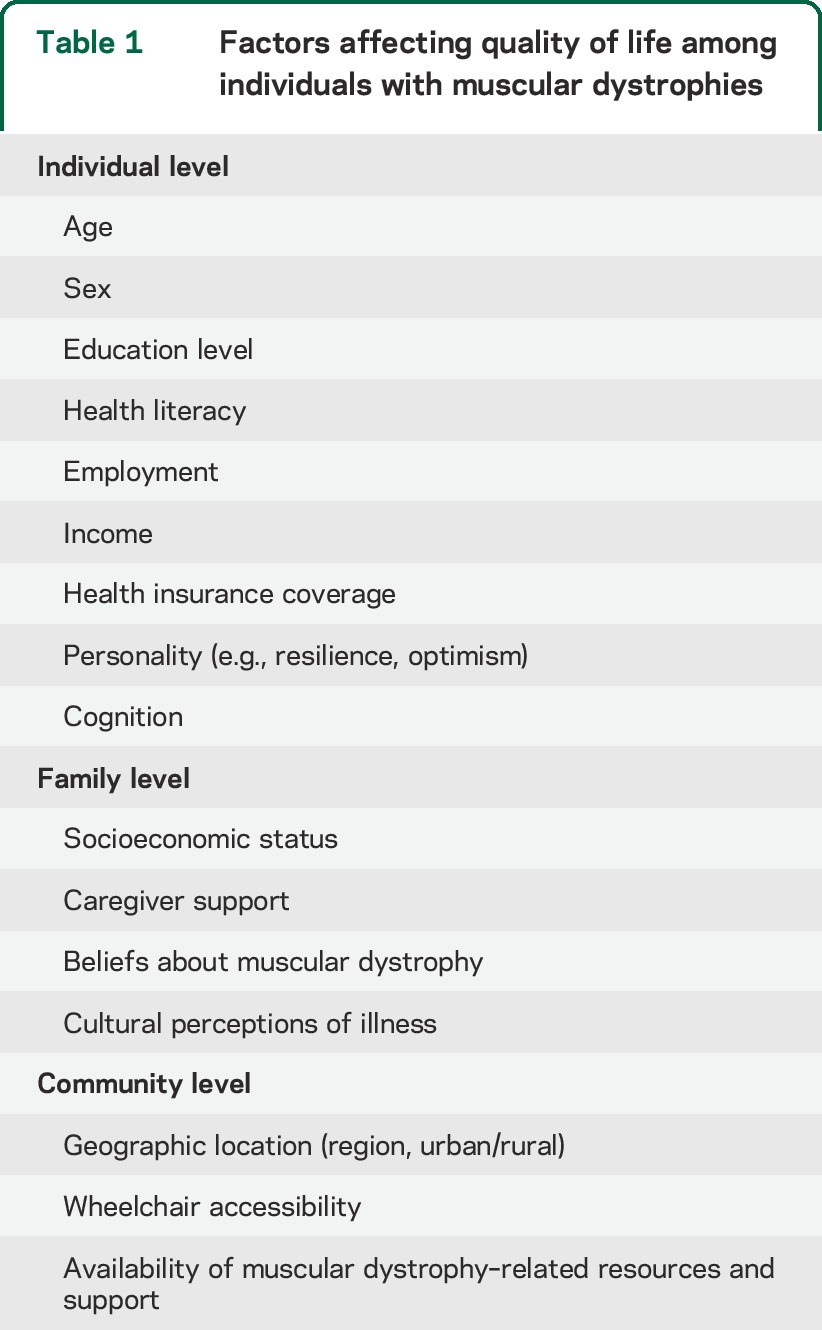
The CMQM also incorporates a Bronfenbrenner ecological systems theory approach15 to account for contextual factors, including the individual, family, and community-level factors shown in table 1. The potential influence of individual and environmental factors on QOL is also acknowledged by other types of models, such as the revised Wilson and Cleary model and the ICF model. Finally, the model accounts for possible changes in QOL across the life course, consistent with life course models16 and addressing concerns of stakeholders about capturing transitions over time in this progressive disease.
Table 1.
Factors affecting quality of life among individuals with muscular dystrophies
QOL construct measures.
The literature review revealed 21 articles covering 15 QOL scales (9 adult and 6 pediatric) applied to individuals with MD (table 2). The content areas and subscales of each scale are shown in table e-2, and their psychometric properties are outlined in table e-3.2–6,17–32
Table 2.
Quality-of-life measures applicable to individuals with MDs
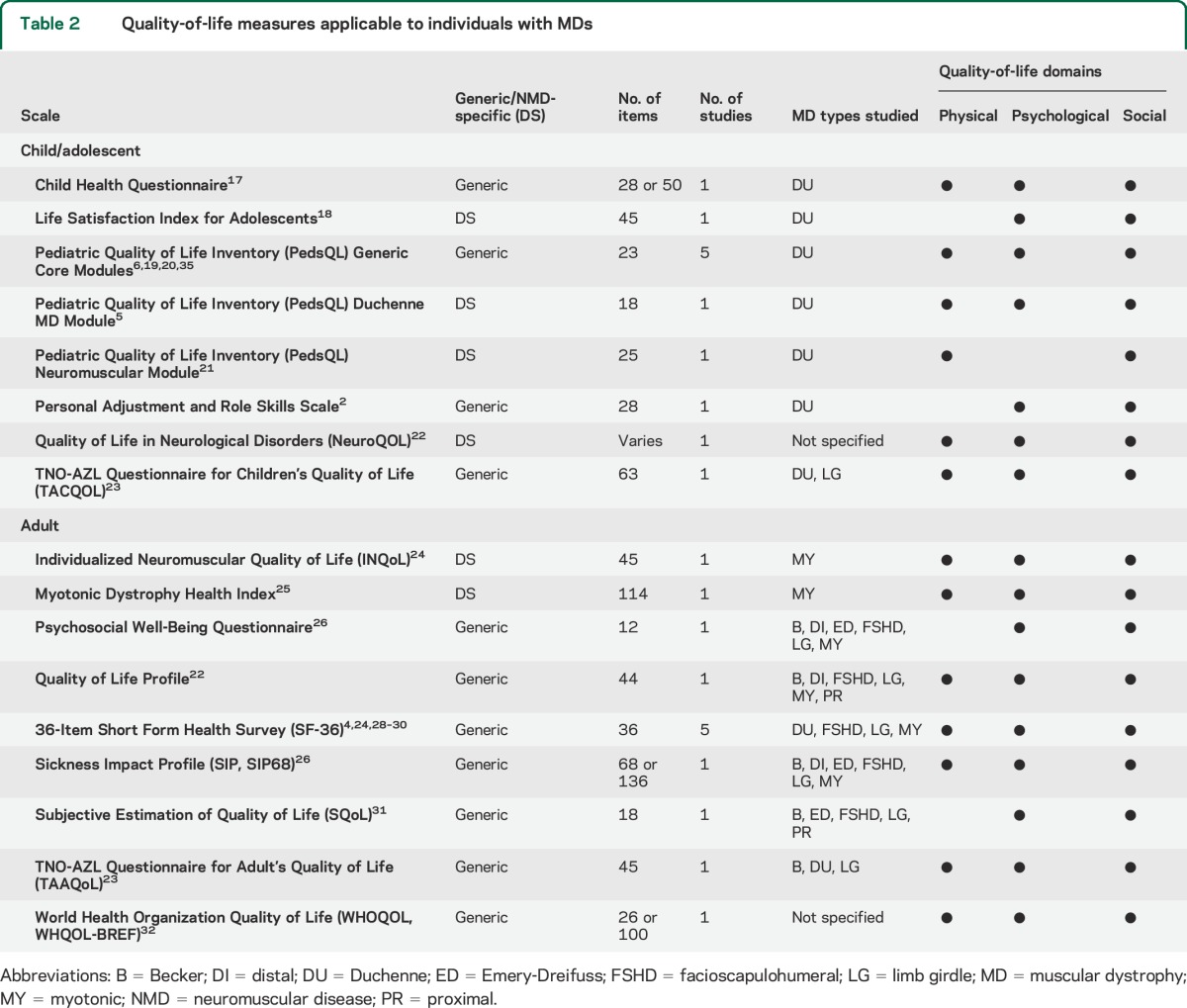
The Medical Outcomes Study 36-Item Short Form Health Survey emerged as the most frequently used generic QOL questionnaire among adults with MD33 while the Individualized Neuromuscular Quality of Life (INQoL) questionnaire was the most frequently used neuromuscular disease–specific measure for adults.
The most frequently used pediatric measure applied to MD, the Pediatric Quality of Life Inventory (PedsQL), assesses QOL among children/adolescents aged 2 to 18 years with chronic health conditions.34,35 Two neuromuscular disease–specific modules have been developed for the PedsQL and administered to children/youth with MD, the Neuromuscular and Duchenne MD modules.
Considerations in measure selection.
In a methodologic report for the Patient Centered Outcomes Research Institute, Acaster et al.36 outlined minimum standards for the design and selection of patient-reported outcome measures. They indicated the following criteria should be considered when selecting measures: (1) content validity, (2) measurement properties and interpretability, (3) patient burden, and (4) the diversity of samples in which the measurement properties were established.
Content validity.
While some existing QOL measures (e.g., NeuroQOL, InQoL, PedsQL) include subscales related to the 3 broad QOL domains (physical, psychological, and social), none captured all of the components within these domains identified by stakeholders in developing the CMQM (figure 3). Within the physical domain, several measures captured common symptoms, such as fatigue, pain, and sleep; however, only the TNO-AZL Questionnaire for Adult's Quality of Life (TAAQoL)37 included a sexual functioning subscale. In the psychological domain, while some scales include related concepts, such as autonomy and self-esteem, none included a self-efficacy subscale, and only the WHOQOL (World Health Organization Quality of Life) had a subscale related to perceived control. Overall, coverage was generally sparsest in the social domain. Several scales included subscales designed to capture overall social functioning or specific roles/daily activities (e.g., employment, leisure), but did not necessarily include all of the social aspects of QOL outlined in the CMQM. For example, none of the measures captured individuals' concerns about the impact of their condition on others. Only disease-specific measures included questions related to stigma.
Measurement properties.
None of the studies reported on responsiveness of the scale to change in clinical condition. Very few studies reported on reliability of the measures among individuals with MD, and those that did generally reported Cronbach α and/or parent-child agreement for pediatric measures2,5,6,17,20–22,31 (table e-3). Validity was primarily assessed by comparing mean scores of those with MD to healthy individuals/norms and/or examining the relationship of the measure with measures of related constructs.
Patient burden.
Patient burden is an important practical consideration in measurement, particularly when assessing QOL among individuals who may be ill or have difficulty in responding because of their condition. As described above, content validity is necessary to obtaining a complete picture of the impact of MD on QOL; however, content validity must be balanced with considerations of patient burden. Table 2 lists the number of items included on each measure to assist researchers and clinicians in assessing the level of patient burden. Some of the measures, such as the Child Health Questionnaire, include short forms that aim to capture the same construct but with fewer items. In addition, the NeuroQOL is an item bank that researchers could use to select items appropriate to their target population.
Diversity of samples.
A primary consideration in measure selection is that the measure is appropriate for, and has been tested among, the target population. In terms of age, the review identified 9 measures for adults, 4 for children/adolescents, and one specifically for adolescents. An additional measure, the NeuroQOL, was developed for children and adults, but it has been tested only among pediatric patients with MD.22
Given variability in disease trajectories and age ranges for the types of MD (figure 1), the most appropriate measure differs based on the type of MD among the target population and whether the validity of the measure has been assessed in that group. All but one of the studies using the pediatric measures focused on individuals with Duchenne MD.2,3,5,6,17–21,23 The studies of adults with MD varied more in terms of including individuals with different types of MD,23,26–28,31,32 although some focused solely on those with myotonic MD.4,24,25,30
Furthermore, the measure should be validated for the language and culture of the target population to avoid potential differences in interpretations. For example, researchers selecting measures for US studies should note that 13 of the 21 studies assessing QOL in MD were conducted outside the United States4,6,17,18,21,23,24,26,27,29–32 (table e-3). Several measures originally developed in the United States have English versions tested among non-MD patients that may also be appropriate for individuals with MD. However, additional translation and validation work may be needed to develop culturally equivalent English versions of scales, such as the TACQOL (TNO-AZL Questionnaire for Children's Quality of Life) and TAAQoL, which include relevant content, but were developed outside the United States.
Finally, it should be noted that the current study focused on measurement of QOL; however, in most cases, these measures would be used in conjunction with measures of other constructs, such as functional status, to fully capture the impact of a treatment or intervention within a clinical trial or research study. Similar considerations regarding measurement properties and burden should be taken when selecting measures of other constructs.
DISCUSSION
The current study revealed gaps in existing models and measures for comprehensively capturing QOL among individuals with MD, suggesting future avenues for research. None of the measures captured all components of QOL outlined in the CMQM, and some concepts, such as concern about impact on others and expectations/aspirations, were not included in any measure. Additional scale development and evaluation work is needed to fill in these gaps. The item banking approach to scale development and the application of computerized adaptive assessment for scale administration would be particularly beneficial by allowing the scale to be tailored to each respondent, thereby minimizing respondent burden. In addition, the approach can also provide scores that may be used to compare across different groups (e.g., healthy vs MD, types of MD, age groups) through the use of item response theory to place items on a common metric. The NeuroQOL item bank used this approach and includes physical, psychological, and social components of QOL; however, only the pediatric version was specifically developed for and tested among individuals with MD.22,38
Beyond expanding existing measures to be more comprehensive, the item banking and computerized adaptive assessment approach could be used to develop brief QOL measures that capture each of the components identified by patients and stakeholders as critical, but are concise enough for administration in settings, such as clinic visits, where a longer version may not be feasible. For example, quality of care is often assessed with functional status measures; however, the development of a brief QOL measure would also allow for the inclusion of QOL in a patient-centered approach to monitoring quality across the continuum of care for individuals with MD.
The current study also revealed that measurement-related research in MD has predominantly focused on those with Duchenne MD. Eleven of the 21 identified studies included individuals with Duchenne MD, although it should be noted that this review included only English-language studies. Further research is needed to determine the validity of measures tested among the Duchenne population for use with individuals who have other types of MD given differences in age at onset, severity of symptoms, and life expectancy across the types of MD (figure 1).
With advancements in treatments and assistive technology, pediatric patients with MD have longer life expectancies than in the past. For example, average life expectancy for children diagnosed with Duchenne MD has improved from the teens to early 20s in the past to now extending into the 20s and 30s.39,40 Youth with MD and their families must now prepare for living as an adult with MD and adapt to the challenging transition from adolescence to adulthood when adolescents/young adults have a desire for increasing autonomy and control over their lives, but may face obstacles to achieving independent living because of the impact of MD. The existing QOL measures were generally designed for either children/adolescents or adults with a few having versions for both. However, none specifically includes questions designed to address the transition from adolescence to adulthood. Measures are needed to understand how individuals and families adapt to the changes during this transition and the impact on their QOL.
Finally, a notable absence in the literature was studies on the responsiveness of QOL measures to changes in condition for individuals with MD. While several studies reported on validity, none described the responsiveness of the scale or identified a clinically meaningful difference in scores. Only one study had a prospective longitudinal design and could examine trajectories of QOL over time, but did not relate changes in QOL to changes in severity of MD.26 Responsiveness is a key psychometric property needed for measures to be used in intervention studies and clinical trials, without which it would be impossible to determine whether a lack of change was attributable to no treatment effect or simply a nonresponsive scale. Longitudinal studies are needed to examine responsiveness of QOL scales to change in individuals with MD and establish clinically meaningful differences regarding overall scale scores and individual QOL domains.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Ms. Rosemarie Kobau (CDC), Dr. Anne Rutkowski (Cure CMD), and Dr. Christopher Rosa (City University of New York) for their valuable input into the model development process.
GLOSSARY
- CMQM
Comprehensive Model of Quality of Life in Muscular Dystrophy
- COSMIN
Consensus-Based Standards for the Selection of Health Measurement Instruments
- HRQOL
health-related quality of life
- ICF
International Classification of Functioning, Disability and Health
- INQoL
Individualized Neuromuscular Quality of Life
- MD
muscular dystrophy
- PedsQL
Pediatric Quality of Life Inventory
- PROMIS
Patient-Reported Outcomes Measurement Information System
- QOL
quality of life
- TAAQoL
TNO-AZL Questionnaire for Adult's Quality of Life
- WHO
World Health Organization
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Bann: study concept and design, analysis and interpretation, manuscript drafting and revision. Dr. Abresch: analysis and interpretation, manuscript revision. Dr. Biesecker: analysis and interpretation, manuscript revision. Dr. Conway: analysis and interpretation, manuscript revision. Dr. Heatwole: analysis and interpretation, manuscript revision. Dr. Peay: analysis and interpretation, manuscript revision. Dr. Scal: analysis and interpretation, manuscript revision. Dr. Strober: analysis and interpretation, manuscript revision. Dr. Uzark: analysis and interpretation, manuscript revision. Dr. Wolff: analysis and interpretation, manuscript revision. Ms. Margolis: analysis and interpretation, manuscript revision. Ms. Blackwell: study concept and design, analysis and interpretation. Ms. Street: study concept and design, analysis and interpretation. Ms. Montesanti: study concept and design, analysis and interpretation. Dr. Bolen: study concept and design, analysis and interpretation.
STUDY FUNDING
Supported by the Centers for Disease Control and Prevention (contract 200-2007-22644/0016). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mercuri E, Muntoni F. Muscular dystrophies. Lancet 2013;381:845–860. 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 2.Hendriksen JG, Poysky JT, Schrans DG, Schouten EG, Aldenkamp AP, Vles JS. Psychosocial adjustment in males with Duchenne muscular dystrophy: psychometric properties and clinical utility of a parent-report questionnaire. J Pediatr Psychol 2009;34:69–78. 10.1093/jpepsy/jsn067. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CM, McDonald DA, Bagley A, et al. Relationship between clinical outcome measures and parent proxy reports of health-related quality of life in ambulatory children with Duchenne muscular dystrophy. J Child Neurol 2010;25:1130–1144. 10.1177/0883073810371509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peric S, Stojanovic VR, Basta I, et al. Influence of multisystemic affection on health-related quality of life in patients with myotonic dystrophy type 1. Clin Neurol Neurosurg 2013;115:270–275. 10.1016/j.clineuro.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Uzark K, King E, Cripe L, et al. Health-related quality of life in children and adolescents with Duchenne muscular dystrophy. Pediatrics 2012;130:e1559–e1566. 10.1542/peds.2012-0858. [DOI] [PubMed] [Google Scholar]
- 6.Bray P, Bundy AC, Ryan MM, North KN, Everett A. Health-related quality of life in boys with Duchenne muscular dystrophy: agreement between parents and their sons. J Child Neurol 2010;25:1188–1194. 10.1177/0883073809357624. [DOI] [PubMed] [Google Scholar]
- 7.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539–549. 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHOQOL: measuring quality of life. Available at: http://www.who.int/mental_health/media/68.pdf. Accessed March 21, 2014.
- 9.Bakas T, McLennon SM, Carpenter JS, et al. Systematic review of health-related quality of life models. Health Qual Life Outcomes 2012;10:134. 10.1186/1477-7525-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995;273:59–65. [PubMed] [Google Scholar]
- 11.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Scholarsh 2005;37:336–342. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. How to use the ICF: a practical manual for using the International Classification of Functioning, Disability and Health (ICF): exposure draft for comment. Available at: http://www.who.int/classifications/drafticfpracticalmanual.pdf. Accessed October 6, 2014.
- 13.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–1194. 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Preamble to the Constitution of the World Health Organization as Adopted by the International Health Conference, New York, 19–22 June, 1946; Signed on 22 July 1946 by the Representatives of 61 States (Official Records of the World Health Organization, No. 2, p. 100) and Entered Into Force on 7 April 1948. New York: WHO; 1948. Available at: http://www.who.int/governance/eb/who_constitution_en.pdf. Accessed October 6, 2014. [Google Scholar]
- 15.Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- 16.Swanson ME. Need for the life course model for spina bifida. Pediatr Clin North Am 2010;57:893–901. 10.1016/j.pcl.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Baiardini I, Minetti C, Bonifacino S, et al. Quality of life in Duchenne muscular dystrophy: the subjective impact on children and parents. J Child Neurol 2011;26:707–713. 10.1177/0883073810389043. [DOI] [PubMed] [Google Scholar]
- 18.Simon VA, Resende MB, Simon MA, Zanoteli E, Reed UC. Duchenne muscular dystrophy: quality of life among 95 patients evaluated using the Life Satisfaction Index for Adolescents. Arq Neuropsiquiatr 2011;69:19–22. [DOI] [PubMed] [Google Scholar]
- 19.Bendixen RM, Senesac C, Lott DJ, Vandenborne K. Participation and quality of life in children with Duchenne muscular dystrophy using the International Classification of Functioning, Disability, and Health. Health Qual Life Outcomes 2012;10:43. 10.1186/1477-7525-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim Y, Velozo C, Bendixen RM. The level of agreement between child self-reports and parent proxy-reports of health-related quality of life in boys with Duchenne muscular dystrophy. Qual Life Res 2014;23:1945–1952. 10.1007/s11136-014-0642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Jiang L, Hong S, Cheng L, Kong M, Ye Y. Reliability and validity of the Chinese version of the Pediatric Quality of Life Inventory™ (PedsQL™) 3.0 neuromuscular module in children with Duchenne muscular dystrophy. Health Qual Life Outcomes 2013;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JS, Nowinski C, Victorson D, et al. Quality-of-life measures in children with neurological conditions: pediatric Neuro-QOL. Neurorehabil Neural Repair 2012;26:36–47. 10.1177/1545968311412054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grootenhuis MA, de Boone J, van der Kooi AJ. Living with muscular dystrophy: health related quality of life consequences for children and adults. Health Qual Life Outcomes 2007;5:31. 10.1186/1477-7525-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansone VA, Ricci C, Montanari M, Apolone G, Rose M, Meola G. Measuring quality of life impairment in skeletal muscle channelopathies. Eur J Neurol 2012;19:1470–1476. 10.1111/j.1468-1331.2012.03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heatwole C, Bode R, Johnson N, et al. The myotonic dystrophy health index: initial evaluation of a new outcome measure. Muscle Nerve 2014;49:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natterlund B, Gunnarsson LG, Ahlstrom G. Disability, coping and quality of life in individuals with muscular dystrophy: a prospective study over five years. Disabil Rehabil 2000;22:776–785. [DOI] [PubMed] [Google Scholar]
- 27.Natterlund B, Ahlstrom G. Activities of daily living and quality of life in persons with muscular dystrophy. J Rehabil Med 2001;33:206–211. [DOI] [PubMed] [Google Scholar]
- 28.Abresch RT, Carter GT, Jensen MP, Kilmer DD. Assessment of pain and health-related quality of life in slowly progressive neuromuscular disease. Am J Hosp Palliat Care 2002;19:39–48. 10.1177/104990910201900109. [DOI] [PubMed] [Google Scholar]
- 29.Kohler M, Clarenbach CF, Boni L, Brack T, Russi EW, Bloch KE. Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med 2005;172:1032–1036. 10.1164/rccm.200503-322OC. [DOI] [PubMed] [Google Scholar]
- 30.Laberge L, Mathieu J, Auclair J, Gagnon E, Noreau L, Gagnon C. Clinical, psychosocial, and central correlates of quality of life in myotonic dystrophy type 1 patients. Eur Neurol 2013;70:308–315. [DOI] [PubMed] [Google Scholar]
- 31.Bostrom K, Ahlstrom G. Quality of life in patients with muscular dystrophy and their next of kin. Int J Rehabil Res 2005;28:103–109. [DOI] [PubMed] [Google Scholar]
- 32.Jovanovic M, Lakicevic M, Stevanovic D, Milic-Rasic V, Slavnic S. Community-based study of health-related quality of life in spinal cord injury, muscular dystrophy, multiple sclerosis, and cerebral palsy. Disabil Rehabil 2012;34:1284–1290. 10.3109/09638288.2011.641659. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998;51:903–912. [DOI] [PubMed] [Google Scholar]
- 34.Connolly MA, Johnson JA. Measuring quality of life in paediatric patients. Pharmacoeconomics 1999;16:605–625. [DOI] [PubMed] [Google Scholar]
- 35.Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 generic core scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res 2011;63(suppl 11):S420–S430. 10.1002/acr.20637. [DOI] [PubMed] [Google Scholar]
- 36.Acaster S, Cimms T, Lloyd A. The design and selection of patient-reported outcomes measures (PROMs) for use in patient centered outcomes research: development of a methodological standards report (topic #3) for Patient Centered Outcomes Research Institute. Available at: http://www.pcori.org/assets/The-Design-and-Selection-of-Patient-Reported-Outcomes-Measures-for-Use-in-Patient-Centered-Outcomes-Research1.pdf. Accessed July 10, 2014.
- 37.Fekkes M, Bruil J, Vogels T, Verrips GH. TAAQOL Manual Leiden Center for Child Health and Pediatric LUMC-TNO. 2004. Available at: https://www.tno.nl/downloads/vragenlijsten_01032012.pdf. Accessed October 8, 2014. [Google Scholar]
- 38.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012;78:1860–1867. 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 2002;12:926–929. [DOI] [PubMed] [Google Scholar]
- 40.Passamano L, Taglia A, Palladino A, et al. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol 2012;31:121–125. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.