Abstract
Objective
An inflammatory response after cardiac surgery is associated with worse clinical outcomes, but recent trials to attenuate it have been neutral. We evaluated the association between systemic inflammatory response syndrome (SIRS) and mortality after transcatheter (TAVR) and surgical aortic valve replacement (SAVR) for aortic stenosis (AS) and evaluated whether diabetes influenced this relationship.
Methods
Patients (n=747) with severe AS treated with TAVR (n=264) or SAVR (n=483) between 1/2008 and 12/2013 were included and 37% had diabetes mellitus. SIRS was defined by 4 criteria 12 to 48 hours after AVR: 1) white blood cell count <4 or >12; 2) heart rate >90; 3) temperature <36 or >38°C; or 4) respiratory rate >20. Severe SIRS was defined as meeting all 4 criteria. The primary endpoint was 6-month all-cause mortality (60 deaths occurred by 6 months). Inverse propensity weighting (IPW) was performed on 44 baseline and procedural variables to minimize confounding.
Results
Severe SIRS developed in 6% of TAVR patients and 11% of SAVR patients (p=0.02). Six-month mortality tended to be higher in those with severe SIRS (15.5%) versus those without (7.4%) (p=0.07). After adjustment, severe SIRS was associated with higher 6-month mortality (IPW adjusted HR 2.77, 95% CI 2.04–3.76, p<0.001). Moreover, severe SIRS was more strongly associated with increased mortality in diabetic (IPW adjusted HR 4.12, 95% CI 2.69–6.31, p<0.001) than non-diabetic patients (IPW adjusted HR 1.74, 95% CI 1.10–2.73, p=0.02) (interaction p=0.007). The adverse effect of severe SIRS on mortality was similar after TAVR and SAVR.
Conclusion
Severe SIRS was associated with a higher mortality after SAVR or TAVR. It occurred more commonly after SAVR and had a greater effect on mortality in diabetic patients. These findings may have implications for treatment decisions in patients with AS, may help explain differences in outcomes between different AVR approaches, and identify diabetic patients as a high risk sub-group to target in clinical trials with therapies to attenuate SIRS.
Keywords: aortic valve stenosis, aortic valve replacement, inflammation, diabetes mellitus, outcomes
INTRODUCTION
Cardiac surgery can stimulate a systemic inflammatory response that has deleterious consequences.[1–3] This is the rationale for clinical trials to attenuate the inflammatory response.[4 5] The Dexamethasone for Cardiac Surgery (DECS) trial failed to meet its primary endpoint, but steroids improved some secondary endpoints.[4] In the recent Steroids in Cardiac Surgery (SIRS) trial, approximately 7,500 patients were administered intravenous methylprednisolone or placebo during any surgery that required cardiopulmonary bypass.[5] A clinical benefit was not observed in the SIRS trial in terms of reduced mortality and morbidity; instead, there was evidence for an increased risk of myocardial infarction in patients receiving intravenous steroids.[6] Whether this is due to the wrong anti-inflammatory strategy or the failure to identify a sub-group of patients who may benefit is unclear.
The incidence and effect of systemic inflammatory response syndrome (SIRS) on mortality after surgical aortic valve replacement (SAVR) for aortic stenosis (AS) has not been studied. One recent report demonstrated that the development of SIRS after transcatheter AVR (TAVR) adversely affects short and long-term survival.[7] Related to this, diabetes mellitus is a pro-inflammatory state that may influence the development, severity, or effect of SIRS after AVR.[8] Diabetes is known to adversely affect outcomes after TAVR and SAVR.[9 10] A recent post-hoc analysis of the PARTNER trial, however, suggested that high-risk patients with AS and diabetes may do better when treated with TAVR compared to SAVR.[11] We hypothesized that these findings may be explained, in part, by: 1) a higher incidence of SIRS after SAVR than TAVR; and 2) the combination of SIRS and diabetes leads to worse clinical outcomes. Accordingly, we examined the incidence of SIRS after SAVR and TAVR in patients with AS and evaluated whether its effect on mortality was influenced by the presence of diabetes.
METHODS
Patient population
We retrospectively included all patients ≥40 years of age with severe AS (indexed aortic valve area ≤0.6 cm2/m2 or transvalvular mean gradient >40 mmHg or peak velocity >4 m/sec) treated with isolated SAVR or TAVR between January 2008 and December 2013 at Barnes Jewish Hospital in St. Louis, Missouri. All TAVR procedures were performed with a balloon expandable Edwards SAPIEN valve under general anesthesia. We excluded patients who had a concomitant surgical procedure (eg. coronary bypass, mitral valve repair), endocarditis, or a valve-in-valve TAVR and also excluded patients who died during their procedure as our objective was to evaluate the association between SIRS (that developed after the procedure) and mortality. The study complied with the Declaration of Helsinki and was approved by the local institutional review board from which a waiver of written informed consent was obtained given the retrospective design of the study.
Clinical data
Clinical variables were obtained by chart abstraction. The publicly available Society of Thoracic Surgeons (STS) website (www.riskcalc.sts.org) provided definitions for the clinical variables and allowed for the calculation of the STS risk score for each patient. The STS defines diabetes according to criteria from the American Diabetes Association.[12] The clinical characteristics, medication usage, laboratory values, and procedural and post-procedural data and events are routinely obtained and entered at or near the time of the procedure into our institution’s database and submitted to the national STS database. The SIRS criteria were defined according to existing guidelines: 1) white blood cell (WBC) count <4 or >12 (109/L); 2) heart rate (HR) >90 beats per minute; 3) temperature <36 or >38°C; or 4) respiratory rate >20 per minute.[13 14] To minimize spurious findings, we did not evaluate for the occurrence of these criteria during the first 12 post-operative hours given the numerous medication and ventilation changes made in the immediate post-operative setting; we evaluated between 12 and 48 hours after the patient left the operating room. Also, to avoid the influence of a potentially misleading, isolated vital sign, we considered the HR and respiratory rate criteria met only if there were two or more recordings above the designated threshold. While the standard definition of SIRS is the occurrence of two or more of these criteria,[13] given the frequency of an inflammatory response after cardiac surgery we hypothesized that a more severe SIRS phenotype (characterized by more criteria being met and indicative of a more marked systemic inflammatory response) might identify a higher risk group of patients in this clinical scenario.
Statistical analysis
The incidence of individual SIRS criteria (WBC, HR, temperature, and respiratory rate) and definitions based on number of criteria met (≥2, ≥3, or 4) were compared between AVR treatment groups using Fisher’s exact test. Those who met 4 SIRS criteria were considered to have “severe SIRS.” Comparisons of baseline clinical, procedural, and post-procedural characteristics between those with and without severe SIRS were conducted with Student’s two sample t-test or Fisher’s exact test for continuous and categorical data, respectively. For non-normal and ordinal data, median (1st, 3rd quartiles) were provided as summary statistics and the between-group comparison was conducted using the Kruskal-Wallis test.
Outcomes were evaluated using inverse probability weighting (IPW) methods to achieve balance between groups in order to adjust for factors known or hypothesized to be associated with mortality in this patient population. This method allows for adjustment of many more variables than are commonly included in multivariable models to more adequately adjust for potential confounders without developing adjusted models that are overfit.[15 16] Using logistic regression, propensity for having severe SIRS was determined using 44 baseline and procedural variables (Online supplement). Propensity for having the individual SIRS criteria was evaluated similarly. Standardized differences were calculated to determine covariate balance before and after IPW. Difference in mortality between those with versus without severe SIRS (or meeting versus not meeting the individual criteria) was evaluated by the hazard ratio produced from a Cox proportional hazards model and using robust standard errors as appropriate for IPW analysis. Surgery date was used as the start time and follow-up was completed through February 7, 2014. Our primary endpoint was 180-day mortality. This time point was chosen as relatively few deaths occur by 30 days and the effect of SIRS on 1-year mortality is likely to be diluted by other co-morbidities. A separate model was built to examine the association between SIRS and mortality according to AVR type (TAVR versus SAVR). The difference between AVR treatment groups was evaluated by the interaction between SIRS and AVR type. A similar model was built to evaluate patients with and without diabetes.
Secondary outcomes were also evaluated and included initial ICU hours, ICU readmission, and length of hospital stay. Initial ICU hours and length of hospital stay were log-transformed and evaluated through linear regression. Beta coefficients represent the difference between SIRS groups of the log-transformed variable. Exponentiation of the beta coefficient yields the difference in the geometric mean of ICU hours or length of stay between groups. Difference in ICU readmission (yes versus no) was determined by the odds ratio (OR) produced from a logistic regression model. Linear and logistic regression models were developed using generalized estimating equations to obtain robust standard error estimates. Comparisons for TAVR versus SAVR and diabetic versus non-diabetic were conducted for all secondary outcomes by developing models containing the corresponding SIRS interaction. A two-sided p-value <0.05 was considered significant. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
There were 747 patients included in the analysis, including 483 treated with SAVR and 264 treated with TAVR (transfemoral [n=117], transapical [n=102], and transaortic [n=45]). The mean age was 75 years, 46% were female, and 37% were diabetic (38% of diabetic patients were treated with insulin). The STS score for patients undergoing TAVR (10.5±5.6) was higher than for those undergoing SAVR (4.1±4.0) (p<0.001). Two or more SIRS criteria were met in 84% of the population and this was not associated with 6-month mortality (HR 1.22, 95% CI 0.58–2.56, p=0.61). Likewise, three or more SIRS criteria were met in 45% of the population and this was not associated with 6-month mortality (HR 1.06, 95% CI 0.64–1.77, p=0.81). In contrast, severe SIRS (4 SIRS criteria met) occurred in 9.5% of the population and was associated with a higher mortality at 6 months (HR 2.25, 95% CI 1.17–4.32, p=0.015).
Characteristics of patients with severe SIRS
Compared to those without severe SIRS, those who developed severe SIRS after valve replacement were younger and had a lower prevalence of cerebrovascular disease, higher baseline hemoglobin and white blood cell count, and more frequently were on dialysis (Table 1). There were no differences in sex, BMI, ejection fraction, or glomerular filtration rate, and no differences in the prevalence of coronary disease, diabetes, atrial arrhythmia, immunocompromised state, chronic corticosteroid use, smoking, or lung disease. Those with severe SIRS were more likely to have been treated with SAVR than TAVR, had longer cardiopulmonary bypass times, and more often had an intra-aortic balloon pump inserted pre-operatively or intra-operatively (Table 2). There was no difference in the use of intra-operative blood products.
Table 1.
Baseline Clinical Characteristics
| No Severe SIRS (n=676) |
Severe SIRS* (n=71) |
p-value | |
|---|---|---|---|
| Age (years) | 75 ±12 | 71 ± 12 | 0.014 |
| Female | 46 | 41 | 0.45 |
| BSA (m2) | 1.96±0.29 | 2.01± 0.26 | 0.14 |
| BMI (kg/m2) | 29.5±7.8 | 30.3±6.5 | 0.46 |
| STS score | 4.8 (2.1, 9.4) | 3.9 (1.6, 8.0) | 0.10 |
| Diabetes | 38 | 34 | 0.52 |
| Insulin treated | 15 | 10 | 0.37 |
| Hypertension | 85 | 77 | 0.12 |
| Coronary disease | 58 | 55 | 0.71 |
| Number of diseased vessels (0/1/2/3) | 50/16/9/25 | 55/13/14/18 | 0.34 |
| Prior myocardial infarction | 25 | 24 | 1.00 |
| Prior coronary artery bypass surgery | 28 | 21 | 0.26 |
| Prior percutaneous coronary intervention | 26 | 30 | 0.48 |
| Prior valve surgery | 6 | 4 | 0.79 |
| Prior sternotomy | 32 | 24 | 0.22 |
| NYHA class III/IV | 66 | 69 | 0.69 |
| Cardiogenic shock | 0 | 3 | 0.025 |
| Atrial arrhythmia | 27 | 18 | 0.12 |
| Smoker | 13 | 15 | 0.58 |
| Chronic lung disease (moderate/severe) | 20 | 24 | 0.35 |
| Immunocompromised state | 8 | 6 | 0.50 |
| Peripheral arterial disease | 37 | 32 | 0.52 |
| Cerebrovascular disease | 26 | 13 | 0.014 |
| Prior stroke | 10 | 8 | 0.84 |
| Laboratory values | |||
| Hemoglobin (g/dL) | 12.0±1.7 | 12.6±2.1 | 0.05 |
| Hematocrit (%) | 35.9± 5.1 | 37.7±6.0 | 0.017 |
| Creatinine (mg/dL), median (Q1, Q3) | 1.0 (0.8, 1.2) | 0.9 (0.8, 1.2) | 0.13 |
| Glomerular filtration rate (mL/min/1.73m2) | 70.2±26.6 | 74.5±31.3 | 0.21 |
| White blood cell count (109/L) | 7.1±2.1 | 8.0±2.2 | <0.001 |
| Albumin (g/dL) | 4.1±0.4 | 4.1±0.4 | 0.86 |
| Medications | |||
| Aspirin | 69 | 68 | 0.79 |
| Anti-platelet within 5 days | 3 | 0 | 0.25 |
| B-blockers | 57 | 52 | 0.53 |
| ACE-I or ARB (within 48 hours) | 40 | 35 | 0.45 |
| Lipid lowering | 72 | 69 | 0.68 |
| Statin | 66 | 65 | 0.9 |
| Steroids (chronic, systemic)** | 9 | 7 | 0.82 |
| Inotropes | 1 | 1 | 0.39 |
| Echocardiography | |||
| Ejection fraction (%) | 55±15 | 56±13 | 0.6 |
| Transvalvular mean gradient (mmHg) | 43±14 | 43±14 | 0.95 |
| Moderate or severe AR | 15 | 24 | 0.06 |
| Moderate or severe MR | 15 | 8 | 0.16 |
| Moderate or severe TR | 9 | 1 | 0.035 |
| Pulmonary artery systolic pressure (mmHg) | 46±15 | 46±16 | 0.97 |
Data presented as %, mean±SD, or median (25th, 75th percentile).
Severe SIRS is defined by meeting 4/4 SIRS criteria between 12 and 48 hours after valve replacement.
Excludes any one-time administration of corticosteroids peri-operatively.
Data shown as percentages, mean±SD, or median (1st, 3rd quartile). Groups were compared by Student’s two sample t-test or Fisher’s exact test, for continuous and categorical variables, respectively. Non-normal and ordinal variables were compared using the Kruskal-Wallis test.
Table 2.
Procedural and Post-Procedural Characteristics
| No Severe SIRS (n=676) |
Severe SIRS* (n=71) |
p-value | |
|---|---|---|---|
| Procedural | |||
| Type of AVR | 0.019 | ||
| Surgical, # (%) | 428 (63) | 55 (77) | |
| Transcatheter, # (%) | 248 (37) | 16 (23) | |
| Transcatheter approach | 0.38 | ||
| Transfemoral, # (%) | 112 (45) | 5 (31) | |
| Transapical, # (%) | 93 (38) | 9 (56) | |
| Transaortic, # (%) | 43 (17) | 2 (13) | |
| Sternotomy performed (full or partial) | 70 | 80 | 0.07 |
| Cardiopulmonary bypass utilized | 64 | 80 | 0.014 |
| Cardioplegia used | 63 | 77 | 0.019 |
| Cardiopulmonary bypass time (minutes) | 107±33 | 117±38 | 0.036 |
| Aortic crossclamp time (minutes) | 73±20 | 76±22 | 0.4 |
| Intra-op blood products (any) used | 61 | 68 | 0.31 |
| Red blood cells | 56 | 58 | 0.80 |
| Fresh frozen plasma | 21 | 28 | 0.23 |
| Cryoprecipitate | 9 | 11 | 0.53 |
| Platelets | 32 | 34 | 0.69 |
| Fibrinolytics (intra-op) | 59 | 65 | 0.37 |
| IABP inserted (pre- or intra-op) | 3 | 13 | 0.002 |
| Vascular or access site complications | 4 | 4 | 1.0 |
| Intubation time >24 hours | 4 | 8 | 0.12 |
| Post-procedural | |||
| Stroke within 24 hours of AVR | 1 | 1 | 0.39 |
| Acute kidney injury | 16 | 25 | 0.044 |
| ICU hours | 30.6 (24.0, 72.2) | 46.5 (25.0, 120.2) | 0.010 |
| ICU readmission | 4 | 13 | 0.002 |
| Length of stay (days) | 6.0 (5.0, 9.0) | 8.0 (5.0, 13.0) | 0.004 |
| Death at 30 days | 14 (2%) | 4 (6%) | 0.08 |
Severe SIRS is defined by meeting 4/4 SIRS criteria between 12 and 48 hours after valve replacement.
Data shown as percentages, mean±SD, or median (25th, 75th percentile). Groups were compared by Student’s two sample t-test or Fisher’s exact test, for continuous and categorical variables, respectively. Non-normal and ordinal variables were compared using the Kruskal-Wallis test.
Incidence of SIRS according to AVR procedure
The incidence of each SIRS criteria and increasing combinations of them are shown according to type and route of aortic valve replacement (Table 3). SIRS criteria were more frequently met in those who underwent SAVR compared to TAVR (p<0.05 for all comparisons except temperature). In unadjusted analyses, SAVR was associated with a higher odds of developing severe SIRS (OR 1.99, 95% CI 1.12–3.55, p=0.02). After balancing the SAVR and TAVR groups on 29 baseline characteristics utilizing inverse propensity weighting (Online Supplemental Methods), this relationship was attenuated (IPW adjusted OR 1.89, 95% CI 0.91–3.94, p=0.09). Transfemoral TAVR generally had the lowest incidence of SIRS criteria met in comparison to non-transfemoral TAVR and SAVR. Non-transfemoral TAVR procedures tended to have a somewhat lower incidence of most, but not all, SIRS criteria compared to SAVR. The SIRS criteria for respiratory rate were met in almost all patients regardless of procedural approach.
Table 3.
Incidence of SIRS Criteria in Treatment Sub-Groups
| TAVR (all) (n=264) |
TAVR-TF (n=117) |
TAVR-nonTF (n=147) |
SAVR (n=483) |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| TAVR (all) vs. SAVR |
TAVR- TF vs. TAVR- nonTF |
TAVR- TF vs. SAVR |
TAVR- nonTF vs. SAVR |
|||||
| SIRS criteria | ||||||||
| ≥2 criteria | 194 (73%) | 74 (63%) | 120 (82%) | 435 (90%) | <0.001 | 0.001 | <0.001 | 0.008 |
| ≥3 criteria | 80 (30%) | 18 (15%) | 62 (42%) | 259 (54%) | <0.001 | <0.001 | <0.001 | 0.018 |
| Severe SIRS (4 criteria) | 16 (6%) | 5 (4%) | 11 (7%) | 55 (11%) | 0.019 | 0.31 | 0.024 | 0.22 |
| WBC | 100 (38%) | 21 (18%) | 79 (54%) | 266 (55%) | <0.001 | <0.001 | <0.001 | 0.78 |
| Heart rate | 128 (48%) | 48 (41%) | 80 (54%) | 387 (80%) | <0.001 | 0.035 | <0.001 | <0.001 |
| Temperature | 64 (24%) | 29 (25%) | 35 (24%) | 124 (26%) | 0.72 | 0.89 | 0.91 | 0.75 |
| Respiratory rate | 259 (98%) | 113 (97%) | 146 (99%) | 452 (94%) | 0.007 | 0.17 | 0.27 | 0.004 |
Abbreviations: TAVR, transcatheter aortic valve replacement; TF, transfemoral; SAVR, surgical AVR.
Severe SIRS and mortality
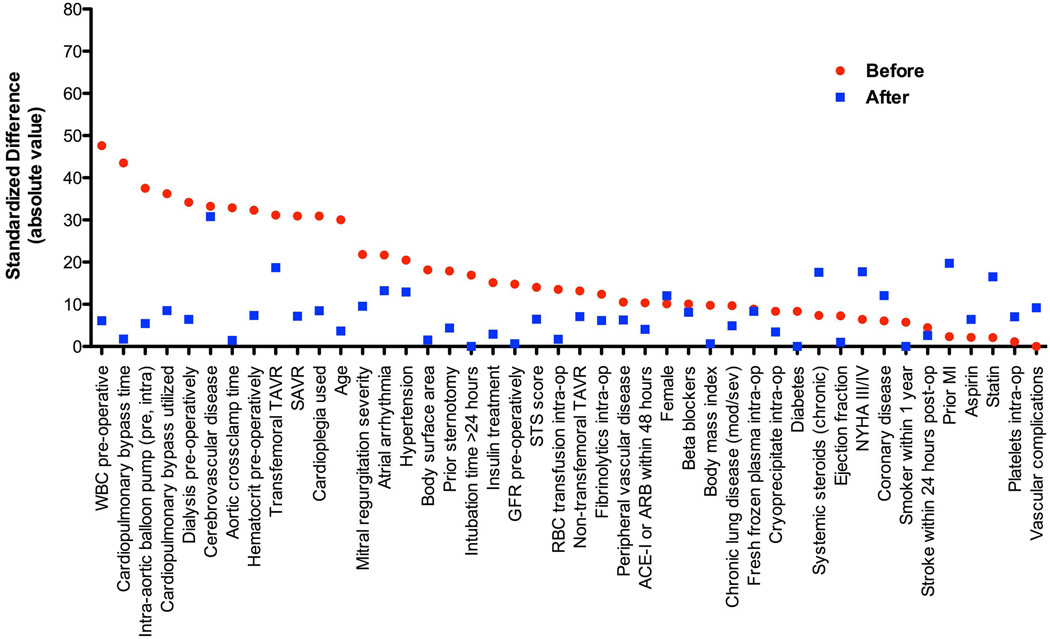
For our primary endpoint of 6-month all-cause mortality, there were 60 deaths in the whole population. There were 18 deaths at 30 days and 88 deaths at 365 days. Six-month mortality tended to be higher in those with severe SIRS (15.5%) versus those without (7.4%) (p=0.07). After inverse propensity weighting to adjust for confounders, the association between severe SIRS and increased 6-month mortality was strengthened (IPW adjusted HR 2.77, 95% CI 2.04–3.76, p<0.001) (Table 4) (Online Figure 1). This association was similar whether patients were treated with TAVR or SAVR (interaction p>0.05). In contrast, severe SIRS had a greater effect on mortality in diabetic (IPW adjusted HR 4.12, 95% CI 2.69–6.31, p<0.001) than non-diabetic patients (IPW adjusted HR 1.74, 95% CI 1.10–2.73, p=0.02) (interaction p=0.007). Similarly, severe SIRS was associated with increased 30-day (IPW adjusted HR 3.86, 95% CI 2.31–6.45, p<0.001) and 1-year mortality (IPW adjusted HR 1.79, 95% CI 1.38–2.33, p<0.001) and more adversely affected diabetic patients at each time point (Table 4). Although most of the 44 baseline and procedural variables were well-balanced between the groups, some were borderline (Figure 1). After further adjusting for AVR type, cerebrovascular disease, NYHA class, prior myocardial infarction, and chronic corticosteroid use in the statistical models, the association between severe SIRS and increased 6-month mortality was strengthened further (adjusted HR 3.90, 95% CI 2.74–5.57, p<0.001) (Online Table 1). As a sensitivity analysis, we evaluated the association between severe SIRS and 6-month mortality in more traditional multivariable models without inverse propensity weighting and our results were similar (Online Table 2).
Table 4.
Impact of SIRS Criteria on Mortality in Propensity Adjusted Analyses
| 6 Month Mortality | 30 Day Mortality | 1 Year Mortality | ||||
|---|---|---|---|---|---|---|
| IPW HR (95% CI) |
p-value | IPW HR (95% CI) |
p-value | IPW HR (95% CI) |
p-value | |
| Severe SIRS* | ||||||
| All AVR | 2.77 (2.04, 3.76) | <.001 | 3.86 (2.31, 6.45) | <.001 | 1.79 (1.38, 2.33) | <.001 |
| TAVR | 3.83 (2.56, 5.73) | <.001 | 7.90 (3.96, 15.78) | <.001 | 2.37 (1.69, 3.32) | <.001 |
| SAVR | 2.14 (1.34, 3.41) | 0.002 | 0.98** (0.40, 2.43) | 0.97 | 1.48 (0.98, 2.25) | 0.06 |
| Diabetics | 4.12 (2.69, 6.31) | <.001 | 6.81 (3.46, 13.43) | <.001 | 3.13 (2.13, 4.60) | <.001 |
| Non-diabetics | 1.74** (1.10, 2.73) | 0.017 | 0.86** (0.32, 2.30) | 0.77 | 0.99** (0.67, 1.46) | 0.96 |
| WBC | ||||||
| All AVR | 2.12 (1.50, 3.00) | <.001 | 1.82 (0.94, 3.52) | 0.08 | 1.53 (1.15, 2.03) | 0.004 |
| TAVR | 2.20 (1.38, 3.51) | <.001 | 1.83 (0.65, 5.13) | 0.25 | 1.56 (1.07, 2.28) | 0.020 |
| SAVR | 2.09 (1.25, 3.50) | 0.005 | 1.82 (0.77, 4.30) | 0.17 | 1.54 (1.00, 2.38) | 0.049 |
| Diabetics | 2.50 (1.58, 3.97) | <.001 | 2.33 (0.86, 6.29) | 0.10 | 2.03 (1.35, 3.05) | <.001 |
| Non-diabetics | 1.70 (1.00, 2.89) | 0.05 | 1.47 (0.60, 3.58) | 0.40 | 1.14 (0.76, 1.72) | 0.52 |
| Heart rate | ||||||
| All AVR | 2.43 (1.59, 3.70) | <.001 | 3.30 (1.43, 7.60) | 0.005 | 1.99 (1.42, 2.80) | <.001 |
| TAVR | 2.27 (1.34, 3.84) | 0.002 | 6.69 (1.59, 28.07) | 0.009 | 1.70 (1.14, 2.55) | 0.010 |
| SAVR | 2.84 (1.40, 5.76) | 0.004 | 1.91 (0.65, 5.61) | 0.24 | 3.02 (1.61, 5.67) | <.001 |
| Diabetics | 2.19 (1.16, 4.16) | 0.016 | 2.13 (0.76, 5.95) | 0.15 | 1.42 (0.85, 2.35) | 0.18 |
| Non-diabetics | 2.50 (1.42, 4.40) | 0.001 | 5.60 (1.30, 24.18) | 0.021 | 2.50 (1.58, 3.94) | <.001 |
| Temperature | ||||||
| All AVR | 0.86 (0.59, 1.24) | 0.40 | 0.87 (0.53, 1.75 | 0.69 | 0.69 (0.50, 0.94) | 0.018 |
| TAVR | 0.71 (0.43, 1.19) | 0.20 | 0.56 (0.20, 1.55) | 0.26 | 0.63 (0.42, 0.95) | 0.026 |
| SAVR | 1.04 (0.61, 1.75) | 0.90 | 1.40 (0.50, 3.88) | 0.52 | 0.76 (0.47, 1.21) | 0.25 |
| Diabetics | 0.84 (0.49, 1.42) | 0.51 | 1.00 (0.37, 2.67) | 0.99 | 0.75 (0.47, 1.21) | 0.24 |
| Non-diabetics | 0.86 (0.52, 1.43) | 0.56 | 0.74 (0.27, 2.02) | 0.56 | 0.64 (0.43, 0.96) | 0.032 |
Severe SIRS is defined by meeting 4/4 SIRS criteria between 12 and 48 hours after valve replacement.
Indicate interaction p<0.05.
Abbreviations: IPW, inverse propensity weighting; others as in Table 3
Figure 1. Covariate balancing between those with and without severe SIRS.
Covariate balancing is shown between those with versus without severe SIRS (4 criteria met) before and after inverse propensity weighting (IPW).
Individual SIRS criteria and mortality
To better understand which SIRS criteria were associated with mortality, we analyzed the relationship between individual SIRS criteria and mortality. After adjustment, meeting the SIRS WBC criteria alone was associated with a higher 6-month mortality (IPW adjusted HR 2.12, 95% CI 1.50–3.00, p<0.001) (Table 4) (Online Figure 1). In a sensitivity analysis in which we excluded patients with a pre-procedure WBC <4 or >12, the results were unchanged. Similarly, meeting the SIRS HR criteria alone was associated with a higher 6-month mortality (IPW adjusted HR 2.43, 95% CI 1.59–3.70, p<0.001) (Table 4) (Online Figure 2). For both WBC and HR alone, there were no significant interactions observed between the SIRS criteria met and AVR type or diabetes. There was no significant relationship between meeting the SIRS temperature criteria and 6-month mortality (Table 4) (Online Figure 3). We did not evaluate the relationship between the SIRS respiratory rate criteria and mortality because it was met in almost all patients.
SIRS and secondary clinical outcomes
Secondary clinical outcomes were also evaluated, including ICU time, ICU readmission, and total hospital length of stay (Table 5). Severe SIRS was associated with more frequent ICU readmissions (adjusted HR 5.93, 95% CI 1.84–19.09, p=0.003) and meeting the WBC or HR criteria alone was associated with longer ICU and hospital stays (Table 5).
Table 5.
Impact of SIRS Criteria on Other Clinical Outcomes in Propensity Adjusted Analyses
| Initial ICU Hours | ICU Readmission | Length of Hospital Stay | ||||
|---|---|---|---|---|---|---|
| IPW β-estimate (95% CI) |
p-value | IPW HR (95% CI) |
p-value | IPW β-estimate (95% CI) |
p-value | |
| Severe SIRS* | ||||||
| All AVR | 0.19 (−0.08, 0.46) | 0.17 | 5.93 (1.84, 19.09) | 0.003 | 0.09 (−0.10, 0.27) | 0.35 |
| TAVR | 0.38 (−0.09, 0.86) | 0.11 | 8.61 (1.17, 63.33) | 0.035 | 0.14 (−0.15, 0.42) | 0.36 |
| SAVR | 0.076 (−0.246, 0.398) | 0.64 | 4.67 (1.33, 16.34) | 0.016 | 0.05 (−0.17, 0.27) | 0.65 |
| Diabetics | 0.22 (−0.29, 0.74) | 0.39 | 9.84 (1.55, 62.45) | 0.015 | 0.14 (−0.26, 0.54) | 0.50 |
| Non-diabetics | 0.17 (−0.16, 0.49) | 0.32 | 3.85 (1.03, 14.39) | 0.045 | 0.06 (−0.13, 0.24) | 0.57 |
| WBC | ||||||
| All AVR | 0.26 (0.10, 0.41) | <.001 | 1.20 (0.53, 2.73) | 0.66 | 0.10 (0.01, 0.20) | 0.033 |
| TAVR | 0.34 (0.11, 0.57) | 0.003 | 1.45 (0.41, 5.19) | 0.57 | 0.11 (−0.05, 0.28) | 0.19 |
| SAVR | 0.20 (0.14, 0.39) | 0.035 | 1.09 (0.38, 3.09) | 0.87 | 0.10 (−0.02, 0.21) | 0.10 |
| Diabetics | 0.36 (0.10, 0.63) | 0.007 | 1.27 (0.36, 4.50) | 0.71 | 0.18 (0.03, 0.33) | 0.018 |
| Non-diabetics | 0.19 (0.01, 0.36) | 0.035 | 1.17 (0.41, 3.36) | 0.78 | 0.06 (−0.07, 0.18) | 0.36 |
| Heart rate | ||||||
| All AVR | 0.29 (0.14, 0.43) | <.001 | 0.81 (0.20, 3.24) | 0.76 | 0.16 (0.06, 0.26) | 0.002 |
| TAVR | 0.15 (−0.02, 0.32) | 0.08 | 1.49 (0.38, 5.78) | 0.57 | 0.15 (0.01, 0.29) | 0.038 |
| SAVR | 0.36 (0.16, 0.56) | <.001 | 0.61 (0.11, 3.53) | 0.58 | 0.16 (0.04, 0.28) | 0.008 |
| Diabetics | 0.39 (0.14, 0.64) | 0.002 | 1.52 (0.33, 6.93) | 0.59 | 0.18 (0.03, 0.33) | 0.016 |
| Non-diabetics | 0.22 (0.05, 0.40) | 0.012 | 0.66 (0.12, 3.46) | 0.62 | 0.14 (0.01, 0.26) | 0.035 |
| Temperature | ||||||
| All AVR | −0.07 (−0.93, 0.35) | 0.35 | 1.92 (0.88, 4.21) | 0.10 | 0.02 (−0.09, 0.11) | 0.77 |
| TAVR | −0.05 (−0.23, 0.13) | 0.58 | 1.06 (0.26, 4.270) | 0.93 | −0.03 (−0.18, 0.11) | 0.66 |
| SAVR | −0.09 (−0.28, 0.11) | 0.40 | 2.55 (0.97, 6.70) | 0.06 | 0.04 (−0.09, 0.16) | 0.57 |
| Diabetics | 0.03 (−0.24, 0.29) | 0.85 | 1.80 (0.51, 6.37) | 0.36 | 0.12 (−0.06, 0.29) | 0.18 |
| Non-diabetics | −0.13 (−0.30, 0.03) | 0.12 | 2.00 (0.74, 5.42) | 0.17 | −0.05 (−0.17, 0.07) | 0.39 |
Severe SIRS is defined by meeting 4/4 SIRS criteria between 12 and 48 hours after valve replacement.
Initial ICU hours and length of hospital stay were log-transformed and evaluated through linear regression. Beta coefficients represent the difference between SIRS groups of the log-transformed variable.
Abbreviations: IPW, inverse propensity weighting; others as in Table 3.
DISCUSSION
We found that a severe SIRS phenotype was associated with higher 30-day, 6-month, and 1-year all-cause mortality in patients with severe AS treated with SAVR or TAVR. SIRS occurred more commonly after SAVR than TAVR and had a greater effect on mortality in diabetic patients. Meeting the WBC or HR criteria individually was also associated with increased mortality. In addition, SIRS was associated with increased resource utilization in terms of a longer ICU stay, more frequent readmission to the ICU, and a longer hospital stay. Importantly, these relationships were observed after extensive adjustment for potential confounders. These findings may have implications for treatment decisions in patients with AS and may help explain differences in outcomes between different AVR approaches. Further, these findings identify diabetic patients as a high-risk sub-group to target in clinical trials with therapies to attenuate SIRS. This is particularly relevant in light of recent neutral trials utilizing corticosteroids to mitigate inflammation after cardiac surgery as a means to improve clinical outcomes.
While the occurrence of an inflammatory response after cardiac surgery is widely recognized,[1 2] the commonly employed SIRS criteria have not been applied to patients undergoing AVR; therefore, the incidence and severity of this inflammatory response in such patients is unknown. It is important to note that in applying these criteria to this clinical setting in which an inflammatory response is common, we have allowed for the possibility that a stricter definition of SIRS (i.e. more criteria met than the traditional two) may be required to identify an association between the severity of an inflammatory response and clinical outcomes.[13] Given the numerous factors that could confound the relationship between SIRS and mortality, we adjusted for variables including diabetes, insulin treatment, use of cardiopulmonary bypass and cross-clamp time, severity of co-morbidities, and intra-operative blood products administered (44 baseline and procedural variables in total) to minimize confounding.
Sinning et al. recently reported the occurrence of SIRS after TAVR and found that it was associated with increased short and long-term mortality.[7] They defined SIRS as the presence of two or more criteria.[13] The incidence of SIRS in their study was 40%, whereas 73% of our TAVR population met two or more criteria. The WBC and HR criteria were met in 41% and 21% in their population, respectively, compared to 38% and 48% in our TAVR population. There were several important procedural differences between our TAVR populations that could have influenced the development of SIRS. Those in the study described by Sinning et al. were all treated percutaneously via a transfemoral approach using the self-expanding CoreValve without general anesthesia. In contrast, our TAVR population was treated with an alternative access approach in 56% of cases, transfemoral cases were generally performed with a surgical cut-down, and all procedures were performed with a balloon expandable Edwards SAPIEN valve under general anesthesia. Importantly, they linked the development of SIRS (≥2 criteria) with increased circulating levels of the inflammatory cytokines interleukin 6, interleukin 8, and C-reactive protein. The prevalence of diabetes in their study was not reported, the patient population and number of events smaller, and their multivariable adjustment more limited.
Clinical Implications
Our observation that the SIRS criteria (individually and collectively) occur more commonly after SAVR than TAVR may underlie differences observed in outcomes after these procedures. While the PARTNER trial showed no difference in mortality between TAVR and SAVR,[17] the recently reported CoreValve trial demonstrated decreased 1-year mortality after TAVR compared to SAVR in high risk patients with AS.[18] Interestingly, in a post-hoc analysis of the PARTNER trial, diabetic patients were noted to have decreased 1-year mortality when treated with TAVR compared to SAVR.[11] Our finding that severe SIRS is more strongly associated with mortality in diabetic patients may help explain this result. Taken together, these observations may tend to favor a transcatheter approach when either approach would be a reasonable option, particularly in those with diabetes. However, our findings are too preliminary to make definitive recommendations regarding procedural approach based on the incidence and hazard of severe SIRS. Certainly, despite our findings, there are scenarios when for a diabetic patient SAVR would be preferable to TAVR such as for the diabetic patient with left ventricular dysfunction, multivessel coronary disease and severe AS. More studies are needed to better understand the mechanisms of inflammation in the peri-operative setting and how procedural approach and diabetes intersect with these processes to affect clinical outcomes.
Insofar as an inflammatory response after AVR is associated with a worse outcome, it is important to consider what steps might be taken to limit or blunt this response. For patients undergoing TAVR, Sinning et al. found that the number of pacing runs and major vascular complications were associated with the development of SIRS.[7] Incorporating a comparison of our two studies, one might also hypothesize that alternative access approaches, a surgical cutdown for transfemoral cases, and the use of general anesthesia may also increase the incidence of SIRS after TAVR. Fortuitously, the movement toward smaller profile devices and a more “minimalist” procedural approach (conscious sedation, no mechanical ventilation, etc.) will allow for more transfemoral procedures (which tended to have the lowest incidence of severe SIRS) with less of the factors that may induce an inflammatory response.[19]
For patients undergoing SAVR, attenuating the inflammatory response after cardiac surgery has been a longstanding goal and the rationale for large randomized clinical trials testing the use of intravenous corticosteroids.[4 5] Steroids may not be the appropriate anti-inflammatory intervention or the patient sub-group(s) with the most potential benefit have not been targeted. Although diabetes is not associated with an increased risk of SIRS, we have identified diabetic patients as a large sub-group that has a higher adverse event rate when severe SIRS develops. As such, diabetic patients are an attractive target population for investigating the clinical effect of therapeutic interventions designed to attenuate SIRS. In this regard, the sub-group analysis of the recent SIRS trial gave some indication that the effect of corticosteroids might differ based on diabetes status (interaction p=0.13), with the HR for death at 30 days among diabetics favoring treatment with steroids (borderline significance).[6] It is also possible that more selective anti-inflammatory therapies will be more effective at attenuating SIRS and improving clinical outcomes.[3]
Limitations
This was a retrospective analysis, which could have influenced the accuracy of assessing whether SIRS criteria were met in the early post-operative period given that these are determined mostly by vital signs. However, vital signs are recorded in the medical record hourly (or more frequently) while patients are in the ICU and every 2 to 4 hours upon discharge from the ICU to the floor. We made an effort to eliminate the influence of potentially spurious, isolated vital signs on the determination of whether SIRS criteria were met. The respiratory rate criteria was met in almost all patients, potentially limiting the interpretability of our results on the hazard of meeting ≥2 or ≥3 SIRS criteria. Finally, despite extensive multivariable adjustment, unmeasured confounders may have affected our findings.
Conclusion
A severe SIRS phenotype is associated with a higher mortality and increased resource utilization after SAVR and TAVR. It occurs more commonly after SAVR and has a greater effect on mortality in patients with diabetes. These observations may have implications for treatment decisions in patients with AS and may help explain differences in outcomes between different AVR approaches. Further, these findings identify diabetic patients as a high-risk sub-group to target in clinical trials that are investigating therapies to attenuate SIRS.
Supplementary Material
Figure 2. SIRS and mortality after aortic valve replacement.
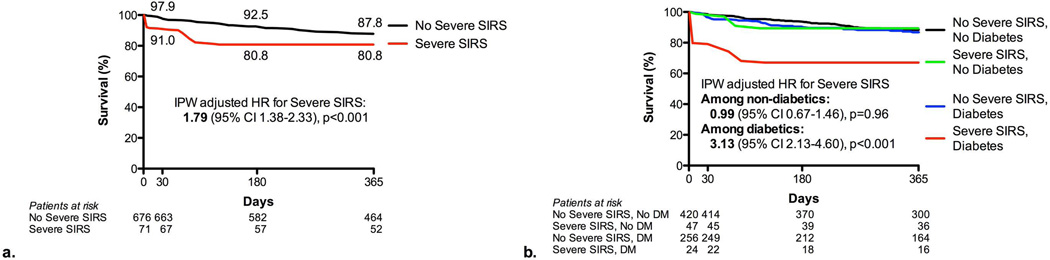
Time-to-event curves are shown based on inverse propensity weighting Kaplan-Meier estimates. Hazard ratios are shown for Cox proportional hazards models utilizing inverse propensity weighting. Comparison between those with and without severe SIRS is shown (a) as well as for those with and without severe SIRS and diabetes (b).
STUDY SUMMARY AND SIGNIFICANCE.
What is already known about this subject?
Cardiac surgery can stimulate a systemic inflammatory response that has deleterious consequences. This is the rationale for recent large randomized clinical trials in cardiac surgery to attenuate the inflammatory response with steroids, but these trials have been neutral.
What does this study add?
Few studies have examined the incidence of a systemic inflammatory immune response (SIRS) after aortic valve replacement (AVR), particularly after transcatheter (AVR). We report the incidence of SIRS after surgical and transcatheter AVR, including different TAVR approaches. We found that diabetes modifies the relationship between a severe SIRS phenotype and mortality; specifically, severe SIRS was more strongly associated with mortality in diabetic patients compared to non-diabetic patients.
How might this impact on clinical practice?
Given the increased hazard of mortality associated with severe SIRS after AVR, which is amplified in patients with diabetes, and the tendency for severe SIRS to develop more frequently after SAVR than TAVR, these issues should be considered in deciding between SAVR versus TAVR in diabetic patients. Further, diabetic patients may be a high-risk sub-group to target in clinical trials testing therapies to attenuate SIRS after cardiac surgery.
Acknowledgements
The authors thank Joel D. Schilling, MD, PhD, and Christopher L. Holley, MD, PhD, for their feedback on the manuscript.
Funding: This work was supported by NIH K23 HL116660 (BRL), the Barnes-Jewish Hospital Foundation (EN), and the Washington University Mentors in Medicine Program (JSG).
Footnotes
Conflicts of interest: Dr. Lindman is a site co-investigator for the PARTNER Trial and has consulted for Gerson Lehrman Group Research. Dr. Zajarias has consulted for Edwards Lifesciences and is a member of the PARTNER Trial Steering Committee. Dr. Damiano has consulted for AtriCure, received grant support from Atricure and Medtronic, and received grant support and speaking fees from Edwards Lifesciences. Dr. Lasala has consulted for Boston Scientific and Direct Flow Medical, received speaking fees from Boston Scientific and St. Jude, and received stock options from Direct Flow Medical. The other authors report no potential conflicts of interest.
References
- 1.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 2.Wan S, DeSmet JM, Barvais L, et al. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:806–811. doi: 10.1016/S0022-5223(96)70068-5. [DOI] [PubMed] [Google Scholar]
- 3.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012;308:1761–1767. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 5.Whitlock R, Teoh K, Vincent J, et al. Rationale and design of the steroids in cardiac surgery trial. Am Heart J. 2014;167:660–665. doi: 10.1016/j.ahj.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock R. Steroids In caRdiac Surgery (SIRS) Trial; Presented at the American College of Cardiology 2014 Scientific Session in Washington DC; http://solaci.org/es/pdfs/acc2014/6_richard_whitlock_slides.pdf. [Google Scholar]
- 7.Sinning JM, Scheer AC, Adenauer V, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. 2012;33:1459–1468. doi: 10.1093/eurheartj/ehs002. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson J, Jovinge S, Niemann A, et al. Relation between plasma tumor necrosis factor-alpha and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1998;18:1199–1202. doi: 10.1161/01.atv.18.8.1199. [DOI] [PubMed] [Google Scholar]
- 9.Halkos ME, Kilgo P, Lattouf OM, et al. The effect of diabetes mellitus on in-hospital and long-term outcomes after heart valve operations. Ann Thorac Surg. 2010;90:124–130. doi: 10.1016/j.athoracsur.2010.03.111. [DOI] [PubMed] [Google Scholar]
- 10.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 11.Lindman BR, Pibarot P, Arnold SV, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Diabetes and Severe Aortic Stenosis at High Risk for Surgery: An Analysis of the PARTNER Trial (Placement of Aortic Transcatheter Valve) J Am Coll Cardiol. 2014;63:1090–1099. doi: 10.1016/j.jacc.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–123. [PubMed] [Google Scholar]
- 15.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate behavioral research. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–1708. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 17.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 18.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 19.Babaliaros V, Devireddy C, Lerakis S, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv. 2014;7:898–904. doi: 10.1016/j.jcin.2014.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.