Abstract
Objective:
We sought to explore whether patients with migraine show heightened interictal intrinsic connectivity within primary sensory networks, the salience network, and a network anchored by the dorsal pons, a region known to be active during migraine attacks.
Methods:
Using task-free fMRI and a region-of-interest analysis, we compared intrinsic connectivity patterns in 15 migraineurs without aura to 15 age- and sex-matched healthy controls, focusing on networks anchored by the calcarine cortex, Heschl gyrus, right anterior insula, and dorsal pons, a region active during migraine attacks. We also examined the relationship between network connectivity, migraine frequency, and sensory sensitivity symptoms.
Results:
Migraineurs showed increased connectivity between primary visual and auditory cortices and the right dorsal anterior insula, between the dorsal pons and the bilateral anterior insulae, and between the right and left ventral anterior insulae. Increased connectivity showed no clinical correlation with migraine frequency or sensory sensitivity.
Conclusions:
Patients with migraine display interictal changes in the topology of intrinsic connections, with greater connectivity between primary sensory cortices, the pons, and the anterior insula, a region involved in representing and coordinating responses to emotional salience.
Migraine is a common, disabling primary headache disorder whose pathophysiology is incompletely understood.1 Migraine attacks often feature an alteration in sensory processing such that normally well-tolerated stimuli become unpleasant, manifesting most frequently as photophobia and phonophobia. These sensory changes may persist between attacks, as migraineurs have lower interictal thresholds for light- and sound-induced discomfort than controls.2,3
Task-free fMRI identifies brain regional ensembles, termed intrinsic connectivity networks (ICNs),4 that exhibit correlated low-frequency blood oxygen level–dependent signal fluctuations. Previous task-free fMRI studies using independent component analysis revealed altered connectivity of the “executive,” “default mode,” and “salience” ICNs in migraineurs, although the direction of changes differed between studies.5,6 Other studies used region-of-interest (ROI) analysis, focusing on pain-related regions such as the periaqueductal gray or amygdala and found increased connectivity in these networks.7–9 Still others have used differences identified with voxel-based morphometry to seed ROI-based connectivity studies.10–13 This approach has identified differences between females and males in cortical thickness and connectivity of the posterior insula and precuneus.12
We studied interictal migraineurs with task-free fMRI to evaluate ICNs relevant to primary sensory processing, nociception, and salience. Given the increased salience of sensory stimuli in migraineurs, we hypothesized that migraineurs would have increased connectivity between the salience network, thought to be involved in identifying homeostatically relevant internal and external stimuli,4 and primary visual and auditory networks, as well as with a region in the dorsal pons previously shown to activate ipsilaterally to pain during spontaneous and triggered migraine attacks.14–17
METHODS
Subjects.
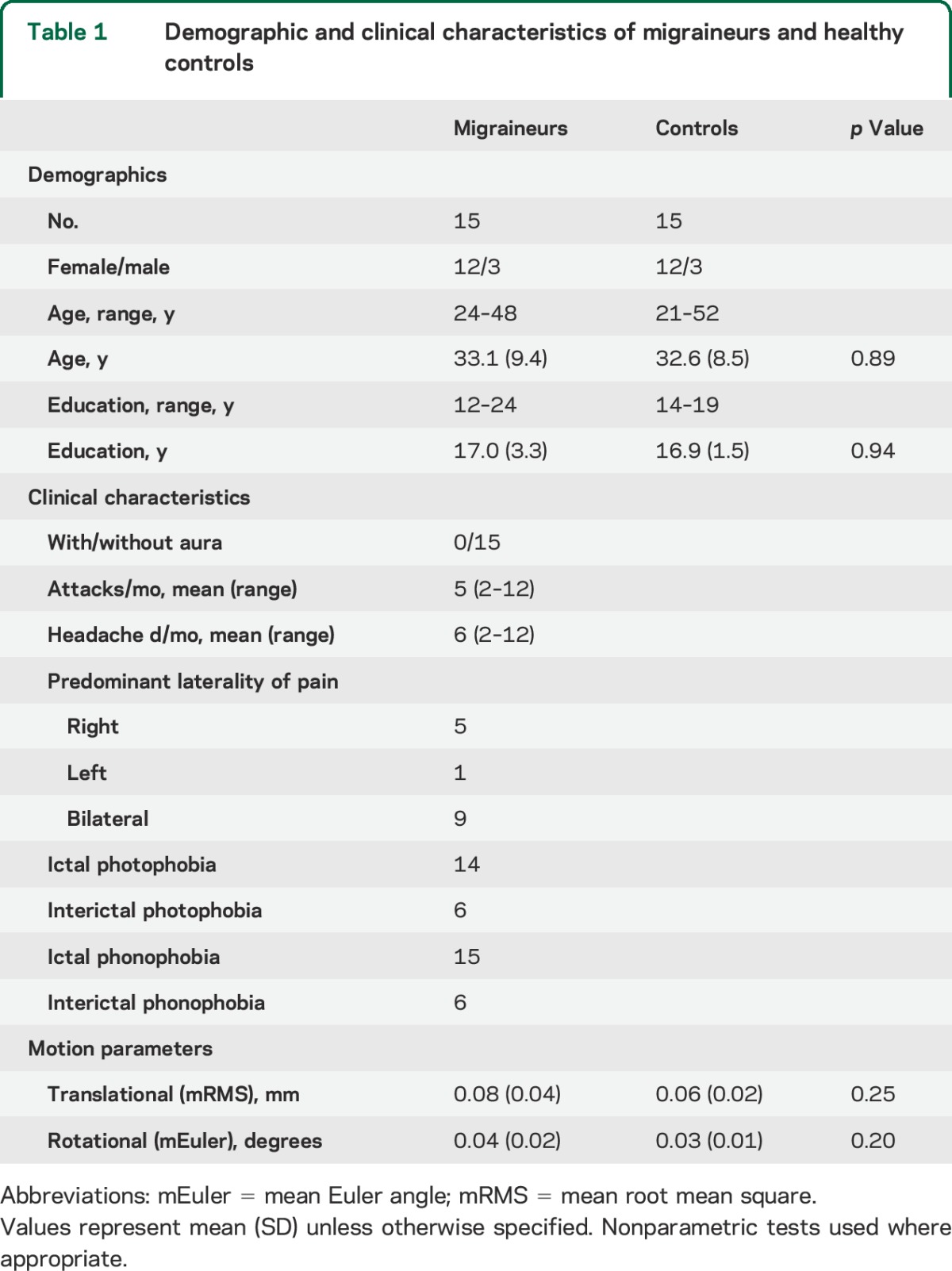
Fifteen subjects between the ages of 21 and 52 years who met International Headache Society criteria for episodic migraine without aura18 and 15 age- and sex-matched healthy controls were included in the study. Control subjects were carefully screened for a personal or family history of migraine or regular or frequent headache. All subjects were right-handed, none had chronic neurologic or psychiatric conditions, and none took daily medications other than vitamins or oral contraceptives. No subjects used analgesics for any reason more than 8 days per month. All subjects were recruited from the community through advertisements and had normal general and neurologic examinations. Migraine subjects were imaged interictally and not within 72 hours before or after an attack. No subject was taking a migraine preventive medication.
Standard protocol approvals, registrations, and patient consents.
Subjects gave written informed consent before participation, and all study procedures were approved by the University of California, San Francisco (UCSF) Committee on Human Research.
Image acquisition.
Subjects were imaged at the UCSF Neuroscience Imaging Center with a Siemens 3T Tim Trio scanner using a 12-channel head coil. Subjects were instructed to lie still with their eyes closed without falling asleep during acquisition of the task-free functional images using a T2*-weighted echo planar imaging sequence. For coregistration, a high-resolution, T1-weighted, volumetric scan was obtained during the same session using a magnetization-prepared rapid acquisition gradient-echo sequence. See e-Methods on the Neurology® Web site at Neurology.org for full image acquisition details.
Image processing.
Functional data were processed and analyzed using SPM8 (Statistical Parametric Mapping; Wellcome Trust Centre for Neuroimaging, London, UK). Functional images were slice time–corrected, spatially realigned, unwarped, coregistered to each subject's own structural T1-weighted image, normalized to the Montreal Neurological Institute (MNI) T1 template space, smoothed with a 4-mm gaussian kernel, and filtered with a bandpass filter for frequencies less than 0.0083 Hz and greater than 0.15 Hz. The functional and structural imaging data for one migraineur with left-sided headaches was flipped along the y-axis, before processing, to correspond with the other migraineurs who experienced either right-sided or bilateral headaches since dorsal pontine activation during migraine attacks is known to be lateralized to the side of pain.17
Subject head motion was assessed using methods detailed in the e-Methods.
fMRI data analysis.
An ROI-based analysis was used, following our previous approaches.19 Based on our a priori hypotheses, seeds were placed in the left calcarine cortex, right Heschl gyrus, right dorsal pons, and right anterior insula, a major node in the salience network.4 Spherical seeds were created with SPM8 by specifying MNI coordinates for the center, which were obtained from the literature as the site of peak activation during relevant tasks7,17,20,21; see e-Methods for details. All cortical ROI seeds had a 4-mm radius. The pontine ROI had a radius of 3 mm to avoid including multiple regions in the pons, which features a denser juxtaposition of functional areas than the chosen cortical areas.
For each ICN analysis, SPM8 was used to calculate the average blood oxygen level–dependent signal intensity of all the voxels in the ROI at each time point over each subject's 8-minute resting-state scan. The resulting time series was used as a covariate of interest in a whole-brain statistical parametric regression analysis, which included regression of nuisance covariates (time series from white matter and CSF, as well as motion parameters), to generate an image representing the ROI's intrinsic functional correlation map for each subject. The global signal was not included as a nuisance covariate based on recent work showing that ICN test-retest reliability is negatively affected by global signal regression and not affected by motion parameters.22 Search volume was limited with an inclusive gray matter mask, and the resulting images from each subject were then entered into a second-level, random-effects analysis to perform 2-sample t tests comparing the connectivity of each network between migraineurs and controls. Results were thresholded using joint probability distribution thresholding23 with joint height and extent thresholds of p < 0.01 and p < 0.05 (for visualization purposes), corrected across the search volume, in keeping with our previous work.19
Clinical data and symptom-imaging correlation analysis.
Migraineurs completed a survey rating ictal and interictal photophobia and phonophobia on a qualitative scale: not at all (0), somewhat (1), quite a bit (2), or unbearable (3). A composite clinical score for each migraineur was calculated by summing the ratings for ictal or interictal photophobia and phonophobia.
Migraineurs exhibited increased insular connectivity with the dorsal pons and right anterior insula, in distinct regions (see the results section). Spearman correlation was used to examine the relationship between migraine frequency and this heightened insular connectivity with both seeds.
Migraineurs exhibited increased connectivity between the calcarine cortex and Heschl gyrus and the right anterior insula, within an overlapping region (see the results section). Spearman correlation was used to examine the relationship between the composite clinical score (for photophobia and phonophobia) and a composite connectivity score representing the average of the connectivity metric from the 2 seeds within the overlapping insular region. For details, see e-Methods.
RESULTS
Subjects.
Subject demographics and clinical characteristics are shown in table 1. Groups showed no significant differences in key demographics or in translational or rotational movement.
Table 1.
Demographic and clinical characteristics of migraineurs and healthy controls
Intrinsic connectivity.
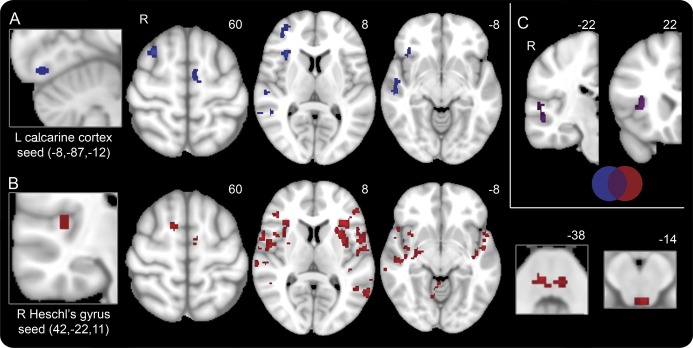
Compared with controls, migraineurs exhibited increased connectivity between the calcarine cortex and Heschl gyrus seeds and the right dorsal anterior insula (dAI, figure 1).
Figure 1. Regions with heightened connectivity to sensory networks in interictal migraineurs compared with healthy controls.
(A) Areas demonstrating increased connectivity with the left calcarine cortex (seed on far left) in migraineurs interictally compared with healthy controls, at a joint height and extent threshold of p = 0.01. Numbers represent Z-slices in Montreal Neurological Institute (MNI) space. (B) Areas demonstrating increased connectivity with the right Heschl's gyrus (seed on far left) in migraineurs interictally compared with healthy controls, at a joint height and extent threshold of p = 0.01. The dorsal pons and periaqueductal gray are shown on the far right. Numbers represent Z-slices in MNI space. (C) Overlap map of areas demonstrating increased connectivity with both the left calcarine cortex (blue) and the right Heschl gyrus (red) in migraineurs interictally compared with healthy controls, at a joint height and extent threshold of p = 0.01. Overlapping areas (purple) are the right dorsal anterior insula and the right middle and superior temporal gyri. Numbers represent Y-slices in MNI space.
In migraineurs, the calcarine cortex also showed greater connectivity with the right frontal operculum, right middle frontal gyrus, superior frontal sulcus (frontal eye field), right superior and middle temporal gyri, left supplementary motor area, and right cerebellum (table 2). Migraineurs further showed increased connectivity between Heschl gyrus and multiple scattered regions including the left anterior insula, the bilateral frontal opercula and temporal lobes, bilateral dorsal pons, periaqueductal gray, right supplementary motor area, left precentral gyrus, as well as the bilateral cerebellum (table 2). No regions showed reduced connectivity to the calcarine cortex or Heschl gyrus in migraineurs.
Table 2.
Peak locations, cluster size, and significance of brain areas with increased connectivity to different regions of interest in migraineurs compared with healthy controls
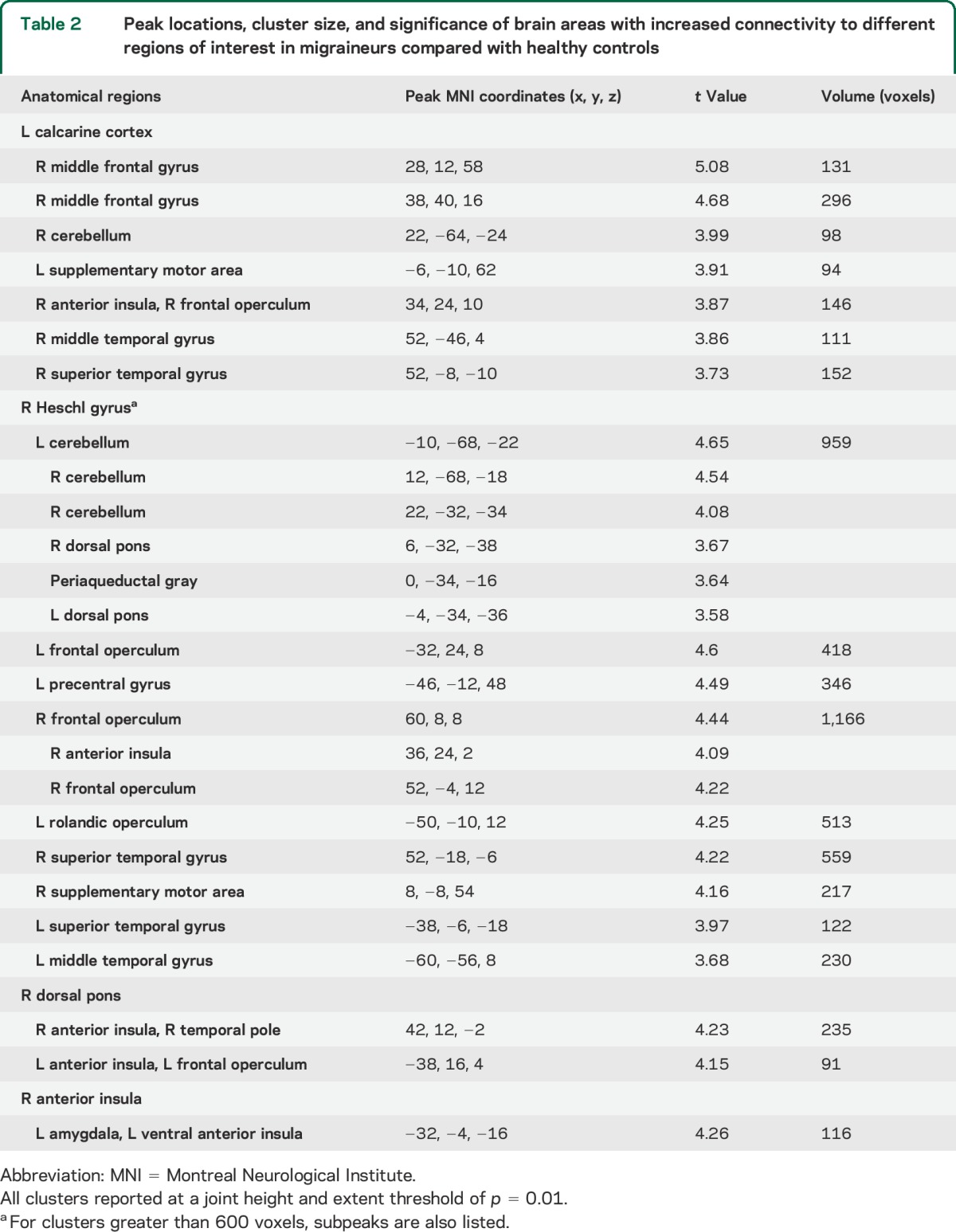
In migraineurs, the dorsal pons showed increased connectivity with the bilateral ventral anterior insula (also referred to as frontoinsular cortex) (figure 2A), a region posterior and ventral to the region demonstrating increased connectivity with the visual and auditory ROIs in migraineurs. In migraineurs, the dorsal pons also had increased connectivity with the right temporal pole, bilateral dAI, and frontal opercula. No regions showed reduced connectivity to the dorsal pons in migraineurs.
Figure 2. Regions with heightened connectivity to the dorsal pons and anterior insula in interictal migraineurs.
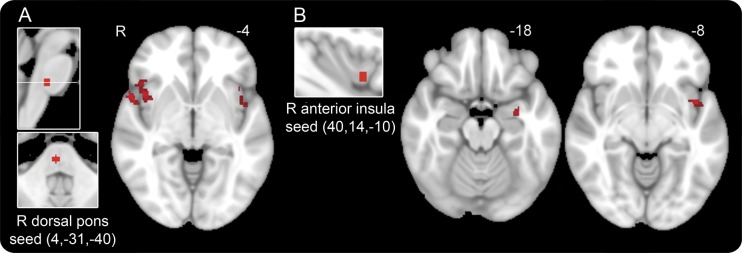
(A) Areas demonstrating increased connectivity with the right dorsal pons (seed on far left), a region that activates during migraine attacks, in interictal migraineurs compared with healthy controls, at a joint height and extent threshold of p = 0.01. Bilateral anterior insula, right temporal pole, and right frontal operculum are shown. Number represents Z-slices in Montreal Neurological Institute (MNI) space. (B) Areas demonstrating increased connectivity with the right ventral anterior insula (seed on left) in interictal migraineurs compared with healthy controls, at a joint height and extent threshold of p = 0.01. Left temporal lobe, amygdala, and midinsula are shown. Numbers represent Z-slices in MNI space.
Seeding the right ventral anterior insula revealed increased connectivity with the left ventral midinsula in migraineurs (figure 2B), as well as with the left temporal lobe and amygdala. There were no regions that showed reduced connectivity with the right anterior insula in migraineurs.
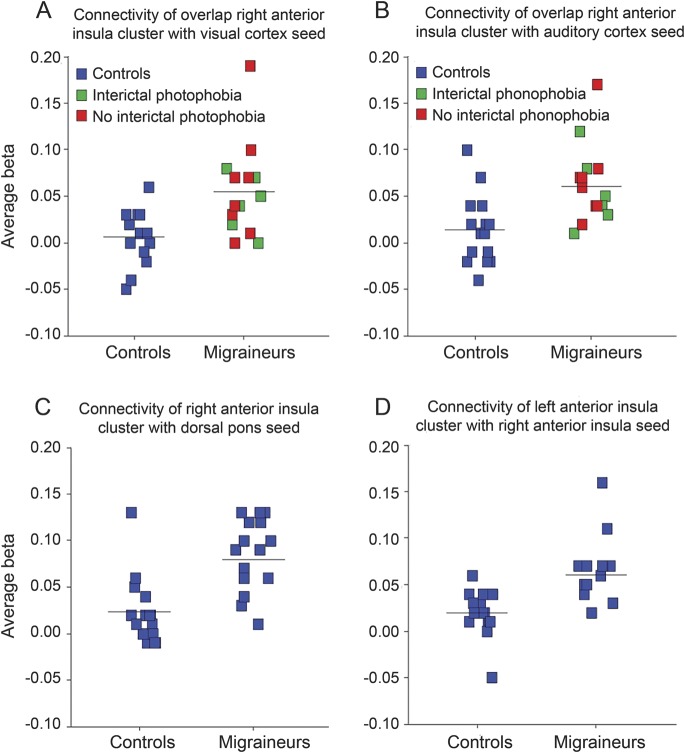
All seeds demonstrated increased connectivity with the insula in migraineurs (figures 1 and 2). Subject-specific scatterplots for mean connectivity within the insular cluster are shown for each seed in figure 3. All findings remained significant after removal of the visualized outliers.
Figure 3. Subject-specific scatterplots of connectivity metrics within anterior insular clusters.
Each square represents the mean β value within the insular cluster of interest for one subject; horizontal line indicates group mean. Subject-specific scatterplots are shown for connectivity within the overlap right dorsal anterior insula cluster (figure 1C, z = 22) from the (A) calcarine cortex–seeded and (B) auditory cortex–seeded maps, (C) within the right anterior insula cluster from the dorsal pons–seeded map (figure 2A), and (D) within the left anterior insula cluster from the right anterior insula–seeded map (figure 2B, z = −8). In A and B, migraineurs are further color-coded according to whether they reported interictal photophobia or phonophobia, respectively.
Clinical correlations.
Migraine frequency (attacks or days per month) showed no significant correlation with mean left insula-to-right insula connectivity or with mean right or left insula-to-dorsal pons connectivity. There was no correlation between the composite clinical score of light and sound sensitivity, ictal or interictal, and the composite right anterior insula-to-visual/auditory ROI connectivity score.
DISCUSSION
Our data reveal that migraineurs have greater interictal connectivity between primary visual and auditory cortices and the right dAI, a region pivotally involved in coordinating responses to nociceptive and other salient internal and external stimuli.4,24,25 The dorsal pons, a region active during migraine attacks, showed increased connectivity with the bilateral anterior insula in migraineurs. Finally, the right ventral anterior insula showed increased connectivity with the left ventral anterior insula in migraineurs. Taken together, these findings suggest that interictal migraineurs have heightened connectivity between modality-selective sensory cortices and regions representing and coordinating responses to emotionally salient stimuli. This finding may yield insights into the disorder and provide a basis for developing novel imaging biomarkers for gauging the impact of therapeutics.
The insula participates in a wide variety of functions24–26 and features known anatomical connectivity to nociceptive and visceral afferent streams, limbic structures, and prefrontal cortex.24,27 The insula is also thought to have an important role in integrating information from these diverse systems.24,28,29 Task-based functional imaging studies and meta-analyses have helped delineate the insula's functional topography.29 Sensory/discriminative aspects of pain activate the dorsal posterior insula, whereas the affective-motivational components activate more anterior insular regions.30 The anterior insula contains 2 specialized nodes within a system whose goal is to represent homeostatic salience and mobilize autonomic, cognitive, and motoric responses that address the identified salient stimulus.4 Whereas the ventral anterior insula has been shown to represent social-emotional-autonomic information,29,31 perhaps as continuous interoceptive “feelings,”24 the dAI may organize and sustain cognitive processing that results from salience.32 Heightened intrinsic connectivity between the dAI and the visual and auditory cortices in migraineurs may thereby set the stage for abnormally intensified responses to sensory information. Increased dAI-to-sensory cortex connectivity could provide a neuroanatomical framework for understanding why visual and auditory stimuli not noxious to controls can become unpleasant to migraineurs and evoke escape responses. In our patient group, nearly all migraineurs reported both photophobia and phonophobia during attacks (see table 1). However, since imaging was performed at least 3 days outside of an attack, the difference in network connectivity that may underlie ictal sensory sensitivity appears to persist interictally, and at a subclinical level, considering that only 6 patients reported interictal photophobia and 6 reported interictal phonophobia.
Multiple studies have shown activation of the dorsal pons during spontaneous and triggered migraine attacks.14–17 This region, hypothesized to correspond to either the locus coeruleus or pontine tegmental neurons close to it,14,16 showed increased connectivity in migraineurs with the ventral anterior insula, an area that activates during interoception and pain and most likely has a general role in representing emotional salience across all stimulus domains.4 This heightened intrinsic functional connection could predispose to migraine attacks or could represent an epiphenomenon of the migrainous state.
Migraineurs exhibited increased connectivity between Heschl gyrus and the dorsal pons near the area active during migraine attacks. Previous work has shown dorsal pontine activation in response to olfactory stimulation during but not between attacks.33 These findings could represent a neuroanatomical basis for the clinical observation that sensory stimuli may exacerbate a migraine attack. The calcarine cortex, however, did not demonstrate increased connectivity with the dorsal pons, and replication of these findings is needed before their significance can be understood.
Previous work has shown increased connectivity in migraineurs between the periaqueductal gray and right anterior insula,7 centered on the ventral anterior insula subregion that is activated by social-emotional-autonomic stimuli. In the present study, this region showed increased connectivity with the left anterior insula in migraineurs. Trait anxiety has been correlated with greater activation of the bilateral anterior insula.34 Migraine is associated with increased risk of anxiety disorders, with an odds ratio between 2.3 and 3.2,35,36 and increased interinsular connectivity could represent a neural trait predisposition to both migraine and anxiety or a state-related consequence of having one or both of these disorders.
Our study was limited by a relatively small sample size, which could have diminished our sensitivity to detect subtle effects within the migraine group, such as symptom-connectivity correlations. Indeed, we found no correlation between a score representing the severity of photophobia and phonophobia, either during or between attacks, and the magnitude of correlation between the visual and auditory networks and the right anterior insula. This negative finding could reflect the lack of variance in the clinical scores because a semiquantitative measure was used; all but one patient reported both ictal photophobia and phonophobia (see table 1). This clinical homogeneity resulted from our use of International Headache Society criteria for episodic migraine, which requires both photophobia and phonophobia or nausea. Larger studies including migraineurs without sensory sensitivity and using continuous measures of sensory sensitivity may reveal novel symptom-connectivity relationships. Future work in other primary headache disorders, such as cluster headache with photophobia, could clarify whether this heightened connectivity is a marker of migrainous biology or simply reflects the presence of sensory sensitivity. Finally, studies examining migraineurs with osmophobia or allodynia would help explore whether heightened connectivity between this anterior insular region and primary sensory cortices might underlie all sensory sensitivities, regardless of modality.
We also detected no correlation between the frequency of migraines and the magnitude of correlation between the dorsal pons and the insulae or between the right and left insulae. Although it is possible that these connections reflect the disease state itself and are therefore not related to disease severity, it is also possible that future larger studies will reveal important symptom-connectivity relationships. Longitudinal studies investigating whether successful treatment with preventive medications is reflected in normalization of this aberrant connectivity could provide additional insight into how these connectivity findings are related to the clinical state. Studies examining migraineurs whose attacks have ceased, as often occurs later in life, could help clarify whether these alterations in connectivity reflect a migraine-related trait or state.
The major strength and novelty of this study is that it interrogated hypotheses derived from clinical features that are distinctive of migraine, rather than more general hypotheses relating to pain processing. Migraine is a complex disorder that is broadly characterized by abnormalities in sensory processing, including nociceptive processing. Our findings support the idea that migraineurs are characterized by heightened and anomalous interictal regional connectivity between networks involved in processing upstream sensory information and those that represent the salience of such stimuli, a finding that provides a new neuroanatomical framework for understanding key clinical features of migraine.
Supplementary Material
GLOSSARY
- dAI
dorsal anterior insula
- ICN
intrinsic connectivity network
- MNI
Montreal Neurological Institute
- ROI
region of interest
- UCSF
University of California, San Francisco
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Amy R. Tso: study concept and design, data acquisition, analysis, and interpretation, drafting and revision of manuscript. Andrew Trujillo: data analysis and interpretation, manuscript revision. Christine C. Guo: data analysis and interpretation. Peter J. Goadsby: study concept and design, revision of manuscript. William W. Seeley: study concept and design, data analysis and interpretation, revision of manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A. Tso, A. Trujillo, and C. Guo report no disclosures relevant to the manuscript. P. Goadsby reports grants and personal fees from Allergan, grants and personal fees from eNeura, personal fees from Autonomic Technologies Inc., grants and personal fees from Amgen, personal fees from Bristol-Myers Squibb, personal fees from AlderBio, personal fees from Pfizer, personal fees from Zogenix, personal fees from Nevro Corp., personal fees from Impax, personal fees from DrReddy, personal fees from Zosano, personal fees from CoLucid, personal fees from Eli Lilly, personal fees from Medtronic, personal fees from Avanir, personal fees from Gore, personal fees from Ethicon, personal fees from Heptares, personal fees from Nupathe, personal fees from Ajinomoto, outside the submitted work. W. Seeley reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med 2002;346:257–270. [DOI] [PubMed] [Google Scholar]
- 2.Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia 1997;17:733–741. [DOI] [PubMed] [Google Scholar]
- 3.Vingen JV, Pareja JA, Storen O, White LR, Stovner LJ. Phonophobia in migraine. Cephalalgia 1998;18:243–249. [DOI] [PubMed] [Google Scholar]
- 4.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo A, Tessitore A, Giordano A, et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia 2012;32:1041–1048. [DOI] [PubMed] [Google Scholar]
- 6.Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One 2012;7:e52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011;70:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjikhani N, Ward N, Boshyan J, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia 2013;33:1264–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwedt TJ, Schlaggar BL, Mar S, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 2013;53:737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed 2013;26:58–64. [DOI] [PubMed] [Google Scholar]
- 11.Yuan K, Zhao L, Cheng P, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 2013;14:836–844. [DOI] [PubMed] [Google Scholar]
- 12.Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain 2012;135:2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012;32:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med 1995;1:658–660. [DOI] [PubMed] [Google Scholar]
- 15.Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–1017. [DOI] [PubMed] [Google Scholar]
- 16.Afridi SK, Giffin NJ, Kaube H, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol 2005;62:1270–1275. [DOI] [PubMed] [Google Scholar]
- 17.Afridi SK, Matharu MS, Lee L, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005;128:932–939. [DOI] [PubMed] [Google Scholar]
- 18.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 19.Gardner RC, Boxer AL, Trujillo A, et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol 2013;73:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alain C, Arnott SR, Hevenor S, Graham S, Grady CL. “What” and “where” in the human auditory system. Proc Natl Acad Sci USA 2001;98:12301–12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drobyshevsky A, Baumann SB, Schneider W. A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage 2006;31:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo CC, Kurth F, Zhou J, et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 2012;61:1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 1997;5:83–96. [DOI] [PubMed] [Google Scholar]
- 24.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–666. [DOI] [PubMed] [Google Scholar]
- 25.Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct 2010;214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 2003;40:655–664. [DOI] [PubMed] [Google Scholar]
- 27.Mesulam MM, Mufson EJ. Insula of the old world monkey: III: efferent cortical output and comments on function. J Comp Neurol 1982;212:38–52. [DOI] [PubMed] [Google Scholar]
- 28.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 29.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 2010;214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science 2004;303:1157–1162. [DOI] [PubMed] [Google Scholar]
- 31.Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neurosci Lett 2009;457:66–70. [DOI] [PubMed] [Google Scholar]
- 32.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007;104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 2011;77:476–482. [DOI] [PubMed] [Google Scholar]
- 34.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164:318–327. [DOI] [PubMed] [Google Scholar]
- 35.Breslau N, Davis GC, Andreski P. Migraine, psychiatric disorders, and suicide attempts: an epidemiologic study of young adults. Psychiatry Res 1991;37:11–23. [DOI] [PubMed] [Google Scholar]
- 36.Zwart JA, Dyb G, Hagen K, et al. Depression and anxiety disorders associated with headache frequency. The Nord-Trøndelag Health Study. Eur J Neurol 2003;10:147–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.