Abstract
Objective:
To evaluate whether delayed appearance of intraventricular hemorrhage (dIVH) represents an independent entity from intraventricular hemorrhage (IVH) present on admission CT or is primarily related to the time interval between symptom onset and admission CT.
Methods:
A total of 282 spontaneous intracerebral hemorrhage (ICH) patients, admitted February 2009–March 2014 to the neurological intensive care unit of a tertiary care university hospital, were prospectively enrolled in the ICH Outcomes Project. Multivariate logistic regression was used to determine associations with acute mortality and functional long-term outcome (modified Rankin Scale).
Results:
A cohort of 282 ICH patients was retrospectively studied: 151 (53.5%) had intraventricular hemorrhage on initial CT scan (iIVH). Of the remaining 131 patients, 19 (14.5%) developed IVH after the initial CT scan (dIVH). The median times from symptom onset to admission CT were 1.1, 6.0, and 7.4 hours for the dIVH, iIVH, and no IVH groups (Mann-Whitney U test, dIVH vs iIVH, p < 0.001) and median time from onset to dIVH detection was 7.2 hours. The increase in ICH volume following hospital admission was larger in dIVH than in iIVH and no IVH patients (mean 17.6, 0.2, and 0.4 mL). After controlling for components of the ICH score and hematoma expansion, presence of IVH on initial CT was associated with discharge mortality and poor outcome at 3, 6, and 12 months, but dIVH was not associated with any of the outcome measures.
Conclusions:
In ICH patients, associated IVH on admission imaging is commonly encountered and is associated with poor long-term outcome. In contrast, dIVH on subsequent scans is far less common and does not appear to portend worse outcome.
Intracerebral hemorrhage (ICH) is associated with high mortality and unfavorable outcome.1 In order to improve clinical outcomes, research efforts have primarily been directed towards understanding modifiable factors that worsen outcome, e.g., hematoma expansion after hospital admission.2,3 One recent study showed that delayed intraventricular hemorrhage (dIVH; intraventricular hemorrhage [IVH] that is not present on the initial admission CT but happens during hospitalization) occurs in up to every fifth ICH patient without IVH on initial CT and is independently associated with in-hospital mortality.4 These results from one single-center study suggest that this subgroup of ICH patients is both at increased risk of unfavorable outcome and a promising target group, in which unfavorable outcome might potentially be prevented by frequent follow-up CTs and aggressive intervention. Before embarking on costly clinical trials to test this hypothesis, we thought it prudent to investigate if these observations can be confirmed in an independent patient sample. We tested the hypothesis that dIVH to a large extent is an artifact of the timing of obtaining the initial CT scan and does not represent a separate clinical entity.
METHODS
Patient selection and data collection.
A total of 401 consecutive patients with spontaneous nontraumatic ICH were admitted to the Neurological Intensive Care Unit at Columbia University Medical Center (CUMC) between February 2009 and March 2014 and prospectively enrolled in an institutional review board–approved study, the ICH Outcomes Project (ICHOP).
Patients were treated according to current guidelines.5 Details of clinical management and imaging analysis are presented in the e-Methods on the Neurology® Web site at Neurology.org. We studied 282 patients with spontaneous ICH. The presumed ICH etiology and presence/absence of IVH were determined by consensus among treating physicians during a weekly meeting.
Patients with ICH attributed to trauma or hemorrhagic conversion of an ischemic stroke were not included in ICHOP. Patients excluded from ICHOP are described in the e-Methods. From the available subjects included in ICHOP, we excluded a priori 50 patients with arteriovenous malformation–associated ICH from our study, and 48 patients whose admission CT was later than 48 hours after symptom onset, as well as an additional 21 patients who did not receive an admission CT scan, resulting in a final cohort of 282 patients with spontaneous, nontraumatic ICH.
dIVH was defined as any newly occurring IVH during hospitalization, i.e., IVH that was not present on admission CT but was present on any CT scan thereafter. Demographics (patient age, sex, ethnicity), clinical variables (e.g., Glasgow Coma Scale [GCS] score, hospital complications), and outcome measures were prospectively recorded by a dedicated study investigator. Missing outcome data were imputed using a predefined SPSS algorithm (SPSS Inc., Chicago, IL) in order to account for sampling bias.
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Board at CUMC approved the study protocol. Written informed consent was obtained from patients or, if neurologically impaired, from family members.
Neuroimaging.
Per hospital protocol, patients underwent a noncontrast CT scan on admission, followed by CT scans at 12 and 24 hours after admission. Most comatose patients or patients with a high ICH score on admission underwent additional CT scans 4–6 hours after admission. Additional CT scans were obtained in case of clinical worsening.
Parenchymal hemorrhage size (ICH) and IVH volumes were measured semiautomatically (MIPAV software, NIH, Bethesda, MD). Hematoma volume change after admission was calculated using a follow-up CT (≥24 hours after admission CT) as reference. Outcome was determined using the modified Rankin Scale (mRS). Methodologic details are presented in the supplementary material.
Outcome assessment.
Mortality (score of 6 on the mRS) was determined on hospital discharge or postbleed day 14, whichever occurred first. Follow-up mRS scores at 3, 6, and 12 months after the ICH were determined by trained study staff using standardized telephone interviews. Poor outcome was defined as a mRS score of 4–6.
Statistical analysis.
All analyses were performed using SPSS version 21 software package and R (version 3.0.2, R Project). Data are expressed either as median and interquartile range (IQR) or mean and SD. Mann-Whitney U and Student t tests were used to assess the differences between groups for non-normally and normally distributed data, respectively. To assess correlations between baseline variables (for more than 2 groups), we used Pearson χ2 and the independent samples Kruskal-Wallis tests (for non-normally distributed data) and one-way analysis of variance (for normally distributed data), as appropriate. In the multivariate logistic regression analysis, we included those binary input variables included in the ICH score6 and hematoma expansion as continuous variable. A p value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the 3 study subgroups are shown in table 1 and table e-1. Table e-2 shows a direct comparison of the baseline characteristics of patients with initial and dIVH. A total of 151 of the patients had IVH on admission CT scan (initial IVH [iIVH]). In 19 (14.5%) of the patients with no IVH on initial CT, IVH was seen on follow-up CT scan. The median time from symptom onset to detection of dIVH was 7.2 hours (IQR 3.4–13.2), and the median time between the initial imaging study and the follow-up study showing IVH was 4.7 hours (IQR 2.4–10.0).
Table 1.
Baseline characteristics of patients with IVH on initial CT, delayed IVH, and no IVH

In 14 out of 19 patients, dIVH was detected until 7.4 hours after the initial CT (second scan after admission), in 2 patients 11 hours (both third scan after admission), in 1 patient 21 hours (second scan), and in 2 patients 34 and 36 hours (both third scan) after the initial CT scan. Among the 5 patients with late IVH discovery (>8 hours after admission CT), 2 were coagulopathic, 2 had hyperglycemic crises potentially associated with intraventricular ICH expansion,7 and in the remaining patient no potential cause of dIVH could be found. Two of 5 died during hospitalization; one was dead at 12 months. The remaining 2 had mRS scores of 3 and 4 at 12 months follow-up.
The median time from symptom onset to first CT scan was shorter in dIVH patients (1.1 hours) than in iIVH patients (6.0 hours) and no IVH patients (7.4 hours) (figure, A; Mann-Whitney U test iIVH vs dIVH groups, p < 0.001). Hematoma volumes on initial CT were similar between iIVH and dIVH patients (median 25.9 mL in iIVH, 30.0 mL in dIVH, and 9.7 mL in no IVH; figure, B). However, the mean ICH increase from initial CT to follow-up CT was far larger in dIVH patients than in the other 2 groups (no IVH 0.4 mL, iIVH 0.2 mL, dIVH 17.6 mL; t test dIVH vs no dIVH: p < 0.001; figure, C). dIVH patients underwent external ventricular drainage less often than iIVH patients (21.1% vs 44.4%). The rate of hematoma surgery was similar between both IVH groups (13.2% in iIVH and 10.5% in dIVH patients). The median IVH volume in the dIVH group was 2.7 mL (IQR 1.1–8.8 mL) and in the iIVH group 6.5 mL (IQR 2.3–21.1 mL). This difference was significant on statistical testing (p = 0.025).
Figure. CT timing and hematoma volumes in patients with initial, delayed, and no intraventricular hemorrhage.
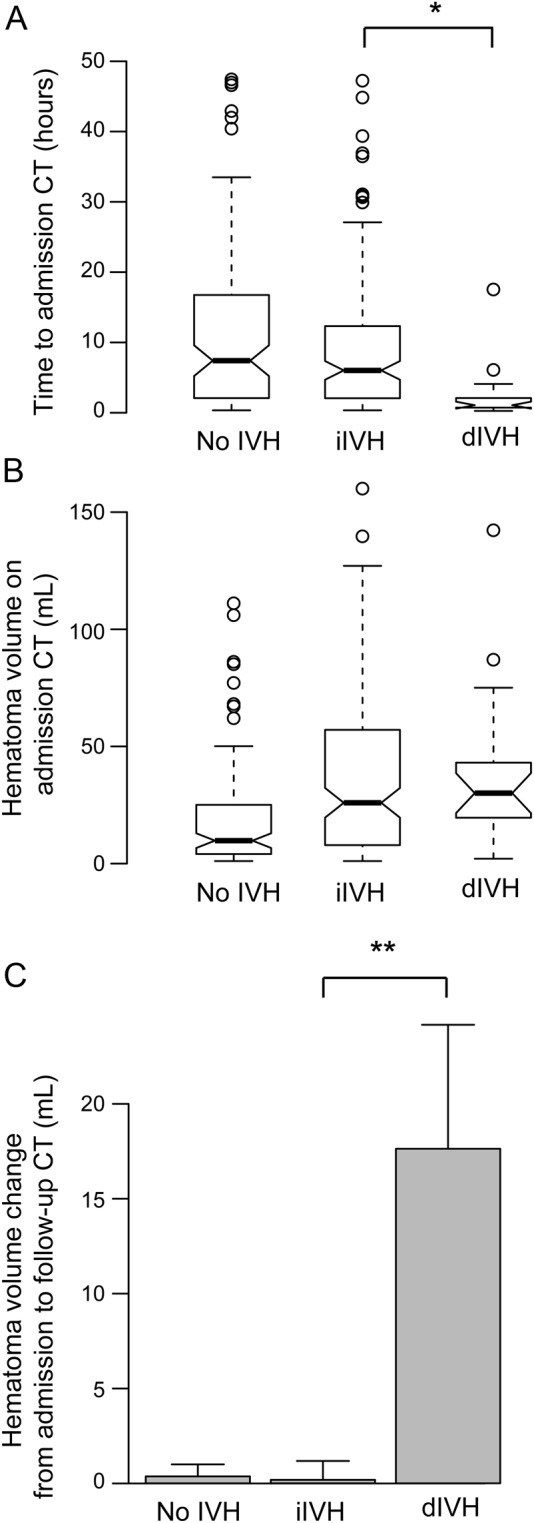
(A) Time from symptom onset to admission CT. (B) Hematoma volume on admission CT scan. (C) Change of hematoma volume from admission CT to follow-up CT (>24 hours after admission CT). dIVH = patients with intraventricular hemorrhage on follow-up CT; iIVH = patients with intraventricular hemorrhage on admission CT; no IVH = patients without intraventricular hemorrhage. */** = significant difference on statistical testing.
The short onset to admission CT time in the 19 dIVH patients was plausible in all cases: 6 cases of ICH occurred in-hospital, 11 experienced symptom onset at home in the presence of relatives (in 3/11 in the presence of health care professionals), and 2 patients had symptom onset while in the emergency department accompanying a relative.
A total of 72%, 71%, and 72% of patients in our cohort had poor outcome (mRS 4–6) at 3, 6, and 12 months after the ICH, respectively. After controlling for age (>80 years), admission hematoma size (≥30 mL), decreased level of consciousness (GCS < 13), hematoma expansion, and infratentorial hematoma location,4,6 the presence of IVH on initial CT was associated with 14-day mortality (odds ratio [OR] 3.95, confidence interval [CI] 1.79–8.72; p = 0.001) and poor outcome at 3 months (OR 2.84, CI 1.42–5.66; p = 0.003), 6 months (OR 2.16, CI 1.13–4.12; p = 0.02), and 12 months (OR 2.7, CI 1.37–5.33; p = 0.004). dIVH was not significantly associated with any of these outcome measures (table 2, table e-3). There was a trend for worse functional outcome at 3 months for those with dIVH (OR 8.38, CI 0.86–81.35; p = 0.07). However, when including both the time from symptom onset to the initial CT and the presence of dIVH as independent variables in the multivariate model, dIVH was not associated but timing of CT (OR 2.48, CI 1.44–4.28) was associated with poor 3-month functional outcome (table e-4).
Table 2.
Multivariate regression analysis of poor outcome (modified Rankin Scale score 4–6) at 12 months

DISCUSSION
Our study confirms that ICH-associated IVH that occurs after hospital admission is not uncommon (14.5% compared to 21% in a previous study4) but is far less frequent than IVH on admission CT. dIVH was predominantly seen in those who underwent very early admission CT scans. Two characteristics of the dIVH group, the markedly shorter onset to CT time and greater hematoma expansion, suggest that dIVH patients represent a specific subgroup of ICH patients with associated IVH, and that the categorization into initial and dIVH is mainly driven by CT timing, which confirms our initial hypothesis.
Comparing our cohort with the one studied by Maas and coworkers,4 we found similar baseline characteristics with higher rates of atrial fibrillation, heart disease, and a higher rate of anticoagulation before admission (p = 0.08) in dIVH when compared to iIVH patients. Accordingly, our dIVH patients had a higher partial thromboplastin time on admission compared to iIVH patients (p = 0.03). In both studies, the initial serum glucose levels were higher among iIVH patients compared to the other groups, which has been described to be associated with intraventricular extension of intracerebral hemorrhages.7 Expectedly and in line with the previous study, patients who never developed IVH were less likely to have a deep location (thalamus/basal ganglia) of the hematoma, both dIVH and iIVH patients had similar hematoma baseline volumes, and hematoma extension was overwhelmingly larger in patients with dIVH. In both studies, ventriculostomy was less frequent among dIVH compared to iIVH patients. However, dIVH patients in our study had a lower rate of surgical hematoma evacuation (11% and 30%, respectively), which might reflect different neurosurgical practice at both study sites.
Notably, and in contrast with the previous study, we did not find an association between dIVH and acute or long-term outcome. Although this finding was consistent over the outcome measures at different time points (discharge and 3, 6, and 12 months after), it is important to note that neither the present cohort (n = 282) nor the one in the previous study (n = 216) are large enough to demonstrate or reject an association between dIVH and outcome.4 The fact that Maas et al.4 found dIVH to be associated with mortality and poor outcome and we did not is most likely a consequence of insufficient power in both studies. Studies in larger patient cohorts are needed to conclusively answer this question.
Both hematoma expansion and early hospital admission are known to be more frequent in stroke patients with poor initial clinical status and low GCS score.8–10 Thus, short latency between symptom onset and hospital admission is likely a surrogate marker for low GCS score, which in turn is a strong predictor of mortality and poor outcome. Accordingly, the trend level association of dIVH and poor outcome at 3 months did not prevail when tested in a model with CT timing (CT ≤8 hours vs CT >8 hours after symptom onset) and hematoma expansion as covariates. The previous study neither quantified nor controlled for the time from symptom onset to initial imaging.
Furthermore it is of note that dIVH in our cohort was detected 2 hours earlier than in the cohort described previously, which we believe is due to differing local guidelines. In the previous study, patients per protocol were supposed to get a follow-up CT after 6 hours (the exact timing of CT scans was not separately reported for those with iIVH and dIVH in this study), while at our institution those with large hematomas or poor clinical state tend to receive follow-up imaging earlier than those with small bleeds and clinically stable patients.
Bias introduced by variable imaging time relative to the onset of acute disease is a well-known phenomenon. Examples in ICH research include the spot sign that may or may not be detectable, depending on the time interval from symptom onset to CT angiography,11 estimation of ICH size that is strongly confounded by hematoma expansion,12 and the quantification of perihematomal edema size, which is markedly dynamic during the first 14 days after the ICH.13 Conversely, imaging may be used to guide therapeutic decisions in the absence of a reliable neurologic history, provided that imaging characteristics can be correlated well enough with changes of the underlying pathophysiology over time.14 In order to control for bias arising from timing of imaging studies, clinical data acquisition has to be thorough and CT time in relation to preceding and subsequent events has to be documented and accounted for in the analysis. In our study, a dedicated investigator gathered all available data including history information for each patient. The onset time was documented after discussion with the treating attending and fellow taking all information into account. Future studies investigating dIVH should ideally prospectively also include only patients with a reliable history regarding bleed onset.
The strengths of our study include a relatively large sample size and a prospective mode of data collection. Furthermore, all CT scans were evaluated in an interdisciplinary meeting. Limitations might arise from a less rigid imaging protocol in comparison to the previous study.4 Our imaging protocol did not include routine follow-up CTs before 12 hours after admission, since awake and cooperative patients can be sufficiently evaluated by neurologic examination alone. Importantly, because all patients received an admission and follow-up CT scan, we do not believe we underestimated the incidence of IVH after hospital admission. Though a longer latency to follow-up CT after admission may prolong the time to dIVH detection, it would most likely not prevent dIVH detection.
It is conceivable that ICH patients with IVH may be subdivided into 2 clinically meaningful groups: one that develops IVH simultaneously with the ICH, and one in which IVH occurs after the ICH as a consequence of hematoma expansion. Our findings of CT timing–dependent detection of IVH illustrate the latter pathomechanism. However, the significantly smaller IVH volumes in the dIVH group compared to the iIVH group indicate that possibly another mechanism of dIVH, e.g., rebleeding in coagulopathic patients during the hospital course, might account for some dIVH cases, possibly leading to smaller IVH volumes than in patients who develop IVH initially.
Our findings challenge recent observations associating dIVH with acute mortality. Moreover, they suggest that the timing of obtaining the admission CT scan is the main driver for categorizing IVH into early vs delayed. Future studies should test these hypotheses in larger patient samples in order to account for the overall relatively low incidence of dIVH. The CT timing aspect of intraventricular expansion in ICH should ideally be studied in an ICH cohort with a short onset to CT time (e.g., ≤2 hours).
Supplementary Material
GLOSSARY
- CI
confidence interval
- CUMC
Columbia University Medical Center
- dIVH
delayed intraventricular hemorrhage
- GCS
Glasgow Coma Scale
- ICH
intracerebral hemorrhage
- ICHOP
ICH Outcomes Project
- iIVH
initial IVH
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Scale
- OR
odds ratio
Footnotes
Editorial, page 970
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jens Witsch drafted, revised, and gave final approval for the manuscript and conducted the statistical analysis. Eliza Bruce provided suggestions for revising the manuscript and gave final approval. Emma Meyers provided suggestions for revising the manuscript and gave final approval. Angela Velazquez provided suggestions for revising the manuscript and gave final approval. J. Michael Schmidt provided suggestions for revising the manuscript and gave final approval. Sureerat Suwatcharangkoon provided suggestions for revising the manuscript and gave final approval. Sachin Agarwal provided suggestions for revising the manuscript and gave final approval. Soojin Park provided suggestions for revising the manuscript and gave final approval. M. Cristina Falo provided suggestions for revising the manuscript and gave final approval. E. Sander Connolly provided suggestions for revising the manuscript and gave final approval. Jan Claassen drafted, revised, and gave final approval for the manuscript.
STUDY FUNDING
Dr. Witsch's work is supported by the German Research Council (DFG, research scholarship Wi 4300/1-1).
DISCLOSURE
J. Witsch, E. Bruce, E. Meyers, and A. Velazquez report no disclosures relevant to the manuscript. J. Schmidt is a consultant of Orsan Medical Technologies. S. Suwatcharangkoon, S. Agarwal, S. Park, and M. Falo report no disclosures relevant to the manuscript. E. Connolly serves as a Section Editor for Neurosurgery and on the editorial boards of the Journal of Neurosurgery and World Neurosurgery; holds patent(s) re: Role of P-selectin and Complement in Stroke; and receives publishing royalties for Handbook of Neurosurgery (Thieme, 2010). J. Claassen received honoraria from serving on the Advisory Board of Actelion for study development. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 2014;71:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer SA, Davis SM, Skolnick BE, et al. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke 2009;40:833–840. [DOI] [PubMed] [Google Scholar]
- 4.Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology 2013;80:1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemphill JC, III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 7.Appelboom G, Piazza MA, Hwang BY, et al. Severity of intraventricular extension correlates with level of admission glucose after intracerebral hemorrhage. Stroke 2011;42:1883–1888. [DOI] [PubMed] [Google Scholar]
- 8.Huttner HB, Kohrmann M, Tognoni E, et al. Clinical severity predicts time to hospital admission in patients with spontaneous intracerebral hemorrhage. Cerebrovasc Dis 2008;25:533–538. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Kirmani JF, Sayed MA, et al. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology 2005;64:2115–2120. [DOI] [PubMed] [Google Scholar]
- 10.Delcourt C, Huang Y, Arima H, et al. Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology 2012;79:314–319. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 2007;68:889–894. [DOI] [PubMed] [Google Scholar]
- 12.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 13.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 2011;42:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomalla G, Fiebach JB, Østergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP). Int J Stroke 2014;9:829–836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


