Abstract
Background
Provider-based research networks, such as the National Cancer Institute’s Community Clinical Oncology Program (CCOP), have been shown to facilitate the translation of evidence-based cancer care into clinical practice. As such, we compared utilization of laparoscopy and partial nephrectomy among patients with early-stage kidney cancer according to exposure to CCOP-affiliated providers.
Methods
Using linked SEER-Medicare data, we identified patients with T1aN0M0 kidney cancer treated with nephrectomy from 2000–2007. For each patient, we determined receipt of care from a CCOP physician or hospital and treatment with laparoscopy or partial nephrectomy. Adjusting for patient characteristics (e.g., age, gender, marital status) and other organizational features (e.g., community hospital, NCI-designated cancer center), we used multivariable logistic regression to estimate the association between each surgical innovation and CCOP affiliation.
Results
Over the study interval, we identified 1,578 (26.8%) patients treated by a provider with CCOP affiliation. Trends in laparoscopy and partial nephrectomy utilization remained similar between affiliated and non-affiliated providers (p≥0.05). Adjusting for patient characteristics, organizational features, and clustering, we noted no association between CCOP affiliation and the use of laparoscopy (OR 1.11, 95% CI 0.81–1.53) or partial nephrectomy (OR 1.04, 95% CI 0.82–1.32) despite the relatively higher receipt of these treatments in academic settings (p-values<0.05).
Conclusions
At a population-level, patients treated by providers affiliated with CCOP were no more likely to receive at least one of two surgical innovations for treatment of their kidney cancer, indicating perhaps a more limited scope to provider-based research networks as they pertain to translational efforts in cancer care.
Keywords: kidney neoplasm, translation research, diffusion of innovation, provider-based research networks, laparoscopy, nephrectomy
Introduction
While continued scientific advancement remains critical, the real-world dissemination and implementation of new medical knowledge plays an essential role in improving health for patients with cancer.1 Accordingly, key stakeholders, including the Institute of Medicine and the National Institutes of Health, have endorsed strategies designed to enhance the transfer of academic discovery into clinical practice.1–4 Provider-based research networks (PBRN), such as the National Cancer Institute’s Community Oncology Research Program (NCORP) and, its predecessor, the Community Clinical Oncology Program (CCOP)—act as one potential mechanism. These bidirectional collaborations between academic research centers and community physicians work to diversify clinical trial enrollment and have further facilitated the delivery of evidence-based care to patients with colon and breast malignancies, successfully traversing the “blue highways” of cancer research.3, 5–7
Although promising, data supporting CCOP as a conduit for dissemination and implementation have focused on interventions that also utilized these networks to complete trial enrollment.6–8 In genitourinary oncology, minimally invasive and nephron-sparing surgery mark two technological advances offering potential benefits to patients with early-stage kidney cancer. When compared to open radical nephrectomy, laparoscopy affords more rapid convalescence while nephron-sparing (i.e., partial nephrectomy) better preserves renal function, thereby reducing long-term renal insufficiency.9–13 Unlike examples in colon and breast cancer, data supporting these interventions come largely from single institution or population-based observational studies rather than large-scale, multi-institutional clinical trials. As such, it remains unclear whether the avenues provided by CCOP would facilitate the adoption of these new treatments now featured in evidence-based guidelines for T1N0M0 kidney cancer.14
In this context, we used linked SEER-Medicare data to examine the relationship between CCOP—a network of 3,500 physicians and 390 hospitals in 34 states—and the utilization of laparoscopy and partial nephrectomy among patients with early-stage kidney cancer during a period of provider adoption.15, 16 By evaluating this potential translation mechanism, we can begin to optimize dissemination strategies for new technology in the care of patients with malignant conditions.
Methods
Data Source
We used linked data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Medicare & Medicaid Services to identify patients diagnosed with non-urothelial T1aN0M0 kidney from 2000 through 2007. SEER is a population-based cancer registry that collects data regarding incidence, treatment, and mortality representative of the US population.17 The Medicare program provides primary health insurance for 97% of the US population aged 65 or older.18 Successful linkage with CMS claims is achieved for over 90% of Medicare patients whose cancer-specific data are tracked by SEER.18
Study cohort and utilization of laparoscopic or partial nephrectomy
After identifying a preliminary cohort of 11,696 patients, we excluded patients enrolled in a Medicare managed care plan or without continuous enrollment in Medicare from 12 months prior to 6 months following surgery (or until death) to yield 7,911 patients. Next, we used a validated algorithm to determine the specific surgical procedure for each subject based on inpatient and physician claims using International Classification of Diseases, 9th revision, Clinical Modification and Current Procedural Terminology codes.19 After excluding patients with claims for ablative therapies, we identified a final analytic cohort of 5,894 patients treated with one of four procedures: open radical nephrectomy, open partial nephrectomy, laparoscopic radical nephrectomy, or laparoscopic partial nephrectomy. For the purpose of our analyses, we created two binary indicator variables for laparoscopic nephrectomy (i.e., radical and partial) and partial nephrectomy (i.e., open and laparoscopic), respectively.
Provider-based research network exposure variables
To explore the relationship with provider-based research networks, these data were then linked through the unique identifiers on the claims to physician and hospital CCOP network data from NCI’s CCOP program. As described previously,6, 7 we used the Unique Physician Identification Number (UPIN) or hospital identifier on Medicare claims to identify physicians and hospitals affiliated with CCOP. We defined CCOP exposure as treatment by any CCOP affiliated physician or hospital during the index procedure claim.
As secondary exposure variables, we further created binary variables for each of the following organizational factors: 1) NCI-designated cancer center; 2) NCI Cooperative Groups with kidney cancer portfolios (e.g., American College of Surgeons Oncology Group, Eastern Cooperative Oncology Group, Southwest Oncology Group); and 3) community hospital with limited or no affiliation with medical schools.
Patient-level covariates
For each patient, we used SEER data to determine age, gender, geography, race, marital status, year of cancer diagnosis and tumor grade. We also measured pre-existing comorbidity by using a modification of the Charlson index to identify co-morbid conditions from inpatient and physician claims submitted during the 12 months prior to the index admission for kidney cancer surgery.20 In addition, we utilized the Medicare/Medicaid indicator of dual eligibility and a census-tract level estimate of high school education divided into equally-sized quartiles within each SEER region as measures of socioeconomic status.21, 22
Statistical Analysis
We first described the relationship between patient and organization characteristics and receipt of laparoscopy and partial nephrectomy using chi-squared testing. We then measured annual rates of laparoscopic and partial nephrectomy from 2000 through 2007 by dividing the number of patients treated with each approach, respectively, by the total number of patients treated surgically in each year and compared time trends according to CCOP affiliation.
Next, we fit separate multivariable logistic regression models to estimate the association between CCOP affiliation and each surgical approach (i.e., laparoscopy and partial nephrectomy). In each model, we controlled for patient characteristics (i.e., age, race, gender, marital status, socioeconomic position, comorbidity, tumor grade, year of diagnosis, and SEER region) and organizational factors (i.e., NCI-designated cancer center, NCI Cooperative Group membership, and community hospital status). Additionally, we used Generalized Estimation Equations (GEE) to account for the clustering of patients treated within the same hospitals.
Sensitivity Analyses
To assess the robustness of our findings, we performed additional sensitivity analyses. First, because our findings depend on our definition for CCOP affiliation, we developed a second continuous measure for the degree of CCOP exposure.6, 7 We defined a window of exposure time to represent the period in which patients may seek advice from physicians regarding choice of surgical procedure. As kidney cancer is diagnosed primarily by cross-sectional imaging, we defined the exposure time as the interval between abdominal computed tomography or magnetic resonance imaging and surgery. Because 60 percent of the study cohort had more than one imaging claim prior to surgery, we tested various exposure-time windows. Within each window, the proportion of claims from CCOP-affiliated physicians or hospitals out of all claims (CCOP and non-CCOP, cancer and non-cancer) was calculated to capture the degree of CCOP exposure. Second, since CCOP affiliation may be associated with other organizational features, especially NCI-designated cancer center and/or Cooperative Groups, we also refitted our models with the following organizational variables: 1) CCOP affiliation only; and 2) CCOP affiliation and community hospital.
All statistical testing was 2-sided, completed using SAS version 9.2 (SAS Institute, Cary, NC), and carried out at the 5% significance level. This study was deemed exempt by the Institutional Review Board at the University of North Carolina, Chapel Hill.
Results
From 2000 through 2007, we identified 2,090 (35.5%) and 1,759 (29.8%) patients with early-stage kidney cancer treated with laparoscopic nephrectomy and partial nephrectomy, respectively. As reported in Table 1, treatment with laparoscopic rather than open nephrectomy was associated with older age, higher socioeconomic status, more recent treatment years, and lower comorbidity score (p<0.05, Table 1). Those treated with partial as opposed to radical nephrectomy were more likely to be less than 75 years old, male, married, and treated in the latter portion of the study period (p<0.05, Table 1).
Table 1.
Patient and organizational characteristics according to receipt of laparoscopy and nephron-sparing surgery
| Covariate | Laparoscopic Nephrectomy (N=2,090) % |
Open Nephrectomy (N=3,804) % |
P- value |
Partial Nephrectomy (N=1759) % |
Radical Nephrectomy (N=4135) % |
P- value |
|---|---|---|---|---|---|---|
| Age at diagnosis | ||||||
| 65–69 | 24 | 24 | 0.045 | 29 | 22 | <0.001 |
| 70–74 | 27 | 30 | 32 | 27 | ||
| 75–79 | 28 | 28 | 26 | 28 | ||
| 80+ | 21 | 19 | 12 | 23 | ||
| Female | 45 | 44 | 0.209 | 41 | 46 | <0.001 |
| Race | ||||||
| Caucasian American | 89 | 89 | 0.469 | 88 | 89 | 0.214 |
| African American | 7 | 7 | 7 | 7 | ||
| Others | 4 | 3 | 4 | 3 | ||
| Married | 62 | 62 | 0.920 | 65 | 60 | <0.001 |
| Dual eligibility for Medicare/Medicaid | 12 | 14 | 0.017 | 13 | 13 | 0.569 |
| Non-high school graduate | ||||||
| Bottom Quartile | 29 | 24 | <0.001 | 29 | 24 | 0.002 |
| 2nd Quartile | 26 | 25 | 26 | 25 | ||
| 3rd Quartile | 24 | 25 | 24 | 25 | ||
| Top Quartile | 21 | 26 | 22 | 25 | ||
| Tumor Grade | ||||||
| Well differentiated | 17 | 17 | <0.001 | 19 | 16 | <0.001 |
| Moderately differentiated | 44 | 41 | 42 | 42 | ||
| Poorly differentiated / Undifferentiated | 19 | 16 | 14 | 18 | ||
| Unknown | 20 | 26 | 25 | 23 | ||
| Comorbidity Score ≥ 1 | 54 | 58 | 0.003 | 55 | 57 | 0.306 |
| Year of diagnosis | ||||||
| 2000 | 2 | 14 | <0.001 | 7 | 11 | <0.001 |
| 2001 | 4 | 14 | 8 | 12 | ||
| 2002 | 8 | 14 | 10 | 13 | ||
| 2003 | 12 | 13 | 12 | 13 | ||
| 2004 | 16 | 13 | 16 | 14 | ||
| 2005 | 18 | 11 | 15 | 13 | ||
| 2006 | 19 | 10 | 16 | 12 | ||
| 2007 | 21 | 10 | 16 | 13 | ||
| SEER Region | ||||||
| San Francisco | 4 | 3 | <0.001 | 4 | 3 | <0.001 |
| Connecticut | 9 | 6 | 7 | 7 | ||
| Detroit | 10 | 7 | 8 | 8 | ||
| Hawaii | 1 | 1 | 1 | 1 | ||
| Iowa | 6 | 8 | 7 | 8 | ||
| New Mexico | 1 | 3 | 1 | 2 | ||
| Seattle | 6 | 4 | 5 | 4 | ||
| Utah | 2 | 2 | 3 | 2 | ||
| Atlanta and Rural Georgia | 2 | 2 | 2 | 2 | ||
| San Jose | 2 | 2 | 2 | 2 | ||
| Los Angeles | 7 | 7 | 8 | 6 | ||
| Greater California | 16 | 16 | 14 | 16 | ||
| Kentucky | 10 | 10 | 10 | 10 | ||
| Louisiana | 8 | 8 | 7 | 8 | ||
| New Jersey | 17 | 20 | 21 | 18 | ||
| CCOP affiliation | 27 | 27 | 0.827 | 26 | 27 | 0.656 |
| Cooperative Group | 84 | 74 | <0.001 | 84 | 75 | <0.001 |
| NCI-designated Cancer Center | 13 | 8 | <0.001 | 19 | 6 | <0.001 |
| Community Hospital | 50 | 61 | <0.001 | 45 | 63 | <0.001 |
Abbreviations: SEER – Surveillance, Epidemiology, and End Results; CCOP – Community Clinical Oncology Program; NCI – National Cancer Institute.
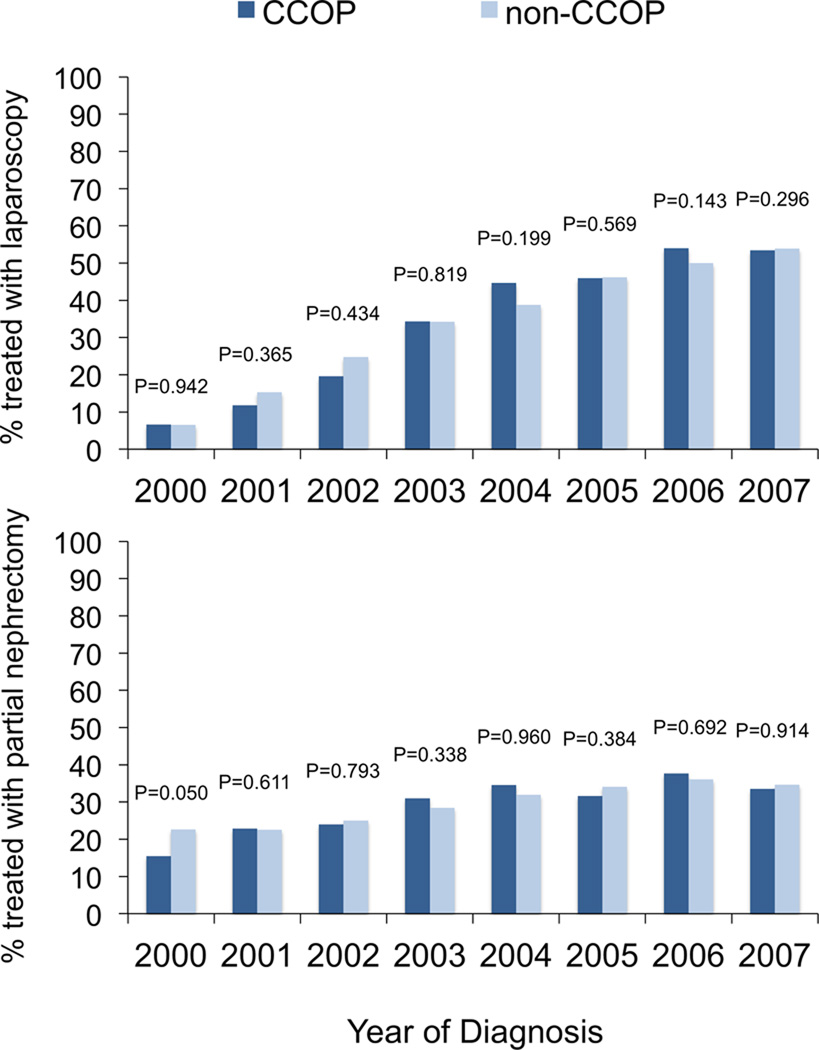
CCOP-affiliated physicians or hospitals treated 1,578 (26.8%) patients over the study interval. Utilization of laparoscopy and partial nephrectomy did not differ by CCOP exposure (p=0.827 and p=0.656, respectively). These technologies were also more common at NCI centers, cooperative group-affiliated organizations, and non-community hospitals (Table 1). Figure 1 depicts annual rates of laparoscopic nephrectomy and partial nephrectomy stratified by CCOP affiliation, demonstrating similar trends between groups.
Figure 1.
Proportion of patients treated with laparoscopy (A) and the proportion of patients treated with partial nephrectomy (B) according to CCOP affiliation. Proportions are derived from the number of patients treated with either laparoscopy or partial nephrectomy divided by the number of patients treated surgically for each given year. Temporal trends compared using chi-squared testing.
Adjusting for patient and organizational characteristics, we found no significant association between CCOP affiliation and either use of laparoscopic (OR 1.11, 95% CI 0.81–1.53) or partial nephrectomy (OR 1.04, 95% CI 0.82–1.32). In contrast, patients were more likely to received laparoscopic nephrectomy in cooperative group-affiliated hospitals (OR 1.59, 95% CI 1.19–2.11) or non-community hospitals (OR 1.35, 95% CI 1.06–1.69). Similarly, patients more frequently underwent nephron-sparing surgery at an NCI-designated cancer center (OR 2.88, 95% CI 2.07–4.01) or non-community hospital (OR 1.43, 95% CI 1.15–1.79). Our findings did not substantively change when considering the degree of CCOP affiliation nor when excluding potentially collinear organizational factors from our models.
Discussion
Laparoscopic and nephron-sparing surgery are widely accepted as two transformative surgical innovations in genitourinary oncology that offer enhanced recovery and reduced renal morbidity, respectively, for patients undergoing kidney surgery.9, 10, 12, 13 Multiple professional organizations (e.g., American Urological Association, European Association of Urology) now advocate for partial nephrectomy as the preferred treatment for patients with early-stage kidney cancer followed by laparoscopic surgery for those requiring radical nephrectomy.14, 23 Despite these recommendations, many patients presenting with renal malignancies continue to undergo cancer treatment without the benefit of these technological advances,24 highlighting the need for more effective delivery of what many consider to be higher-quality kidney cancer care.
Unlike the enhanced dissemination of evidence-based practices seen with colon and breast cancer,6, 7 exposure to CCOP was not associated with delivery of recommended care among a sample of Medicare beneficiaries with kidney cancer. Consistent with the existing literature, we found that patients treated in an academic research setting (i.e., NCI-designated cancer center or Cooperative Group member) were more likely to receive laparoscopic and/or partial nephrectomy.15, 16, 25 However, these higher usage rates did not translate into greater utilization among patients cared for by physicians and hospitals affiliated with this cancer-specific PBRN. Taken together, these findings suggest that—despite CCOP’s available infrastructure—persistent disconnect exists between academic centers and community practices, at least as it pertains to new surgical technologies in kidney cancer.
Paradoxically, one challenge in advancing surgical innovations through CCOP may lie within the program’s deep roots in clinical trial development and implementation. Since its inception in 1983, CCOP has operated with a fundamental principle of engaging community providers in clinical trial design and subsequent translation into clinical practice.5 Accounting for nearly one third of NCI-sponsored clinical trial participants, CCOP has been involved with a diverse array of experimental studies including large-scale trials in breast and colon cancer.38, 39 As such, CCOP has been well-positioned to propagate newly-established, evidence-based practices in these cancer populations.5–7, 26, 27
However, owing to a variety of externalities, the evolution of new surgical technologies may proceed in a manner where randomized control trials become impractical, unethical, or inopportune.28–30 Accordingly, the IDEAL paradigm has been proposed for the evaluation and diffusion of surgical innovations. Herein, developmental studies, prospective registries, quasi-experimental designs, and long-term monitoring are embraced in addition to randomized control studies.31 Because the data supporting laparoscopic and partial nephrectomy have come primarily from single institution studies and population-level observational data,9–13 CCOP may not have been fully activated. In fact, survey data shortly following our study interval suggest that many community urologists, compared to their academic counterparts, considered partial nephrectomy to be preferable in only a subset of indicated cases, underscoring potential issues related to knowledge transfer.32, 33
Additionally, without the support and experience gained through trial participation, community providers may not be as well prepared to readily deliver new, effective cancer treatments. For laparoscopy and partial nephrectomy, physician training and experience serve as major determinants of technology adoption.15, 25, 34 For laparoscopic surgery, in particular, utilization appears to be strongly linked to a surgeon’s medical training, more so than his or her practice setting.15 In this context, lower use of laparoscopic and/or partial nephrectomy may be potentially appropriate if community urologist lack the requisite knowledge or technical expertise. Therefore, with clinical trials serving as the nexus for community–academic engagement, CCOP may not be as well suited to either disseminate or implement evidence-based surgical therapies that follow the IDEAL paradigm.
Our findings should be considered in the context of several limitations. First, as with any observational study, these data remain vulnerable to residual selection bias and/or unmeasured confounding. For example, discrepancies in tumor anatomy or surgical appropriateness may influence treatment selection and bias our findings. It can be argued, however, that such factors would affect treatment in an NCI-designated cancer center or Cooperative Group in a similar manner. Second, our measure for CCOP affiliation focuses on the physician and hospital performing the surgical procedure. Exposure to a CCOP-affiliated provider or facility prior to but not during the surgical episode may potentially influence treatment utilization. However, we found no difference when extending the exposure window or when accounting for the degree of CCOP exposure. Third, the geographic footprints of SEER and NCI’s CCOP may not overlap precisely. That being said, CCOP sites exist in approximately three quarters of SEER registries nationwide, suggesting robust exposure at the population-level.
These limitations notwithstanding, our findings have important implications for the delivery of quality cancer care. In response to the shifting landscape of US health care, the National Cancer Institute has bolstered its investment in PBRNs, replacing CCOP with the larger and more integrated Community Oncology Research Program (NCORP). Utilizing much of the preexisting infrastructure from CCOP, this newly established research platform aims to advance both clinical trial science and cancer care delivery research to improve health for patients with cancer. As part of this effort, NCORP will broaden its research agenda to include both longitudinal, observational data and post-treatment surveillance.35 Moving forward then, NCORP may become ideally positioned to rigorously assess surgical innovations according to the IDEAL paradigm. In turn, with more active engagement, NCORP may better facilitate the efficient dissemination of a broader array of new technologies.
Furthermore, with NCORP moving into cancer care delivery research, its extensive network may be well suited for large-scale, provider-based collaboratives focused on cancer care quality. Utilizing transparent, equitable, and bidirectional exchanges similar to CCOP and now NCORP, these entities actively disseminate, implement, and monitor care redesign. Examples in urology have enhanced the uptake of evidence-based guidelines for the radiographic evaluation of prostate cancer and intravesical therapy in bladder cancer. By coupling knowledge transfer with quality improvement, these efforts have overcome provider- and practice-level barriers to achieve high levels of recommendation adherence.36–38 With quality metrics already established through the American Society of Clinical Oncology’s Quality Oncology Practice Initiative, NCORP may extend the initial benefits generated by this voluntary program and further address residual quality gaps.39, 40 So while CCOP was not associated with accelerated care delivery in the context of new kidney cancer surgeries, recent developments may expand the capacity of CCOP’s successor, NCORP, and other PBRNs to efficiently disseminate and effectively implement new surgical technologies in cancer care.
Table 2.
Multivariable Models - Generalized Estimating Equation
| Covariate | Laparoscopy | Partial Nephrectomy | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age at diagnosis | ||||
| 65–69 | Ref | Ref | ||
| 70–74 | 0.98 (0.84–1.14) | 0.749 | 0.90 (0.78–1.05) | 0.179 |
| 75–79 | 1.08 (0.93–1.26) | 0.324 | 0.74 (0.63–0.86) | <0.001 |
| 80+ | 1.24 (1.03–1.49) | 0.023 | 0.44 (0.36–0.54) | <0.001 |
| Female | 1.07 (0.95–1.20) | 0.268 | 0.84 (0.73–0.97) | 0.015 |
| Race | ||||
| Caucasian American | Ref | Ref | ||
| African American | 0.89 (0.73–1.10) | 0.285 | 0.94 (0.72–1.21) | 0.607 |
| Others | 1.15 (0.86–1.54) | 0.345 | 1.02 (0.73–1.42) | 0.897 |
| Married | 0.94 (0.84–1.06) | 0.326 | 1.02 (0.88–1.19) | 0.790 |
| Dual eligibility for Medicare/Medicaid | 0.89 (0.74–1.07) | 0.213 | 1.00 (0.80–1.24) | 0.970 |
| Non-high school graduate | ||||
| Bottom Quartile | Ref | Ref | ||
| 2nd Quartile | 0.93 (0.79–1.09) | 0.353 | 0.94 (0.81–1.10) | 0.466 |
| 3rd Quartile | 0.87 (0.75–1.02) | 0.089 | 0.93 (0.77–1.13) | 0.489 |
| Top Quartile | 0.87 (0.74–1.02) | 0.088 | 0.88 (0.73–1.06) | 0.167 |
| Tumor Grade | ||||
| Well differentiated | 0.90 (0.73–1.13) | 0.370 | 1.77 (1.40–2.24) | <0.001 |
| Moderately differentiated | 0.86 (0.73–1.01) | 0.061 | 1.30 (1.07–1.58) | 0.009 |
| Poorly differentiated / Undifferentiated | Ref | Ref | ||
| Unknown | 0.82 (0.69–0.98) | 0.030 | 1.56 (1.26–1.94) | <0.001 |
| Comorbidity Score ≥ 1 | 0.85 (0.76–0.96) | 0.008 | 1.00 (0.88–1.14) | 0.985 |
| Year of diagnosis | ||||
| 2000 | Ref | Ref | ||
| 2001 | 2.38 (1.51–3.76) | <0.001 | 1.11 (0.83–1.47) | 0.484 |
| 2002 | 4.28 (2.76–6.65) | <0.001 | 1.25 (0.92–1.71) | 0.156 |
| 2003 | 7.40 (4.84–11.31) | <0.001 | 1.53 (1.15–2.03) | 0.003 |
| 2004 | 9.88 (6.59–14.81) | <0.001 | 1.87 (1.40–2.50) | <0.001 |
| 2005 | 11.79 (7.94–17.51) | <0.001 | 1.83 (1.38–2.42) | <0.001 |
| 2006 | 14.74 (9.81–22.15) | <0.001 | 2.22 (1.64–3.01) | <0.001 |
| 2007 | 16.63 (11.14–24.82) | <0.001 | 1.93 (1.44–2.60) | <0.001 |
| SEER Region | ||||
| San Francisco | 1.70 (0.99–2.92) | 0.055 | 1.29 (0.81–2.04) | 0.284 |
| Connecticut | 1.37 (0.88–2.13) | 0.162 | 0.81 (0.52–1.28) | 0.370 |
| Detroit | 1.73 (1.04–2.87) | 0.033 | 0.77 (0.51–1.15) | 0.205 |
| Hawaii | 0.66 (0.29–1.47) | 0.306 | 0.94 (0.49–1.78) | 0.845 |
| Iowa | 0.76 (0.47–1.25) | 0.283 | 0.73 (0.49–1.08) | 0.117 |
| New Mexico | 0.81 (0.42–1.56) | 0.523 | 0.97 (0.54–1.73) | 0.919 |
| Seattle | 1.35 (0.81–2.26) | 0.255 | 1.39 (0.88–2.20) | 0.153 |
| Utah | 0.83 (0.45–1.54) | 0.563 | 1.48 (0.88–2.48) | 0.140 |
| Atlanta and Rural Georgia | 1.42 (0.62–3.26) | 0.404 | 0.86 (0.46–1.62) | 0.643 |
| San Jose | 0.92 (0.43–1.99) | 0.837 | 0.15 (0.54–2.47) | 0.717 |
| Los Angeles | 1.15 (0.77–1.72) | 0.503 | 1.10 (0.75–1.60) | 0.634 |
| Greater California | 1.26 (0.86–1.84) | 0.242 | 0.94 (0.69–1.28) | 0.691 |
| Kentucky | 1.02 (0.66–1.57) | 0.922 | 1.03 (0.70–1.51) | 0.897 |
| Louisiana | 1.24 (0.78–1.98) | 0.369 | 1.03 (0.71–1.48) | 0.883 |
| New Jersey | Ref | Ref | ||
| CCOP affiliation | 1.11 (0.81–1.53) | 0.513 | 1.04 (0.82–1.32) | 0.741 |
| Cooperative Group | 1.59 (1.19–2.11) | 0.002 | 1.20 (0.96–1.50) | 0.118 |
| NCI-designated Cancer Center | 1.49 (0.88–2.50) | 0.136 | 2.88 (2.07–4.01) | <0.001 |
| Community Hospital | 0.74 (0.59–0.94) | 0.012 | 0.70 (0.56–0.87) | 0.001 |
Abbreviations: SEER – Surveillance, Epidemiology, and End Results; CCOP – Community Clinical Oncology Program; NCI – National Cancer Institute.
Acknowledgments
This research was supported by NCI grants 5R01CA124402 and 5P01CA142538, and NCI Contract HHSN261200800726P (WC). Additional support provided by funding from the VA Office of Academic Affiliations through the VA/Robert Wood Johnson Clinical Scholars Program (HT); The American Cancer Society (SW, MN); The National Institutes of Health (AS, WC); The Urology Care Foundation / Astellas (MN); and the University Cancer Research Fund (AM, TK, AS, SW, WC, MN).
Dr. Nielsen has served as a consultant to The American College of Physicians and Grand Rounds, both unrelated to the present work.
Footnotes
The authors have no other financial disclosures.
References
- 1.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.Institute of Medicine. Bridging the Gap Between Practice and Research. Washington, D.C: National Academy Press; 1998. [Google Scholar]
- 3.Westfall JM, Mold J, Fagnan L. Practice-based research--"Blue Highways" on the NIH roadmap. JAMA : the journal of the American Medical Association. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 4.Zerhouni E. Medicine. The NIH Roadmap. Science. 2003;302(5642):63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 5.Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116(19):4440–4449. doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer AM, Reeder-Hayes KE, Liu H, et al. Differential receipt of sentinel lymph node biopsy within practice-based research networks. Medical care. 2013;51(9):812–818. doi: 10.1097/MLR.0b013e31829c8ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter WR, Meyer AM, Wu Y, et al. Translating research into practice: the role of provider-based research networks in the diffusion of an evidence-based colon cancer treatment innovation. Medical care. 2012;50(8):737–748. doi: 10.1097/MLR.0b013e31824ebe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. The lancet oncology. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf JS, Merion RM, Leichtman AB, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation. 2001;72(2):284–290. doi: 10.1097/00007890-200107270-00021. [DOI] [PubMed] [Google Scholar]
- 10.Dunn MD, Portis AJ, Shalhav AL, et al. Laparoscopic versus open radical nephrectomy: a 9-year experience. J Urol. 2000;164(4):1153–1159. [PubMed] [Google Scholar]
- 11.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. The Journal of urology. 2000;163(2):442–445. [PubMed] [Google Scholar]
- 12.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. The lancet oncology. 2006;7(9):735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112(3):511–520. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 14.Novick AC, Campbell SC, Belldegrun A, et al. Guideline for Management of the Clinical Stage 1 Renal Mass. [accessed July 3, 2014]; Available from URL: http://www.auanet.org/education/guidelines/renal-mass.cfm. [Google Scholar]
- 15.Filson CP, Banerjee M, Wolf JS, Jr, Ye Z, Wei JT, Miller DC. Surgeon characteristics and long-term trends in the adoption of laparoscopic radical nephrectomy. The Journal of urology. 2011;185(6):2072–2077. doi: 10.1016/j.juro.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SG, Penson DF, Pabla B, et al. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. The Journal of urology. 2012;187(3):816–821. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 17.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121. [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Services Research. 2009;9:92. doi: 10.1186/1472-6963-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Medical care. 2002;40(8 Suppl) doi: 10.1097/00005650-200208001-00003. IV-19-25. [DOI] [PubMed] [Google Scholar]
- 22.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Medical care. 2009;47(7):765–773. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]
- 23.Ljungberg B, Hanbury DC, Kuczyk MA, et al. Renal cell carcinoma guideline. European urology. 2007;51(6):1502–1510. doi: 10.1016/j.eururo.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 24.Smaldone MC, Kutikov A, Egleston B, et al. Assessing performance trends in laparoscopic nephrectomy and nephron-sparing surgery for localized renal tumors. Urology. 2012;80(2):286–291. doi: 10.1016/j.urology.2012.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DC, Daignault S, Wolf JS, et al. Hospital characteristics and use of innovative surgical therapies among patients with kidney cancer. Medical care. 2008;46(4):372–379. doi: 10.1097/MLR.0b013e31816099a7. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 27.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 28.Wilson CB. Adoption of new surgical technology. BMJ. 2006;332(7533):112–114. doi: 10.1136/bmj.332.7533.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escarce JJ. Externalities in hospitals and physician adoption of a new surgical technology: an exploratory analysis. J Health Econ. 1996;15(6):715–734. doi: 10.1016/s0167-6296(96)00501-2. [DOI] [PubMed] [Google Scholar]
- 30.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374(9695):1089–1096. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 31.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 32.Breau RH, Crispen PL, Jenkins SM, Blute ML, Leibovich BC. Treatment of patients with small renal masses: a survey of the American Urological Association. The Journal of urology. 2011;185(2):407–413. doi: 10.1016/j.juro.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 33.Weight CJ, Crispen PL, Breau RH, et al. Practice-setting and surgeon characteristics heavily influence the decision to perform partial nephrectomy among American Urologic Association surgeons. BJU international. 2013;111(5):731–738. doi: 10.1111/j.1464-410X.2012.11112.x. [DOI] [PubMed] [Google Scholar]
- 34.Miller DC, Saigal CS, Banerjee M, Hanley J, Litwin MS. Diffusion of surgical innovation among patients with kidney cancer. Cancer. 2008;112(8):1708–1717. doi: 10.1002/cncr.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCaskill-Stevens W, Clauser SB. NCORP Overview: National Cancer Institute. 2013 [Google Scholar]
- 36.Miller DC, Murtagh DS, Suh RS, Knapp PM, Dunn RL, Montie JE. Establishment of a urological surgery quality collaborative. The Journal of urology. 2010;184(6):2485–2490. doi: 10.1016/j.juro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Miller DC, Murtagh DS, Suh RS, et al. Regional collaboration to improve radiographic staging practices among men with early stage prostate cancer. The Journal of urology. 2011;186(3):844–849. doi: 10.1016/j.juro.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 38.Barocas DA, Liu A, Burks FN, et al. Practice based collaboration to improve the use of immediate intravesical therapy after resection of nonmuscle invasive bladder cancer. The Journal of urology. 2013;190(6):2011–2016. doi: 10.1016/j.juro.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(25):6233–6239. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- 40.Campion FX, Larson LR, Kadlubek PJ, Earle CC, Neuss MN. Advancing performance measurement in oncology: quality oncology practice initiative participation and quality outcomes. Journal of oncology practice / American Society of Clinical Oncology. 2011;7(3 Suppl):31s–35s. doi: 10.1200/JOP.2011.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]