Abstract
Dietary ingestion of persistent organic pollutants (POPs) correlates with developing obesity. Obesity alters metabolism, induces an inflammatory tissue microenvironment, and is also linked with diabetes and breast cancer risk/promotion of the disease. However, no direct evidence exists exploring the correlation among all three of these factors (POPs, obesity, and breast cancer). Herein, we present current correlative studies suggesting a causal link between POPs exposure through diet and their bioaccumulation in adipose that promotes the development of obesity and ultimately influences breast cancer development and/or progression. Furthermore, as endocrine disruptors, POPs can potentially interfere with hormonally responsive tissue functions causing dysregulation in hormone signaling and cell function. This review highlights the critical need for advanced in vitro and in vivo model systems to understand the complex relationship between obesity, POPs, breast cancer, and, more importantly, to delineate their multifaceted molecular, cellular, and biochemical mechanisms. Comprehensive in vitro and in vivo studies directly testing the observed correlations as well as detailing their molecular mechanisms are vital to cancer research and, ultimately, public health.
Keywords: Breast Cancer, Persistent Organic Pollutants, Obesity, Metabolism
Introduction
Persistent Organic Pollutants (POPs), a group of chemicals such as organochlorine pesticides (OCP) and polychlorinated biphenyls (PCBs), are ubiquitous pollutants found globally. These chemicals have common properties of persistence including lipophilicity, toxicity, and bioaccumulation (Lee, et al. 2010; Woodwell 1967). Due to their high lipophilic properties, POPs (organo-hydrocarbons with chlorine, fluoride and bromine groups) are most notably resistant to degradation in both humans and the environment, thus proving to be a bigger threat to global health (Yu, et al. 2011). With their half-lives varying from months to decades due to their high lipid solubility that resist biological, photolytic and chemical degradation, POPs are also semi-volatile allowing them to evaporate into the atmosphere and deposit back to the earth in precipitates (Thundiyil, et al. 2007). The Stockholm Convention on POPs was adopted on May 22nd, 2001 and entered into force on May 17th 2004 with the goal of managing, cleaning and eliminating the production of POPs in the environment. In the US, production and distribution of many POPs have been banned under the Safe Chemicals Act of 2011. However, according to the Environmental Protection Agency and other regulatory sources, chronic POPs exposure continues due to contamination in the food chain, both in artificial and natural environments, and their continued use in under-developed countries with little to no regulation (La Merrill, et al. 2013; Lee et al. 2010; Ljunggren, et al. 2014; Noyes, et al. 2009; Ritter, et al. 2002; Weber, et al. 2008). POPs accumulation in adipose tissues has been proposed as the likely stimuli for their pathological manifestations. While non-lipophilic POPs are not the focus of this review, it is important to note that compounds such as perfluorooctane sulfonate and perfluorooctanoic acid, commonly used in industry as flame-retardants and fabric protectors, do not accumulate in fatty tissue but bioaccumulate due to their extremely stable carbon–fluorine bonds. These POPs have been shown to bind to proteins ultimately interfering with their function (Corsini, et al. 2014; DeWitt, et al. 2012; Domingo 2012; Jones, et al. 2003). Exposure to any type of POPs has the potential for long-term disruption of metabolic, immune, and endocrine system functions. Consequently, POPs are gaining research and public health attention due to their strong link to type 2 diabetes, metabolic syndrome, and cancer, each of which are intimately linked with obesity (Lee et al. 2010; Park, et al. 2014b; Prieto-Hontoria, et al. 2011). Human exposure to POPs occurs through consumption of seafood and livestock and because POPs are water insoluble and not easily metabolized, their levels accumulate in adipose tissue (Lee et al. 2010; Woodwell 1967).
As endocrine disruptors, POPs interfere with hormonally responsive tissue functions via dysregulation in hormone signaling and cell function (Lara, et al. 2012; Meeker 2012; Pombo and Castro-Feijoo 2005; Rylander, et al. 2014; Solomon and Schettler 2000). Specific to this review, dysregulation of estrogen, progesterone, and prolactin, important breast development hormones, can cause a multitude of growth dysfunctions including fibrocystic breast disease and/or breast cancer (Hovey, et al. 2002; Vonderhaar 1988). Breast development is unique compared to other major organs as the majority of development occurs after birth. Rudimentary epithelial and stromal growth is isometric until the onset of puberty, whereupon allometric growth of the epithelial ducts and supportive stromal tissues occur (Hassiotou and Geddes 2013). Post-puberty, breast tissue continues to proliferate and regress in response to hormonal-stimuli during each menstruation cycle. Depending on the woman’s life choices, subsequent key developmental stages may include pregnancy- and lactation- and post-lactation associated remodeling. Ultimately, every woman experiences post-menopausal involution, initiated via declining ovarian function and subsequent decreased circulating estrogen and progesterone. Atrophy and regression of glandular epithelium with a concurrent increase in adipose and a reduced elasticity of the supporting connective tissue are observed (Hassiotou and Geddes 2013). In sum, breast tissue is highly dynamic throughout a woman’s life, and during any of these unique stages, a disruption in the delicate balance of growth factor and hormonal signaling between the stroma and epithelium has the potential to promote disease.
The global obesity epidemic compounds any predisposition to breast disease due to changes in hormone and growth factor signaling. Obesity and metabolic syndrome promotes an inflammatory microenvironment, thereby disrupting tissue homeostasis (Park et al. 2014b). Inflammatory microenvironment is known to promote aggressive breast cancer cell behaviors and affect tumor subtype, ultimately leading to poor prognosis and decreased survival (Casbas-Hernandez, et al. 2014; Ham and Moon 2013; Nguyen, et al. 2013; Rose and Vona-Davis 2014; Sun, et al. 2012). Consumption and bioaccumulation of POPs in adipose enhances the obesity-driven dysregulation of cell function, as POPs accumulation is hypothesized to promote obesity (Lee et al. 2010; Woodwell 1967). Thus, the potential exists for POPs and obesity-related factors to work together in a self-reinforcing mode to magnify the effects of endocrine disruption and altered cell signaling, thereby increasing the risk of developing breast cancer or existing tumors progressing into more aggressive subtypes. Herein, we highlight the potential interaction between POPs accumulation, development of obesity, and breast cancer.
Adipose Tissue and POPs Localization
Each adipose tissue depot (serum, subcutaneous, visceral, and inter-muscular) has differing endocrine, metabolic, and cell signaling functions (Bloor and Symonds 2014; Gilsanz, et al. 2013; La Merrill et al. 2013; Rosenwald and Wolfrum 2014). Women are unique in that they possess an additional, highly dynamic and functional source of adipose in their breast tissue. Within each of these adipose depots resides a multitude of different cell subtypes. White adipocyte tissue (WAT), found mainly around the waist and thighs, have a single lipid droplet and function as energy stores that secret various hormones with endocrine and immune functions. Residing in WAT are brown adipocytes derived from precursor cells differing from classical brown adipocytes that are closer to the white adipocyte cell lineage termed “brite adipocytes.” These brite cells appear at anatomical sites corresponding to WAT after thermogenic stimuli (Giralt and Villarroya 2013). Conversely, brown adipocyte tissue (BAT) is primarily involved in body temperature maintenance (non-shivering thermogenesis) and body adiposity. In adult humans, BAT is mainly found around the cervical-supraclavicular area, contains many small lipid droplets, a high number of mitochondria, higher oxygen consumption, high capillary perfusion and sympathetic nervous system innervation (Bloor and Symonds 2014; Gilsanz et al. 2013; Rosenwald and Wolfrum 2014; Seale, et al. 2009; Seale and Lazar 2009). Lipid droplet proteins have been shown to regulate BAT metabolism in adult mice while the role of human BAT metabolism, as well as their overall function, needs further analyses to fully appreciate their physiological roles (Giralt and Villarroya 2013; Seale et al. 2009; Seale and Lazar 2009). Of note, poor BAT activity in humans correlates with ageing, BMI and measures of metabolic disease, and the study of adipocyte transdifferentiation mechanisms may lead to therapeutic strategies against metabolic syndrome and cellular metabolic deregulation observed in breast cancer (Giordano, et al. 2014; Giralt and Villarroya 2013; Seale et al. 2009; Seale and Lazar 2009).
In women, a large source of WAT can be found in breast tissue. Adipose tissue comprises the majority of breast volume and is altered due to puberty, pregnancy, lactation, and menopause (Hassiotou and Geddes 2013). During puberty, periductal connective tissue is generated in breast adipose tissue with thickening and elongation of the ductal system. In pregnancy and lactation, active glandular tissue doubles in relation to adipose tissue but reverts once lactation ceases. Starting around age 40, ductal and lobular breast tissue begins to atrophy with involution of glandular tissue that is replaced by fat and connective tissue. Both systemic and local growth factors direct this continual remodeling of the gland. Breast adipocyte secretion of adipokines, cytokines, and hormones such as estrogen, has been linked to numerous biological processes including cell proliferation, communication, immune response, apoptosis, and cell metabolism (Park et al. 2014b). Estrogens also regulate adipose deposition, adipogenesis and adipocyte differentiation. Any deregulation in the production or distribution of estrogens can potentially lead to disease or breast cancer (Park, et al. 2014a; Planey, et al. 2014). Moreover, it has been shown that adipocytes can be manipulated by cancer cells to promote tumor invasion, characterized by adipocyte hypertrophy and the accumulation of neutrophils and macrophages in adipose tissue, creating an imbalance and poor prognosis for cancer patients (Park et al. 2014b; Prieto-Hontoria et al. 2011).
Adipose tissue contains high levels of aromatase, an enzyme that converts androgens to estrogen (Simpson 2003). In obesity, estrogen levels have been shown to be increased in postmenopausal women but decreased in premenopausal women (De Pergola and Silvestris 2013; Suba 2013). These obesity driven changes in estrogen regulation are suggested to play a role in the complex relationship to premenopausal and postmenopausal breast cancer, and the clinical behavior of the disease. A meta-analysis from seven cohort studies reported a direct link between obesity and an elevated risk of breast cancer in postmenopausal women, with the inverse being true in premenopausal women (van den Brandt, et al. 2000). However, obesity is correlated with advanced disease at diagnosis and poor prognosis at any age (Stephenson and Rose 2003; van den Brandt et al. 2000). Furthermore, excessive weight gain during pregnancy, fetal exposure to high fat diet, high birth weight, and postnatal exposure to high fat diets during lactation have each been shown to increase breast cancer susceptibility and development in adults in carcinogen-induced rat model studies (De Assis and Hilakivi-Clarke 2006; de Oliveira Andrade, et al. 2014; Hilakivi-Clarke and de Assis 2006; Hilakivi-Clarke, et al. 2005; Hilakivi-Clarke, et al. 2004). Collectively these data suggest that in addition to increased risk of cancer later in life, childhood obesity and obesity-related intrauterine factors may ultimately increase breast cancer risk in women.
A recent study focused on understanding the effects on POPs exposure on adipose tissue demonstrated exposure of human preadipocytes to PCB126 affected their ability to differentiate into mature adipocytes. PCB126 stimulated intrinsic changes in cell function by decreasing transcript levels of a key regulator of adipogenesis, PPARγ (Gadupudi, et al. 2014). The ability of preadipocytes to mature is integral to development and homeostasis. POP exposure compromising the ability of preadipocytes to differentiate in children and adolescents has the potential to alter their ability to form normal adipose tissue, ultimately leading to dysfunction and disease. In adults, chronic POPs exposure may alter their ability to replace mature adipocytes caused by cell death, again potentially leading to dysfunctional adipose tissue (Gadupudi et al. 2014).
Two well-studied sources of adipose tissue are visceral and subcutaneous, each having unique physiological and clinical features. In a study investigating POPs content of these sources, Korean patients (n = 50) undergoing surgery for gallbladder or liver lesions had samples isolated and tested for a set of POPs congeners (Kim, et al. 2014). POPs accumulation were correlated with both sources of adipose. However, researchers found five to 10 times higher absolute concentration of PCB congeners in visceral (VAT) versus subcutaneous adipose tissue (SAT). A pattern also emerged in patients with diabetes, showing a set of OCPs and PCB congeners significantly correlated with VAT (Kim et al. 2014). The authors propose that these correlations may be due to biological properties of the VAT adipocytes as these cells have enhanced sensitivity to lipolysis, are more metabolically active, and have increased insulin resistance compared to SAT. Given the emerging complex biological roles of adipose, it is important to ascertain whether POPs distribute equally throughout all adipose sources in the body or are preferentially localized.
In a parallel study, Yu and colleagues analyzed ten PCB congeners and OCPs in serum levels of both lean and obese subjects. Serum samples, visceral and subcutaneous adipose biopsies were taken from subjects during laparotomy and evaluated. Overall, higher levels of OCPs were found in VAT while PCBs accumulated more readily in SAT (Yu et al. 2011). Variations were contributed to exposure level, BMI, and genetic differences of the individual highlighting the fact that POPs-containing food sources vary from each geographical region, and within ethnicities. This study is very limited (n=7) with only one woman included in this report, and despite gender-specific adipose distribution, limited studies have directly observed gender differences with respect to POPs accumulation. A recent, more comprehensive study evaluated the accumulation of 13 types of POPs and in VAT and SAT from Portuguese obese (>35 BMI) bariatric surgery patients (n=189) of which 166 were females (Pestana, et al. 2014). While gender and breast adipose was also not specifically studied in this report, the data confirm those found in the Kim and Yu et al. studies. Pestana and colleagues show POPs were prevalent in this obese population (96.3% of detection on both tissues), their abundance increased with age and duration of obesity. An increase in POPs deposition in VAT was observed, a positive correlation between POP levels and the presence of metabolic syndrome, and a relation of higher POP levels with lower weight loss in older patients (Pestana et al. 2014).
While none of these studies focus on gender differences, women tend to have overall higher adipose levels, with the majority localized to the hips and thighs (Karastergiou, et al. 2012) and a significant amount in breast tissue, while men tend to exhibit a preferential accumulation of abdominal adipose. Limited information exists on breast adipose tissue and POPs accumulation. Three methods papers have directly demonstrated POPs accumulation in breast adipose tissue; however, no analysis on health or etiology of disease was performed. The first report validated the use of chromatography-time-of-flight mass spectrometry (GC-TOF MS) for screening anthropogenic organic contaminants in 40 human breast adipose tissues show that both target and non-target approaches detected pollutants including p,p′-DDE, hexachlorobenzene (HCB), and several PCBs, polyaromatic hydrocarbons (PAHs), 5-di-tert-butyl-4-hydroxy-toluene (BHT) and its metabolite 3,5-di-tert-butyl-4-hydroxybenzaldehyde (BHT-CHO), and dibenzylamine, N-butyl benzenesulfonamide (N-BBSA) (Hernandez, et al. 2009). The second report by the same authors used a multiresidue method for the quantification and confirmation of 30 organohalogenated compounds in human breast tissue samples (Medina, et al. 2009). Lastly, the presence of prevalent dioxins, furans, PCBs, OCPs, and brominated diphenyl ethers in both breast and abdominal adipose tissues of 21 women were reported. These results show that, with some notable exceptions, measurements in breast and abdominal adipose tissues were correlated and that concentrations of POPs in one tissue could be derived from measurements in the other tissue (Petreas, et al. 2004). Again, no functional studies on the effects of these POPs on cell behavior were performed, however these data highlight the accumulation of the potentially disruptive POPs in the breast microenvironment.
In support of lipid-specific accumulation, the levels of PCBs uptake were directly correlated with triglyceride levels in adipocyte lipid droplets (Bourez, et al. 2012; Bourez, et al. 2013). Lipid droplets are organelles composed of a phospholipid monolayer and a lipid ester core that occupy a large portion of cell volume in many cells including mammalian, plant, and microorganisms. Obesity, characterized by excessive adipocytes, is essentially a disease of lipid droplet excess (Walther and Farese 2009). To identify rate of absorption and localization of PCBs into cells, Bourez et al. assayed the well-characterized 3T3-L1 murine cell model system for adipogenesis, as well as primary murine embryonic adipocytes by exposing them to a cocktail of PCB congeners. After 90 minutes of incubation, the majority of all congeners were recovered inside the differentiated adipocytes, and not the control pre-differentiated fibroblasts. After 24 hours, the intracellular PCB accumulation was almost exclusively recovered in the high triglyceride-containing lipid droplet fraction of the adipocytes compared to the membrane fraction (Bourez et al. 2012), indicating that PCB accumulation in adipocytes is dependent on the triglyceride content of the adipose, and highlighting the potential for greater risk in accumulation of POPs in obese individuals. A limitation of this study is the use of murine cells and cell lines. It is unknown if human pre-adipocytes would have a comparable cellular differentiation process in the presence of the PCB congeners, or if human adipocytes would display similar uptake patterns of the congeners used in the study.
Complement to these studies, Gutleb and colleagues developed a method to visualize cellular localization and semi-quantitatively calculate concentrations of halogenated POPs via secondary ion mass spectrometry (Gutleb, et al. 2012). Using a human adrenocortical carcinoma and a rat pituitary tumor cell line, bromine-containing POP tetrabromobisphenol A (TBBPA; commonly used flame retardant) and the fluorine POP perfluorooctane sulfonate (PFOS; a fabric protector) were localized to the lipid bilayer of cell membranes (Gutleb et al. 2012). Again, a limitation to this study is the lack of normal/non-transformed human cells and tissues to observe POPs incorporation and localization. It is unknown if the effects of POPs localization in tumor cells would be applicable to normal human epithelial cells, given the well-documented altered cellular metabolism and membrane lipid content in cancer cells (Baumann, et al. 2013; Ibarguren, et al. 2014; Siddiqui, et al. 2007; Zaidi, et al. 2013). To date, no additional studies have investigated such detailed molecular localization of POPs in human tissues and/or cell culture model systems.
This would be of particular interest in breast tissue, as the specific mechanisms of fatty acid uptake in breast epithelium during various human developmental stages (puberty, pregnancy, lactation, involution) have not been as well identified compared to rodents and livestock (Barber, et al. 1997; McManaman 2014). Lipophilic POPs would be hypothesized to be potentially endocytosed by cells via LPL and CD36, two proposed mediators of breast lipid endocytosis (Kuemmerle, et al. 2011; McManaman 2014). The significant increase in epithelial cell lipid uptake during lactation to produce milk proteins would suggest a potential route of transmission of POPs from mother to the developing child.
In a developmental study on prenatal exposure to POPs, Valvi et al. (2014) collected information on pregnant women and their infants (n=1285) in a population-based birth cohort study. POP concentrations (DDT, DDE, HCB, β-hexachlorocyclohexane, and the PCB congeners 28, 52, 101, 118, 138, 153, and 180) were measured in serum collected from the pregnant women in the 7th and 26th week of pregnancy (Valvi, et al. 2014). The infant model included, age, sex, gestational age, breast feeding duration, and several maternal characteristics. The researchers found that prenatal exposure to DDE and HCB was associated with rapid growth in the first 6 months and overweight infants at 14 months. This suggests a direct link of childhood obesity and prenatal exposure to POPs. Interestingly, prenatal growth was not influenced by PCB exposure (Valvi et al. 2014) suggesting different mechanisms for various POPs. Human exposure to POPs is unavoidable, however to understand tissue-specific differences in POPs metabolism, as well as cell-type specific reactions and localization, further studies are needed.
Studies investigating the mechanisms of POPs cellular uptake and localization in breast cells (epithelium vs. adipose) would further advance our understanding of breast and lactation physiology as well as cellular lipid metabolism. Moreover, detailing the alterations in POPs uptake and localization in cancer cells and their tumor microenvironment, compared to healthy breast epithelium and supportive stroma, may provide further insight into the transition of cellular lipid metabolism and deregulation of cellular respiration during tumorigenesis.
Obesity and POPs
Adipose tissue is classified as a metabolically active endocrine organ and obesity-induced dysregulation stimulates an enhanced inflammatory tissue microenvironment, including altered secretion of chemokines/cytokines (monocyte chemotactic protein 1, interleukin-6 and TNF-alpha) and adipokines (haptogolin, PAI, leptin, resistan, visfatin, adiponectin and VEGF) (Myre and Imbeault 2014; Prieto-Hontoria et al.). Obesity-stimulated production of these factors is directly correlated to conditions such as chronic inflammation, cardiovascular disease, atherosclerosis, cancer (including breast cancer), and lipotoxicity (Prieto-Hontoria et al. 2011). Moreover, emerging research suggests a strong relationship between POPs, obesity, and the development and/or progression of disease (Bourez et al. 2012; Bourez et al. 2013; La Merrill et al. 2013; Ljunggren et al. 2014; Magliano, et al. 2014; Myre and Imbeault 2014; Pereira-Fernandes, et al. 2014; Taylor, et al. 2013). The “obesogen hypothesis” postulates that chemical pollutants (obesogens) promote obesity by disrupting appetite controls and promoting adipocyte hypertrophy and hyperplasia, thereby causing weight gain (Baillie-Hamilton 2002; Decherf and Demeneix 2011; Grun and Blumberg 2006; Pereira-Fernandes et al. 2014). The link between dysregulated adipose tissue in lipid metabolism, cancer, cardiovascular disease and diabetes has been extensively researched (Matsuda and Shimomura 2013; Prieto-Hontoria et al. 2011; Schwab, et al. 2014). The molecular mechanisms of POPs signaling in driving the development or progression of these diseases remain to be fully understood.
Dirinck and colleagues assessed the association between serum POPs levels and the prevalence of obesity in a cross-sectional study of 98 obese and 47 lean adults (Dirinck, et al. 2011). BMI, glucose tolerance, adipose storage deposits, and measurement of waist and hip circumference were recorded, and serum levels were analyzed for lipophilic PCB congeners 153, 138, 180, and 170, and for the hydrophilic OCPs: dichloro-diphenyl-dichloroethylene (pp-DDE) and β-hexachlorocyclohexane (βHCH). Results show as BMI increased, the serum levels of highly lipophilic congeners decreased. The converse was true for the less lipophilic congeners (Dirinck et al. 2011). The authors suggest that the lipophilic POPs are preferably stored in adipose tissue and a higher percentage of body fat will lead to fast and efficient storage, with lower serum levels as a consequence. While not discussed here, this inverse relationship has been observed by others (Agudo, et al. 2009; Wolff, et al. 2005). In the Dirinck study, serum βHCH had a significant positive correlation with BMI in both genders. In females, fat mass percentage and increased waist circumference had a significant negative correlation with all PCBs, while only PCB 180 and 170 had a negative correlation with waist and fat mass percentage in males. When analyzing the abdominal fat distribution in more detail, a significant negative correlation between the PCBs and abdominal fat was almost solely due to subcutaneous fat mass. In contrast, the positive correlation of βHCH in serum was observed regardless of adipose distribution and localization (Dirinck et al. 2011). While no tissue samples were taken to directly measure POPs levels in adipose tissue depots, these data show a correlation of obesity and serum POPs levels and, highlight gender differences with adipose localization and serum POPs levels.
Herein it should be stated that BMI is the most commonly used method to characterize obesity and is routinely applied in estimating body fat (adiposity), yet this method is inaccurate when based solely on weight and height, excluding total body fat. In addition, BMI provides little to no information on overall health status or disease severity, cannot be generalized among different ethnic groups or gender, and is especially inaccurate in individuals with lean body mass (Green and Duffull 2004; WHO 2000; Whyte, et al. 2014). The limitations of BMI as a risk assessment tool are recognized, and there is continuing interest in identifying substitute or complementary indicators linking adiposity and disease risk. For example, visceral adiposity plays a key role in the metabolic disorders associated with obesity, yet the lack of a practical method to assess visceral fat in routine examinations precludes its use as a screening tool for the general population. Developing simple and reliable methods to assess body fat compartments should be an important priority of obesity research (Caballero 2007).
A common source of lipophilic POPs exposure in humans is the consumption of fish, especially fatty fish such as salmon. In an effort to directly test the accumulation of POPs from dietary consumption and its downstream effects on insulin resistance and metabolism, Ruzzin and colleagues investigated various high fat diets in rats (Ruzzin, et al. 2010). Male rats were fed a high fat diet of crude salmon oil (HFC) containing high levels of POPs or refined salmon oil (HFR) with significantly lower POPs levels. After 28 days, rats fed the HFC had significantly higher concentrations of VAT or abdominal obesity, and elevated levels of diacylglycerol, triacylglycerol and total cholesterol compared to the HFR rats. Moreover, HFC-fed rats developed insulin resistance, impaired lipid and glucose metabolism, and hepatosteatosis, confirming that chronic exposure to POPs severely impairs insulin sensitivity and contributes to abdominal obesity in male rats (Ruzzin et al. 2010). Parallel studies in female rats were not performed, thus the effects of estrogens and potential gender-specific consequences of this study are unknown. In this same study, in vitro exposure of adipocytes to a cocktail of POPs showed a significant inhibition of insulin-dependent glucose uptake, providing further evidence of the risk of insulin resistance and metabolic disorders associated with the ingestion of POPs.
POPs have been shown to cause metabolic disruption. The Pestana study focused on assessing the association between POPs and this imbalance. VAT and SAT adipose tissue were collected from obese (>35 BMI) bariatric surgery patients (n=189) of whom 166 were females, and tested them for 13 POPs (Pestana et al. 2014). Higher concentrations of POPs were found in VAT; however, detectable quantities of all compounds were found in virtually all patients. VAT acts as a highly metabolic active tissue with susceptibility to lipolysis and thereby causes POP release (Roos, et al. 2013). Metabolic syndrome was found in 65.8% of the cohorts and in this subset, total level of POPs in both VAT and SAT were higher. Notably the researchers found that the metabolic abnormalities had a correlation with dysglycaemia, high blood pressure, and cardiovascular risk (Pestana et al. 2014). This study highlights the link between obesity and dysregulated adipose tissue due to POPs exposure underlining the need for more research into the mechanisms behind the dysregulation.
POPs, Obesity, and Breast Cancer
Obesity-driven changes in the normal and cancerous breast microenvironment, alterations in metabolism, and release of signaling molecules such as endocrine, growth, and inflammatory mediators are known to increase breast cancer risk as well as promote breast cancer progression (Harvey, et al. 2011; Nalabolu, et al. 2014; Sundaram, et al. 2013). However, studies investigating a direct mechanistic link between breast cancer, POPs accumulation, and obesity are remarkably lacking despite the strong correlation between POPs, obesity, and disease (Prieto-Hontoria et al. 2011; Taylor et al. 2013; Zeliger 2013a, b).
While a correlation between high levels of POPs and breast cancer suggests that POPs may be a risk factor for breast cancer (Bonefeld-Jorgensen, et al. 2011; Cabaravdic 2006; Lemaire, et al. 2006; Warner, et al. 2002), a direct relationship and corresponding biochemical mechanisms remain relatively undefined. The majority of associations between POPs and breast cancer risk have been attributed to the estrogenic effects of particular POPs on breast lesions. Basal-like tumors are characterized by lack of ER expression, an expression signature similar to the basal/myoepithelium, and are reported to have characteristics similar to those tumors arising from BRCA1 germline mutations carriers. They are unresponsive to endocrine-targeted therapies such as tamoxifin and aromatase inhibitors, and are associated with almost twice the risk of death when compared to ER positive tumors (Dunnwald, et al. 2007). Whether POPs affect both ER positive and ER negative breast cancer subtypes needs further investigation. In an attempt to address POPs effects on breast cancer cell proliferation, Aube et al. (2011) exposed four breast cancer cell lines to a cocktail of 15 different organochlorine compounds frequently found in the serum of women all around the world. ER positive MCF-7, T47D, and CAMA-1, and the ER negative MDA-MB231 were tested. Results show the non-hormone dependent MDA-MB231 cell line had decreased proliferation in the presence of the OC cocktail, and ER positive T47D cells had no response. Low levels of exposure to the OC cocktail induced significant proliferation in the MCF7 cells, while high levels were inhibitory (Aube, et al. 2011). The OC cocktail also had the capacity to stimulate the proliferation of CAMA-1 cells, however this was only in the presence of exogenous 17β-estradiol and dihydrotestosterone. Clearly more research is needed to understand these results and potential clinical implications. Defining the effects of POPs on the various breast cancer subtypes have never been directly addressed, but are critical to fully understanding whether POPs differentially influence the behavior or promotion of various breast cancer subtypes.
In a comprehensive report focused on estrogen receptor tested 49 POPs for ER alpha and beta (ERα/ERβ) activation or inhibition via transfection of a luciferase-based reporter construct in HeLa cells (Lemaire et al. 2006). Fifteen of the POPs tested stimulated ERα-mediated transcription in a dose-dependent manner, and five were capable of activating ERβ. Antagonistic activities toward ERα and ERβ were also shown in select POPs. Most interestingly, chlordecone and methoxychlor, the most effective antagonist compounds for ERβ, were strong agonists for ERα. These data underscore the complexity of POPs activation of ER signaling. Although ER activation potential of the POPs was documented in reporter assays, the results were not confirmed in breast cancer cells and the effects of these POPs on cell behavior were not investigated (Lemaire et al. 2006). In another study, the effects of the POP DDT and its metabolites on ERα expressing MCF7 and ERα-negative MDA-MB231 cell lines were investigated. Notably, the POPs tested had strong opposing results on the two cell lines, with the invasive potential of the MCF7 cells enhanced while the invasion of MDA-MB-231 cells significantly reduced (Lemaire et al. 2006). While the estrogenic effects of POPs on breast cancer cells are apparent, the POPs induced inflammatory microenvironment and their paracrine effects on ER negative or any breast cancer subtype remain to be defined.
A study released in 2011 focused on the observation that California has some of the highest breast cancer rates in the world, and some of the highest body burden levels of polybrominated diphenyl ethers (PBDE; flame retardants common in plastics, electronics and building materials), with levels ranging tenfold higher than those reported among European and Asian populations. The authors postulated that the estrogenic effects of PBDE might influence breast cancer risk (Petreas, et al. 2011). Breast biopsies of women from the San Francisco Bay area were analyzed for five major PBDE congeners. Results suggested that elevated BDE-154 correlated with breast cancer risk, but sample size was limited. Overall the data provided no significant evidence of an association between breast tissue concentrations of PBDE and breast cancer risk, though the breast tissue levels of PBDE were the highest ever reported. Study limitations were a significant factor, including that the levels of PBDEs were measured at/near the time of diagnosis and therefore may not be representative of lifetime exposures or exposures during potentially critical windows of susceptibility during earlier life. Second, the control group included women with proliferative benign breast lesions and evidence suggests these women are at increased risk for breast cancer (Aroner, et al. 2010; Collins, et al. 2006; Schnitt 2001). Third, PBDE levels are inversely correlated with age and recruitment resulted in a skewed age distribution such that controls were significantly younger than cases. Moreover, it is of note that out of the 78 cases and 56 controls only a total of seven women had a BMI over 30. As presented herein, high BMI combined with POPs is a factor worthy of investigation, but not adequately represent by this cohort (Petreas et al. 2011).
Until the 1970s, the Inuit population of Greenland and Canada held one of the lowest breast cancer rates in the world. No cases were reported before 1967, however a surge of 193 cases was reported between 1969 and 1997 (Bonefeld-Jorgensen 2010; Bonefeld-Jorgensen et al. 2011; Nielsen and Hansen 1980). Congruently, the Inuit population exhibited some the highest serum concentrations of POPs globally. This bioaccumulation has been proposed to be a direct result from the high dietary consumption of fats from fish, seal, whale, polar bear and seabirds leading researchers to theorize the high concentration of POPs found within tissues was related to the recent rise in breast cancer cases (Bonefeld-Jorgensen 2010). To further investigate, serum levels of breast cancer patients were analyzed for POPs between 2000 and 2003. Results showed significantly higher levels of perfluorinated compounds (PFCs), as well as double the median level of perfluorooctane sulfonate (PFOs) and sum of perfluorsulfonated acids (sumPFSA) in breast cancer patients compared to controls. No difference was observed in the serum levels of OCPs and PCBs. However, when PCBs were subdivided, significantly higher concentrations were seen in the highest quartiles. Collectively, these data indicate high levels of POPs are directly related increased breast cancer rates in the Inuit population. As studies continue to show increasing concentrations of select POPs in artic mammals and fish, it is critical to understand the molecular mechanism contributing to cancer risk or progression to provide better guidelines for targeted therapies and preventative measures (Butt, et al. 2010; Letcher, et al. 2010; Sonne 2010).
In the past decade, the role of the cancer microenvironment has come to the forefront of research. It is now well documented that the surrounding stromal cells, including adipocytes, fibroblasts, immune, and endothelial cells, have a significant effect on breast cancer progression (Casbas-Hernandez, et al. 2011; Sundaram et al. 2013). As the majority of breast tissue is adipose, and numerous reports have shown dramatic effects of POPs on the adipose, coculture studies observing the direct effects of POPs on adipocytes and subsequent paracrine effect on breast cancer cell behavior are necessary. Ex vivo studies using breast cancer cell cocultures with adipocytes are an excellent starting point for comprehensive paracrine studies on POPs influence on cancer cell behavior. Salamah and colleagues (Salameh, et al. 2013) isolated rat mammary fatpads, devoid of epithelium, and cultured these glands in vitro. The cultured tissues were injected with the CRL1743 rat mammary tumor epithelial cells and their migration and tumorigenesis documented. Results using coherent anti-Stokes Raman scattering (CARS) microscopy showed the morphological outgrowths mimicked cancer cell morphology in vivo and highlighted the potential for future studies. This model also provides a platform to develop paracrine cell interaction studies investigating the effects of POPs incorporation during various metabolic states (i.e. high fat/western vs. lean diet). A second 3D model system, similar to a skin culture system, demonstrated the interactions between pre-adipocytes, adipocytes, fibroblasts, and breast cancer cells (Delort, et al. 2013). Contact with breast cancer cells enhanced the differentiation of pre-adipocytes into adipocytes, and completely modified their transcriptional programs by increasing the expression of genes involved in cell proliferation (cyclinD1, MAPK), angiogenesis (MMP9, VEGF) and hormonal signaling (ESR1, IL6). Moreover, another 3D model of differentiated T3T-L1 adipocytes with normal murine mammary epithelial cells demonstrated that adipocyte-rich stroma induces branching through paracrine signals, including hepatocyte growth factor secretion, and may serve as a good model for observations during pubertal development (Pavlovich, et al. 2010). The addition of POPs could show potential alterations in branching morphogenesis or initiation of pre-cancerous lesions during puberty. This issue is imperative as it directly relates to contemporary issues involving childhood obesity and cancer risk later in life.
Serum levels of leptin, a key modulator in regulating energy intake and expenditure, is increased in breast cancer patients and has been implicated in the association between high BMI and breast cancer risk (Romero-Figueroa Mdel, et al. 2013). Leptin also transactivates key breast cancer promoting targets, including HER2, ERα, EGFR, and IGF-I receptors. Notably, correlations between POPs and leptin deregulation are strong (Pereira-Fernandes et al. 2014; Saxena, et al. 2008). Perfluorooctane sulfonate (PFOS), which belongs to the degradation product of many perfluorinated POPs, was fed to maternal rats and the effects of ingestion were investigated on the offspring in adulthood (Lv, et al. 2013). During gestation and lactation, offspring were indirectly exposed to 0.5 mg/kg/day or 1.5 mg/kg/day PFOS, respectively, from gestation day 0 to postnatal day 21. The offspring exhibited low body weight until weaning and displayed signs of a pre-diabetic state including elevated fasting serum leptin levels, elevated insulin and impaired glucose tolerance, regardless of exposure levels. These results suggested that developmental exposure to PFOS may contribute to glucose and lipid metabolic disorder in adulthood.
Further highlighting the potential differential gender impact, a clinical-based analysis of adipose depots with POPs accumulation found a significant correlation between high leptin serum concentrations and several PCBs in women, whereas no correlations were found in men (Pereira-Fernandes et al. 2014). Though breast tissue was not analyzed, in visceral fat a significant correlation was found between leptin, POPs and obesity in women. Leptin gene expression was also positively correlated with key POPs, such as CB180 and BDE153. These data indicate a potential interaction between POPs and leptin signaling, however, definitive studies testing whether circulating POPs disrupts leptin signaling remain to be performed. Further extending these observations on changes in leptin levels in response to POPs to whether or not they influence breast cancer tumorigenesis also remains unexamined.
POPs levels are exponentially higher in adipose tissue among obese people than normal weight individuals (Airaksinen, et al. 2011), and obesity causes adipose tissue to become inflamed thus promoting dysfunction (Prieto-Hontoria et al. 2011). POPs exposure and bioaccumulation in tissue also promotes deregulated adipokines, including adiponectin (Kim, et al. 2010; Pereira-Fernandes et al. 2014). For example, adiponectin expression in VAT correlated negatively with the PCB congener CB138, indicating that POPs could have an influence on obesity-related disorders by suppressing the expression of adiponectin, a protective adipokine (Pereira-Fernandes et al. 2014). Another study demonstrated in vitro the effects of another PCB congener, CB77, on the suppression of adiponectin expression of mature adipocytes (Arsenescu, et al. 2008). Furthermore, in overweight and obese individuals, adiponectin is downregulated and inversely associated with sex steroids (Duggan, et al. 2011), all of which are markers for increased risk of breast cancer risk in pre- and post-menopausal women. Whether POPs stimulate a decrease in adiponectin specifically in breast tissue is unknown, and the direct relationship between POPs exposure driving a reduction in adiponectin and the subsequent effects on breast cancer cell behavior has not been investigated. However, adiponectin inhibits important metastatic properties such as adhesion, invasion, and migration of breast cancer cells. Consequently, breast tumors arising in a low adiponectin environment are associated with a more aggressive phenotype (Hwang, et al. 2013; Saxena and Sharma 2010). Thus, further investigation as to whether POPs would stimulate more aggressive breast cancer subtypes via suppression of adiponectin is warranted.
Conversely, a recent study by Salisbury et al. (2013) implicated an inverse correlation between POPs and breast cancer incidence in the binding and activation of the aryl hydrocarbon receptor (AhR) (Salisbury, et al. 2013). AhR is a diverse transcription factor involved in regulating xenobiotic metabolism as well as genes relevant to obesity and inflammation (Beischlag, et al. 2008; Bourdon, et al. 2010; Kerley-Hamilton, et al. 2012; La Merrill et al. 2013; Myre and Imbeault 2014). The AhR affects signaling pathways including the retinoic acid receptor, ER and the NFkB (Beischlag et al. 2008). In their study, AhR signaling activation-enhanced proliferation of ER positive breast cancer cell lines (MCF7 and T47D) via IGF-II-containing adipocyte conditioned media. Conversely TCDD, a highly specific AhR agonist and POP, blocked IGF-II signaling and cell proliferation (Salisbury et al. 2013) indicating that select POPs in the local microenvironment may inhibit cancer cell growth via activation of AhR. However, the paracrine effects of POPs in the tissue microenvironment must be investigated in combination with tumor cell growth. A study using human multipotent adipose-derived stem cells found that exposure to the AhR ligands TCDD and PCB congener 126 significantly regulated genes involved in inflammatory/immune response, cancer, and metabolism pathways (Kim, et al. 2012). This was observed in both precursor and adipocytes, and demonstrated that AhR activation was the major pathway inducing these changes via inhibition of AhR using the AhR antagonist α-naphthoflavone. Regulation of the inflammatory pathway via TCDD and PCB-126 was also observed in wildtype but not AhR-knockout mice. While this study did not directly observe the paracrine effect of the adipose on tumor cells, the pathways activated in the precursor and adipose suggest potential augmentation of tumorigenesis.
Another potential mechanism of action for POPs and obesity on breast tumorigenesis is through the action of fatty acid synthase (FASN), an enzyme responsible for producing endogenous fatty acids. This enzyme has been shown to be necessary for proliferation and survival effects of numerous malignant cancers promoting tumor aggression (Puig, et al. 2009). Specific to breast cancer, studies have shown significantly elevated levels of FASN in vitro and in vivo, and have directly correlated this enhanced expression with enhance aggressive breast cancer cell behaviors, enhanced tumor progression and poor prognosis (Hilvo, et al. 2011; Hopperton, et al. 2014; Hunt, et al. 2007; Khan, et al. 2014). FASN is also linked to the accumulation of visceral fat, the development of type 2 diabetes and obesity (Berndt, et al. 2007). Obesity generates free fatty acids that in turn elevates tissue levels FASN and gives cancer cells heightened de novo lipogenesis (Hunt et al. 2007). This cyclic observation highlights a molecular mechanism for obesity driving breast cancer. To tie in POPs with this mechanism, we highlight a study focused on determining whether the antioxidant glutathione was important in protecting liver tissue from TCDD-mediated toxicity and development of nonalcoholic fatty liver disease with associated steatosis (Chen, et al. 2012). Results show TCDD stimulates liver toxicity and altered lipid metabolism via several lipid metabolism genes, including FASN. Gclm(−/−) knockout mice, a model of liver glutathione deficiency, were protected from liver steatosis and correspondingly had significantly decreased FASN expression levels. This data suggests that altered lipid metabolism, in part by FASN function, is induced via the POPs TCDD. It would be of interested to determine whether POPs similarly function in part through FASN to alter lipid metabolism in breast tissue, thereby potentially promoting breast tumorigenesis.
Lifetime exposure to POPs through dietary intake would, over time, result in continual increases of bioaccumulation in adipose tissue due to POPs lipophilic nature. It is also of note that age is the most significant risk factor in developing breast cancer (Carriot, et al. 2014). It could be hypothesized that POPs bioaccumulation with age could additively/synergistically promote breast cancer progression. The collective interaction of inflammation is with altered local hormone production and altered immune function as seen with age (Chung, et al. 2008; Priyanka, et al. 2013). In post-menopausal women, a significant amount of estrogen is supplied by adipose tissue (Huang, et al. 1997). By adding a lifetime exposure to POPs, bioaccumulation in adipose could have severe consequences, potentially further compounded by obesity. It is important to ward off this increased risk as 70% of breast cancers are estrogen receptor positive and higher levels of estrogen increases risk (Carriot et al. 2014; Santen, et al. 2014).
Socioeconomic Impact of POPs and Breast Cancer
Multiple epidemiological studies indicate a worse prognosis for women who are obese at the time of breast cancer diagnosis compared with non-obese patients (Protani, et al. 2010). A meta-analysis of 43 studies of obesity and breast cancer revealed that obese patients were 33% more likely than non-obese patients to die of breast cancer (Protani et al. 2010). Socioeconomic status has been classically known to impact on diet, with low level of education and moderate physical activity level, cost per calorie as important mediators identified in the socioeconomic status-BMI relationship (Dunneram and Jeewon 2013; Fokeena and Jeewon 2012). Previous trends have shown an increase in BMI and metabolic syndrome in the populations of higher socioeconomic strata among developing countries (Caballero 2007). But, the impact of socioeconomic status on obesity has been shifting, affecting a broader range of societies, and is suggested to be due to increased consumption of sweetened beverages, and increased availability of low-cost, energy-dense foods in urban areas of developing countries (Caballero 2007). Recent studies show obesity is rising among middle-aged (Caballero 2007; Dunneram and Jeewon 2013) and postmenopausal women (Bhurosy and Jeewon 2013; Caballero 2007) and adolescents of low socioeconomic status in middle-income countries in Africa, as well as urban and rural areas in the poorest countries of sub-Saharan Africa and South Asia to populations in countries with higher income levels (Bhurosy and Jeewon 2014; Popkin, et al. 2012). Similarly, another socioeconomic study across 22 western European countries reported that obesity (BMI>30) was more common among women of lower education level (Mackenbach, et al. 2008).
With respect to breast cancer mortality, African-American women suffer disproportionately compared to other racial/ethnic groups (DeSantis, et al. 2014; Jemal, et al. 2007; Newman, et al. 2002) and this has been related to their increased prevalence of high fat/poor diet, obesity and metabolic syndrome. Both social and biological mechanisms are contributory, including a higher prevalence of aggressive basal-like, “triple-negative” breast cancers in young, premenopausal African-American women (Boyle 2012). This subtype does not express detectable levels of ER, PR and does not over-express the growth factor Her2. Therefore, this subtype is deemed to be unresponsive to anti-estrogenic compounds, currently no effective targeted therapies exist, and patient survival is poor (Sorlie, et al. 2001). Even when African-American women (of any age) are diagnosed with ‘good prognosis’ Luminal A breast cancers, they fare worse than Caucasian women with the same subtype (Murray, et al. 2010). Correspondingly, the prevalence of metabolic syndrome is higher among Luminal breast cancers in postmenopausal women [OR 1.37 (95% CI 1.07–2.80) P=0.03] and most notably, BMI alone was associated to Luminal A subtype breast cancer risk [OR 1.12 (95% CI 0.96–2.196 p = 0.2] (Capasso, et al. 2014). Unfortunately race was not addressed in this study.
Social disparities research show the percentage of POPs to be at its highest in women without a formal education and at its lowest in affluent women (Arrebola, et al. 2013; Freire, et al. 2011; Gasull, et al. 2013; Wilcox, et al. 2013). In low socioeconomic ethnic-based communities, the availability of healthy food is inconsistent at best. Fresh fruits and vegetables are somewhat non-existent or too expensive to feed a family. A poor diet of processed/fast foods and fatty meats all contribute to the obesity epidemic prevalent in the African-American community increasing their susceptibility to hypertension, cardiovascular disease, diabetes, and obesity (Caprio, et al. 2008a, b; Hebert, et al. 2013; Wilcox et al. 2013). In a study by Chandran et al (2012), African-American women were shown to be less physically active, overweight or obese, and have a higher intake of total cholesterol and fat than other ethnicities (Chandran, et al. 2012). In addition to dietary choices and food availability, a multitude of factors influence the observed disparities in obesity. For example, a study involving the Jackson Heart Study participants (N = 2,881) reported that travel for healthier food options was inhibited due to safety reasons and perceived neighborhood safety (Pham do, et al. 2014). African American women who strongly disagreed their neighborhood was safe from crime had a higher BMI compared to women who felt safe, and premenopausal African American women who felt most unsafe had significantly higher BMI, waist circumference, and volumes of visceral and total adipose tissue than those who felt safe (Pham do, et al. 2014). These data highlight that in addition to dietary factors, emotional stress may play a role in the promotion of obesity in these communities. It has also been shown that African-American women have a higher burden of PCDD/PCDFs compared to other ethnicities (Reynolds, et al. 2005). These women do not have a higher incidence of breast cancer but, more importantly, they have the worst prognosis and survival rates (Chandran et al. 2012). If it is true that lower socioeconomic classes have higher levels of POPs and POPs accumulates in adipose tissue, then it can be hypothesized that the poor prognosis of African-American women and breast cancer could be attributed to the level of POPs in their adipose tissue.
Model Systems
To study breast development, deregulation and cancer, mouse and rat animal models are frequently used. Numerous murine models have been developed to study normal human breast development and cancer, including the most widely used human xenograft models. In these models, human breast epithelium, stromal and/or tumor cells are transplanted orthotopically into immunocompromised mice that do not reject human cells (Proia and Kuperwasser 2006; Richmond and Su 2008). The three-dimensional tissue organization, whole body metabolism, hormone and growth factor interactions are a highlighted benefit to understanding post-pubertal breast development and function compared to limited in vitro co-culture studies. However, significant limitations include cross-species complications in signaling molecules and the lack of immune cell interactions, a known critical component of tumorigenesis and progression. Knockout mouse models are useful in studying the effects of a single gene during disease. Two knockout mouse models have been used to study the effects of POPs in vivo, though we were unable to find any studies involving mammary function and POPs interactions. The AhR-knockout mouse model was used to test the effects of TCDD on epididymal adipose tissue compared to wild type controls. Male mice were treated with a non-acute toxic dose of TCDD and results demonstrated that pre-adipocytes and adipocytes are targets of POPs and that one of the main pathways activated was inflammation. Activation of the inflammatory pathways was not observed in AhR-knockout, thus this model demonstrated that a major pathway of TCDD was via AhR activation (Kim et al. 2012). The Apo E−/− mouse model has also been used to study POPs in vivo confirming, or highlighting, the synergistic effects of POPs on atherosclerosis (Conklin, et al. 2010; Shan, et al. 2014). Another model for studying human breast cancer are genetically engineered mouse (GEM) models, in which mice carry the same mutation within the endogenous locus, the gene is expressed within the specific cell types that occur in human tumors, and is silent during embryogenesis (Shoushtari, et al. 2007). Although knockout and GEM models are highly useful for evaluating the effects of specific mutation, deletion or gene amplification of one or two genes during breast or tumor progression, they usually cannot fully reproduce the genetic complexity of human tumors. However, it cannot be argued that many advances have been gained from these models and they will continue to provide valuable data to the field.
Potentially one of the most significant limitations to using mouse models is the morphological and structural differences among the epithelium and stroma in murine fat pad compared to human breast. The mouse mammary gland contains large depots of adipose laced with small amounts of interspersed connective tissue (Parmar and Cunha 2004). The functional lobular units of the mouse gland are embedded within the fat pad, and have a considerable amount of space between the minimally branched ducts. In contrast, the lobular units of the human breast are surrounded by loose intralobular connective tissue, consisting primarily of fibroblasts. This intralobular stroma is subsequently surrounded by a more compact interlobular stroma, which detaches the lobules and intralobular stroma from any substantial direct contact with the adipose tissue (Fleming, et al. 2008). Thus, alterations in breast cellular communication in response to obesity in humans compared to mice may substantially vary, given the differences in amount and proximity of the adipose to the epithelium. Stemming from the observations that these stromal subtypes differ in their physical location in relation to epithelial lobules, and that epithelial/stromal interactions can promote or inhibit tumorigenesis, animal models that better resemble human breast structure are critically needed to relate experimental studies investigating collective metabolic, developmental and environmental interactions.
Rats have been highlighted as a more appropriate model due to the similarities in ductal and lobular mammary structures, spatial proximity to fibroblasts and adipose, total adipose content, as well as hormone and paracrine cell interactions (Shepel and Gould 1999; Vargo-Gogola and Rosen 2007). One slight developmental variation in rats is a constant growth of lobular and ductal structures into the adipose; human lobular structures do not extend into fat tissue and ductal structures grow along connective tissue septa (Russo 1983; Russo, et al. 1990). However, a significant advantage of using rat models is the close association of rat and human breast cancer genes and risk alleles (Sanders and Samuelson 2014). Moreover, in contrast to mouse models, chemically induced rat mammary tumors are generally hormone-dependent adenocarcinomas. For this reason, the rat mammary carcinogenesis models have been utilized extensively to investigate hormone-dependent breast cancer and the protective role of pregnancy in breast cancer (Vargo-Gogola and Rosen 2007). Rat models are also popular for metabolic and nutritional studies, and historically chemically induced tumors have been evaluated in correlation with diet-induced obesity. Collectively, these studies have shown that 7, 12, dimethylbenz [a]anthracene (DMBA) and N-nitrosomethylurea (NMU) induced mammary tumors had enhanced proliferation in obese rats compared to normal weight controls (Chan, et al. 1977; Hakkak, et al. 2005; Hakkak, et al. 2007; Lautenbach, et al. 2009). It is of note that the use of more relevant studies to human health and breast cancer, such as dietary POPs accumulation, their contribution to obesity, mammary adipose/stromal function together with mammary cancers have not been studied in the rat model.
Conclusions
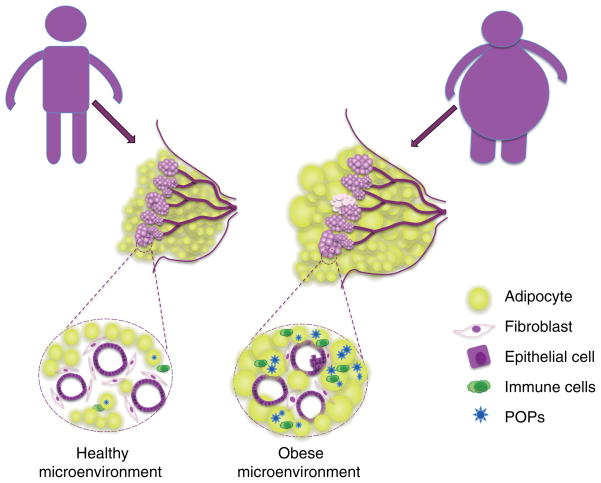
In conclusion, numerous correlative studies suggest a strong association between POPs exposure through diet and their bioaccumulation in adipose promotes the development of obesity and ultimately influences breast cancer development and/or progression (Figure 1). There is a critical need for advanced model systems and pioneering in vitro and in vivo studies to understand the complex relationship between these causative factors and, more importantly, to delineate their multifaceted molecular, cellular, and biochemical mechanisms.
Figure 1. Potential mechanisms for POPs in the promotion of breast cancer.
Obesity-associated endocrine and metabolic mediators are suspected to play a role in oncogenesis by modifying both systemic nutrient metabolism and the nutrients available locally in the supportive stromal tissue. Excessive adipose in obese women also serve as storage depots for POPs obtained from dietary sources. The accumulated POPs stimulate additional adipocyte proliferation and recruitment of immune cells, leading to an inflammatory microenvironment. Collectively, these factors may promote breast cancer development and/or direct cancer cell phenotypes.
Acknowledgments
Funding
This work was supported by funding from the National Cancer Institute (U54 CA156735 and R21 CA175783) and the National Institute of General Medical Sciences (SC2 GM102012). This work was supported in part by the Intramural Research Program of the NIH, NCI.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Agudo A, Goni F, Etxeandia A, Vives A, Millan E, Lopez R, Amiano P, Ardanaz E, Barricarte A, Chirlaque MD, et al. Polychlorinated biphenyls in Spanish adults: determinants of serum concentrations. Environ Res. 2009;109:620–628. doi: 10.1016/j.envres.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care. 2011;34:1972–1979. doi: 10.2337/dc10-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroner SA, Collins LC, Schnitt SJ, Connolly JL, Colditz GA, Tamimi RM. Columnar cell lesions and subsequent breast cancer risk: a nested case-control study. Breast Cancer Res. 2010;12:R61. doi: 10.1186/bcr2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrebola JP, Pumarega J, Gasull M, Fernandez MF, Martin-Olmedo P, Molina-Molina JM, Fernandez-Rodriguez M, Porta M, Olea N. Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ Res. 2013;122:31–37. doi: 10.1016/j.envres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aube M, Larochelle C, Ayotte P. Differential effects of a complex organochlorine mixture on the proliferation of breast cancer cell lines. Environ Res. 2011;111:337–347. doi: 10.1016/j.envres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- Barber MC, Clegg RA, Travers MT, Vernon RG. Lipid metabolism in the lactating mammary gland. Biochim Biophys Acta. 1997;1347:101–126. doi: 10.1016/s0005-2760(97)00079-9. [DOI] [PubMed] [Google Scholar]
- Baumann J, Sevinsky C, Conklin DS. Lipid biology of breast cancer. Biochim Biophys Acta. 2013;1831:1509–1517. doi: 10.1016/j.bbalip.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt J, Kovacs P, Ruschke K, Kloting N, Fasshauer M, Schon MR, Korner A, Stumvoll M, Bluher M. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- Bhurosy T, Jeewon R. Food habits, socioeconomic status and body mass index among premenopausal and post-menopausal women in Mauritius. J Hum Nutr Diet. 2013;26(Suppl 1):114–122. doi: 10.1111/jhn.12100. [DOI] [PubMed] [Google Scholar]
- Bhurosy T, Jeewon R. Overweight and Obesity Epidemic in Developing Countries: A Problem with Diet, Physical Activity, or Socioeconomic Status? Scientific World Journal. 2014;2014:964236. doi: 10.1155/2014/964236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav. 2014 doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC. Biomonitoring in Greenland: human biomarkers of exposure and effects - a short review. Rural Remote Health. 2010;10:1362. [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Long M, Bossi R, Ayotte P, Asmund G, Kruger T, Ghisari M, Mulvad G, Kern P, Nzulumiki P, et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: a case control study. Environ Health. 2011;10:88. doi: 10.1186/1476-069X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon JA, Bazinet TM, Arnason TT, Kimpe LE, Blais JM, White PA. Polychlorinated biphenyls (PCBs) contamination and aryl hydrocarbon receptor (AhR) agonist activity of Omega-3 polyunsaturated fatty acid supplements: implications for daily intake of dioxins and PCBs. Food Chem Toxicol. 2010;48:3093–3097. doi: 10.1016/j.fct.2010.07.051. [DOI] [PubMed] [Google Scholar]
- Bourez S, Le Lay S, Van den Daelen C, Louis C, Larondelle Y, Thome JP, Schneider YJ, Dugail I, Debier C. Accumulation of polychlorinated biphenyls in adipocytes: selective targeting to lipid droplets and role of caveolin-1. PLoS One. 2012;7:e31834. doi: 10.1371/journal.pone.0031834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourez S, Van den Daelen C, Le Lay S, Poupaert J, Larondelle Y, Thome JP, Schneider YJ, Dugail I, Debier C. The dynamics of accumulation of PCBs in cultured adipocytes vary with the cell lipid content and the lipophilicity of the congener. Toxicol Lett. 2013;216:40–46. doi: 10.1016/j.toxlet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- Butt CM, Berger U, Bossi R, Tomy GT. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ. 2010;408:2936–2965. doi: 10.1016/j.scitotenv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- Cabaravdic M. Xenoestrogen effects of chemical compounds: influence on the breast cancer. Med Arh. 2006;60:97–100. [PubMed] [Google Scholar]
- Capasso I, Esposito E, de Laurentiis M, Maurea N, Cavalcanti E, Botti G, Petrillo A, Montella M, D’Aiuto M, Coppola C, et al. Metabolic syndrome-breast cancer link varies by intrinsic molecular subtype. Diabetol Metab Syndr. 2014;6:105. doi: 10.1186/1758-5996-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprio S, Daniels SR, Drewnowski A, Kaufman FR, Palinkas LA, Rosenbloom AL, Schwimmer JB. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment. Obesity (Silver Spring) 2008a;16:2566–2577. doi: 10.1038/oby.2008.398. [DOI] [PubMed] [Google Scholar]
- Caprio S, Daniels SR, Drewnowski A, Kaufman FR, Palinkas LA, Rosenbloom AL, Schwimmer JB. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment: a consensus statement of Shaping America’s Health and the Obesity Society. Diabetes Care. 2008b;31:2211–2221. doi: 10.2337/dc08-9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriot J, Jamali M, Chacron MJ, Cullen KE. Statistics of the vestibular input experienced during natural self-motion: implications for neural processing. J Neurosci. 2014;34:8347–8357. doi: 10.1523/JNEUROSCI.0692-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casbas-Hernandez P, Fleming JM, Troester MA. Gene expression analysis of in vitro cocultures to study interactions between breast epithelium and stroma. J Biomed Biotechnol. 2011;2011:520987. doi: 10.1155/2011/520987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casbas-Hernandez P, Sun X, Roman-Perez E, D’Arcy M, Sandhu R, Hishida A, McNaughton KK, Yang XR, Makowski L, Sherman ME, et al. Tumor Intrinsic Subtype is Reflected in Cancer-Adjacent Tissue. Cancer Epidemiol Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-14-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PC, Head JF, Cohen LA, Wynder EL. Influence of dietary fat on the induction of mammary tumors by N-nitrosomethylurea: associated hormone changes and differences between Sprague-Dawley and F344 rats. J Natl Cancer Inst. 1977;59:1279–1283. doi: 10.1093/jnci/59.4.1279. [DOI] [PubMed] [Google Scholar]
- Chandran U, Hirshfield KM, Bandera EV. The role of anthropometric and nutritional factors on breast cancer risk in African-American women. Public Health Nutr. 2012;15:738–748. doi: 10.1017/S136898001100303X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Krishan M, Nebert DW, Shertzer HG. Glutathione-deficient mice are susceptible to TCDD-Induced hepatocellular toxicity but resistant to steatosis. Chem Res Toxicol. 2012;25:94–100. doi: 10.1021/tx200242a. [DOI] [PubMed] [Google Scholar]
- Chung JH, Seo AY, Chung SW, Kim MK, Leeuwenburgh C, Yu BP, Chung HY. Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res Rev. 2008;7:126–136. doi: 10.1016/j.arr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. The influence of family history on breast cancer risk in women with biopsy-confirmed benign breast disease: results from the Nurses’ Health Study. Cancer. 2006;107:1240–1247. doi: 10.1002/cncr.22136. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Barski OA, Lesgards JF, Juvan P, Rezen T, Rozman D, Prough RA, Vladykovskaya E, Liu S, Srivastava S, et al. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol Appl Pharmacol. 2010;243:1–12. doi: 10.1016/j.taap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Luebke RW, Germolec DR, DeWitt JC. Perfluorinated compounds: emerging POPs with potential immunotoxicity. Toxicol Lett. 2014;230:263–270. doi: 10.1016/j.toxlet.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Ann N Y Acad Sci. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- de Oliveira Andrade F, Fontelles CC, Rosim MP, de Oliveira TF, de Melo Loureiro AP, Mancini-Filho J, Rogero MM, Moreno FS, de Assis S, Barbisan LF, et al. Exposure to lard-based high-fat diet during fetal and lactation periods modifies breast cancer susceptibility in adulthood in rats. J Nutr Biochem. 2014;25:613–622. doi: 10.1016/j.jnutbio.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decherf S, Demeneix BA. The obesogen hypothesis: a shift of focus from the periphery to the hypothalamus. J Toxicol Environ Health B Crit Rev. 2011;14:423–448. doi: 10.1080/10937404.2011.578561. [DOI] [PubMed] [Google Scholar]
- Delort L, Lequeux C, Dubois V, Dubouloz A, Billard H, Mojallal A, Damour O, Vasson MP, Caldefie-Chezet F. Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3D adipose equivalent model. PLoS One. 2013;8:e66284. doi: 10.1371/journal.pone.0066284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40:300–311. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, Van Gaal L. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring) 2011;19:709–714. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Health risks of dietary exposure to perfluorinated compounds. Environ Int. 2012;40:187–195. doi: 10.1016/j.envint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Duggan C, Irwin ML, Xiao L, Henderson KD, Smith AW, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R, McTiernan A. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. 2011;29:32–39. doi: 10.1200/JCO.2009.26.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunneram Y, Jeewon R. A Scientific Assessment of Sociodemographic Factors, Physical Activity Level, and Nutritional Knowledge as Determinants of Dietary Quality among Indo-Mauritian Women. J Nutr Metab. 2013;2013:572132. doi: 10.1155/2013/572132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JM, Long EL, Ginsburg E, Gerscovich D, Meltzer PS, Vonderhaar BK. Interlobular and intralobular mammary stroma: genotype may not reflect phenotype. BMC Cell Biol. 2008;9:46. doi: 10.1186/1471-2121-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokeena WB, Jeewon R. Is there an association between socioeconomic status and body mass index among adolescents in Mauritius? Scientific World Journal. 2012;2012:750659. doi: 10.1100/2012/750659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Amaya E, Fernandez MF, Gonzalez-Galarzo MC, Ramos R, Molina-Molina JM, Arrebola JP, Olea N. Relationship between occupational social class and exposure to organochlorine pesticides during pregnancy. Chemosphere. 2011;83:831–838. doi: 10.1016/j.chemosphere.2011.02.076. [DOI] [PubMed] [Google Scholar]
- Gadupudi G, Gourronc FA, Ludewig G, Robertson LW, Klingelhutz AJ. PCB126 inhibits adipogenesis of human preadipocytes. Toxicol In Vitro. 2014 doi: 10.1016/j.tiv.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull M, Pumarega J, Rovira G, Lopez T, Alguacil J, Porta M. Relative effects of educational level and occupational social class on body concentrations of persistent organic pollutants in a representative sample of the general population of Catalonia, Spain. Environ Int. 2013;60:190–201. doi: 10.1016/j.envint.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Hu HH, Kajimura S. Relevance of brown adipose tissue in infancy and adolescence. Pediatr Res. 2013;73:3–9. doi: 10.1038/pr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170:R159–171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Gutleb AC, Freitas J, Murk AJ, Verhaegen S, Ropstad E, Udelhoven T, Hoffmann L, Audinot JN. NanoSIMS50 - a powerful tool to elucidate cellular localization of halogenated organic compounds. Anal Bioanal Chem. 2012;404:2693–2698. doi: 10.1007/s00216-012-6066-8. [DOI] [PubMed] [Google Scholar]
- Hakkak R, Holley AW, Macleod SL, Simpson PM, Fuchs GJ, Jo CH, Kieber-Emmons T, Korourian S. Obesity promotes 7,12-dimethylbenz(a)anthracene-induced mammary tumor development in female zucker rats. Breast Cancer Res. 2005;7:R627–633. doi: 10.1186/bcr1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkak R, MacLeod S, Shaaf S, Holley AW, Simpson P, Fuchs G, Jo CH, Kieber-Emmons T, Korourian S. Obesity increases the incidence of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in an ovariectomized Zucker rat model. Int J Oncol. 2007;30:557–563. [PubMed] [Google Scholar]
- Ham M, Moon A. Inflammatory and microenvironmental factors involved in breast cancer progression. Arch Pharm Res. 2013;36:1419–1431. doi: 10.1007/s12272-013-0271-7. [DOI] [PubMed] [Google Scholar]
- Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- Hassiotou F, Geddes D. Anatomy of the human mammary gland: Current status of knowledge. Clin Anat. 2013;26:29–48. doi: 10.1002/ca.22165. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Wirth M, Davis L, Davis B, Harmon BE, Hurley TG, Drayton R, Angela Murphy E, Shivappa N, Wilcox S, et al. C-reactive protein levels in African Americans: a diet and lifestyle randomized community trial. Am J Prev Med. 2013;45:430–440. doi: 10.1016/j.amepre.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Portoles T, Pitarch E, Lopez FJ. Searching for anthropogenic contaminants in human breast adipose tissues using gas chromatography-time-of-flight mass spectrometry. J Mass Spectrom. 2009;44:1–11. doi: 10.1002/jms.1538. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–348. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Luoto R, Huttunen T, Koskenvuo M. Pregnancy weight gain and premenopausal breast cancer risk. J Reprod Med. 2005;50:811–816. [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Wang C, Kalil M, Riggins R, Pestell RG. Nutritional modulation of the cell cycle and breast cancer. Endocr Relat Cancer. 2004;11:603–622. doi: 10.1677/erc.1.00665. [DOI] [PubMed] [Google Scholar]
- Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- Hopperton KE, Duncan RE, Bazinet RP, Archer MC. Fatty acid synthase plays a role in cancer metabolism beyond providing fatty acids for phospholipid synthesis or sustaining elevations in glycolytic activity. Exp Cell Res. 2014;320:302–310. doi: 10.1016/j.yexcr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- Hunt DA, Lane HM, Zygmont ME, Dervan PA, Hennigar RA. MRNA stability and overexpression of fatty acid synthase in human breast cancer cell lines. Anticancer Res. 2007;27:27–34. [PubMed] [Google Scholar]
- Hwang MS, Yu N, Stinson SY, Yue P, Newman RJ, Allan BB, Dornan D. miR-221/222 targets adiponectin receptor 1 to promote the epithelial-to-mesenchymal transition in breast cancer. PLoS One. 2013;8:e66502. doi: 10.1371/journal.pone.0066502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarguren M, Lopez DJ, Escriba PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta. 2014;1838:1518–1528. doi: 10.1016/j.bbamem.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem. 2003;22:2639–2649. doi: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley-Hamilton JS, Trask HW, Ridley CJ, Dufour E, Ringelberg CS, Nurinova N, Wong D, Moodie KL, Shipman SL, Moore JH, et al. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Environ Health Perspect. 2012;120:1252–1259. doi: 10.1289/ehp.1205003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Aljarbou AN, Aldebasi YH, Faisal SM, Khan MA. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014;38:765–772. doi: 10.1016/j.canep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Kim BM, Rhee JS, Hwang UK, Seo JS, Shin KH, Lee JS. Dose- and time-dependent expression of aryl hydrocarbon receptor (AhR) and aryl hydrocarbon receptor nuclear translocator (ARNT) in PCB-, B[a]P-, and TBT-exposed intertidal copepod Tigriopus japonicus. Chemosphere. 2014;120C:398–406. doi: 10.1016/j.chemosphere.2014.07.099. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, Steffes M, Lee DH. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010;118:370–374. doi: 10.1289/ehp.0901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clement K, Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120:508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, Pettus JR, Froehlich HM, Memoli VA, Morganelli PM, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clement K, Birnbaum LS, Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121:162–169. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara LA, Duarte AA, Reis RM, Vieira CS, Rosa-e-Silva AC. Endocrine disrupters: potential risk factors affecting sexual function in both men and women. J Sex Med. 2012;9:941–942. doi: 10.1111/j.1743-6109.2011.02596.x. [DOI] [PubMed] [Google Scholar]
- Lautenbach A, Budde A, Wrann CD, Teichmann B, Vieten G, Karl T, Nave H. Obesity and the associated mediators leptin, estrogen and IGF-I enhance the cell proliferation and early tumorigenesis of breast cancer cells. Nutr Cancer. 2009;61:484–491. doi: 10.1080/01635580802610115. [DOI] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118:1235–1242. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006;79:1160–1169. doi: 10.1016/j.lfs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, Sonne C, Verreault J, Vijayan MM, Gabrielsen GW. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ. 2010;408:2995–3043. doi: 10.1016/j.scitotenv.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Ljunggren SA, Helmfrid I, Salihovic S, van Bavel B, Wingren G, Lindahl M, Karlsson H. Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environ Int. 2014;65:93–99. doi: 10.1016/j.envint.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, Wei J, Lin Y, Jiang Y, Wang Y, et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28:532–542. doi: 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- Mackenbach JP, Stirbu I, Roskam AJ, Schaap MM, Menvielle G, Leinsalu M, Kunst AE. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Loh VH, Harding JL, Botton J, Shaw JE. Persistent organic pollutants and diabetes: A review of the epidemiological evidence. Diabetes Metab. 2014;40:1–14. doi: 10.1016/j.diabet.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- McManaman JL. Lipid transport in the lactating mammary gland. J Mammary Gland Biol Neoplasia. 2014;19:35–42. doi: 10.1007/s10911-014-9318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina CM, Pitarch E, Portoles T, Lopez FJ, Hernandez F. GC-MS/MS multi-residue method for the determination of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in human breast tissues. J Sep Sci. 2009;32:2090–2102. doi: 10.1002/jssc.200800737. [DOI] [PubMed] [Google Scholar]
- Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166:E1–7. [PubMed] [Google Scholar]
- Murray MJ, Saini HK, van Dongen S, Palmer RD, Muralidhar B, Pett MR, Piipari M, Thornton CM, Nicholson JC, Enright AJ, et al. The two most common histological subtypes of malignant germ cell tumour are distinguished by global microRNA profiles, associated with differential transcription factor expression. Mol Cancer. 2010;9:290. doi: 10.1186/1476-4598-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myre M, Imbeault P. Persistent organic pollutants meet adipose tissue hypoxia: does cross-talk contribute to inflammation during obesity? Obes Rev. 2014;15:19–28. doi: 10.1111/obr.12086. [DOI] [PubMed] [Google Scholar]
- Nalabolu MR, Palasamudram K, Jamil K. Adiponectin and Leptin Molecular Actions and Clinical Significance in Breast Cancer. Int J Hematol Oncol Stem Cell Res. 2014;8:31–40. [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Mason J, Cote D, Vin Y, Carolin K, Bouwman D, Colditz GA. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Fredlund E, Zhao W, Perou CM, Balmain A, Mao JH, Barcellos-Hoff MH. Murine microenvironment metaprofiles associate with human cancer etiology and intrinsic subtypes. Clin Cancer Res. 2013;19:1353–1362. doi: 10.1158/1078-0432.CCR-12-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen NH, Hansen JP. Breast cancer in Greenland--selected epidemiological, clinical, and histological features. J Cancer Res Clin Oncol. 1980;98:287–299. doi: 10.1007/BF00410791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED. The toxicology of climate change: environmental contaminants in a warming world. Environ Int. 2009;35:971–986. doi: 10.1016/j.envint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Park J, Cho SY, Lee SB, Son H, Jeong H. Obesity is associated with higher risk of prostate cancer detection in a biopsy population in Korea. BJU Int. 2014a;114:891–895. doi: 10.1111/bju.12600. [DOI] [PubMed] [Google Scholar]
- Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014b;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437–458. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- Pavlovich AL, Manivannan S, Nelson CM. Adipose stroma induces branching morphogenesis of engineered epithelial tubules. Tissue Eng Part A. 2010;16:3719–3726. doi: 10.1089/ten.tea.2009.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Fernandes A, Dirinck E, Dirtu AC, Malarvannan G, Covaci A, Van Gaal L, Vanparys C, Jorens PG, Blust R. Expression of obesity markers and Persistent Organic Pollutants levels in adipose tissue of obese patients: reinforcing the obesogen hypothesis? PLoS One. 2014;9:e84816. doi: 10.1371/journal.pone.0084816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestana D, Faria G, Sa C, Fernandes VC, Teixeira D, Norberto S, Faria A, Meireles M, Marques C, Correia-Sa L, et al. Persistent organic pollutant levels in human visceral and subcutaneous adipose tissue in obese individuals--depot differences and dysmetabolism implications. Environ Res. 2014;133:170–177. doi: 10.1016/j.envres.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Petreas M, Nelson D, Brown FR, Goldberg D, Hurley S, Reynolds P. High concentrations of polybrominated diphenylethers (PBDEs) in breast adipose tissue of California women. Environ Int. 2011;37:190–197. doi: 10.1016/j.envint.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreas M, Smith D, Hurley S, Jeffrey SS, Gilliss D, Reynolds P. Distribution of persistent, lipid-soluble chemicals in breast and abdominal adipose tissues: lessons learned from a breast cancer study. Cancer Epidemiol Biomarkers Prev. 2004;13:416–424. [PubMed] [Google Scholar]
- Pham do Q, Ommerborn MJ, Hickson DA, Taylor HA, Clark CR. Neighborhood safety and adipose tissue distribution in African Americans: the Jackson Heart Study. PLoS One. 2014;9:e105251. doi: 10.1371/journal.pone.0105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planey SL, Kumar R, Arnott JA. Estrogen receptors (ERalpha versus ERbeta): friends or foes in human biology? J Recept Signal Transduct Res. 2014;34:1–5. doi: 10.3109/10799893.2013.853188. [DOI] [PubMed] [Google Scholar]
- Pombo M, Castro-Feijoo L. Endocrine disruptors. J Pediatr Endocrinol Metab. 2005;18(Suppl 1):1145–1155. doi: 10.1515/jpem.2005.18.s1.1145. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011;1807:664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Priyanka HP, Sharma U, Gopinath S, Sharma V, Hima L, ThyagaRajan S. Menstrual cycle and reproductive aging alters immune reactivity, NGF expression, antioxidant enzyme activities, and intracellular signaling pathways in the peripheral blood mononuclear cells of healthy women. Brain Behav Immun. 2013;32:131–143. doi: 10.1016/j.bbi.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1:206–214. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- Puig T, Porta R, Colomer R. Fatty acid synthase: a new anti-tumor target. Med Clin (Barc) 2009;132:359–363. doi: 10.1016/j.medcli.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Hurley SE, Petreas M, Goldberg DE, Smith D, Gilliss D, Mahoney ME, Jeffrey SS. Adipose levels of dioxins and risk of breast cancer. Cancer Causes Control. 2005;16:525–535. doi: 10.1007/s10552-004-7840-5. [DOI] [PubMed] [Google Scholar]
- Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech. 2008;1:78–82. doi: 10.1242/dmm.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter L, Solomon K, Sibley P, Hall K, Keen P, Mattu G, Linton B. Sources, pathways, and relative risks of contaminants in surface water and groundwater: a perspective prepared for the Walkerton inquiry. J Toxicol Environ Health A. 2002;65:1–142. doi: 10.1080/152873902753338572. [DOI] [PubMed] [Google Scholar]
- del Romero-Figueroa MS, de Garduno-Garcia JJ, Duarte-Mote J, Matute-Gonzalez G, Gomez-Villanueva A, De la Cruz-Vargas J. Insulin and leptin levels in obese patients with and without breast cancer. Clin Breast Cancer. 2013;13:482–485. doi: 10.1016/j.clbc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Roos V, Ronn M, Salihovic S, Lind L, van Bavel B, Kullberg J, Johansson L, Ahlstrom H, Lind PM. Circulating levels of persistent organic pollutants in relation to visceral and subcutaneous adipose tissue by abdominal MRI. Obesity (Silver Spring) 2013;21:413–418. doi: 10.1002/oby.20267. [DOI] [PubMed] [Google Scholar]
- Rose DP, Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors. 2014;40:1–12. doi: 10.1002/biof.1109. [DOI] [PubMed] [Google Scholar]
- Rosenwald M, Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3:4–9. doi: 10.4161/adip.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J. Basis of cellular autonomy in the susceptibility to carcinogenesis. Toxicol Pathol. 1983;11:149–166. doi: 10.1177/019262338301100207. [DOI] [PubMed] [Google Scholar]
- Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander L, Rignell-Hydbom A, Tinnerberg H, Jonsson BA. Trends in human concentrations of endocrine disruptors: possible reasons and consequences. J Epidemiol Community Health. 2014;68:4–5. doi: 10.1136/jech-2012-201508. [DOI] [PubMed] [Google Scholar]
- Salameh TS, Le TT, Nichols MB, Bauer E, Cheng J, Camarillo IG. An ex vivo co-culture model system to evaluate stromal-epithelial interactions in breast cancer. Int J Cancer. 2013;132:288–296. doi: 10.1002/ijc.27672. [DOI] [PubMed] [Google Scholar]
- Salisbury TB, Morris GZ, Tomblin JK, Chaudhry AR, Cook CR, Santanam N. Aryl hydrocarbon receptor ligands inhibit igf-ii and adipokine stimulated breast cancer cell proliferation. ISRN Endocrinol. 2013;2013:104850. doi: 10.1155/2013/104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Samuelson DJ. Significant overlap between human genome-wide association-study nominated breast cancer risk alleles and rat mammary cancer susceptibility loci. Breast Cancer Res. 2014;16:R14. doi: 10.1186/bcr3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. 2014 doi: 10.1016/j.steroids.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Saxena NK, Sharma D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell Adh Migr. 2010;4:358–362. doi: 10.4161/cam.4.3.11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitt SJ. Benign breast disease and breast cancer risk: potential role for antiestrogens. Clin Cancer Res. 2001;7:4419s–4422s. discussion 4411s–4412s. [PubMed] [Google Scholar]
- Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, Becker W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. 2014:58. doi: 10.3402/fnr.v58.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes. 2009;58:1482–1484. doi: 10.2337/db09-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Wang J, Huang F, Lv X, Ma M, Du Y. Augmented atherogenesis in ApoE-null mice co-exposed to polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2014;276:136–146. doi: 10.1016/j.taap.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Shepel LA, Gould MN. The genetic components of susceptibility to breast cancer in the rat. Prog Exp Tumor Res. 1999;35:158–169. doi: 10.1159/000062012. [DOI] [PubMed] [Google Scholar]
- Shoushtari AN, Michalowska AM, Green JE. Comparing genetically engineered mouse mammary cancer models with human breast cancer by expression profiling. Breast Dis. 2007;28:39–51. doi: 10.3233/bd-2007-28105. [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Harvey KA, Zaloga GP, Stillwell W. Modulation of lipid rafts by Omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract. 2007;22:74–88. doi: 10.1177/011542650702200174. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Solomon GM, Schettler T. Environment and health: 6. Endocrine disruption and potential human health implications. CMAJ. 2000;163:1471–1476. [PMC free article] [PubMed] [Google Scholar]
- Sonne C. Health effects from long-range transported contaminants in Arctic top predators: An integrated review based on studies of polar bears and relevant model species. Environ Int. 2010;36:461–491. doi: 10.1016/j.envint.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003;45:1–16. doi: 10.1207/S15327914NC4501_1. [DOI] [PubMed] [Google Scholar]
- Suba Z. Circulatory estrogen level protects against breast cancer in obese women. Recent Pat Anticancer Drug Discov. 2013;8:154–167. doi: 10.2174/1574892811308020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, Troester MA. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, Jacobs D, Kohrle J, Lee DH, Rylander L, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thundiyil JG, Solomon GM, Miller MD. Transgenerational exposures: persistent chemical pollutants in the environment and breast milk. Pediatr Clin North Am. 2007;54:81–101. ix. doi: 10.1016/j.pcl.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goni F, Grimalt JO, Llop S, Marina LS, Vizcaino E, et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity (Silver Spring) 2014;22:488–496. doi: 10.1002/oby.20603. [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Regulation of development of the normal mammary gland by hormones and growth factors. Cancer Treat Res. 1988;40:251–266. doi: 10.1007/978-1-4613-1733-3_12. [DOI] [PubMed] [Google Scholar]
- Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, Needham L, Patterson D, Brambilla P. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2002;110:625–628. doi: 10.1289/ehp.02110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch E, Holoubek I, Lloyd-Smith M, Masunaga S, et al. Dioxin- and POP-contaminated sites--contemporary and future relevance and challenges: overview on background, aims and scope of the series. Environ Sci Pollut Res Int. 2008;15:363–393. doi: 10.1007/s11356-008-0024-1. [DOI] [PubMed] [Google Scholar]
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- Whyte MB, Velusamy S, Aylwin SJ. Disease severity and staging of obesity: a rational approach to patient selection. Curr Atheroscler Rep. 2014;16:456. doi: 10.1007/s11883-014-0456-7. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Sharpe PA, Turner-McGrievy G, Granner M, Baruth M. Frequency of consumption at fast-food restaurants is associated with dietary intake in overweight and obese women recruited from financially disadvantaged neighborhoods. Nutr Res. 2013;33:636–646. doi: 10.1016/j.nutres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Britton JA, Teitelbaum SL, Eng S, Deych E, Ireland K, Liu Z, Neugut AI, Santella RM, Gammon MD. Improving organochlorine biomarker models for cancer research. Cancer Epidemiol Biomarkers Prev. 2005;14:2224–2236. doi: 10.1158/1055-9965.EPI-05-0173. [DOI] [PubMed] [Google Scholar]
- Woodwell GM. Toxic substances and ecological cycles. Sci Am. 1967;216:24–31. doi: 10.1038/scientificamerican0367-24. [DOI] [PubMed] [Google Scholar]
- Yu GW, Laseter J, Mylander C. Persistent organic pollutants in serum and several different fat compartments in humans. J Environ Public Health. 2011;2011:417980. doi: 10.1155/2011/417980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeliger HI. Lipophilic chemical exposure as a cause of cardiovascular disease. Interdiscip Toxicol. 2013a;6:55–62. doi: 10.2478/intox-2013-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeliger HI. Lipophilic chemical exposure as a cause of type 2 diabetes (T2D) Rev Environ Health. 2013b;28:9–20. doi: 10.1515/reveh-2012-0031. [DOI] [PubMed] [Google Scholar]