Abstract
Background
Patients with chronic rhinosinustis (CRS) have significant quality-of-life (QOL) improvements following endoscopic sinus surgery (ESS). These improvements remain stable and persist between 6-months and 20-months as measured by the Rhinosinusitis Disability Index and the Chronic Sinusitis Survey. There has yet to be an evaluation of the longitudinal stability of the Sinonasal Outcome Test-22 (SNOT-22) after ESS in patients with CRS.
Methods
Adults with medically recalcitrant CRS who were considered surgical candidates were enrolled in a prospective, multi-center, observational cohort study from February 2011 to February 2013. Baseline evaluation of subjects included assessment of clinical characteristics, measures of CRS-specific disease severity, and QOL evaluation using the SNOT-22. Subjects were then re-evaluated at approximately 6-month, 12-month, and 18-month intervals postoperatively. Data was analyzed using repeated measures ANOVA with Bonferroni corrections for matched pairwise comparisons.
Results
110 patients completed baseline evaluations and follow-up for all three postoperative time points. Significant improvement in SNOT-22 scores was seen between baseline and 6-months across both SNOT-22 total and subdomain scores (p<0.001). There was no statistically significant difference between the 6-month, 12-month, and 18-month time points in the total SNOT-22 score or its domains (p≥0.125) for both the entire cohort or subgroups (p≥0.077).
Conclusions
Postoperative improvement in CRS-specific QOL and symptom severity, as measured by the SNOT-22, suggest stability and durability between 6-months and 18-months. Further study on the longitudinal stability of the SNOT-22 past the 18-month timeframe will help further refine clinical study of CRS and provide further understanding of temporal improvements following ESS.
MeSH Key Words: sinusitis, therapeutics, quality of life, outcome assessment, endoscopy
INTRODUCTION
The interval change in patient quality of life (QOL) continues to be the most widely used metric of investigation to quantify outcomes after treatment for CRS.1 Accurate determination of the minimum time interval required to capture significant and clinically meaningful change allows for efficient use of limited study resources. Multi-institutional prospective cohort data has shown that two CRS disease-specific QOL measures, the Rhinosinusitis Disability Index (RSDI) and the Chronic Sinusitis Survey (CSS), are stable between 6- and 20-months after endoscopic sinus surgery (ESS).2 Additionally, a longitudinal investigation comparing patients with CRS electing surgical versus medical management demonstrated QOL stability using these same outcome measures.3
The Sinonasal Outcome Test-22 (SNOT-22) has emerged as a commonly used CRS-specific QOL metric and has been evaluated as the primary outcome in prospective studies.4–6 Longitudinal SNOT-22 data has similarly been reported across a variety of disease processes (e.g., CRS, anterior skull base neoplasms, cystic fibrosis associated sinusitis);7–11 however, the majority of these studies are retrospective in nature and derived from a single-institution experience. One notable exception is the English and Welsh cohort, which has investigated five year follow-up SNOT-22 scores undergoing ESS with and without nasal polyposis.4 Even though that investigation incorporated one of the largest real-life cohorts utilizing the SNOT-22 instrument, the study was limited and potentially biased by varied responses rates for each longitudinal time point. Additionally, surgical extent is notoriously more conservative in the National Health System as is acknowledged in prior reporting of this cohort as well as reflected in the operative times when compared to North American surgical standards.12
The present study seeks to characterize the longitudinal trends of post-intervention SNOT-22 scores however have yet to be prospectively evaluated to assess whether symptoms of CRS stabilize in a similar fashion in a North American patient population. Furthermore, the SNOT-22 instrument has been since found to contain five distinct sub-domains, which are differentially associated with treatment selection and are variably impacted by treatment interventions.13 The goal of the present study is to prospectively investigate whether patients report similar symptom improvement and stability after surgical management, as measured by SNOT-22 total and sub-domain scores, and to identify a minimal length of time required to achieve that improvement.
MATERIALS and METHODS
Patient Population and Inclusion Criteria
Adult patients with a confirmed diagnosis of CRS, as determined by the American Academy of Otolaryngology—Head and Neck Surgery Foundation consensus guidelines,14 were prospectively enrolled into an on-going observational cohort study across five academic rhinology practices (Oregon Health & Science University {Portland, OR.} the Medical University of South Carolina {Charleston, SC.}, Stanford University {Palo Alto, CA.}, University of Calgary {Calgary, Alberta, Canada},). Preliminary findings from this cohort have been previously reported.5,6,15–20 Inclusion criteria also required prior treatment with at least a 2-week course of broad-spectrum or culture directed antibiotics in addition to either nasal steroid spray for at least 3-weeks or at least a 7-day course of systemic steroids.
Participants that failed to improve with medical therapy were identified as candidates for ESS and were enrolled following counseling surrounding treatment options. Participants electing to proceed with ESS were included in the final analysis. Surgical management plans were tailored by the enrolling surgeon at each site based on reported disease burden and both radiologic and visual assessments of disease severity and location.
Study Data Collection and Management
Study participants were required to complete baseline surveys and provide informed consent in English. Demographic, medical comorbidity, and disease severity measures were collected during the initial screening and enrollment visit. All study data was de-identified and collected at each site using standardized clinical research forms, and manually transferred to a centralized database (Access 2007; Microsoft Inc., Redmond, WA.) for further analysis. Central review and study coordination was conducted at OHSU (eIRB #7198) while the Institutional Review Board at each enrollment site provided annual review and approval for all stud protocols.
Baseline evaluations included completion of the Sinonasal Outcome Test (SNOT-22),21 Lund-Kennedy nasal endoscopy scoring22 and Lund-Mackay scoring of computed tomography (CT) of the sinuses.23 Both endoscopy and CT scoring was performed by the enrolling surgeon at each site. Follow-up evaluations consisted of asking patients to complete the SNOT-22 instrument at 6-months, 12-months, and 18-months postoperatively either during routine follow-up appointments or via follow-up reminder mailings using the United States Postal Service with self-addressed return envelopes. Concurrent follow-up endoscopy examinations, with associated Lund-Kennedy scores, were collected at 6-month, 12-month, and 18-month intervals when feasible.
Exclusion Criteria
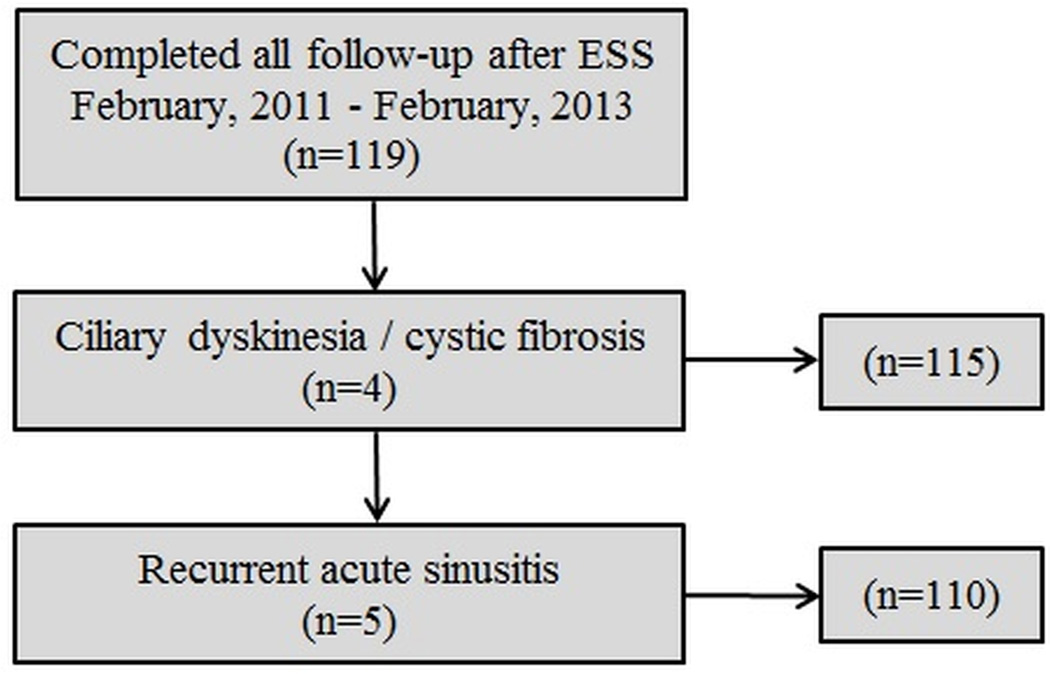
Subjects diagnosed with a current exacerbation of either recurrent acute sinusitis or CRS associated with either comorbid ciliary dyskinesia or cystic fibrosis were excluded from the final study cohort due to the heterogeneity of those underlying disease processes. Subjects were excluded from final analyses if they failed to complete baseline and all three follow-up evaluations at the appropriate intervals or if they had not yet entered into the 18-month follow-up window.
Primary Outcome Measurements
The SNOT-22 is a validated, 22-item treatment outcome measure applicable to chronic sinonasal conditions (©2006, Washington University, St. Louis, MO, USA). Higher scores on the SNOT-22 survey items suggest worse patient functioning or symptom severity (total score range: 0–110). Scoring is conducted via Likert scale responses whereas 0=”No problem”, 1=”Very mild problem”, 2=”Mild or slight problem”, 3=”Moderate problem”, 4=”Severe problem”, and 5=”Problem as bad as it can be”. Individual sub-domain scores of the SNOT-22 were operationalized based upon prior analysis of the SNOT-22 that revealed 5 underlying sub-domains.19 The 22-items of the SNOT-22 survey were re-categorized and summarized into five distinct domains including: rhinologic symptoms, extra-nasal rhinologic symptoms, ear/facial symptoms, psychological dysfunction, and sleep dysfunction (Table 1).
Table 1.
Categorized survey items for separate domains of the SNOT-22 instrument
| SNOT-22 Domains: | Survey Items: | Score Range: |
|---|---|---|
| Rhinologic Symptoms | #1, #2, #3, #6, #21, #22 | 0–30 |
| Extra-Nasal Rhinologic Symptoms | #4, #5, #6 | 0–15 |
| Ear/Facial Symptoms | #2, #7, #8, #9, #10 | 0–25 |
| Psychological Dysfunction | #14, #15, #16, #17, #18, #19, #20 | 0–35 |
| Sleep Dysfunction | #11, #12, #13, #14, #15 | 0–25 |
SNOT-22, 22-item Sinonasal Outcome Test
Statistical Analysis
All statistical analyses were completed using a commercially available software application (SPSS v.22; IBM Corporation, Armonk, NY.). Descriptive analysis was used to evaluate means [standard deviations] and frequencies of all demographic, medical comorbidity, and disease severity measures. Two-sided matched pair t tests were used to determine significant changes in continuous SNOT-22 scores between two time points. Repeated measures analysis of variance (ANOVA) with a Greenhouse-Geisser correction was used to test within-subjects effects using either Level III or Level IV models. When F-tests identified overall significant associations, post hoc Bonferroni adjustments were used to evaluate matched pairwise comparisons between time points. All statistical analysis assumed a 0.050 error probability.
RESULTS
Final Study Population
Between February 2011, and February, 2013 a total of 119 participants met inclusion criteria and completed all follow-up evaluations (Figure 1). A total of 9 subjects were excluded due to either ciliary dyskinesia, cystic fibrosis related sinusitis, or recurrent acute rhinosinusitis leaving 110 subjects for final analysis. Baseline demographics, medical comorbidities and disease severity measures are described in Table 2. Mean follow-up times for all three required 6-, 12-, and 18-month interval visits were 6.3[1.3], 12.3[1.6], and 18.7[1.9] months, respectively.
Figure 1.
Final cohort selection after inclusion and exclusion criteria
Table 2.
Baseline patient characteristics for subjects electing endoscopic sinus surgery (n=110)
| Demographics: | Mean [SD] | N (%) |
|---|---|---|
| Age (years) | 56.7 [12.2] | |
| Males | 50 (45.5) | |
| Females | 60 (54.5) | |
| White / caucasian | 99 (90.0) | |
| Hispanic / Latino | 6 (5.5) | |
| Medical comorbidity: | ||
| Asthma | 39 (35.5) | |
| Nasal polyposis | 41 (37.3) | |
| Allergies (skin prick / RAST) | 40 (36.4) | |
| ASA intolerance | 10 (9.1) | |
| Depression | 19 (17.3) | |
| Current tobacco smoker | 3 (2.7) | |
| Previous sinus surgery | 59 (53.6) | |
| Baseline disease severity: | ||
| Computed tomography score | 12.8 [6.0] | |
| Endoscopy score | 6.2 [3.9] | |
| SNOT-22 total score | 52.8 [19.8] | |
| Rhinologic symptoms | 16.4 [6.4] | |
| Extra-nasal rhinologic symptoms | 9.0 [3.3] | |
| Ear / facial symptoms | 9.3 [5.1] | |
| Psychological dysfunction | 15.3 [8.7] | |
| Sleep dysfunction | 13.3 [7.3] |
SD, standard deviation; RAST, radioallergosorbent testing; ASA, acetylsalicyclic acid; SNOT-22, 22-item Sinonasal Outcome Test
Quality of life Outcome Trends
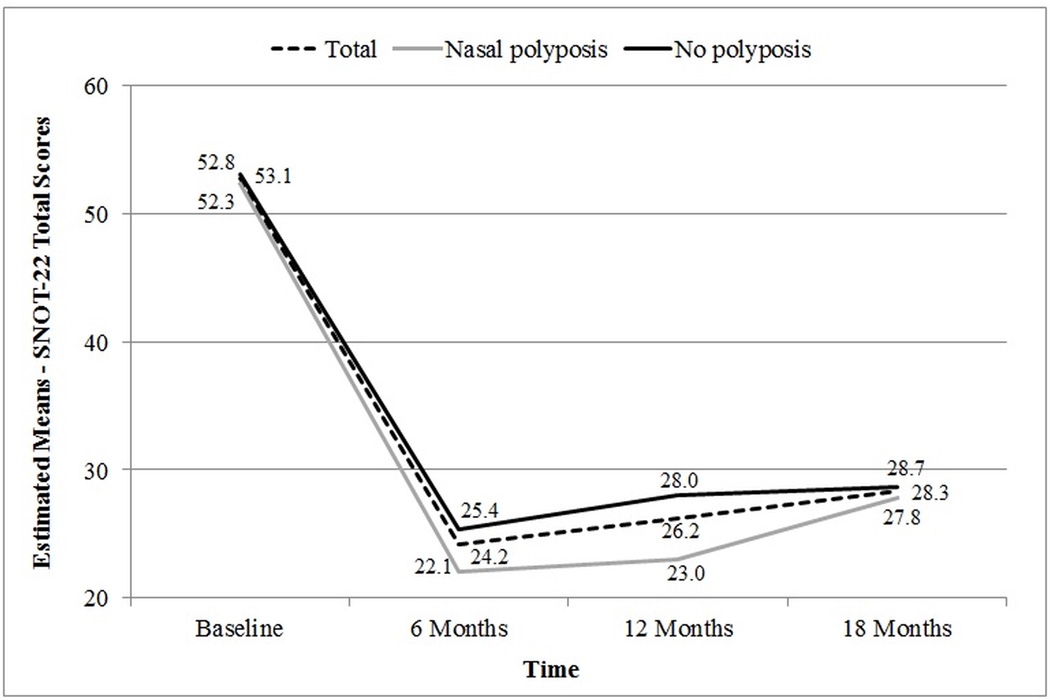
Longitudinal trends in average SNOT-22 aggregate scores for all subjects were found to significantly improve (F(2.43)=99.36; p<0.001) within the first 6-months after surgical intervention (Figure 2). Aggregate scores remained statistically similar between 6-, 12-, and 18-month follow-up (p≥0.061). Statistically significant improvements in SNOT-22 total scores were also found between baseline and 6-months post-intervention (Table 3) for all study subgroups with the exception of current tobacco smokers potentially due to limited sample size (n=3).
Figure 2.
Longitudinal trends in mean SNOT-22 aggregate scores for subjects with and without nasal polyposis.
Table 3.
Mean SNOT-22 total scores between baseline and 6-month follow-up time points (n=110)
| Characteristics: | Baseline Mean [SD] |
6 Month Follow-up Mean [SD] |
p-value |
|---|---|---|---|
| Males | 44.9 [17.3] | 23.3 [17.4] | <0.001 |
| Females | 59.4 [19.5] | 24.9 [17.4] | <0.001 |
| Asthma | 54.2 [21.5] | 24.2 [15.6] | <0.001 |
| Nasal polyposis | 52.3 [22.8] | 22.1 [14.4] | <0.001 |
| Allergies (skin prick / RAST) | 55.8 [18.4] | 24.6 [19.2] | <0.001 |
| ASA intolerance | 60.4 [23.7] | 30.7 [18.9] | 0.011 |
| Depression | 58.3 [14.7] | 31.1 [20.4] | <0.001 |
| Current tobacco smoker | 50.0 [7.0] | 9.0 [13.0] | 0.109 |
| Previous sinus surgery | 54.6 [19.4] | 29.0 [19.7] | <0.001 |
SD, standard deviation; RAST, radioallergosorbent testing; ASA, acetylsalicyclic acid; SNOT-22, 22-item Sinonasal Outcome Test
Global comparisons of post-treatment follow-up SNOT-22 total scores revealed no significant differences between 6-month, 12-month, and 18-month mean scores for any of the study subgroups with the exception of those study participants with nasal polyposis (22.1[14.4], 23.1[17.0], 27.8[19.6], at 6-,12-,18-months, respectively; F(1.92)=3.63; p=0.033). Post hoc pairwise comparisons revealed however that the average increase in SNOT-22 total scores in that subgroup between 6-month and 18-months was not significantly different at the conventional 0.050 alpha level (p=0.077; Table 4).
Table 4.
Mean post-treatment scores for SNOT-22 total scores (n=110)
| Characteristics: | 6 Month Follow-up Mean [SD] |
12 Month Follow-up Mean [SD] |
18 Month Follow-up Mean [SD] |
F-test statistic |
df | p-value |
|---|---|---|---|---|---|---|
| Males | 23.3 [17.4] | 24.8 [19.7] | 27.3 [18.3] | 1.82 | 1.97 | 0.168 |
| Females | 24.9 [17.4] | 27.4 [21.7] | 29.2 [21.5] | 1.85 | 1.97 | 0.163 |
| Asthma | 24.2 [15.6] | 26.8 [22.1] | 29.5 [22.0] | 2.26 | 1.89 | 0.115 |
| Nasal polyposis | 22.1 [14.4] | 23.1 [17.0] | 27.8 [19.6] | 3.63 | 1.92 | 0.033 |
| Allergies (skin prick / RAST) | 24.6 [19.2] | 26.4 [22.5] | 25.3 [20.1] | 0.32 | 1.95 | 0.724 |
| ASA intolerance | 30.7 [18.9] | 37.0 [28.3] | 35.4 [23.0] | 0.70 | 1.41 | 0.467 |
| Depression | 31.1 [20.4] | 29.1 [20.7] | 32.4 [17.3] | 0.31 | 1.53 | 0.676 |
| Current tobacco smoker | 9.0 [13.0] | 14.0 [18.5] | 18.0 [19.1] | 0.28 | 1.13 | 0.672 |
| Previous sinus surgery | 29.0 [19.7] | 31.0 [22.1] | 32.7 [21.5] | 1.25 | 1.99 | 0.289 |
SD, standard deviation; df, degrees of freedom; RAST, radioallergosorbent testing; ASA, acetylsalicyclic acid; SNOT-22, 22-item Sinonasal Outcome Test
Similar to overall SNOT-22 results, repeated measures ANOVA determined that mean SNOT-22 scores significantly improved (p<0.001) between baseline and 6-month follow-up for all five sub-domains (Table 5), while post hoc Bonferroni corrections found no significant differences between any two follow-up time points for rhinologic symptom scores (p≥0.212), extra-nasal rhinologic symptom scores (p≥0.597), ear/facial symptom scores (p≥0.125), psychological dysfunction scores (p≥0.374), or sleep dysfunction scores (p≥0.286).
Table 5.
Longitudinal trends in mean SNOT-22 sub-domain scores following endoscopic sinus surgery (n=110)
| SNOT-22 Sub-domains: | Baseline Mean [SD] |
6 Month Follow-up Mean [SD] |
12 Month Follow-up Mean [SD] |
18 Month Follow-up Mean [SD] |
F-test statistic |
df | p-value |
|---|---|---|---|---|---|---|---|
| Rhinologic symptoms | 16.4 [6.4] | 7.4 [6.0] | 7.8 [6.3] | 8.5 [6.1] | 94.66 | 2.33 | <0.001 |
| Extra-Nasal Rhinologic Symptoms | 9.0 [3.3] | 4.1 [3.5] | 4.3 [3.5] | 4.6 [3.6] | 101.99 | 2.54 | <0.001 |
| Ear/Facial Symptoms | 9.3 [5.1] | 4.1 [3.9] | 4.6 [4.6] | 5.0 [4.5] | 60.11 | 2.60 | <0.001 |
| Psychological Dysfunction | 15.3 [8.7] | 7.0 [7.0] | 7.6 [7.9] | 8.1 [7.8] | 55.84 | 2.29 | <0.001 |
| Sleep Dysfunction | 13.3 [7.3] | 6.8 [6.0] | 7.4 [6.9] | 7.9 [6.4] | 48.74 | 2.41 | <0.001 |
SD, standard deviation; df, degrees of freedom; SNOT-22, 22-item Sinonasal Outcome Test
Endoscopy Score Trends
Subjects with endoscopy examinations at all four study time points (n=24) were evaluated for longitudinal trends in mean Lund-Kennedy staging scores. Subjects were found with a mean baseline endoscopy score of 6.1[4.3], a 6-month mean score of 4.4[2.9], a 12-month mean score of 3.5[2.2], and 18-month mean score of 4.3[3.1]. While testing of within-subjects effects found a global improvement (F(2.17)=3.69; p=0.029), pairwise comparisons were only able to identify significant improvement between baseline and 12-month scores.
DISCUSSION
This investigation was able to prospectively identify improvement durability and stability of SNOT-22 score between 6-month and 18-months following ESS. There were no significant differences in SNOT-22 total scores between 6-month and 18-month follow-up time points. Similarly, sub-domains of the SNOT-22 were not significantly different between 6-month and 18-month following surgical intervention. When SNOT-22 scores were stratified by medical comorbidities, which have been previously associated with diminished QOL improvement, average survey scores remained stable over time. Mean SNOT-22 aggregate scores did increase, on average, between 6-month, 12-month, and 18-months by approximately 4 score units, however that was not statistically significant nor does it reflect a minimal clinically important difference which has been found in prior study to approximate 9 points.21
Although there has been prior investigation using the RSDI and CSS to track longitudinal trends CRS-specific QOL measures in a North American prospective cohort study,2 there has yet to be such an investigation into the SNOT-22. It was a common assumption that the SNOT-22 also has longitudinal stability beyond 6-months due to known correlations between the RSDI and SNOT-22.5,6,19,20,24 The present study serves as evidence that total and subdomain scores of the SNOT-22, as well as the symptoms they measure, have inherent postoperative stability between 6-months and 18-months in patients with CRS undergoing ESS. This data suggests that 6 month SNOT-22 data could be considered a surrogate for 12–18 month SNOT-22 scores. Short-term follow-up (6–24 months) in many cases can be beneficial due to increased cost associated with longer-term study and standard patient attrition in academic, tertiary practices. However, we acknowledge that this does not abrogate the need for well-designed long-term QOL outcomes. Ideally, given the chronicity of CRS, studies including follow-up between 5 – 10 years would allow for a more accurate and complete understanding of the efficacy of a given intervention as well as its impact on revision surgery rates.
A prospective clinical trial of CRS utilizing follow-up procedures greater than 18-months can be a challenging endeavor, but clinicians routinely encounter patients that underwent prior ESS many years previously. One retrospective cohort study investigating the rate of revision surgery in subjects with CRS with nasal polyposis found that the mean time to revision surgery was on average approximately 5 years.25 The present study identified that study participants with nasal polyposis had a rising burden of disease indicated by higher mean SNOT-22 scores, and although achieved statistical significance did not reach a clinically meaningful change over time, which has been reported to be approximately 9.0 points on the SNOT-22.21 However, in the context of the findings on long-term follow-up it’s conceivable that our data is demonstrating manifestations of rising sinonasal inflammation and polyp recurrence. Taken together, this data highlights the importance of identifying treatment strategies that might reduce the rates of revision surgery or at least increase the interval between surgical interventions in the subtype of CRS. Due to the lifetime impact of CRS, we need to develop innovative strategies to accurately investigate the long-term outcomes (e.g. between 5 – 10 years) following CRS-related interventions.
Extrapolation of these findings needs to be carefully considered by the clinician. The data presented here represent sample means, which may not reflect an individual’s particular course which may fall on either side of these sample means at a given time point. Furthermore, subjects with CRS can experience relapsing and remitting symptoms that may not have been captured at the studied time points.14 Some individuals may experience early (less-than 6-months) worsening of quality-of-life and need additional revision surgery, such as in the event of adverse healing that results in obstructed, symptomatic sinuses. In this event, nothing would be gained from waiting until 6 months.
These findings should also be carefully applied to future clinical study. For example, the longitudinal trends of the SNOT-22 have yet to be elucidated for continued medical therapy without surgical intervention as a treatment comparison cohort. There have been calls for long-term prospective studies for continued medical therapy,26 but ‘long-term’ has yet to be defined in an academic, tertiary care setting. Although, a difference of ~4 points on the aggregate SNOT-22 scores between 6-months and 18-months is not clinically meaningful on a 110 point scale, larger cohort sample size might achieve greater differences through regression to the mean. With regards to the subdomains of the SNOT-22 there has never been any description of what constitutes a clinically meaningful difference. Future study direction should also focus on QOL changes within the 6-month follow-up period using various shorter intervals (e.g., 1-week, 2-weeks, 1-month, 2-month, 3-month, etc). It is probable that even 6-months is gratuitously long and a higher resolution of early follow-up would also allow for investigation into interventions and comorbidities associated with expedited recovery after endoscopic sinus surgery.
CONCLUSION
Following ESS, SNOT-22 scores assessed at 6-month intervals significantly improve and remain stable up to 18 months after surgery. Further study on the longitudinal stability of the SNOT-22 past the 18-month timeframe will help further refine clinical study of CRS and provide further understanding of temporal improvements following ESS. Furthermore, study of an earlier time frame will help clarify the rate at which disease-specific QOL recovers after ESS.
Acknowledgments
Financial Disclosures: Timothy L. Smith, Jess C. Mace, Jeremiah A. Alt, and Zachary M. Soler are supported for this investigation by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD (R01 DC005805; PI/PD: TL Smith). Zachary M. Soler is also supported by another NIDCD grant (R03 DC013651-01) which is not affiliated with this investigation. Timothy L. Smith is a consultant for IntersectENT, Inc (Menlo Park, CA.), which is not affiliated with this investigation.
Footnotes
Conflict(s) of Interest: None
REFERENCES
- 1.Rudmik L, Smith TL. Quality of life in patients with chronic rhinosinusitis. Curr Allergy Asthma Rep. 2011;11(3):247–252. doi: 10.1007/s11882-010-0175-2. [DOI] [PubMed] [Google Scholar]
- 2.Soler ZM, Smith TL. Quality-of-life outcomes after endoscopic sinus surgery: How long is long enough? Otolaryngol Head Neck Surg. 2010;143(5):621–625. doi: 10.1016/j.otohns.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith TL, Kern R, Palmer JN, Schlosser R, Chandra RK, Chiu AG, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. Int Forum Allergy Rhinol. 2013;3(1):4–9. doi: 10.1002/alr.21065. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins C, Slack R, Lund V, Brown P, Copley L, Browne J. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009;119(12):2459–2465. doi: 10.1002/lary.20653. [DOI] [PubMed] [Google Scholar]
- 5.DeConde AS, Mace JC, Alt JA, Schlosser RJ, Smith TL, Soler ZM. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014;4(9):725–733. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeConde AS, Mace JC, Alt JA, Soler ZM, Orlandi RR, Smith TL. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol. 2015;5(1):36–45. doi: 10.1002/alr.21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransom ER, Doghramji L, Palmer JN, Chiu AG. Global and disease-specific health-related quality of life after complete endoscopic resection of anterior skull base neoplasms. Am J Rhinol Allergy. 2012;26(1):76–79. doi: 10.2500/ajra.2012.26.3713. [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas JG, da Fonseca VMG, Chen VG, Itamoto CH, Silva CAP da, Gregório LC, et al. Long-term outcomes of endoscopic sinus surgery for chronic rhinosinusitis with and without nasal polyps. Braz J Otorhinolaryngol. 2013;79(3):306–311. doi: 10.5935/1808-8694.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ElBadawey MR, Alwaa A, ElTaher M, Carrie S. Quality of life benefit after endoscopic frontal sinus surgery. Am J Rhinol Allergy. 2014;28(5):428–432. doi: 10.2500/ajra.2014.28.4063. [DOI] [PubMed] [Google Scholar]
- 10.Savastano V, Bertin S, Vittori T, Tripodi C, Magliulo G. Evaluation of chronic rhinosinusitis management using the SNOT-22 in adult cystic fibrosis patients. Eur Rev Med Pharmacol Sci. 2014;18(14):1985–1989. [PubMed] [Google Scholar]
- 11.Zhang Z, Adappa ND, Doghramji LJ, Chiu AG, Lautenbach E, Cohen NA, et al. Quality of life improvement from sinus surgery in chronic rhinosinusitis patients with asthma and nasal polyps. Int Forum Allergy Rhinol. 2014 doi: 10.1002/alr.21406. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Browne JP, Hopkins C, Slack R, et al. Health-related quality of life after polypectomy with and without additional surgery. Laryngoscope. 2006;116(2):297–302. doi: 10.1097/01.mlg.0000198338.05826.18. [DOI] [PubMed] [Google Scholar]
- 13.DeConde AS, Mace JC, Bodner T, Hwang PH, Rudmik L, Soler ZM, Smith TL. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld RM, Andes D, Neil B, Cheung D, Eisenberg S, Ganiats TG, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 15.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123(10):2341–2346. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(9):693–701. doi: 10.1002/alr.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123(10):2364–2370. doi: 10.1002/lary.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alt JA, Mace JC, Buniel MCF, Soler ZM, Smith TL. Predictors of Olfactory Dysfunction in Rhinosinusitis Using the Brief Smell Identification Test. Laryngoscope. 2014;124(7):E259–E266. doi: 10.1002/lary.24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140(8):712–719. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeConde AS, Barton MD, Mace JC, Smith TL. Can sinus anatomy predict quality of life outcomes and operative times of endoscopic frontal sinus surgery? Am J Otolaryngol. 2014 doi: 10.1016/j.amjoto.2014.08.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 22.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 23.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 24.Quintanilla-Dieck L, Litvack JR, Mace JC, Smith TL. Comparison of disease-specific quality-of-life instruments in the assessment of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2(6):437–443. doi: 10.1002/alr.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu AW, Ting JY, Platt MP, Tierney HT, Metson R. Factors affecting time to revision sinus surgery for nasal polyps: a 25-year experience. Laryngoscope. 2014;124(1):29–33. doi: 10.1002/lary.24213. [DOI] [PubMed] [Google Scholar]
- 26.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]