Abstract
Objectives:
To determine whether vascular and demographic factors predict worsening disability up to 8 years after lacunar stroke.
Methods:
SPS3 (Secondary Prevention of Small Subcortical Strokes) was a clinical trial in lacunar stroke patients with annual assessment of disability using the Older Americans Resources and Survey instrumental activities of daily living (IADL) scale (range 0–14). Generalized estimating equations modeled the likelihood of disability (IADL <14) over time, adjusting for demographics, medical risk factors, cognition, mood, stroke location, and geographic region in univariate and multivariable models. IADL assessments after recurrent stroke were censored. We stratified by study region and age quartile.
Results:
Among 2,820 participants, mean age was 63.4 years (SD 10.8), 63% were male, 36% had diabetes, 90% hypertension, and 10% prior stroke. Mean follow-up was 3.7 years. In multivariable models, female sex, education, diabetes, nonregular alcohol use, prior stroke, Cognitive Abilities Screening Instrument score, depression, mild cognitive impairment, and stroke location were associated with disability. The youngest age quartile had decreased odds of disability over time (odds ratio 0.90 per year, 95% confidence interval 0.85–0.95), whereas the oldest age quartile had increased odds (2.20, 95% confidence interval 1.75–2.75). Americans and Latin Americans had >2-fold greater odds of disability per year compared with Spaniards (p < 0.0001).
Conclusions:
In lacunar stroke patients, older age was associated with worsening long-term disability, even without recurrence. Worse long-term function was associated with diabetes, cognitive status, and prior stroke, and regional differences may be attributable to variations in health care delivery or scale interpretation.
The course of disability after the initial 3- to 6-month recovery period after stroke is not well characterized because of short-term follow-up and single measurements of disability.1–6 There is a great need to examine patient-centered outcomes such as disability after stroke, because an exclusive focus on event-based outcomes such as mortality or vascular events may underestimate stroke burden.
In a prior study, there was a steeper decline among those with lower compared with higher socioeconomic status.7 However, all ischemic stroke subtypes were included (n = 525), of which only 135 were lacunar strokes. These small numbers limited the ability to model the long-term disability course after lacunar stroke, which comprises about 25% of ischemic stroke8,9 and typically occurs at younger ages compared with other stroke subtypes.10
The Secondary Prevention of Small Subcortical Strokes (SPS3) Study provides a large phenotypically pure sample of MRI-verified lacunar strokes ideal for characterizing the trajectory of long-term prognosis, up to 8 years after stroke. In a prior analysis of quality of life (QOL) in SPS3,11 we found a slight annual increase in QOL overall, and age, level of education, and prior stroke were associated with changes in QOL over time. SPS3 also collected data on disability at multiple follow-up time points. Herein, we describe the course of disability after lacunar stroke and identify risk factors associated with worse outcomes. We hypothesized that vascular risk factors predict lower function and that there is an ongoing decline in function after lacunar stroke.
METHODS
The SPS3 Study was a randomized, multicenter clinical trial among lacunar stroke patients, testing different antiplatelet regimens and different antihypertensive treatment targets and with annual assessments of disability. Details of the study design and results of both intervention arms have been published elsewhere.10,12,13 Subjects were eligible if they had “a clinical lacunar stroke syndrome or subcortical transient ischemic attack (TIA) in the 6 months before enrollment with confirmation by MRI, no clinical or radiological evidence of cortical involvement, and no surgically amenable ipsilateral carotid artery disease or major-risk cardioembolic sources.”14 Lacunar stroke was defined as 1 of 13 syndromes modified from Fisher criteria.10 Patients were randomized, factorially, to 1 of 2 antiplatelet interventions and 1 of 2 target levels of blood pressure control. We included all SPS3 participants with ≥1 follow-up poststroke, reflecting up to 8 years of follow-up.
Standard protocol approvals, registrations, and patient consents.
The SPS3 Study was approved by the institutional review boards of all participating centers, and all patients provided written informed consent. The clinical trial registration identifier was NCT00059306 (http://www.clinicaltrials.gov).
Baseline assessment.
Demographics, behavioral risk factors, and medical history before the qualifying stroke were collected. Race and ethnicity were determined by self-report, modeled after the 2000 US Census. Participants from Spain were categorized as non-Hispanic white and those from Latin America as Hispanic.10
History of hypertension was defined by ≥1 of the following: (1) hypertension recorded in medical records for ≥1 year, (2) medical record or self-reported use of ≥1 antihypertensive medication and/or adjustment to achieve blood pressure control, and (3) elevated blood pressure sustained for ≥3 months. History of diabetes was defined by self-reported history, chronic fasting serum glucose elevation >120 mg/dL, or chronic requirement for hypoglycemic medication. Coronary artery disease was defined as history of myocardial infarction, angina, revascularization procedure, or heart failure. Hyperlipidemia was defined as current treatment with lipid-lowering medication or laboratory data confirming fasting hyperlipidemia.15 Before entry, patients were screened for cognitive dysfunction with the Mini-Mental State Examination,16 and patients with scores 2 SDs below the mean for age and education were excluded. An SPS3-certified examiner administered blinded, detailed neuropsychological testing including tests for episodic memory, visuoconstruction, perceptual speed, motor dexterity, verbal fluency, attention, and executive functioning, as detailed in previous publications.17 Mild cognitive impairment (MCI) was determined to be present when one had a z score of −1.5 or less in ≥1 test domain.17 A standardized neurologic examination was performed, and the modified Rankin score was assessed.
MRI of the brain, ECG, echocardiography, and standard laboratory blood tests were performed on all patients. Stroke location was defined as thalamus (referent group), basal ganglia/internal capsule, corona radiata/centrum semiovale, or brainstem (pons/medulla/midbrain/cerebellum), as in previous publications.18 Imaging of the cervical and intracranial arteries was performed with magnetic resonance or CT angiography.
Prospective follow-up.
All participants were seen monthly for the first 3 months after enrollment and then every 3 months. After July 2004, at 3 months after enrollment, at 1 year, and annually thereafter up to 8 years, disability was assessed with the Older Americans Resources and Services instrumental activities of daily living (IADL) scale.19 Since recruitment occurred over an 8-year period, there was a range of follow-up time; the average number of IADL assessments was 3.9 (SD 1.9), and the average follow-up time in SPS3 as a whole was 3.7 (SD 2.0) years. The IADL scale comprises 7 activities (using the telephone, getting to places out of walking distance, shopping, meal preparation, housework, medication, and paying bills) on which an individual's performance ability is scored on a 3-level scale (unable = 0; with some help = 1; without help = 2). The overall score is an unweighted sum of scores on individual items, with a range of 0 to 14, with 14 signifying no deficits in IADLs. Because of a skewed score distribution, the IADL scale was dichotomized into no disability (14) vs disability (<14).
Depression was assessed at 3 months with the 9-item Patient Health Questionnaire (PHQ-9),20 a scale that assesses the 9 DSM depression criteria. If ≥2 of 9 symptoms were present more than half of the days, including the anhedonia or depressed mood item, depression was defined as present.18
Statistical analysis.
Descriptive means and SDs were calculated for continuous variables and proportions for categorical variables, and median and quartiles were additionally calculated for IADL scores. We fit generalized estimating equations models with a compound symmetric covariance structure to estimate odds ratios (ORs) for which factors were associated with any disability (IADL score <14), both on average and over time. There were no interactions between antiplatelet treatment assignment and time (p value 0.36) and no interactions between blood pressure treatment assignment and time (p value 0.77). Hence, since there was no effect of treatment assignment on disability for either arm, all analyses were performed in the entire enrolled cohort.
We assessed whether variables were associated with trends in disability over time, represented as interactions between each variable and time. We first fit univariable models, then individual models of interactions with time without multivariable adjustment. We then fit a multivariable model that included each of the baseline factors associated with disability, as well as significant interactions from univariate models. Nonsignificant terms were then removed one-by-one (beginning with the least significant) until all remaining terms were statistically significant. The final model excluded nonsignificant interactions with time and nonsignificant main effects. All statistical tests were 2-sided, and analyses were performed using SAS, version 9.3 (Cary, NC).
Time was treated as a continuous variable, since it had linear properties in the data. Functional assessments in the first 6 months after stroke were not included in the analysis, since our interest was in the long-term course of disability. Models were run both with and without censoring of recurrent stroke events occurring during follow-up, and results were similar; the uncensored models are presented here.
Covariates measured at baseline were chosen based on epidemiologic relevance and included demographics (age at qualifying stroke, sex, race/ethnicity [non-Hispanic white, Hispanic, African American, and other], education [any college vs other], marital status), medical risk factors (diabetes, hypertension, tobacco use [current smoker vs no smoking], regular alcohol use, and coronary artery disease [myocardial infarction, angina, congestive heart failure, coronary artery bypass grafting/percutaneous transluminal coronary angioplasty/coronary stent]), body mass index, stroke prior to qualifying event, baseline age-, race-, and education-adjusted z score on the Cognitive Abilities Screening Instrument (CASI), stroke location, MCI, and baseline modified Rankin Scale score. We also included the time between the qualifying event and the measurement of the first IADL (time since qualifying stroke). The correlations among the parameters in our final model were typically less than 0.10, and none were larger than 0.5, reducing concern about collinearity.
Because of 2-way interactions between time and age, in a secondary analysis, we fit models stratified by age quartile (<55.5, 55.5–63.0, 63.0–72.0, and >72.0 years). Because of interactions between region and time, stratified models were also fit by geographic region (North America, Spain, and Latin America).
RESULTS
A flowchart of overall trial participation and completion has been previously published.12 Baseline characteristics of the 2,820 participants with ≥1 IADL measurement, who formed the cohort for these analyses, are summarized in table 1. At least 3 IADL assessments were available for 1,956 participants, and mean follow-up was 3.7 (SD 2.0) years. At 3 months after enrollment, mean IADL score was 12.5 (SD 2.5, interquartile range 12–14), with 43% being disabled (figure).
Table 1.
Baseline characteristics of study population
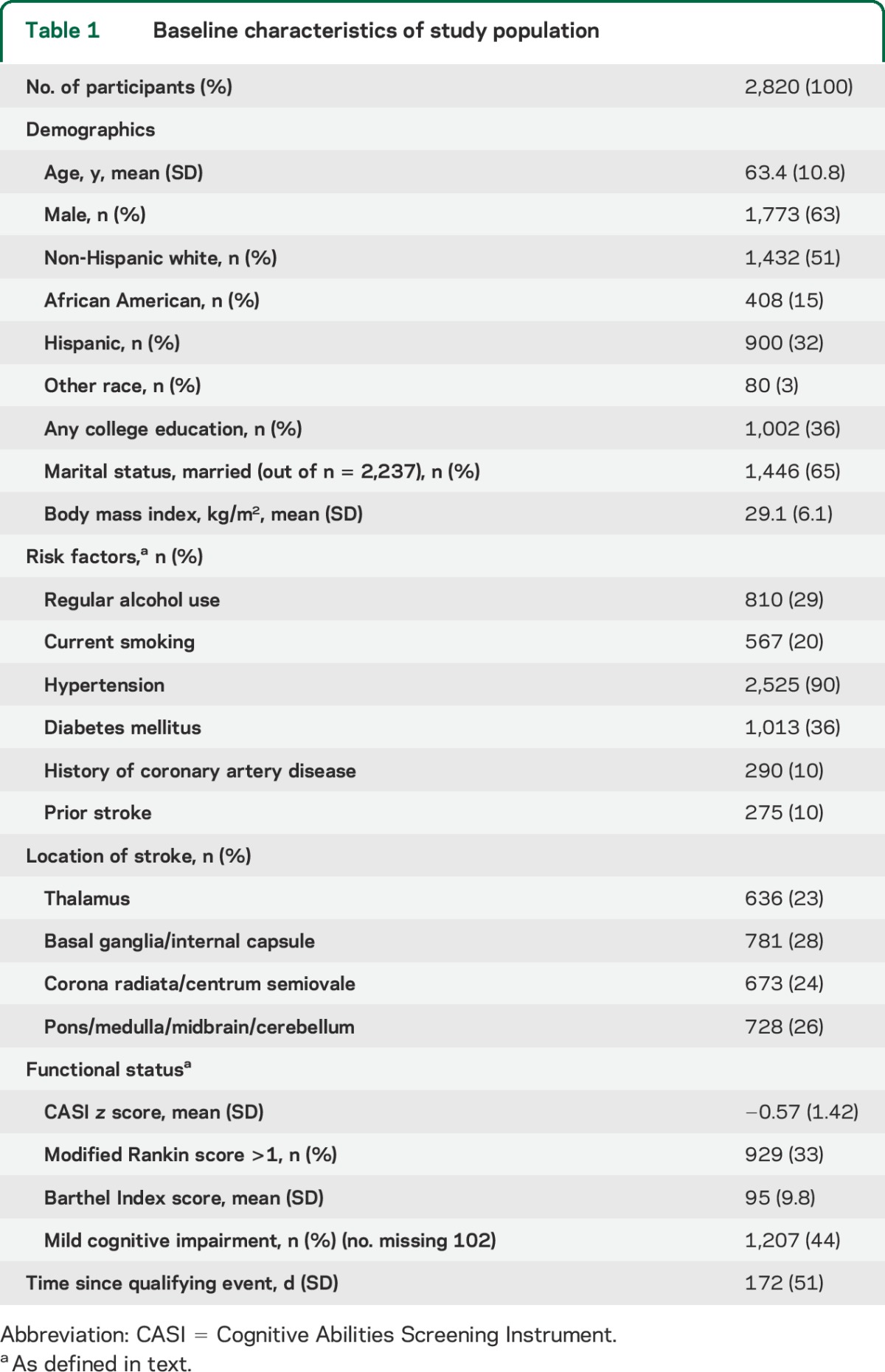
Figure. Distribution of instrumental activities of daily living (IADL) scores at 3 months.
In univariate analysis, male sex, white race, any college education, married status, current smoking, and regular alcohol use were associated with a decreased odds of disability, whereas vascular risk factors (diabetes, hypertension, coronary artery disease, prior stroke, higher body mass index, and depression) were associated with an increased odds of disability. There was significant heterogeneity by region, and the odds of disability was lowest among those in Spain (OR 0.5, 95% confidence interval [CI] 0.4–0.6), followed by those in North America (reference category), and then those in Latin America (2.3, 2.0–2.7). Brainstem stroke location was associated with the highest odds of disability. A higher baseline Rankin score, lower CASI score, and MCI were significantly associated with increased odds of disability. Significant interactions were observed between time and age, smoking status, geographic region, and depression, indicating that each of these factors was associated with a decline in function over time.
Table 2 presents the results of the multivariable model incorporating significant predictors of baseline disability and of change in function over time. Female sex, lower education, diabetes, nonregular alcohol use, prior stroke, CASI score, depression, MCI, and stroke location were associated with baseline disability.
Table 2.
Multivariable model of predictors of disability (IADL score <14)a
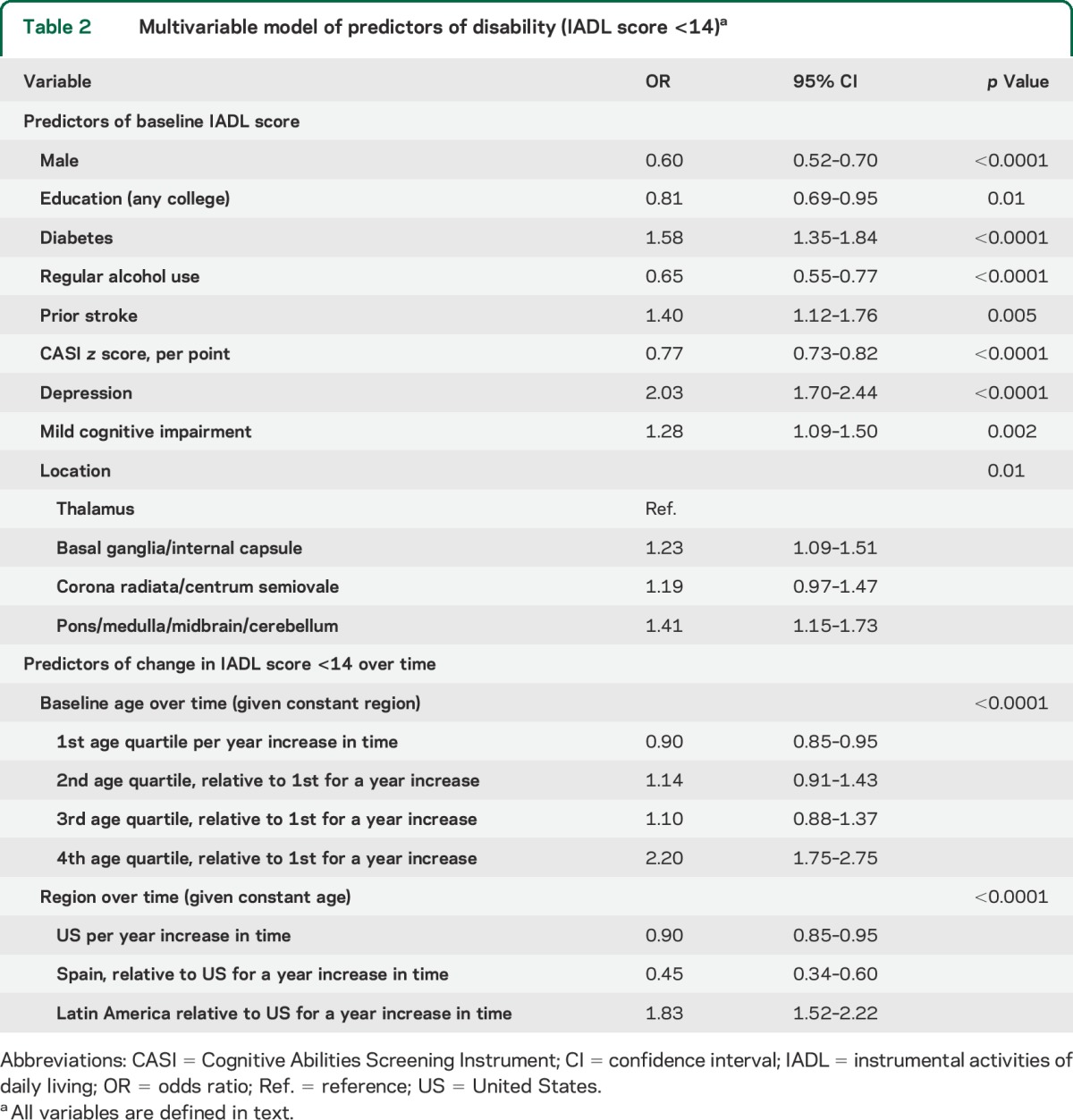
The youngest age quartile had decreased odds of disability over time (OR 0.90 per year, 95% CI 0.85–0.95), whereas the oldest age quartile had increased odds of disability over time relative to the youngest (2.20, 1.75–2.75). There was heterogeneity by region (p for interaction <0.0001): Americans and Latin Americans had ≥2-fold greater odds of disability per year compared with Spaniards. No other factors were associated with a decline in function over time.
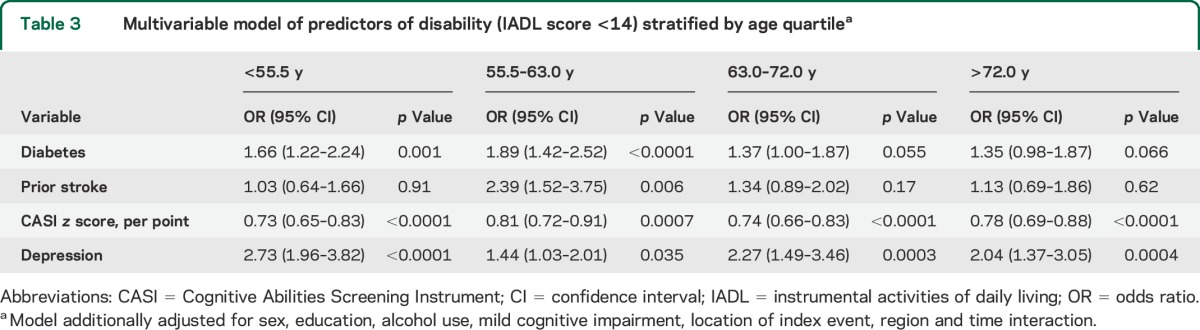
In age-stratified analyses, the impact of diabetes on average disability was higher in the lowest age quartile (OR 1.66, 95% CI 1.22–2.24) compared with the highest age quartile (1.35, 0.98–1.87, table 3). The impact of CASI score was consistent and significant in all age quartiles, and the impact of depression was highest in the youngest age quartile but was significant at all ages.
Table 3.
Multivariable model of predictors of disability (IADL score <14) stratified by age quartilea
DISCUSSION
In this clinical trial of lacunar stroke patients, we found high average functional scores, reflecting relatively mild disability compared with prior studies among all stroke subtypes.7,21,22 However, loss of ≥1 IADL was common, occurring in 43% of patients at 3 months after enrollment and 38% at 1 year. Lacunar strokes are caused by occlusion of small penetrator arteries in the deep structures of the brain, including the brainstem and basal ganglia.23 The types of vessels involved and the anatomy of injury lead generally to milder deficits that have, on average, favorable recovery. However, the accumulation of lacunar infarcts and white matter disease over time has been associated with subtle motor and cognitive deficits.24 Cognitive function is closely linked to IADLs, which, compared with activities of daily living, are higher-level functional activities that require executive functioning and memory as well as motor and ambulatory abilities. In this study, MCI was prevalent and was associated with worse overall function, and there was a strong link between CASI score and function in all age quartiles.
We found that vascular risk factors and depression were associated with greater disability in fully adjusted models. Factors such as diabetes and smoking may cause subclinical or clinical vascular events such as myocardial infarction or stroke,25,26 or may cause nonvascular conditions that affect disability, such as diabetic neuropathy. Depression has been previously linked to disability and vascular events.27 It is thought that depression not only causes decreased motivation, self-care, and social participation, but also is associated with inflammatory states, metabolic changes, and autonomic dysregulation that may lead to neurologic deficits that affect function. Stroke location in the basal ganglia, internal capsule, and posterior circulation structures was associated with greater disability than thalamic location, which is expected considering the isolated sensory loss typical of thalamic strokes compared with other locations, which involve greater motor disability and functional impairment.
A large hospital-based study conducted in Taiwan28 used semiparametric extrapolation methods to estimate the duration of disability after different stroke subtypes. The duration of severe physical functional disability was least among lacunar stroke patients compared with other subtypes, but starting around 5 years after stroke, there was a steady increase in estimated disability among lacunar stroke patients. In the Oxford Vascular Study registry, among 618 index ischemic stroke cases, 40% of survivors had modified Rankin scores >2 at 5 years, but subtype-specific data were unavailable.29 In the Atherosclerosis Risk in Communities Study,30 among 987 first strokes (183 lacunar) with a median 5.3 years of follow-up, survival was highest for lacunar infarcts (90.5%) and all-cause readmission was the lowest (41.2%) compared with other stroke subtypes.
We found that several factors were associated with a downward slope in functional trajectory over time, particularly prior stroke, which had a significant effect on functional change among all age quartiles. There are several possible mechanisms by which multiple strokes may cause decline in function over time. There could be an accumulation of deficits in gait, continence, and cognition. Stroke recurrence may reflect worse control of risk factors that could result not only in clinical strokes but also subclinical infarcts, which are prevalent,25 cause cognitive impairment,26,31 and would likely worsen function. Alternatively, ischemic stroke may lead to ongoing nonischemic damage in surrounding brain regions due to changes in inflammatory profiles32,33 that may persist years after stroke.34 Such changes may promote neurodegeneration or impair recovery mechanisms that would accelerate loss of function.
We also found heterogeneity in function by region: Americans and Latin Americans had ≥2-fold greater odds of disability per year compared with Spaniards. Regional differences may have been attributable to geographic variations in health care delivery or scale interpretation. A prior study among 30 stroke centers in Spain showed a lower than expected stroke recurrence rate of uncertain cause.35 Among Spaniards aged 75 years or older, there is a high prevalence of disability that is related to medical and social factors,36 and the results here may reflect the younger age of the cohort.
There are several weaknesses of this study. As a post hoc analysis of a clinical trial, the results of this study may not be generalizable to other populations, since these trial participants received regular medical care and study medication. Also, we did not perform cross-validation of the models in a different cohort, and further study in other populations would assess for consistency of these results. Also, the multicenter design of the study allows for greater generalization of results but may have introduced geographic confounders of the relationships we observed.
This study highlights the need to examine patient-centered outcomes such as disability, because an exclusive focus on event-based outcomes such as mortality or vascular events may underestimate the burden of stroke, especially over the long term. Also, we found that multiple strokes had an independent effect on decline in function, suggesting that preventing recurrent stroke may prevent not only discrete vascular events but also improve an individual's function over the long term. The use of patient-centered outcomes highlights the fact that treating stroke should not be focused solely on preventing events, but also maximizing functional status. Furthermore, targeting vascular risk factors such as diabetes and mood disorders such as depression after lacunar stroke may have a long-term effect not only on vascular events but also functional status. Further study is needed in lacunar stroke patients to better define the relationships among vascular risk factors, cognitive and mood disorders, and long-term functional status.
Supplementary Material
GLOSSARY
- CASI
Cognitive Abilities Screening Instrument
- CI
confidence interval
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- IADL
instrumental activities of daily living
- MCI
mild cognitive impairment
- OR
odds ratio
- PHQ-9
Patient Health Questionnaire, 9-item
- QOL
quality of life
- SPS3
Secondary Prevention of Small Subcortical Strokes
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Oscar Benavente, Robert Hart, Pablo Pergola, Santiago Palacio, Irma Castro, Arlene Farias, Ana Roldan, Carlos Kase, Irene Gavras, Helena Lau, Matt Ogrodnik, Nancy Allen, Irene Meissner, John Graves, Deb Herzig, Jody Covalt, Brett Meyer, Christy Jackson, Paul Gamble, Nancy Kelly, Janet Warner, Jo Bell, Bart Demaerschalk, Michael Hogan, Daniel Wochos, Judith Wieser, Barbara Cleary, Lori Wood, Joseph Hanna, Thomas Zipp, Scott Bailey, Dana Cook, Alice Liskay, Dana Simcox, Joan Kappler, David Anderson, Richard Grimm, Donna Brauer, Creed Pettigrew, Anand Vaishnov, Peter Sawaya, Anna Fowler, Nedda Hughes, Johnya Rice, Kathy Vanderpool, Salvador Cruz-Flores, H Douglas Walden, Eve Holzemer, Sunitha Santhakumar, Renee Van Stavern, Seemant Chatuverdi, John Flack, Flicia Mada, David Wiseman, Elizabeth Berlow, Julie Klinker, David Chiu, Addison Taylor, Larry Katz, Bruce Coull, Lien Howard, Mina Malekniazi, Melissa VanSkiver, Denise Bruck, Stacey Redman, William Logan, David Carpenter, Sally Schroer, Angelo Katramados, Brian Silver, Jerry Yee, Krisy Aiello, Kathleen Wilson, Sharon McCarthy, Bhuvaneswari Dandapani, C Peter Spies, Carole Vasile, Betty Anthony, Jennifer Ferguson, Sharon Krubel, Amanda Synman, Natalie Andrews, Howard Kirshner, Craig Sussman, Diane Brown, Mitch Elkind, Russell John Crew, Jai Radhakrishan, Tania E. Corporan, Julisa Diaz, Rebeca Aragon, Andrew Slivka, Dan Spetie, Julie Agriesti, Peggy Notstein, Dean Naritoku, Richard Zweifler, Michael Culpepper, Mel Parnell, Robin Yunker, Kelly Boots, Renay Drinkard, Rachel Backlin, Curtis Benesch, John Bisognano, Ann Leonhardt, Justine Zentner, Molly Hildreth, Mark Johnson, Yinghui Liu, Robert Goldsteen, April Blair, Gregg Wright, Naomie Gathua, Diane Book, Sunu Eapen, Clarence Grimm, Barbara Blaney, Stephanie Rozman, Linda Gaertner, Erin Bradenburg, Laura Loomis, Jolene Monarch- Cotton, Jean Ravavelli-Meyer, Anna Golembieski, Scott W. Burgin, Joshua Hollander, Walter Polashenski, Patricia Wallace, Cheryl Weber, Helmi Lutsep, Don Girard, Kali Seisler, Megan Cingel, Megan Ross, Rachel Stone, Darren Larsen, Ann Doherty, David Lefkowitz, Levy Pavel, Nancy Buchheimer, Sara Vaughn, Emily Smith, Jean Satterfield, David Tirschwell, Christine Logar, Michael Ryan, Glenn Schubert, Patricia Tanzai, Tom Mirsen, Susan McAllister, Arnaud Bastien, Patricia Niblack, James Frey, Carol Darbonne, Percy Karanjia, Narayana Murali, Richard Dart, Kathleen Mancl, Richard Atkinson, Roger Lieberman, Teresa Carter, Pat Zrelak, Nola Kenney, Michael Jacoby, David Jones, Jeffrey DeFrancisco, Theresa Hamm, José Romano, Gustavo Ortiz, Maria del Carmen Lichtenberger, Rafael Linas, Steven Kravet, Janice Alt, Sophia Sundararajan, Mahboob Rahman, Tom Horvath, David Korosec, Chris Murphy, Jason Greenberg, Laura Lennihan, Marjorie King, Laura Tenteromano, Michael Frankel, Joyce Doyle, Janet Braimah, Lorainne Pereira, Marilou Ching, Robert Sawyer, Kathy Parkes, Cheryl Conover, Erfan Albakri, German Ramirez, Stephenie Segal, Kathy Taylor, Judy Jackson, Kathy Helgason, Maureen Hillmann, Robert Ringel, Nicholas Fleming, Bunny Mckown, Stanley Cohen, Robert Jenders, Ravinder Singh, Minh-Thu La, Khanhphong Trinh, Jesse Weinberger, Lewis Wright, Dorothy Burch, Ronnie Horowitz, Steven Atlas, Sandra Augustine, Renee Van Stavern, Angela Brown, Jannie Serna, Jill Newgent, Julie Naylor, Laura Carpenter, Tanya Warwick, Steven Stoltz, Rebekah Garcia, Michael Scheck, Linda Chadwick, James Fleck, Myron Weinberger, Alison Sears, Chris Fanale, Lenden Neeper, Paula Fisk, Stanley Cohen, Daniel Sabry, Ron Phoenix, David Tong, Madelleine Garcia, Ariane Mackey, Jovette Morin, Annette Haché, Claudette Lessard, Robert Côté, Laurence Green, Lisa Wadup, Anne-Marie Fontaine, Gordon Gubitz, Rosario Rebello, Tim Dean, Yvette Reidy, Mukul Sharma, Grant Stotts, Heather Clark, Melodie Mortensen, Jenniffer Sauve, Doris Sharma, Michelle Savage, Jeffrey Minuk, Luc Trudeau, Claudia Schanz, Leo Berger, Sylvain Brunet, Johanne Pontbriand, Martine Mainville, Denise Racicot, Michael Hill, Karyn Fischer, Andrea Cole-Kaskayne, Carol Keeney, Ashfaq Shuab, Khurshid Khan, Naeen Dean, Frederika Herbert, Karen Kastelic, Edwin Javier Pretell, José Valdivia, Marissa Pretell, Oscar del Brutto, Rocio Santibáñez, Joffre Lara, Mauricio Zambrano, Antonio Arauz, G Amin Cervantes, Adolfo Leyva, Itzel Camacho, José Luis Ruiz Sandoval, Eduardo Salcido Vásquez, Carmen Ruiz, Carlos Cantú Brito, Margarita Fernández, Juan Fernándo Góngora-Rivera, Juan Manuel Escamilla, Joaquín Moxica, Genny Arciniega, Wendy Joana Gonzalez, Gonzalo Matamala, Helmut Goecke, Marcela Parra, Jessica Pozo, Jorge Tapia, Ivan Esteban Godoy, Marcela Valdes, Conrado Estol, Cecilia Peralta, Adriana Ellenberg, Daniela Chezzio, Manuel M. Fernández Pardal, Hernan Trimachi, Pablo Bonardo, Julieta Mazziotti, Raul Carlos Rey, Gustavo Caruso, Luciana Melamud, Sandra Lepera, Ana Paula Stilman, Sebastian Ameriso, Ramiro Sánchez, Guadalupe Bruera, Javier Moschini, Maria J. Ramírez, José A. Bueri, Sebastián Sevilla, Bruno de Ambrosi, Gabriela Marinsalta, Francisco Rubio, Yurek Krupinski, Ana Carvajal, Jaume Roquer, Ana Oliveras Serrano, Jordi Jiménez Conde, Ana Rodríguez, Gemma Romeral, Adrià Arboix, Antoni Pelegrí, Lorena Blanco, David Cánovas Vergé, Jordi Estela Herrero, Ana Gómez, Lorena Blanco, Joaquín Serena Leal, Mar Castellanos, Verónica Cruz, Mercè Cepeda, Meritxell Gomis, Juan Arenillas, Antonio Dávalos, Ana Suñol, Silvia Reverté, José Lluis Martí-Villalta, Sergio Martínez, Rebeca Marín, José Castillo Sánchez, Miguel Blanco González, Manuel Rodríguez, Isabel Jiménez, Jaime Rodríguez, Exuperio Díez Tejedor, Patricia Martínez, Blanca Fuentes, Lluis Soler Singla, Ernest Balaguer, Joan Izquierdo, Cristina Soler, Maria Armenteros, Oscar Benavente, Robert Hart, Pablo Pergola, Alexander Shepherd, Ana Roldan, Marie-France Benavente, Carole White, Camilla Robu, Che Kelly, Robert Talbert, Eduardo Martinez, Carlos Bazan, Gabriela Pergola, Lesly Pearce, Raymond Costello, Claudia Jacova, Luisa Camelia, Crystal Mendoza, Brandy Pratt, B. Kin, Steve Holliday, Leslie McClure, Christopher Coffey, Jeff Szychowski, George Howard, Charles Katholi, Yu Zhang, Kalyani Peri, Charles Allcorn, Richard Mailhot, Lisa Irby, Fekisha Guyton, Mary Jo Sewell, Robert Ringer, Dennis Raisch, Dave Hunt, Francisco Rubio, Clara M. Rosso, Ariadna Martin, Mireia Sanllorente, Celso Arabetti, José Luis Fernández, Maria Julia Cremona, Ana Capece, Oscar Benavente, Robert Hart, Marie Benavente, Christopher Coffey, Robin Conwit, Leslie McClure, Pablo Pergola, Ana Roldan, Jeff Szychowski, Robert Talbert, Oscar Benavente, David Anderson, Antonio Arauz, Marie Benavente, Christopher Coffey, Robin Conwit, Robert Côté, Bart Demaerschalk, Oscar del Brutto, Mitchell Elkind, Robert Hart, Carlos Kase, Leslie McClure, Claudia Moy, Pablo Pergola, Ana Roldan, Mukul Sharma, Jeff Szychowski, Robert Talbert, Carole White, Carole White, Oscar Benavente, Curtis Benesch, Christopher Coffey, Robin Conwit, John Graves, Richard Grimm, Gordon Gubitz, Steve Holliday, Helena Lau, Claudia Moy, Lesly Pearce, Jeff Szychowski, Maria Aguilar, David Sherman, Elaine Nasco, Joe Blackshear, Mina Chung, Pierre Fayad, Brian Gage, Clay Johnston, Walter Kernan, Enrique Leira, Jose Merino, Gustavo Roman, Cathy Sila, Gene Sung, Carl van Walraven, Richard Zweifler, Barney Stern, Robin Conwit, Claudia Moy, K. Michael Welch, William Clarke, Jeffrey Cutler, Karen Furie, Karen Johnston, and Matthew Mayo
AUTHOR CONTRIBUTIONS
Dhamoon: involved in drafting/revising the manuscript for content, including medical writing for content, study concept, and analysis or interpretation of data. McClure: involved in revising the manuscript for content, including medical writing for content, study design, and analysis or interpretation of data. White: involved in revising the manuscript for content, including medical writing for content, and analysis or interpretation of data. Lakshminarayan: involved in revising the manuscript for content, including medical writing for content, and interpretation of data. Benavente: involved in revising the manuscript for content, including medical writing for content, study concept and design, analysis or interpretation of data. Elkind: involved in revising the manuscript for content, including medical writing for content, study design, analysis or interpretation of data.
STUDY FUNDING
NIH/National Institute of Neurological Disorders and Stroke (U01 NS38529-04A1); Sanofi/BMS (USA) with drug donation for the study.
DISCLOSURE
M. Dhamoon is supported by NIH/National Institute of Neurological Disorders and Stroke (NINDS) [K23NS079422]. L. McClure was supported by NIH/NINDS (U01 NS38529-04A1). C. White reports no disclosures. K. Lakshminarayan was supported by NIH 1K23NS051377, 1R21NS075608, CDC 1U58DP000857, AHRQ 1R21HS021794-01; St. Jude Medical. O. Benavente was supported by NIH/NINDS (U01 NS38529-04A1); Sanofi/BMS (USA) with drug donation for the study. M. Elkind was supported by diaDexus, Inc., BMS-Sanofi; NIH/NINDS (R01 NS050724, NS048134, P50 NS049060, R27 NS029993, R01 NS55809, R01 NS062820); BMS-Pfizer Partnership, Janssen Pharmaceuticals, Daiichi-Sankyo, Biogen IDEC; Jarvik Heart; Organon/Merck; Novartis; and American Academy of Neurology. No financial relationships relevant to the manuscript topic. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dombovy ML, Sandok BA, Basford JR. Rehabilitation for stroke: a review. Stroke 1986;17:363–369. [DOI] [PubMed] [Google Scholar]
- 2.de Groot-Driessen D, van de Sande P, van Heugten C. Speed of finger tapping as a predictor of functional outcome after unilateral stroke. Arch Phys Med Rehabil 2006;87:40–44. [DOI] [PubMed] [Google Scholar]
- 3.Pan SL, Lien IN, Yen MF, Lee TK, Chen TH. Dynamic aspect of functional recovery after stroke using a multistate model. Arch Phys Med Rehabil 2008;89:1054–1060. [DOI] [PubMed] [Google Scholar]
- 4.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology 2007;68:1583–1587. [DOI] [PubMed] [Google Scholar]
- 5.Johnston M, Pollard B, Morrison V, MacWalter R. Functional limitations and survival following stroke: psychological and clinical predictors of 3-year outcome. Int J Behav Med 2004;11:187–196. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen HS, Reith J, Nakayama H, Kammersgaard LP, Raaschou HO, Olsen TS. What determines good recovery in patients with the most severe strokes? The Copenhagen Stroke Study. Stroke 1999;30:2008–2012. [DOI] [PubMed] [Google Scholar]
- 7.Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke 2009;40:2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735–2740. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe CD, Rudd AG, Howard R, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry 2002;72:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benavente OR, White CL, Pearce L, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) Study. Int J Stroke 2011;6:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhamoon MS, McClure LA, White CL, Lau H, Benavente O, Elkind MS. Quality of life after lacunar stroke: the Secondary Prevention of Small Subcortical Strokes Study. J Stroke Cerebrovasc Dis 2014;23:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White CL, Szychowski JM, Roldan A, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. J Stroke Cerebrovasc Dis 2013;22:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17.Jacova C, Pearce LA, Costello R, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol 2012;72:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White CL, McClure LA, Wallace PM, et al. The correlates and course of depression in patients with lacunar stroke: results from the Secondary Prevention of Small Subcortical Strokes (SPS3) Study. Cerebrovasc Dis 2011;32:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS Multidimensional Functional Assessment Questionnaire. J Gerontol 1981;36:428–434. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–1744. [DOI] [PubMed] [Google Scholar]
- 21.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke 2002;33:1034–1040. [DOI] [PubMed] [Google Scholar]
- 22.Kojima S, Omura T, Wakamatsu W, et al. Prognosis and disability of stroke patients after 5 years in Akita, Japan. Stroke 1990;21:72–77. [DOI] [PubMed] [Google Scholar]
- 23.Bailey EL, Smith C, Sudlow CL, Wardlaw JM. Pathology of lacunar ischemic stroke in humans: a systematic review. Brain Pathol 2012;22:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards JD, Jacova C, Sepehry AA, Pratt B, Benavente OR. A quantitative systematic review of domain-specific cognitive impairment in lacunar stroke. Neurology 2013;80:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2002;33:21–25. [DOI] [PubMed] [Google Scholar]
- 26.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2002;33:2376–2382. [DOI] [PubMed] [Google Scholar]
- 27.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013;11:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung MC, Hsieh CL, Hwang JS, Jeng JS, Wang JD. Estimation of the long-term care needs of stroke patients by integrating functional disability and survival. PLoS One 2013;8:e75605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luengo-Fernandez R, Paul NL, Gray AM, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013;44:2854–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones SB, Sen S, Lakshminarayan K, Rosamond WD. Poststroke outcomes vary by pathogenic stroke subtype in the Atherosclerosis Risk in Communities Study. Stroke 2013;44:2307–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hachinski V. World Stroke Day 2008: “little strokes, big trouble.” Stroke 2008;39:2407–2420. [DOI] [PubMed] [Google Scholar]
- 32.Zaremba J, Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand 2001;104:288–295. [DOI] [PubMed] [Google Scholar]
- 33.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009;15:192–199. [DOI] [PubMed] [Google Scholar]
- 34.Theodorou GL, Marousi S, Ellul J, et al. T helper 1 (Th1)/Th2 cytokine expression shift of peripheral blood CD4+ and CD8+ T cells in patients at the post-acute phase of stroke. Clin Exp Immunol 2008;152:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purroy F, Jimenez Caballero PE, Gorospe A, et al. Prediction of early stroke recurrence in transient ischemic attack patients from the PROMAPA study: a comparison of prognostic risk scores. Cerebrovasc Dis 2012;33:182–189. [DOI] [PubMed] [Google Scholar]
- 36.Virues-Ortega J, de Pedro-Cuesta J, del Barrio JL, et al. Medical, environmental and personal factors of disability in the elderly in Spain: a screening survey based on the International Classification of Functioning. Gac Sanit 2011;25(suppl 2):29–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.