Abstract
Immunotherapeutic strategies are promising approaches for the treatment of follicular lymphoma (FL). However, their efficacy may be limited by immunosuppressive elements in the immune system and tumor microenvironment. Therefore, strategies to reverse the effects of the immunosuppressive elements are needed. We observed that regulatory T cells (Tregs) were increased in the peripheral blood at diagnosis and persisted in high numbers after induction of clinical remission with a cyclophosphamide and doxorubicin-containing chemotherapy regimen in FL patients. High levels of peripheral blood Tregs prior to therapy were associated with decreased progression-free survival in FL patients treated with either chemotherapy or combination immunotherapy that targeted CD20 and PD-1 with monoclonal antibodies rituximab and pidilizumab, respectively. Intratumoral and peripheral blood Tregs potently suppressed autologous antitumor effector T cells in FL. However, the effects of FL Tregs could be reversed by triggering Toll-like receptors (TLR) with TLR ligands Pam3CSK4 (TLR 1/2), flagellin (TLR 5), and CpG-B (TLR 9), and/or OX40. The TLR ligands synergized with each other as well as OX40 signaling to inhibit Tregs. Furthermore, they restored the function of FL tumor-specific effector T cells. Our results suggest that a state of tolerance exists in FL patients at diagnosis and after induction of clinical remission, and agents that activate TLRs 1/2, 5, and 9, and OX40 may serve as adjuvants to enhance the efficacy of antitumor immunotherapeutic strategies and preventive vaccines against infectious diseases in these patients.
Keywords: Regulatory T cells, Toll-like receptors, OX40, lymphoma, immunotherapy
INTRODUCTION
Active immunotherapy is a promising approach for the treatment of follicular lymphoma (FL), an incurable B-cell non-Hodgkin lymphoma.1 Several studies demonstrated that therapeutic vaccination with autologous tumor-derived idiotype protein induces humoral and cellular immune responses in FL patients and was associated with induction of molecular remissions and improved disease-free survival when administered in the setting of minimal residual disease.2–6 However, the efficacy of therapeutic vaccination in patients with gross disease has been disappointing with generally low clinical response rates.5 One potential reason might be that the generation and magnitude of antitumor effector T cells after vaccination, may be opposed by any of several immune checkpoints that have been found in tumor microenvironments and the immune system of cancer patients with gross disease. Among the many immunosuppressive elements, forkhead box P3 (Foxp3)+ regulatory T cells (Tregs) are considered one of the most potent suppressors of effector T cells.7 In FL, Tregs are markedly increased in number in the peripheral blood and tumor microenvironment at diagnosis.8–11 Whether Tregs decline in number after induction of clinical remission has not been investigated. Also, while FL Tregs were demonstrated to potently suppress polyclonally activated autologous T cells,8–10 their effect on autologous antitumor effector T cells has not been studied.
In retrospective studies, increased numbers of Foxp3+ cells within the tumor assessed by immunohistochemistry were associated with improved survival in FL,12–14 a result contradictory to the suppressive function of Tregs and to findings in non-hematopoietic cancers.7 In contrast, by focusing on the architectural pattern of Foxp3+ cells in the tumor microenvironment rather than their numbers, Farinha et al. found that intrafollicular localization of Tregs correlated with shorter survival in advanced stage FL patients treated uniformly with multiagent chemotherapy and radiation.14 In mouse models, depletion of Tregs correlated with decreased lymphoma burden15, 16 and in vitro studies demonstrated the suppressive function of FL Tregs.8–10 Together, these results support the notion that Tregs are immunosuppressive in FL and that they may adversely affect clinical outcome after treatment with immunotherapeutic agents such as vaccines and after certain chemotherapies. Therefore, development of therapeutic strategies that reverse the immunosuppressive effects of Tregs is necessary to improve clinical outcome in FL.
Accumulating evidence from the literature suggests that the suppressive function of intratumoral Tregs can be modulated by A) triggering Toll-like receptors (TLRs) and co-stimulatory molecules (OX40, 4-1BB and GITR), or B) blocking the activities of co-inhibitory molecules within the B7/CD28 family and TNF/TNFR family. TLRs are abundantly expressed by antigen-presenting cells (APC) and T cells.17 They recognize unique molecular structures of pathogens to distinguish “infectious non-self” from “self” antigens, and therefore constitute a critical immune sensing system. Activation of TLRs on APCs by microbes induces maturation of APCs, which secrete pro-inflammatory cytokines leading to the modulation of effector T cell and Treg functions. Of the nine known TLR ligands (TLRLs) in humans, a few have been reported to block the immunosuppressive function of Tregs, directly or indirectly through activating dendritic cells (DCs);18 however, TLRLs could also promote the suppressive effects of Tregs.19, 20 It is possible that TLRLs block the function of Tregs at an early stage of innate immune responses, but promote their function at a later stage to control tissue damage and autoimmunity. Therefore, a combination of a TLRL with another agent that could eliminate the Treg-promoting effects associated with TLR triggering would be critical for cancer immunotherapy. Our recent studies and those from others suggest that triggering OX40 by OX40 agonists could fulfill this objective, because OX40 signaling could 1) promote effector T cell function and T cell memory by promoting T cell survival and clonal expansion;21 2) shut down the generation and the function of interleukin 10 (IL-10)-producing Tregs (Tr1);22 3) block TGF-β and antigen-driven conversion of naïve CD4+ T cells into CD25+Foxp3+ Tregs;23 4) completely inhibit the function or survival of Foxp3+ natural occurring Tregs (nTregs);24 and 5) induce changes in the tumor stroma, including a decrease in the number of macrophages and myeloid-derived suppressor cells.25
Here, we evaluated changes in Treg numbers in FL patients treated uniformly with a standard chemotherapy and determined the effects of Tregs on autologous FL-specific antitumor effector T cells. In addition, we assessed whether peripheral blood Treg numbers have prognostic value in FL patients treated with chemotherapy or immunotherapy. We also determined whether triggering TLRs and OX40 could reverse the immunosuppressive functions of FL Tregs.
MATERIALS AND METHODS
Patient samples and therapy
All blood and tissue samples were obtained after written informed consent from patients through an institutional review board-approved protocol. Lymph node biopsies were obtained from patients with FL at the time of their initial diagnosis prior to therapy. Tonsils were obtained from children who had undergone elective tonsillectomy. The use of tonsils with benign follicular hyperplasia as controls for FL has generally been accepted in the scientific literature.26 Surgical samples were processed into single-cell suspension and cryopreserved. Patients were treated uniformly with prednisone, doxorubicin, cyclophosphamide, and etoposide (PACE) chemotherapy6 and six to twelve months after completion of chemotherapy, patients received autologous tumor-derived idiotype vaccination as previously described.2, 6 Physical examination; computed tomography scans of chest, abdomen, and pelvis; and bone marrow examination were performed prior to chemotherapy, after cycle four and every two cycles thereafter until completion of chemotherapy. Thereafter, physical examination and CT scans were performed every 6 months until relapse. Tumor response categorized as complete response (CR), CR unconfirmed (CRu), partial response (PR), stable disease (SD), or progressive disease, was assessed according to the International Workshop Response Criteria for Non-Hodgkin’s Lymphoma.27 The details of therapy for FL patients treated with rituximab and pidilizumab, an anti-PD-1 blocking monoclonal antibody, were recently reported.28
Reagents and cell lines
TLR ligands: Pam3CSK4, PGN, LTA, Poly(I:C), CL075, CL097, LPS, Flagellin (FLA-ST), Flagellin (FLA-ST ultrapure grade), R848, CpG-A (ODN2216), CpG-B (ODN2006) and CpG-C (ODN M362) were purchased from Invivogen. Poly (G3) was purchased from Sigma. Mycobacterium tuberculosis (MT) killed bacteria and Bordetella pertussis (BP) inactivated bacteria were purchased from BD Diagnostic Systems. Yellow fever vaccine (YFVAX) was a generous gift from Dr. B. Pullendran, Emory Vaccine Center at Yerkes National Primate Research Center, Emory University, Atlanta, Georgia, USA. ELISA kits for GM-CSF, IL-2, and IL-12 were purchased from R&D systems. Fetal calf serum and human serum were purchased from Gemini. Carboxyfluorescein succinimidyl ester (CFSE) was purchased from Invitrogen. Mouse fibroblast L cell line (L-cell) and OX40 ligand expressing L cells (L-cell/OX40L) generated in our laboratory were maintained with RPMI cultured medium containing 10% fetal calf serum, 1% Glutamax, and 1% penicillin-streptomycin. FL-specific T-cell lines and clones generated from intratumoral T cells were described previously.29
Antibodies, FACS analysis, and cell sorting
The following antibodies were used for flow cytometry analysis: CD4-APC-Cy7, CD4-PerCP, CD127-PE, CD127-Alexa Fluor® 647, CD25-FITC, CD25-PE-Cy7, CD14-FITC, CD16-FITC, CD20-FITC, CD45RO-PE, CD56-FITC, CD11c-FITC, TCRγδ-FITC (all from BD Pharmingen). Anti-hOX40 antibodies were purchased from eBioscience (ACT35). Anti-Foxp3 staining antibodies (236A/E7, PCH101) were from eBioscience and staining was conducted using Foxp3 fixation-/permeabilization buffer according to manufacturer’s instructions. Flow cytometry was performed on a flow cytometer (FACS Calibur or LSRFortessa, Becton Dickinson). FACS sorting was conducted on a cell sorter (FACS Aria II, BD). Functional grade anti-CD3 (OKT3) was purchased from Centocor Ortho. Antibodies against TLRs 1 were from Abcam Inc (GD2-F4), and 2, 5, 9 from BD Biosciences (EB72-1665,).
Isolation of CD4+ T-Cell subsets from healthy donors
Adult blood buffy coats from healthy donors were obtained from the Gulf Coast Regional Blood Center in Texas. CD4+ T cells were enriched using a CD4+ T-cell isolation kit (Miltenyi Biotec) according to manufacturer’s instructions. We isolated Teffs and Tregs from enriched CD4+ T cells by staining with APC-Cy7-CD4, PE-CD127, PE-Cy7-CD25, APC-CD45RO, PerCP5.5-CD45RA antibodies, and FITC-labeled lineage mixture antibodies against CD14, CD16, CD19, CD56, CD11c, and d-TCR and sorting on a FACSAria. Stained cells were gated on CD4+ and FITC lineage negative populations, and sorted into Tregs (CD4+CD127loCD25hi) and Teffs (CD4+CD127+CD25loCD45RA-CD45RO+).
Isolation of T-cell subsets and APCs from FL tumors
Single cell suspensions of human FL tumors were cultured with IL-2 (300 IU/ml) in RPMI medium supplemented with 10% human serum plus 1% penicillin-streptomycin. CD8+ T cells were depleted by CD8 micro beads (Miltenyi Biotec) and remaining cells were stained and sorted on a FACSAria into two fractions of CD4+CD127loCD25hi and CD4+CD127+CD25lo (Teffs). Tumor cells used as APCs were obtained from suspensions of FL tumors by depleting T cells with CD3 micro beads (Miltenyi Biotec). In selected experiments Tregs were isolated with the CD4+CD25+CD127lo/– Regulatory T Cell Isolation Kit II (Miltenyi Biotec), CD4+CD25- Teffs were purified by depleting CD25+ cells from CD4+ T cells after isolating CD4+ T cells using CD4+ T-cell isolation kit (Miltenyi Biotec), and CD4+CD45RA+ naïve T cells were isolated by Naive CD4+ T cell Isolation Kit II (Miltenyi Biotec).
Isolation of myeloid and plasmacytoid DCs from healthy donors
Dentritic cells were enriched using human Pan-DC enrichment cocktail (STEM Cell Technologies) from buffy coat of healthy adult donors. Myeloid DC (mDCs) and plasmacytoid DC (pDCs) were isolated from enriched DCs by staining with APC-CY7-CD4, APC-CD123, PE-CD11c and FITC-labeled lineage mixture antibodies against CD3, CD14, CD16, CD19, CD20, CD56. Stained cells were gated on FITC lineage negative cells, and sorted into CD4+CD11c-CD123+ pDCs and CD4+CD11chigh mDCs.
Treg suppression assay
Teffs (5–8 × 104/well) and Tregs were derived from peripheral blood or intratumoral T cells from FL patients. CFSE (5 μM) labeled or unlabeled Teffs were cultured with or without autologous Tregs at indicated ratios. Teffs were stimulated with soluble anti-CD3 (0.3 μg/ml) and anti-CD28 antibodies or particles preloaded with anti-CD2, -CD3 and -CD28 antibodies (Treg Suppression Inspector, Miltenyi Biotec) or autologous tumor cells pre-activated with CD40L and IL-4 and irradiated to 3,000 Rads as previously described.3 For evaluating synergy between OX40 and TLR signaling, Teffs were stimulated with 0.5 μg/ml of plate-bound anti-CD3 plus increasing concentrations of plate-bound rhOX40L. Proliferation of Teffs was measured after 4 days either by CFSE dilution or 3H thyimidine incorporation. In selected experiments, Teffs and Tregs were derived from normal donor peripheral blood and cultured with allogeneic T-cell depleted tumor-derived APC irradiated to 2,000 Rads.
Screening of TLR ligands
TLR ligands (TLRL) were screened using allogeneic tumor-derived APC in Treg suppression assay as described above. The following TLRLs and vaccines were tested at 4 different concentrations: a) TLR1/2L: Pam3CSK4 (2, 10, 50, 100 ng/ml); b) TLR2L: PGN (0.1, 0.5, 1, 5 μg/ml) and LTA (0.05, 0.1, 0.5, 1 μg/ml); c) TLR3L or TLR4L: Poly I:C, CL075 and CL097 LPS (0.1, 0.5, 1, 5 μg/ml); d) TLR5L: Flagellin (FLA-ST, 0.05, 0.2, 1, 10 μg/ml); e) TLR7/8L: R848 (10, 20, 100, 500 ng/ml); f) TLR9L: CpG-A (ODN2216), CpG-B (ODN2006), and CpG-C (ODN M362) (0.1, 0.5, 1, 5 μM); g) TLR2L: MT (0.1, 0.5, 2.5, 10 μg/ml); g) TLR4L: BP (0.05, 0.1, 0.5, 1×106 inactivated, whole cell bacteria); and h) TLR2, 7, 8, 9L: YFVAX, (0.05, 0.1, 0.5, 1 × 106 live, attenuated virus).
Statistical analysis
Statistical difference of Tregs between experimental groups was determined by Wilcoxon-Mann-Whitney U test using Prism software (Graphpad Software, Inc). P values < 0.05 were considered statistically significant. Progression-free survival (PFS) was defined as time from initiation of treatment to the time of progression or death, whichever occurred first, or to the time of last contact. Progression-free survival was estimated using the Kaplan-Meier method. Log-rank test was performed to test the difference in survival times between groups. Multivariate regression models were developed to examine the effect of percentage of Tregs adjusted for Follicular Lymphoma International Prognostic Index (FLIPI) score. Regression analyses of PFS data was conducted based on the Cox proportional hazards model. Logistic regression analysis was used to assess the multivariate relationship between percentage of Tregs and the probability of achieving CR/CRu. SAS version 9.1 and S-Plus version 7.0 were used to carry out the computations for statistical analyses.
RESULTS
FL patients had a relative increase in Tregs at diagnosis
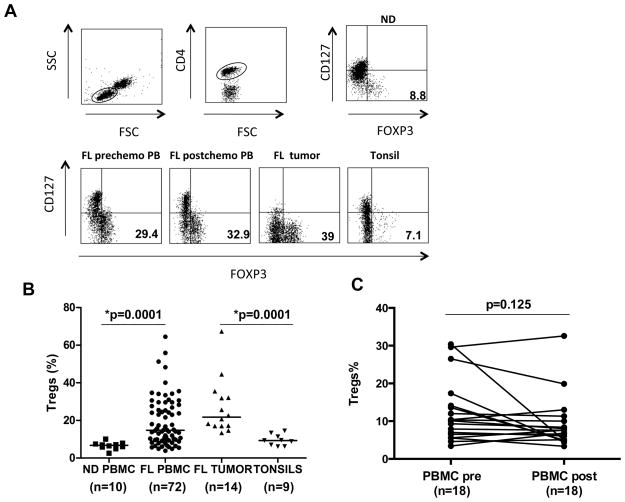
Consistent with previous reports,8, 10, 11 we observed that the percentage of Foxp3+ Tregs was significantly increased both in the peripheral blood (median, 17.3%; range, 6.5–64.5%; n=72; p=0.0001) and at tumor site (median, 21.7%; range, 13.4–67.3%; n=14; p=0.0001) at initial diagnosis in patients with FL as compared with peripheral blood from normal donors (median, 6.7%; range, 2.6–10.1%; n=10) and tonsils (median, 9.32%; range, 6.1–14.5%; n= 9), respectively (Figs. 1A and B). To determine whether the Tregs decrease after induction of clinical remission, we evaluated paired pre and post-chemotherapy peripheral blood samples from 18 FL patients that were uniformly treated into complete remission with PACE chemotherapy (Supplementary Table 1). Although a decrease in Tregs was observed in a few patients, the percentage of Tregs did not differ significantly between pre and post-chemotherapy samples when assessed for the entire group of 18 patients (p=0.125) (Fig. 1C).
Figure 1. Tregs are increased in follicular lymphoma patients at diagnosis and remain elevated after induction of clinical remission with chemotherapy.
A) Gating strategy for Tregs is shown. The CD4+ T cells were gated from the total lymphocyte population identified based on forward and side scatter parameters. Tregs were then identified as CD4+CD127low/-Foxp3+ T cells and the percentage is shown for each sample. B) The percentage of CD4+CD127low/-Foxp3+ Tregs was determined by flow cytometry in PBMC and lymph node biopsies of FL patients and normal donor (ND) PBMC and tonsils. Data are expressed as percentages of CD4+ T cells. Horizontal bars indicate median values for each group. C) The percentage of CD4+CD127low/-Foxp3+ Tregs cells in paired PBMC samples obtained at diagnosis (PBMC pre) and after a median of 12 months (range, 1–16 mo) after completion of the chemotherapy (PBMC post) is shown. P values were calculated by Wilcoxon rank-sum test (B) or Wilcoxon signed-rank test (C).
Intratumoral and peripheral blood Tregs from FL patients were highly immunosuppressive
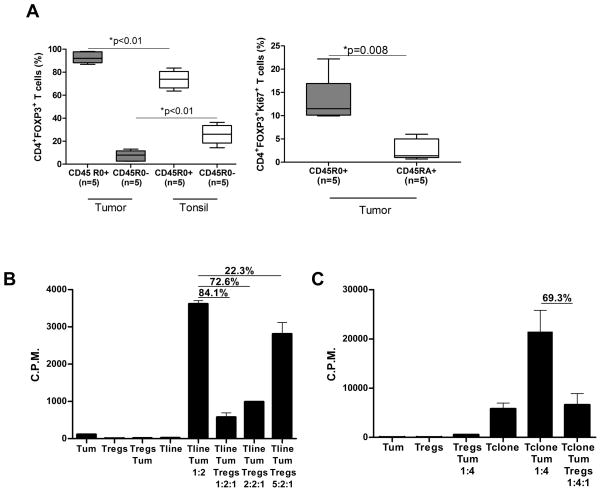
Using multiparametric flow cytometry, we observed that among the intratumoral Tregs, the CD45RO+ Tregs were significantly higher and the CD45RA+ (CD45RO-) naïve Tregs were significantly lower compared with tonsils suggesting that most Tregs in FL tumors were activated.30 Furthermore, Ki67+ Tregs were enriched in the CD45R0+ Tregs compared to the CD45RA+ naïve Tregs (Fig. 2A). Consistent with this, intratumoral Tregs significantly suppressed proliferation of tumor-specific T-cell line and clone in a dose-dependent manner in response to stimulation with autologous tumor (Figs. 2B and C). Similarly, peripheral blood Tregs obtained at initial diagnosis and after induction of clinical remission suppressed polyclonally activated and tumor-induced proliferation of autologous CD4+CD25- effector T cells (Supplementary Figs. 1A and B). Intratumoral Tregs also significantly inhibited the production of TH1 cytokines IL-2, IFN-γ, TNF-α, and GM-CSF from tumor-specific T-cell line and clone (Fig. 2D and Supplementary Fig. 2).29 Together, these results suggested that intratumoral and peripheral blood Tregs in FL patients were activated and suppressed antitumor T cell responses. Importantly, suppression by these Tregs did not require activation in vitro.
Figure 2. Intratumoral Tregs from FL patients are activated and suppress the function of antitumor T cells.
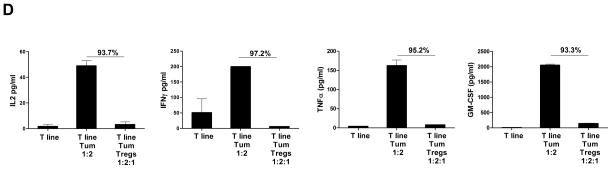
A) Box plots represent percentages of CD45RO+ and CD45R0- Tregs in FL tumor biopsies and tonsil controls (left panel) and of Ki67+ cells in CD45RO+ and CD45RA+ Tregs in FL tumors (right panel) as determined by flow cytometry. P values were calculated by Wilcoxon rank-sum test (left panel) or paired t-test (right panel). B–D) CD4+ tumor-specific T cell line (B, D) and clone (C) derived from FL tumor-infiltrating lymphocytes were incubated with autologous tumor cells for 6 days in the presence or absence of autologous intratumoral Tregs at the indicated ratios. Proliferation was assessed by 3H thymidine incorporation (B, C) and production of cytokines (D) in supernatants was measured by multiplex cytokine assay. The percent inhibition of proliferation or cytokine production in the presence of Tregs is shown in each panel. Data is representative of one of three experiments (B–D).
Peripheral blood Treg number at initial diagnosis correlated with clinical outcome
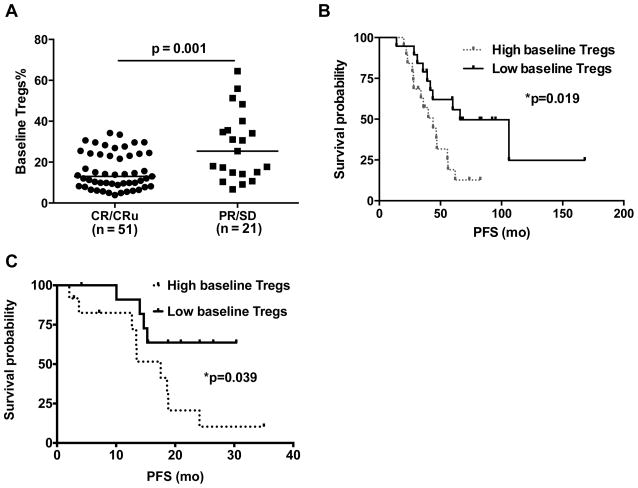
Since Tregs from FL patients suppressed antitumor T cell responses, we evaluated whether the peripheral blood Treg number at initial diagnosis is predictive of clinical outcome. Analysis of data from a cohort of 72 FL patients that were uniformly treated with PACE chemotherapy (Supplementary Table 1) and had available peripheral blood samples for analysis showed that patients that achieved a CR or CRu had a significantly lower baseline level of Tregs (median, 13% of CD4+ T cells) compared with patients that did not achieve a CR/CRu (median 25.4% of CD4+ T cells; p = 0.001) (Fig. 3A). With the adjustment of FLIPI risk score in multivariate logistic regression model, percent of peripheral blood Tregs remained significant. Patients with higher percentage of Tregs had a lower likelihood of achieving CR/CRu than those with lower percentage of Tregs (Odds Ratio = 0.912 (95% CI: 0.863 to 0.965) per unit increase of Tregs; p = 0.001).
Figure 3. High baseline levels of peripheral blood Tregs are associated with poor clinical outcome in FL.
A) Percentages of CD4+CD127low/-Foxp3+ Tregs in peripheral blood at diagnosis in FL patients who achieved complete remission (CR), CR unconfirmed (CRu), partial remission (PR), or stable disease (SD) following PACE chemotherapy are shown. Horizontal bars indicate median values for each group. P value was calculated by Wilcoxon rank-sum test. B and C) Kaplan-Meier survival curves for progression free survival (PFS) in FL patients with high (> median) and low (= median) numbers of Tregs in peripheral blood at baseline and treated with PACE chemotherapy (B) or a combination of rituximab and pidilizumab (C) are shown. P values were calculated by log-rank test.
More importantly, the peripheral blood Treg percentage at baseline was also predictive of PFS in the cohort of 38 patients that were treated uniformly with PACE chemotherapy and idiotype vaccination. Patients with greater than median level of Tregs (> 14.4%) at initial diagnosis had a significantly inferior PFS compared to patients with less than or equal to median level of Tregs (median 43.9 vs. 66.0 months; p = 0.019) (Fig. 3B). With the adjustment of FLIPI risk score in multivariate Cox proportional hazard model, percent of peripheral blood Tregs remained significant. Patients with higher percentage of Tregs had larger hazard of progression than those with lower percentage of Tregs (Hazard Ratio = 1.05 (95% CI: 1.017 to 1.085) per unit increase of Tregs; p = 0.003). Higher numbers of peripheral blood Tregs were also associated with lower PFS in a second cohort of 24 relapsed FL patients treated uniformly with a combination of rituximab, an anti-CD20 monoclonal antibody that targets the tumor B cells and pidilizumab, an anti-PD-1 monoclonal antibody that blocks the co-inhibitory molecule PD-1 on T cells28 (median PFS 17.5 months vs not reached for high vs low baseline Tregs; p = 0.039) (Fig. 3C). Together, these results suggest that high numbers of peripheral blood Tregs are associated with poor prognosis and strategies to reverse their immunosuppressive effects might improve clinical outcome in these patients when used in combination with chemotherapy and/or immunotherapy.
Triggering TLRs broke Treg-mediated tolerance
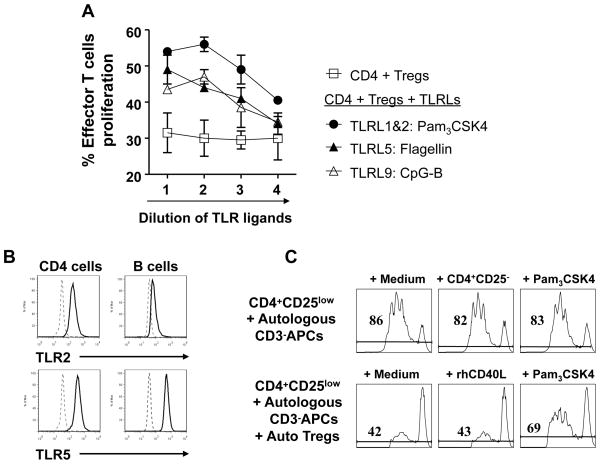
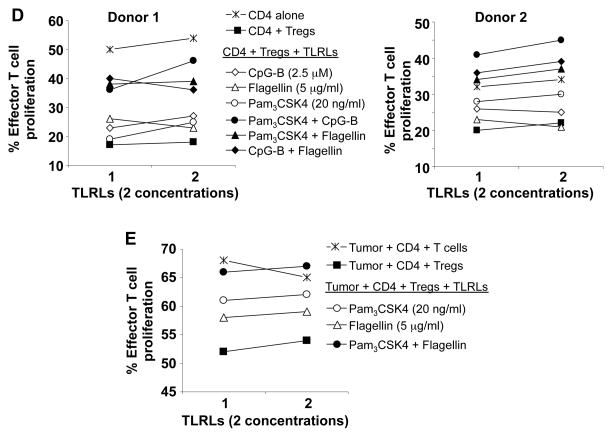
Previous studies showed that TLR ligands could block Treg function either by acting directly on Tregs or indirectly by activating tumor-infiltrating innate immune cells which in turn might enhance the proliferation, survival, and function of effector T cells.17, 18 To investigate which TLR ligand could most potently block Treg function in a microenvironment that mimics human B cell lymphomas, we systematically screened all known human TLR ligands for their ability to block suppressive function of Tregs in the presence of T cell-depleted human lymphoma cells. We found that TLR ligands Pam3CSK4 (ligand for TLR1/2), flagellin (ligand for TLR5), and CpG-B (ligand for TLR9) consistently blocked the suppressive function of healthy donor Tregs and restored the proliferation of effector T cells in a dose-dependent manner (Fig. 4A). However, agonists of TLR2, TLR2\7\8\9 (YFVAX), TLR2/6, TLR3, TLR4, TLR7/8, and TLR8 were ineffective (data not shown). Since TLR9 is expressed by pDCs and B cell lineage including human B lymphoma cells,31 the effect of CpG-B on Tregs may be indirect via tumor-infiltrating pDCs or lymphoma B cells. To determine whether TLR 1/2 and 5 are expressed in effector T or FL tumor B cells, we performed intracellular staining and analyzed by flow cytometry. We observed that TLR2 and 5 were expressed in intratumoral CD4+ T cells, but TLR2 was barely detectable in FL tumor B cells (Fig. 4B), suggesting that Pam3CSK4 may act on T cells. To determine whether TLR ligands block intratumoral Tregs, we tested them in an autologous setting where the Tregs, effector T cells, and tumor cells were isolated from the same FL tumor sample. We found that Pam3CSK4 reversed the suppressive effect of Tregs, while control rhCD40L could not (Fig. 4C). We found that the three TLR ligands, Pam3CSK4, flagellin, and CpG-B, when used at suboptimal doses synergized with each other to block suppressive function of healthy donor Tregs on healthy donor effector T cells stimulated with allogeneic FL-derived APCs (Fig. 4D). We also found that these TLR ligands synergized with each other to block the function of FL-derived intratumoral Tregs on autologous tumor-reactive T cells (Fig. 4E).
Figure 4. Identification of TLR ligands that block the function of FL-derived Tregs.
The ability of TLR ligands (TLRL) to reverse Treg suppression of CD4+CD25- effector T cell proliferation was analyzed by CFSE dilution assay. A) Healthy donor derived effector T cells were cultured with CD3-depleted FL tumor-derived antigen-presenting cells in the presence or absence of healthy donor derived Tregs with or without various doses of TLR 1&2, 5, and 9 agonists as described in materials and methods. Representative results from one of two donors are shown. Proliferation of effector T cells was significantly enhanced in the presence of Pam3CSK4, Flagellin, and CpG-B (P <0.05 compared to control). P values were calculated by two-way ANOVA test. B) Expression of TLR2 and TLR5 in FL tumor cells and intratumoral CD4+ T cells was determined by flow cytometry. Solid and dotted line histograms represent staining with specific antibody or its isotype control, respectively. C), CFSE-labeled intratumoral effector T cells were cultured with autologous CD3-depleted FL tumor-derived antigen-presenting cells in the presence or absence of autologous intratumoral Tregs and Pam3CSK4 (100 ng/ml) or rhCD40L (0.8 μg/ml). The percentage of proliferating effector T cells as determined by CFSE dilution is shown in each plat. D and E) CFSE dilution assays were performed using the allogeneic (D) or autologous (E) systems as in panels A and C, respectively to test synergy between different TLRLs. The concentrations of TLRLs tested were: Pam3CSK4 (20 ng/ml); Flagellin (FLA-ST, 5 μg/ml); and CpG-B (1 μM).
Triggering TLRs did not induce anti-inflammatory cytokine IL-10
Reports from mouse studies suggest that TLR2 ligands such as Pam3CSK4 or Pam2 lipopeptides induce IL-10 secretion and expand CD4+CD25+Foxp3+ Tregs.32, 33 Since induction of IL-10 by TLR ligands may offset their inhibitory effects on Tregs, we evaluated the effects of TLR ligands on different human APCs including FL-derived APCs, PBMCs, mDCs, and pDCs. We found that the three TLR ligands failed to induce significant secretion of IL-10 from all APC types even at high concentrations that were reported to induce significant IL-10 secretion in mice (Supplementary Figs. 3A–C and data not shown). The positive control, LPS an agonist of TLR4, induced IL-10 from PBMCs and mDCs but not from FL-derived APCs or pDCs (Supplementary Figs. 3B and C and data not shown). CpGs are agonists of TLR9 and CpG-A, -B, and -C induced high levels of IFNα from pDCs (Supplementary Fig. 3D). Interestingly, Pam3CSK4 induced significant amount of IFNα from pDCs, when used at 1 μg/ml (Supplementary Fig. 3D), suggesting that at high concentration, it could have an antitumor effect. These results suggested that Pam3CSK4, flagellin, and CpGs do not induce significant amount of IL-10 and IFNα from human APCs at concentrations that block Treg suppressive function.
Triggering OX40 receptor enhanced effector T cell function and blocked Treg suppression
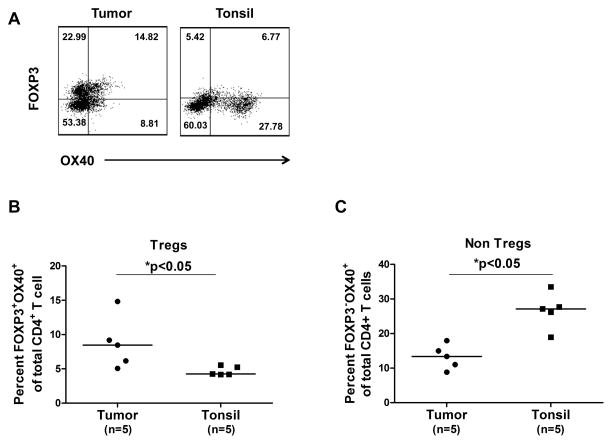
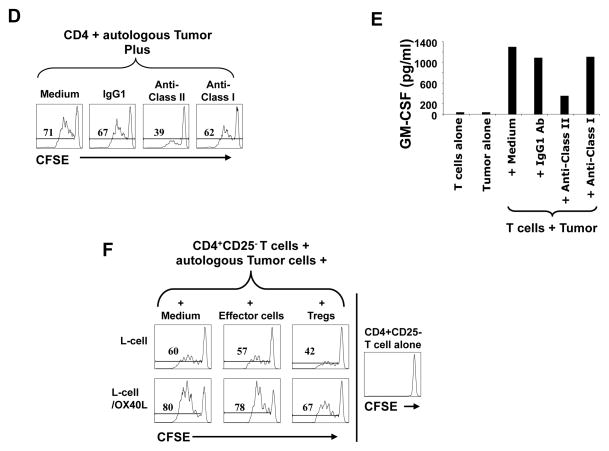
Evidence from our studies and the literature suggests that activating OX40 in T cells could promote effector T cell proliferation, shut down the generation and function of induced Tregs, and completely inhibit the function or survival of Foxp3+ natural Tregs.22, 23, 25, 34 Here, we evaluated whether activating OX40 induces similar effects on FL-derived Tregs and effector T cells. First, we found that OX40 is expressed on both Foxp3+ and Foxp3- CD4+ T cells in FL tumors (Fig. 5A). Interestingly, FL tumors had higher percentage Foxp3+OX40+ cells of total CD4+ T cells compared to those from tonsil. In contrast, FL tumors had lower percentage of Foxp3-OX40+ cells of total CD4+ T cells compared to those from tonsil (Figs. 5B and C). Next, we showed that CD4+CD25- intratumoral effector T cells proliferated and produced GM-CSF in response to autologous FL tumor cells (Figs. 5D and E). The proliferation and cytokine production was inhibited by anti-MHC class II but not anti-MHC class I blocking antibodies suggesting that the CD4+ effector T cells are responding to antigens presented in the context of MHC class II molecules. Lastly, we observed that OX40 triggering by OX40 ligand expressing L cells enhanced antitumor effector T cell proliferation (increased from 60% to 80%) and reversed the suppressive function of intratumoral FL Tregs (effector T cell proliferation increased from 42% to 67%) (Fig. 5F). These results suggest that activating OX40 could restore the function of antitumor effector T cells in the presence of immunosuppressive Tregs from FL tumors.
Figure 5. Triggering OX40 enhanced effector T cell function and blocked Treg suppression in FL.
A–C) Expression of OX40 was determined by flow cytometry on Foxp3+ (A,B) and Foxp3- (A,C) CD4+ T cells in FL tumors and tonsils. Representative data (A) and data from 5 FL tumors and tonsils (B,C) are shown. Horizontal bars indicate median values for each group. P values were calculated by Wilcoxon rank-sum test. D and E) CFSE-labeled (D) or unlabeled (E) intratumoral CD4+CD25- effector T cells were cultured with autologous CD3-depleted FL tumor-derived antigen-presenting cells in the presence or absence of anti-MHC Class I or II antibodies or isotype control antibody. Proliferation was assessed by CFSE dilution after 4 days (D) and GM-CSF production was measured in the culture supernatants after 24 hours (E). F) Effector T cells were stimulated as in panel D and cultured with parental L cells or L cells expressing OX40 ligand in the presence or absence of autologous intratumoral Tregs. Numbers shown in each histogram indicate the percentage of proliferating effector T cells (D, F).
TLR ligands synergized with OX40 signaling to enhance T cell proliferation and block Treg function
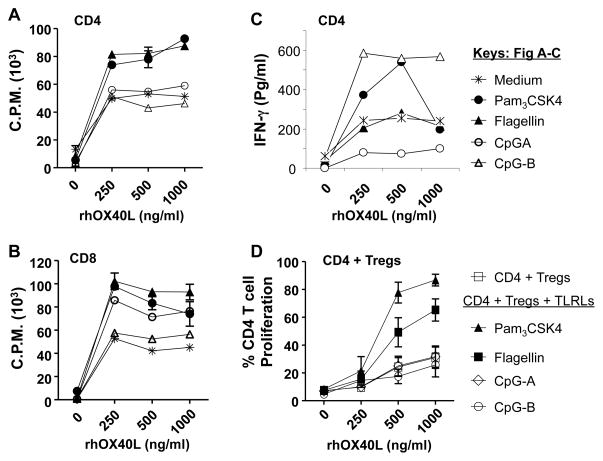
Next, we determined whether OX40 signaling would synergize with TLR signaling to further enhance naïve CD4+ and CD8+ T cell proliferation. We tested for synergy in the presence of suboptimal doses of TLR ligands, a fixed amount of plate-bound anti-CD3, and increasing concentrations of plate-bound rhOX40L. We found that both Pam3CSK4 and flagellin synergized with 250 ng/ml of rhOX40L to enhance the proliferation of polyclonally stimulated CD4+ and CD8+ effector T cells isolated from healthy donors (Figs. 6A and B). There was no further increase in proliferation at higher concentrations of rhOX40L. In addition to enhancement of T cell proliferation, Pam3CSK4 and CpG-B but not flagellin were able to synergize with rhOX40L to increase the production of IFN-γ by CD4+ T cells (Fig. 6C). Next, we determined whether the combination could block natural Treg suppression of autologous naïve CD4+ T cell proliferation. We found that both Pam3CSK4 and flagellin synergized with 1000 ng/ml of rhOX40L to enhance naïve CD4+ T cell proliferation even in the presence of Tregs (Fig. 6D). These results suggest that activating OX40 in combination with TLRLs 1/2 or 5 is very effective in blocking the suppressive function of Tregs and enhancing the proliferation of effector T cells.
Figure 6. OX40 signaling synergizes with TLRLs to enhance T cell proliferation and block Treg function.
A–C) Healthy donor CD4+ (A,C) and CD8+ (B) effector T cells were stimulated with plate-bound anti-CD3 and various concentrations of rhOX40L in the presence or absence of TLRLs as described in materials and methods. The concentrations of TLRL tested were: Pam3CSK4 (100 ng/ml); Flagellin (FLA-ST ultrapure grade, 100 ng/ml); and CpG-A/B (2.5 μM). Proliferation was assessed by 3H thymidine incorporation (A,B) and IFN-γ production was measured in culture supernatants by ELISA (C). D) CFSE-labeled naïve CD4+ T cells were stimulated as in panel A in the presence or absence of autologous natural Tregs isolated from healthy donors and percentage of proliferating CD4+ T cells was determined by CFSE dilution. Data are representative of results from one of three donors tested. Proliferation of effector T cells was significantly enhanced in the presence of Pam3CSK4 and Flagellin (P <0.05 compared to control). P values were calculated by two-way ANOVA test.
DISCUSSION
Here, we showed that Tregs persisted in high numbers in peripheral blood even after induction of complete clinical remission with standard cyclophosphamide and doxorubicin containing chemotherapy. This result was unexpected as metronomic doses of cyclophosphamide were previously shown to reduce the number of Tregs in cancer patients.35 However, it is possible that the higher doses of cyclophosphamide used in our patients may not affect Treg numbers. Like intratumoral Tregs, peripheral blood Tregs were highly immunosuppressive and may potentially get recruited to the tumor site36 and suppress antitumor T cells. Indeed, Navarrete et al recently demonstrated that patients with FL and other indolent B-cell lymphomas have impaired immune responses to hepatitis B vaccination.37 Collectively, these results suggest that a state of peripheral tolerance is present at baseline and after induction of complete clinical remission and strategies that deplete or block Treg function might enhance the efficacy of therapeutic vaccines and other immunotherapeutic strategies including anti-PD-1 antibody therapy28 in FL patients.
In contrast to prior studies where FL Tregs and effector T cells were stimulated polyclonally,8–10 we used a completely autologous system and evaluated the effect of Tregs on effector T cells stimulated with autologous tumor cells. Furthermore, to mimic the tumor microenvironment and to include various types of potentially tolerizing tumor-infiltrating APCs such as dendritic cells, myeloid cells, and other stromal cells, we used autologous T cell-depleted tumor samples as APCs in our assays. Using these stringent conditions, we found that TLRLs Pam3CSK4, Flagellin, and CpG-B as well as OX40 signaling potently inhibited Treg function in FL. However, the mechanism of suppression of Tregs by TLRLs and OX40 signaling may be different. TLR signaling could reduce the function of Tregs by directly acting on Tregs or indirectly via APCs. Activation of TLR2 may promote Treg differentiation into a Th17-like phenotype, induce IL-6 and IL-17, and reduce their suppressive function.38 Activation of TLRs on APCs induces their maturation and leads to secretion of pro-inflammatory cytokines such as IL-6 and IL-1 that may modulate effector T cell and Treg function.39 In contrast, OX40 signaling acts directly on T cells making effector T cells resistant to suppression by Tregs, or blocks Treg function by inhibiting IL-10 secretion or by inducing apoptosis.40 Our results suggest that the agents identified in this study might be useful for reversing the suppressive effects of Tregs and may be used as adjuvants to enhance the efficacy of therapeutic vaccines and possibly other immunotherapeutic and chemotherapeutic strategies in FL. Furthermore, these agents may also enhance the efficacy of preventive infectious disease vaccines in these patients.
In mice, Pam3CSK4 was reported to enhance induction of antigen-specific tolerance via IL-10 secretion.32 However, we were unable to induce significant secretion of IL-10 from human FL tumor samples, PBMCs, mDC, and pDC with Pam3CSK4 at a concentration of 1 μg/ml. Our findings are consistent with other reports that also failed to induce IL-10 secretion with Pam3CSK4 in human PBMCs.41 These results suggest that there is species associated difference between mouse and human in response to Pam3CSK4 and that it might be useful to stimulate effector T cell function and proliferation in humans. In particular, Pam3CSK4 could augment TCR stimulation-induced IL-2 production from CD4+ T cells.42 These results are also supported by the observation that BLP, a TLR1/TLR2 agonist enhanced cytotoxic effector T cell function, blocked Treg function, and led to tumor regressions in mouse models of carcinoma, leukemia, and melanoma.43
Preclinical models indicate that combinations of TLR agonists or the combination of a TLR agonist with other immune modulators are superior to single use in vivo.44, 45 Furthermore, a combination of CpG, anti-CTLA-4, and anti-OX40 treatments cured lymphoma in mice without the need for chemotherapy.16 Our results expand the list of TLR ligands that can be used for FL and suggest that TLR ligands Pam3CSK4, Flagellin, and CpG-B are potent stimulators of effector T cell proliferation and also block suppressive function of Tregs. These TLR ligands synergized with each other to further boost antitumor immune responses. We have previously shown that OX40 signaling can shut down IL-10-production by Tr1 cells.22 Others have shown that OX40 signaling can promote expansion and survival of effector memory T cells as well as block TGF-β-induced Tregs.21, 23 Since TLR ligands may potentially dampen immunity during late phases of the immune response by triggering release of anti-inflammatory cytokines, using them in combination with OX40 signaling may be critical. Indeed, our finding that OX40 activation promotes effector T cell proliferation, inhibits FL Treg function, and synergizes with TLR ligands in blocking Treg function suggests that these reagents may be used for developing combination immunotherapy for FL patients. Whether these agents also suppress the induction of Tregs by lymphoma tumor cells11, 46 needs to be determined.
Here, we also demonstrated for the first time that high numbers of peripheral blood Tregs at baseline was associated with poor response to therapy and/or inferior PFS in FL after chemotherapy or immunotherapy (Fig. 3). If confirmed in additional studies, monitoring peripheral blood Tregs by flow cytometry may serve as a useful prognostic marker for FL patients. The ease of monitoring Tregs through a simple blood test is especially appealing as opposed to the requirement for tissue biopsies for other prognostic factors. Wahlin et al recently showed that higher levels of total CD4+ T cells in blood predicted superior treatment response and overall survival in FL.47 As our results suggest that peripheral blood Tregs may adversely affect clinical outcome, additional studies are needed to fully characterize the prognostic significance of various CD4+ T cell subsets in FL.48
The unfavorable outcome we observed with peripheral blood Tregs is in contradiction with other reports that showed a favorable prognosis with high numbers of Tregs at the tumor site in FL patients.12, 13 A potential explanation for these differing results may be provided based on the recent discovery that Tregs are polarized by various cues in their microenvironment and differentiate into distinct functional subsets to regulate diverse immune cells. For example, Tregs that express transcriptions factors T-bet, IRF4, STAT3, and Bcl6 appear to control TH1-, TH2-, TH17-, and TFH-mediated immune responses, respectively.49–52 Therefore, it is possible that intratumoral Tregs may contain multiple subsets of Tregs: the TH1/TC1-suppressing subset that may exert protumor effects and the follicular regulatory T cell (TFR) subset52, 53 that may inhibit tumor-promoting TFH and also directly suppress tumor B cell proliferation. Thus, high numbers of TFR in the tumor may have antitumor effects and confer a favorable prognosis in FL. In contrast, it is possible that peripheral blood Tregs are predominantly polarized towards suppressing antitumor TH1/TC1 responses and thus confer an unfavorable prognosis.
In conclusion, our results show that high numbers of peripheral blood Tregs at baseline adversely affect clinical outcome in FL patients and a state of peripheral tolerance is present even after induction of clinical remission with standard chemotherapy. Agents that activate TLRs 1/2, 5, and 9, and OX40 can inhibit the immunosuppressive functions of FL Tregs and may be used alone or in combination to develop novel immunotherapeutic strategies and improve clinical outcome in these patients.
Supplementary Material
Novelty and Impact.
We show that intratumoral and peripheral blood regulatory T cells (Tregs) suppress autologous antitumor effector T cells in follicular lymphoma and peripheral blood Treg numbers may serve as a prognostic marker in these patients. Triggering Toll-like receptors and OX40 reversed the immunosuppressive effects of Tregs suggesting that such agents may enhance the efficacy of immunotherapeutic strategies and improve clinical outcome. These agents may also serve as adjuvants for infectious diseases vaccines in these patients.
Acknowledgments
We thank Karen Ramirez and Zhiwei He for cell sorting and support. We thank Melissa Wentz for careful reading of the manuscript. This work was supported by the National Institutes of Health grants R01AI091130 (Y-J.L.); R01CA155143 (S.S.N. and R.E.D.); R21 CA143785 (SSN); Lymphoma SPORE P50CA136411 (K.S.V); Doris Duke Charitable Foundation Clinical Scientist Development Award 2008040 (S.S.N.); Leukemia and Lymphoma Society Translational Research Grant 6255-11 (S.S.N.); The University of Texas MD Anderson Cancer Center Institutional Research Grant Program (S.S.N.); KECK foundation 01, pp-4; and the Leukemia and Lymphoma Society Specialized Center of Research grant 7262-08 (L.W.K.). The Monoclonal Antibody Core Facility and the Flow Cytometry Core Facility at M. D. Anderson Cancer Center are supported by Cancer Center Support Grant P30CA16672 (National Institutes of Health).
Abbreviations
- ANOVA
Analysis of variance
- APC
Antigen-presenting cell
- BLP
Bacterial lipoproteins
- CFSE
Carboxyfluorescein succinimidyl ester
- CR
Complete response
- CRu
Complete response unconfirmed
- CT
Computed tomography
- DC
Dendritic cell
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence activated cell sorting
- FL
Follicular lymphoma
- FLA-ST
Flagellin Salmonella typhimurium
- FLIPI
Follicular Lymphoma International Prognostic Index
- Foxp3
Forkhead box P3
- GITR
Glucocorticoid-induced tumor necrosis factor-related
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IFN
Interferon
- IL
Interleukin
- LPS
Lipopolysaccharide
- mDC
Myeloid DC
- MHC
Major histocompatibility complex
- ND
Normal donor
- nTregs
Natural regulatory T cells
- PACE
Prednisone, doxorubicin, cyclophosphamide, and etoposide
- PBMC
Peripheral blood mononuclear cells
- PD-1
Programmed death 1
- pDC
Plasmacytoid DC
- PFS
Progression-free survival
- PR
Partial response
- rhCD40L
Recombinant human CD40 ligand
- rhOX40L
Recombinant human OX40 ligand
- SD
Stable disease
- TC1
Type 1 cytotoxic T cell
- TCR
T-cell receptor
- TFH
Follicular helper T cells
- TFR
Follicular regulatory T cell
- TGF-β
Transforming growth factor, beta
- TH
T helper
- TLR
Toll-like receptor
- TLRL
Toll-like receptor ligand
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
- Tr1
Type 1 regulatory T cells
- Tregs
Regulatory T cells
Footnotes
Disclosures: The authors declare that there are no conflicts of interests.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–18. [PubMed] [Google Scholar]
- 2.Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL, Jaffe ES, Creekmore SP, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–7. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 3.Neelapu SS, Baskar S, Gause BL, Kobrin CB, Watson TM, Frye AR, Pennington R, Harvey L, Jaffe ES, Robb RJ, Popescu MC, Kwak LW. Human autologous tumor-specific T-cell responses induced by liposomal delivery of a lymphoma antigen. Clin Cancer Res. 2004;10:8309–17. doi: 10.1158/1078-0432.CCR-04-1071. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–26. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 5.Park HJ, Neelapu SS. Developing idiotype vaccines for lymphoma: from preclinical studies to phase III clinical trials. Br J Haematol. 2008;142:179–91. doi: 10.1111/j.1365-2141.2008.07143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, McGaughey DS, Jaffe ES, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–46. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilchey SP, De A, Rimsza LM, Bankert RB, Bernstein SH. Follicular lymphoma intratumoral CD4+CD25+GITR+ regulatory T cells potently suppress CD3/CD28-costimulated autologous and allogeneic CD8+CD25- and CD4+CD25- T cells. J Immunol. 2007;178:4051–61. doi: 10.4049/jimmunol.178.7.4051. [DOI] [PubMed] [Google Scholar]
- 10.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–70. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 11.Ai WZ, Hou JZ, Zeiser R, Czerwinski D, Negrin RS, Levy R. Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. Int J Cancer. 2009;124:239–44. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–64. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 13.Alvaro T, Lejeune M, Salvado MT, Lopez C, Jaen J, Bosch R, Pons LE. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24:5350–7. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- 14.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115:289–95. doi: 10.1182/blood-2009-07-235598. [DOI] [PubMed] [Google Scholar]
- 15.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L, Levy R. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–8. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–52. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 18.Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27:181–9. doi: 10.1038/sj.onc.1210906. [DOI] [PubMed] [Google Scholar]
- 19.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–9. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Davidson TS, Huter EN, Shevach EM. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J Immunol. 2009;183:4458–66. doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–91. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–43. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 24.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–16. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–15. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 26.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, Miller TP, LeBlanc M, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–69. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 28.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, Feng L, Baladandayuthapani V, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. The lancet oncology. 2013 doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee ST, Liu S, Radvanyi L, Sukhumalchandra P, Molldrem JJ, Wieder ED, Hwu P, Liu YJ, Kwak LW, Lizee G, Neelapu SS. A novel strategy for rapid and efficient isolation of human tumor-specific CD4(+) and CD8(+) T-cell clones. J Immunol Methods. 2008;331:13–26. doi: 10.1016/j.jim.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 32.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol. 2005;174:7558–63. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki S, Okada K, Maruyama A, Matsumoto M, Yagita H, Seya T. TLR2-dependent induction of IL-10 and Foxp3+ CD25+ CD4+ regulatory T cells prevents effective anti-tumor immunity induced by Pam2 lipopeptides in vivo. PLoS One. 6:e18833. doi: 10.1371/journal.pone.0018833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–39. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawal S, Chu F, Zhang M, Park HJ, Nattamai D, Kannan S, Sharma R, Delgado D, Chou T, Lin HY, Baladandayuthapani V, Luong A, et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J Immunol. 2013;190:6681–93. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarrete MA, Heining-Mikesch K, Schuler F, Bertinetti-Lapatki C, Ihorst G, Keppler-Hafkemeyer A, Dolken G, Veelken H. Upfront immunization with autologous recombinant idiotype Fab fragment without prior cytoreduction in indolent B-cell lymphoma. Blood. 2011;117:1483–91. doi: 10.1182/blood-2010-06-292342. [DOI] [PubMed] [Google Scholar]
- 38.Nyirenda MH, Sanvito L, Darlington PJ, O’Brien K, Zhang GX, Constantinescu CS, Bar-Or A, Gran B. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–90. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 39.Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–58. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 40.Voo KS, Bover L, Harline ML, Vien LT, Facchinetti V, Arima K, Kwak LW, Liu YJ. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J Immunol. 2013;191:3641–50. doi: 10.4049/jimmunol.1202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor RC, Richmond P, Upham JW. Toll-like receptor 2 ligands inhibit TH2 responses to mite allergen. J Allergy Clin Immunol. 2006;117:1148–54. doi: 10.1016/j.jaci.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Asai H, Iijima H, Matsunaga K, Oguchi Y, Katsuno H, Maeda K. Protein-bound polysaccharide K augments IL-2 production from murine mesenteric lymph node CD4+ T cells by modulating T cell receptor signaling. Cancer Immunol Immunother. 2008;57:1647–55. doi: 10.1007/s00262-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Luo F, Cai Y, Liu N, Wang L, Xu D, Chu Y. TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol. 186:1963–9. doi: 10.4049/jimmunol.1002320. [DOI] [PubMed] [Google Scholar]
- 44.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature immunology. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. The Journal of clinical investigation. 2010;120:607–16. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25 T cells. Blood. 2007;110:2537–44. doi: 10.1182/blood-2007-03-082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wahlin BE, Sundstrom C, Holte H, Hagberg H, Erlanson M, Nilsson-Ehle H, Linden O, Nordstrom M, Ostenstad B, Geisler CH, de Brown PN, Lehtinen T, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4136–44. doi: 10.1158/1078-0432.CCR-11-0264. [DOI] [PubMed] [Google Scholar]
- 48.Christopoulos P, Pfeifer D, Bartholome K, Follo M, Timmer J, Fisch P, Veelken H. Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Blood. 117:3836–46. doi: 10.1182/blood-2010-07-299321. [DOI] [PubMed] [Google Scholar]
- 49.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–6. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature medicine. 2011;17:983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ame-Thomas P, Le Priol J, Yssel H, Caron G, Pangault C, Jean R, Martin N, Marafioti T, Gaulard P, Lamy T, Fest T, Semana G, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011 doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.