Abstract
Background
To investigate the impact of hospitalization for hematopoietic stem cell transplantation (HCT) on patients’ and family caregivers’ (FC) quality of life (QOL) and mood.
Methods
We conducted a longitudinal study of patients hospitalized for HCT and their FC. At baseline (6 days pre-HCT), day+1, and day+8 of HCT, we assessed QOL (Functional Assessment of Cancer Therapy-Bone Marrow Transplantation FACT-BMT), and mood (Hospital Anxiety and Depression Scale HADS). We administered the SF-36 to examine FC QOL [physical component scale (PCS), and mental component scale (MCS)]. To identify predictors of QOL changes, we used multivariable linear mixed models.
Results
We enrolled 97% of eligible patients undergoing autologous (n=30), myeloablative (n=30) or reduced intensity (n=30) allogeneic HCT. Patients’ QOL markedly declined (mean FACT-BMT 109.6 to 96.0, P < 0.0001) throughout hospitalization. The proportion of patients with depression (HADS-Depression> 7) more than doubled from baseline to day+8 (15.6% to 37.8%, P < 0.0001), whereas the proportion of patients with anxiety remained stable (22.2%, P = 0.8). These results remained consistent when data were stratified by HCT type. Baseline depression (β= −2.24, F=42.2, p < 0.0001) and anxiety (β= −0.63, F=4.4, p = 0.03) independently predicted worse QOL throughout hospitalization. FC QOL declined during patient’s hospitalization (PCS: 83.1 to 79.6, P= 0.03, MCS: 71.6 to 67.4, P = 0.04).
Conclusions
Patients undergoing HCT reported a steep deterioration in QOL and substantially worsening depression during hospitalization. Baseline anxiety and depression predicted worse QOL during hospitalization, underscoring the importance of assessing pre-HCT psychiatric morbidity.
Keywords: QOL, Depression, Mood, Family Caregivers, Stem Cell Transplant
Introduction
Patients with hematologic malignancies undergoing HCT receive high-dose chemotherapy with substantial side effects during a prolonged and socially isolating hospitalization.1, 2 While many studies have focused on the morbidity experienced by HCT survivors,2–6 few have examined patients’ QOL and physical and psychological symptoms during their HCT hospitalization.2, 4, 7 Additionally, prior research describing patients’ experience during HCT is limited by cross-sectional and retrospective study designs, as well as the lack of validated, longitudinal assessments of QOL and psychological measures.2, 5, 7–10 Moreover, prior prospective studies were conducted in the 1990s and do not reflect current transplant practices and supportive care measures.7, 8, 11, 12 Data comparing patients’ symptoms and QOL during autologous, myeloablative allogeneic (MAC), or reduced intensity conditioning allogeneic (RIC) HCT are also lacking.
Despite the limited research efforts, there is a general acceptance by transplant clinicians that patients experience the highest degree of distress while hospitalized for HCT.2 Additionally, clinicians often perceive patients’ distress during the transplant to be expected and unmodifiable.1 However, detailed knowledge of patients’ QOL and symptoms during transplantation can 1) inform the design of interventions to improve patients’ experience; 2) better prepare patients for their HCT; and 3) identify subsets of patients who are at the greatest risk of experiencing distress. Lastly, studying patients’ QOL and mood during their hospitalization may identify those at high risk of post-transplant maladjustment and long-term morbidity.7, 13
Family members (family caregivers [FC]) are also substantially impacted by the patients’ hospitalization for HCT. In addition to the burden of witnessing their loved one in distress, FC are confronted with significant disruptions to their personal lives at home and work.14 Although studies have examined the long-term impact of HCT on FC QOL, none have assessed the burden to caregivers during the acute phase of treatment.15
We conducted a prospective longitudinal study to assess the QOL and physical and psychological symptoms experienced by patients and their FC during hospitalization for HCT. We also sought to compare QOL, mood, and fatigue trajectories for patients undergoing autologous, MAC, and RIC HCT. Finally, we identified predictors of patients’ QOL during their HCT in order to identify particularly vulnerable subsets of patients who may benefit from intensive supportive care interventions.
Methods
Participants
We recruited patients with hematologic malignancies admitted for autologous (n=30), MAC (n=30), and RIC HCT (n=30) consecutively for each HCT cohort within 72 hours of admission at Massachusetts General Hospital. Patients (age ≥ 18) with the ability to read questions in English were eligible to participate. We excluded patients with significant psychiatric or co-morbid disease, which the oncologist believed impaired their ability to provide informed consent. We asked enrolled patients to identify a FC (a relative or a friend who either lived with the patient or had in-person contact with him/her at least twice per week) and invited this person to participate in the FC portion of the study. Patients without a willing or available FC were eligible to participate.
Study Design and Procedures
We identified eligible patients with a planned admission for HCT during our weekly Bone Marrow Transplant team meeting. A research assistant obtained permission from the treating oncologist by email to approach eligible patients and their FC. Willing participants provided written informed consent and completed baseline questionnaires at the time of enrollment, six days prior to transplant (day-6 +/−2 days). We administered self-reported measures weekly at the following time points: one day after transplant (day+1+/− 2 days), eight days after transplant (day+8 +/−2 days), and 15 days after transplant (day +15 +/− 2 days). We continued to administer measures weekly until hospital discharge for participants hospitalized longer than 15 days.
Patient-reported measures
Participants completed a demographic questionnaire detailing their age, gender, marital status, income, and education level. We reviewed patients’ electronic health records to obtain their cancer diagnosis, comorbidities, and date of transplant. For each participant, we calculated the HCT Comorbidity Index (HCT-CI)16 at the time of their transplant consultation.
We used the Functional Assessment of Cancer Therapy- BMT (FACT-BMT) to assess patients’ QOL.17 The FACT-BMT contains 47 items that comprise 5 subscales assessing physical, functional, emotional, social well-being, and Bone Marrow Transplant (BMT) specific concerns. Higher total and subscale scores indicate better QOL. We also calculated the Trial Outcome Index (TOI), which is the sum of the physical, functional, and BMT subscales. To assess the prevalence and severity of various symptoms, we examined specific items of the FACT-BMT. We measured patients’ fatigue using the FACT-Fatigue subscale, which consists of 13-items about fatigue symptoms during the past week.18 Lower scores indicate greater fatigue burden.
We measured patients’ anxiety and depression with the 14-item Hospital Anxiety and Depression Scale (HADS). The HADS consists of two subscales assessing anxiety (HADS-Anxiety) and depression (HADS-Depression) symptoms in the past week, with subscale scores ranging from 0 (no distress) to 21 (maximum distress).19 We also assessed mood using the Patient Health Questionnaire 9 (PHQ-9), a nine-item measure that detects symptoms of major depressive disorder according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).20 The HADS subscales and PHQ-9 can also be evaluated continuously with higher scores indicating worse psychological distress.20
Family caregivers-reported measures
We utilized the Medical Outcomes Study 36-item Short Form Health Survey (SF-36), which measures physical functioning, role functioning-physical, bodily pain, general health, vitality, social functioning, role functioning-emotional, and mental health to assess FC QOL.21 We also calculated two summary scores, the Physical Component Scale (PCS) and Mental Component Scale (MCS).21 Finally, FC completed the HADS and PHQ-9 to assess their anxiety and depression.
Attrition and missing data
At our institution, patients undergoing autologous and RIC HCT are hospitalized approximately 1.5 weeks and those undergoing MAC HCT are hospitalized for 2.5–3 weeks after their transplant (day 0). Therefore, we analyzed data up to day +8 for participants undergoing autologous and RIC HCT. For participants undergoing MAC HCT, we also included data from day +15 in the analyses. Only 6.7% (6/90) of patients were missing day +1 and 20% (18/90) were missing day +8 evaluations. For patients undergoing MAC HCT, 30% (9/30) were missing day +15 evaluation. Most (91%) missing evaluations were due to patients’ health deterioration and symptom burden per patients’ self-report, and 9% were due to patients’ preference not to complete the questionnaires longitudinally. We used several methods to compute missing QOL data and compared the results to those without imputations. Imputations involved (1) simple mean imputation and (2) last observation carried forward. We also utilized maximum likelihood estimation for missing data. As our results were similar with the different methods, we used the conservative approach of carrying last value forward to account for all missing patient and FC reported outcomes as data were not missing at random.
Statistical Analysis
We calculated descriptive statistics, including means or medians for continuous variables depending on the normality of the data, and proportions for categorical variables. We compared baseline patient characteristics between the three types of HCT using chi square test for categorical variables and ANOVA for continuous variables. For all analyses, we considered two-sided p-values < 0.05 to be statistically significant.
We computed linear mixed effects models to characterize trajectories of changes in patient outcomes (FACT-BMT, TOI, fatigue, HADS, PHQ-9, and SF-36). Analyses estimated baseline values and rate of change separately for each outcome. Each model was constructed in several steps. Step 1 included a baseline model to estimate intercept and slope random effects for the outcome of interest. In step 2, we added demographic variables (age and HCT-Comorbidity Index) that are known to be associated with HCT type. Finally, in step 3, we added HCT type as a fixed effect variable predicting both outcome of interest and slope of change over time (HCT type X time interaction).
In addition to examining participants’ mood scores continuously as described above, we also transformed scores into dichotomous outcomes reflecting the presence or absence of clinically significant depression and anxiety symptoms ((HADS-subscale score > 7)) and major or other depressive syndromes (PHQ-9). For the PHQ-9, a major or other depressive syndrome is diagnosed if a patient reports at least two of the nine symptoms of depression, with one of the symptoms being anhedonia or depressed mood.20 We used non-linear mixed effects models with binomial distribution adjusting for age and comorbidities to examine the change in these dichotomous outcomes over time.
To identify potential predictors of QOL (TOI), we first tested unadjusted associations of the following baseline variables of interest with QOL scores over time: age, gender, diagnosis, HCT comorbidity index, relationship status, education, income, social well-being, emotional well-being, HADS-Depression, and HADS-Anxiety. Variables that were associated with QOL at P < 0.10 were then used to construct multivariable linear mixed model. We examined slope of decline in QOL using interaction terms (variable of interest X time). We excluded baseline emotional well-being and income from the final model as they appeared to be collinear with baseline depression/anxiety and educational level respectively.
Results
Patient Participants
We enrolled 97% (90/93) of consecutively eligible patients admitted for autologous (n=30), and allogeneic (MAC (n=30), and RIC (n=30)) HCT between 7/1/2012 and 3/10/2014. Table 1 depicts baseline characteristics of study participants. Patients undergoing RIC HCT were more likely to be older and have a higher comorbidity score than those undergoing autologous or MAC HCT [Table 1].
Table 1.
Patients Baseline Characteristics.
| Variable | Autologous (n=30) | MAC Allo (n=30) | RIC Allo (n=30) | All HCT (n=90) | P- Value |
|---|---|---|---|---|---|
|
| |||||
| Age mean (SD) | 56.4 (13.7) | 47.0 (10.2) | 63.9 (14.1) | 58.1(14.4) | P < 0.0001 |
|
| |||||
| Female (%) | 13 (43.3%) | 11 (36.7%) | 13 (43.3%) | 37 (41.1%) | P = 0.83 |
|
| |||||
| Diagnosis | p < 0.0001 | ||||
| ALL | 0 | 6 (20%) | 1 (3.3%) | 7 (7.8%) | |
| AML/MDS | 0 | 23 (76.6%) | 22 (73.3%) | 45 (50.0%) | |
| MF/CML | 0 | 0 | 2 (6.6%) | 2 (2.2%) | |
| Lymphoma | 22 (73.3%) | 1 (3.3%) | 0 | 23 (25.6%) | |
| MM | 8 (26.7%) | 0 | 1 (3.3%) | 9 (10%) | |
|
| |||||
| Race: White | 26 (86.7%) | 27 (90%) | 29 (96.7%) | 82 (91.1%) | P = 0.38 |
|
| |||||
| Relationship status | P = 0.67 | ||||
| Married | 19 (63.3%) | 22 (73.3%) | 19 (63.3%) | 60 (66.7%) | |
| Divorced | 1 (3.3%) | 2 (6.7%) | 4 (13.3%) | 7 (7.8%) | |
| Single | 5 (16.7% | 4 (13.3%) | 3 (10.0%) | 12 (13.3%) | |
| Widowed | 5 (16.7%) | 2 (6.7%) | 4 (36.4%) | 11 (12.2%) | |
|
| |||||
| Education | P = 0.13 | ||||
| High school | 6 (20.0%) | 5 (16.7%) | 12 (40%) | 23 (25.6%) | |
| College | 14 (46.7%) | 19 (63.3%) | 13 (43.3%) | 46 (51.1%) | |
| Post graduate | 10 (33.3%) | 6 (20.0%) | 5 (16.7%) | 21 (23.3%) | |
|
| |||||
| Income | P = 0.37 | ||||
| <25,000 | 3 (10.0%) | 2 (6.7%) | 6 (20.0%) | 11 (12.2%) | |
| 25,000–50,000 | 3 (10.0%) | 5 (16.7%) | 6 (20.0%) | 14 (15.6%) | |
| 51,000–100,000 | 12 (40.0%) | 6 (20.0%) | 9 (30.0%) | 27 (30.0%) | |
| 101,000–150,000 | 7 (23.3%) | 7 (23.3%) | 3 (10.0%) | 17 (18.9%) | |
| >150,000 | 3 (10.0%) | 6 (20.0%) | 3 (10.0%) | 12 (13.3%) | |
| Missing | 2 (6.7%) | 4 (13.3%) | 3 (10.0%) | 9 (10.0%) | |
|
| |||||
| HCT-CI median (SD) | 0 (1.7) | 0.5 (1.3) | 2.5 (1.6) | 1.0 (1.6) | p = 0.002 |
ALL = Acute lymphoblastic leukemia, AML = Acute myeloid leukemia, MDS = Myelodysplastic syndrome, MF = Myelofibrosis, CML = Chronic myeloid leukemia, MM = Multiple myeloma, HCT-CI: HCT comorbidity index, SD = Standard deviation.
Patient-reported QOL, fatigue, physical and psychological symptoms
Patients’ QOL (FACT-BMT, TOI) markedly declined while fatigue (FACT-fatigue) and depression (HADS-Depression, PHQ-9) increased during their hospitalization for HCT [Table 2]. Scoring the HADS categorically revealed similar results as the proportion of patients with clinically significant depressive symptoms more than doubled from baseline to day +8 (15.6% to 37.8%, p = 0.005), whereas the proportion with anxiety symptoms did not change over time (22.2% to 18.9%, p=0.8). Similarly, the proportion of patients meeting diagnostic criteria for major depression or other depressive syndrome as measured by the PHQ9 increased more than fourfold from baseline to day +8 (7.8% to 36.7%, p < 0.0001).
Table 2.
QOL, Fatigue, and Mood by Type of Transplant.
| Variable | Day -6 Mean |
Day +1 Mean |
Day +8 Mean |
Time | Transplant Type | Time X Transplant Type | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| F | P-value | F | P-value | F | P-value | ||||
|
| |||||||||
| FACT-BMT | 37.9 | P < 0.0001 | 2.1 | P= 0.12 | 1.7 | P = 0.15 | |||
| Autologous | 105.8 | 92.1 | 92.6 | ||||||
| MAC allo | 110.4 | 96.1 | 92.0 | ||||||
| RIC Allo | 112.6 | 104.6 | 105.1 | ||||||
| All | 109.6 | 97.6 | 96.0 | ||||||
|
| |||||||||
| FACT-TOI | 54.8 | P < 0.0001 | 2.5 | P = 0.08 | 1.6 | P = 0.18 | |||
| Autologous | 63.3 | 50.9 | 51.6 | ||||||
| MAC Allo | 67.6 | 53.6 | 49.9 | ||||||
| RIC Allo | 69.7 | 60.2 | 58.6 | ||||||
| All | 66.8 | 54.9 | 53.3 | ||||||
|
| |||||||||
| FACT-Fatigue | 32.6 | P < 0.0001 | 2.5 | P = 0.08 | 2.2 | P = 0.07 | |||
| Autologous | 34.4 | 25.4 | 26.3 | ||||||
| MAC Allo | 39.1 | 32.8 | 27.6 | ||||||
| RIC Allo | 36.2 | 31.7 | 28.2 | ||||||
| All | 36.6 | 29.9 | 27.9 | ||||||
|
| |||||||||
| HADS-Depression | 12.8 | P < 0.0001 | 1.2 | P = 0.30 | 0.15 | P = 0.96 | |||
| Autologous | 5.0 | 6.9 | 7.0 | ||||||
| MAC Allo | 4.4 | 6.1 | 6.5 | ||||||
| RIC Allo | 4.0 | 5.7 | 5.0 | ||||||
| All | 4.5 | 6.2 | 6.3 | ||||||
|
| |||||||||
| HADS-Anxiety | 0.6 | P = 0.53 | 1.5 | P = 0.22 | 0.51 | P = 0.73 | |||
| Autologous | 5.3 | 5.8 | 5.1 | ||||||
| MAC Allo | 4.3 | 4.8 | 4.9 | ||||||
| RIC Allo | 4.0 | 3.6 | 3.6 | ||||||
| All | 4.6 | 4.7 | 4.5 | ||||||
|
| |||||||||
| PHQ-9 | 24.2 | P < 0.0001 | 2.3 | P = 0.10 | 1.1 | P = 0.38 | |||
| Autologous | 5.7 | 8.9 | 9.7 | ||||||
| MAC Allo | 4.1 | 5.8 | 8.2 | ||||||
| RIC Allo | 3.9 | 5.5 | 6.3 | ||||||
| All | 4.6 | 6.8 | 8.1 | ||||||
MAC Allo = Myeloablative conditioning allogeneic HCT, RIC Allo = Reduced intensity conditioning allogeneic HCT.
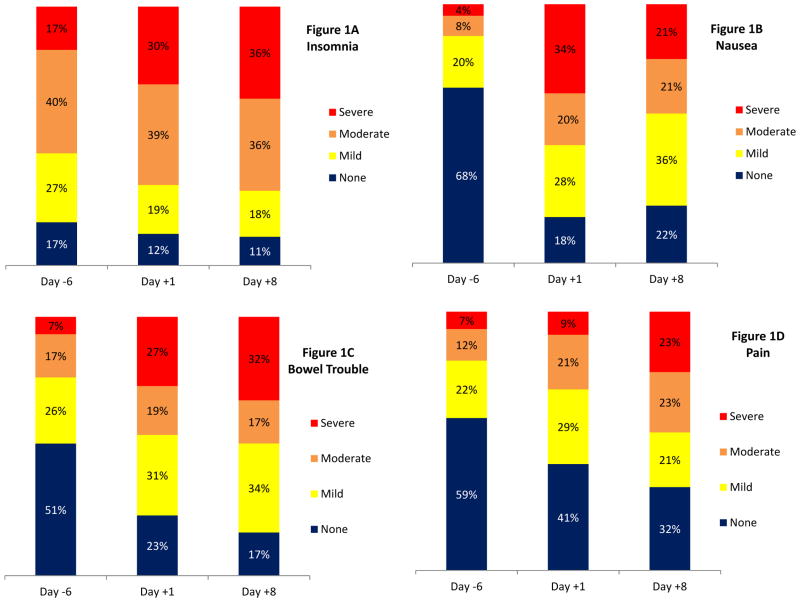
We examined frequencies of specific items of the FACT-BMT to delineate patients’ physical symptoms. Patients most commonly reported moderate to severe insomnia (day+1 = 69%, day+8 = 72%: Figure 1A), nausea (day+1 = 54%, day+8 = 42%; Figure 1B), bowel trouble (day+1 = 46%, day+8 = 49%; Figure 1C), and pain (day+1 = 30%, day+8 = 47%, Figure 1D).
Figure 1. Common Symptoms during HCT Hospitalization.
Figure 1A = Insomnia, Figure 1B = Nausea, Figure 1C = Bowel Trouble, Figure 1D = Pain.
Comparing outcomes between types of HCT
After adjusting for age and comorbidity score, there were no statistically significant differences in QOL, fatigue, or mood between patients undergoing autologous, MAC or RIC HCT [Table 2]. Furthermore, the slope of decline in QOL, fatigue, and psychological symptoms over time did not differ by HCT type.
Predictors of patients-reported QOL
In unadjusted analyses, diagnosis of multiple myeloma, higher baseline depression and anxiety scores, and lower baseline emotional and social well-being scores were all associated with lower QOL, as measured by the TOI [Table 3]. In a multivariable linear mixed model including age, comorbidity score, and all significant covariates, only patients’ baseline depression (β = −2.24, F=42.2, p < 0.0001) and anxiety (β = −0.63, F=4.4, p = 0.03) predicted lower QOL throughout hospitalization. Age (F = 3.32, p = 0.009), post-graduate education (F=2.64, p = 0.03), and lower baseline social-wellbeing (F=4.49, P = 0.01) were predictive of a steeper decline in QOL over time.
Table 3.
Unadjusted Analyses: Predictors of QOL Trajectory during HCT Hospitalization.
| Variable | Day -6 Mean TOI |
Day +1 Mean TOI |
Day +8 Mean TOI |
Variable Effect | Variable X Time Effect | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| F | P-value | F | P-value | ||||
|
| |||||||
| Age | 1.8 | P = 0.16 | 4.9 | P = 0.0006 | |||
| < 40 | 59.1 | 53.9 | 50.9 | ||||
| 40–60 | 68.8 | 51.1 | 49.3 | ||||
| > 60 | 67.5 | 58.5 | 57.7 | ||||
|
| |||||||
| Gender | 0.69 | P = 0.40 | 1.7 | P = 0.18 | |||
| Male | 67.1 | 57.2 | 54.9 | ||||
| Female | 66.5 | 51.6 | 51.1 | ||||
|
| |||||||
| Diagnosis | 4.1 | P = 0.01 | 18.9 | P < 0.0001 | |||
| Lymphoma | 65.0 | 55.5 | 52.9 | ||||
| Multiple Myeloma | 58.4 | 41.4 | 47.3 | ||||
| AML/ALL/MDS | 69.2 | 56.9 | 54.6 | ||||
|
| |||||||
| Relationship status | 0.85 | P = 0.36 | 2.8 | P = 0.06 | |||
| Married | 66.7 | 52.8 | 51.2 | ||||
| Other | 67.1 | 59.1 | 57.6 | ||||
|
| |||||||
| Living Alone | 0.00 | P = 0.98 | 1.1 | P = 0.33 | |||
| Yes | 65.6 | 56.7 | 56.6 | ||||
| No | 67.1 | 54.5 | 52.7 | ||||
|
| |||||||
| Education | 0.41 | P = 0.66 | 3.1 | P= 0.01 | |||
| High School | 64.6 | 58.9 | 52.0 | ||||
| College | 68.5 | 54.8 | 53.8 | ||||
| Post Graduate | 65.7 | 50.8 | 53.9 | ||||
|
| |||||||
| Income | 0.59 | P = 0.56 | 2.6 | P = 0.03 | |||
| < $50,000 | 65.2 | 58.9 | 54.4 | ||||
| $50,000–$100,000 | 68.7 | 56.8 | 57.6 | ||||
| > $100,000 | 67.4 | 50.4 | 51.1 | ||||
|
| |||||||
| Baseline Social Well-Being | β = + 1.1, SE = 0.32 | 13.0 | P = 0.003 | 4.4 | P = 0.01 | ||
|
| |||||||
| Baseline Emotional Well-Being | β = + 1.9, SE= 0.34 | 36.8 | P < 0.0001 | 0.14 | P = 0.87 | ||
|
| |||||||
| Baseline HADS-Depression | β = −2.9, SE = 0.28 | 94.69 | P < 0.0001 | 2.02 | P = 0.13 | ||
|
| |||||||
| Baseline HADS-Anxiety | β = − 1.8, SE = 0.33 | 32.30 | p < 0.0001 | 0.04 | P = 0.96 | ||
|
| |||||||
| Baseline PHQ9 | β = −2.0, SE = 0.22 | 65.40 | P < 0.0001 | 1.8 | P = 0.16 | ||
AML = Acute myeloid leukemia, ALL = Acute lymphoblastic leukemia, MDS = Myelodysplastic syndrome.
Family Caregiver- reported Outcomes
We enrolled 47 FC [Supplemental Table 1] as 23% (n = 21) of patients did not identify a FC that they were willing to have us approach for study participation. The remaining 24% (n = 22) of FC either refused to participate or were not available during the allotted recruitment window to consent for study participation. FC QOL declined over time with a significant decrease in PCS (83.1 to 79.6, P = 0.03) and MCS (71.6 to 67.4, P = 0.04) [Table 4]. We identified declines in physical functioning (P = 0.02), general health (P = 0.0007), vitality (P = 0.003), social functioning (P = 0.004), and role functioning-emotional (P = 0.05) during patients’ hospitalization. Similar to patients, the proportion of FC with depression increased during the hospital course, whereas the proportion with anxiety did not change [Table 4]. The proportion of FC meeting criteria for major depression or other depressive symptoms based on the PHQ9 almost tripled from baseline to day +8 (8.5% to 25.5%, P = 0.03).
Table 4.
Family Caregivers’ QOL and Mood during HCT Hospitalization
| Variable | Day -6 Mean |
Day +1 Mean |
Day +8 Mean |
Longitudinal Analysis P-Value |
|---|---|---|---|---|
| Physical Functioning | 87.3 | 88.7 | 82.4 | P = 0.02 |
| Role- Physical | 84.0 | 77.8 | 75.8 | P = 0.22 |
| Bodily Pain | 82.4 | 82.6 | 79.2 | P = 0.35 |
| General Health | 78.7 | 78.3 | 72.4 | P = 0.0007 |
| Vitality | 59.6 | 59.1 | 53.5 | p = 0.003 |
| Social Functioning | 80.3 | 78.0 | 70.1 | P = 0.004 |
| Role- Emotional | 74.5 | 69.3 | 61.6 | P = 0.05 |
| Mental Health | 72.0 | 73.1 | 70.1 | P = 0.09 |
| Physical Component Score | 83.1 | 80.5 | 79.6 | p = 0.03 |
| Mental Component Score | 71.6 | 71.2 | 67.4 | P = 0.04 |
| HADS-Depression | 3.4 | 3.5 | 4.3 | P = 0.03 |
| HADS-Anxiety | 5.0 | 5.4 | 5.6 | p = 0.22 |
| PHQ9 | 4.6 | 4.0 | 5.3 | P = 0.006 |
Discussion
Patients with hematologic malignancies undergoing autologous and allogeneic HCT experience a marked and dramatic deterioration in QOL and an increase in fatigue and depression. Interestingly, the observed QOL and symptom burden was similar among patients receiving autologous, MAC and RIC HCT suggesting that use of reduced intensity chemotherapy does not necessarily result in a lower symptom burden for patients. Importantly, patients with depression and anxiety at admission reported worse QOL across all time points, underscoring the importance of identifying and addressing psychiatric morbidity prior to HCT.
While this study confirms the universally held belief that the hospitalization period for HCT is highly distressing to patients physically and emotionally, the magnitude of patients’ QOL decline and physical and emotional suffering is sobering. We detected a greater than 12 point drop in mean FACT-BMT QOL scores and climbing rates of depression up to 36.7% during the hospital stay. As a five point change in the FACT-BMT is considered clinically significant17, these results highlight the severity of QOL decline seen in this population. Furthermore, the deterioration in QOL, fatigue, and mood occurred rapidly within one week of hospitalization and persisted at the time of discharge. This study calls attention to the critical need for supportive care interventions to enhance the QOL and care of patients undergoing HCT.
Assuming that the drastic QOL decline and large symptom burden observed in patients undergoing HCT is a natural and unmodifiable part of the transplantation process is simply unacceptable. Several of our findings support the notion that patients’ symptoms are modifiable. First, we identified pain, fatigue, bowel disturbances, insomnia, nausea, and depression as the most prominent symptoms contributing to the QOL decline. Many if not all of these symptoms are treatable and can be improved by intensive supportive care measures.22–24 Second, we identified baseline depression and anxiety as strongly predictive of patients’ QOL throughout their hospitalization. Therefore, patients with higher pre-transplant depression and anxiety represent a particularly vulnerable population who may benefit from pre-emptive interventions to optimize their coping strategies during the transplantation process.
Interestingly, we also identified patients who were highly educated, middle-aged and had lower baseline social well-being as a particularly vulnerable population with a steeper decline in QOL during their hospitalization. Other studies have shown a worse QOL decrement in middle-aged patients,25, 26 which has been attributed to the balance between patients’ expectations and personal demands leading to more difficulty coping with the effect of their disease.25 It remains unclear why highly educated patients experienced a steeper decline in QOL compared to those less educated, and this will require further validation and exploration in future work. It is plausible that patients’ educational background may impact their illness perception, prognostic understanding, symptom burden, and/or transplant experience. Thus, middle-aged and highly educated patients, especially those lacking social supports appear to be an at-risk population who may benefit from interventions designed to optimize social supports and establish realistic expectations during transplantation.
To our knowledge, this is the first study to demonstrate the impact of patients’ hospitalization for HCT on their FC. FC experienced significant decline in vitality, social functioning, emotional role functioning, and increasing rates of depression during their loved ones’ hospitalization. In addition to the emotional toll, FC also reported significant deterioration in their physical functioning and general health. The decline in FC QOL during a relatively short period of time further corroborates the importance of focusing on the hospitalization period when supporting the FC. Highlighting the burden of caregiving during the transplant process is a crucial first-step to developing strategies to improve FC QOL and mood.
Several of our findings are consistent with prior studies examining outcomes in patients undergoing HCT. In a study of patients undergoing autologous and MAC HCT between 1994 and 1997, anxiety symptoms declined, while depression increased during hospitalization.7 However, patients in our study had higher rates of depression, which may be due to differences in transplant practices and heterogeneity in the patient population. Few prior studies compared outcomes among patients receiving various types of HCT.7, 10, 27 In one study, patients undergoing MAC HCT reported better physical status and energy compared to those undergoing autologous HCT.7 Another showed higher physical functioning in patients undergoing RIC HCT compared to those undergoing autologous HCT.10 In contrast, our findings suggest that patients undergoing autologous, MAC, or RIC HCT had a similar rapid deterioration in QOL, mood, and fatigue over time. One potential explanation for this is the heterogeneity of the patient populations receiving the different types of transplants. Patients undergoing autologous HCT are more heavily pre-treated with multiple cycles of chemotherapy prior to transplantation, compared to allogeneic HCT recipients, which may add to their cumulative symptom and psychological burden over time. Furthermore, patients undergoing RIC HCT are often older with significant comorbidities compared to autologous and MAC HCT patients, which may exacerbate their QOL deterioration and symptom burden during HCT. With the advent of RIC HCT and better supportive care measures, many hoped that the symptom burden during HCT would decline. However, our findings emphasize the need in the modern era for intensive supportive care interventions to address the symptoms and QOL decline seen in patients undergoing all types of HCT.
Our study has several important limitations. First, we included a small sample of mostly white patients drawn from a single transplant center and thus, our findings may not be generalizable to minority groups, patients in other geographic areas or transplant centers with different practices. Second, by including a small sample of patients undergoing different types of HCT, our ability to detect meaningful differences in the outcomes of interest by type of transplant may be limited. Third, the use of imputation methods may introduce bias as patients with missing data are more likely to be symptomatic and have lower QOL scores. However, we utilized a conservative approach that underestimates patients’ QOL and symptom burden over time and biases results towards the null hypothesis.
In this study, we demonstrate a rapid and marked deterioration in QOL and a rise in fatigue and depression for patients with hematologic malignancies during their hospitalization for autologous, MAC, and RIC HCT. Patients’ FC also suffer physically and emotionally during their loved ones’ hospitalization. As baseline anxiety and depression are identified as important predictors of QOL, addressing pre-transplant psychiatric morbidity may have important clinical implications on patients’ QOL and adaptation during the transplantation process. Most importantly, the magnitude of physical and psychological distress experienced by patients and families during the hospitalization must become a focus for intensive interventions to improve the QOL and care for this vulnerable population.
Supplementary Material
Acknowledgments
Funding: This work was supported by funds from the MGH Cancer Center and K24 CA 181253 (Temel).
Footnotes
Financial Disclosures:
The NIH grant information is: K24 CA 181253
References
- 1.Lee SJ, Joffe S, Kim HT, et al. Physicians’ attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104(7):2194–200. doi: 10.1182/blood-2003-07-2430. [DOI] [PubMed] [Google Scholar]
- 2.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiss TL, Abdolell M, Jamal N, Minden MD, Lipton JH, Messner HA. Long-term medical outcomes and quality-of-life assessment of patients with chronic myeloid leukemia followed at least 10 years after allogeneic bone marrow transplantation. J Clin Oncol. 2002;20(9):2334–43. doi: 10.1200/JCO.2002.06.077. [DOI] [PubMed] [Google Scholar]
- 4.Braamse AM, Gerrits MM, van Meijel B, et al. Predictors of health-related quality of life in patients treated with auto- and allo-SCT for hematological malignancies. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.130. [DOI] [PubMed] [Google Scholar]
- 5.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology. 2009;18(2):113–27. doi: 10.1002/pon.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–43. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 7.Prieto JM, Atala J, Blanch J, et al. Patient-rated emotional and physical functioning among hematologic cancer patients during hospitalization for stem-cell transplantation. Bone Marrow Transplant. 2005;35(3):307–14. doi: 10.1038/sj.bmt.1704788. [DOI] [PubMed] [Google Scholar]
- 8.Prieto JM, Blanch J, Atala J, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20(7):1907–17. doi: 10.1200/JCO.2002.07.101. [DOI] [PubMed] [Google Scholar]
- 9.Meyers CA, Weitzner M, Byrne K, Valentine A, Champlin RE, Przepiorka D. Evaluation of the neurobehavioral functioning of patients before, during, and after bone marrow transplantation. J Clin Oncol. 1994;12(4):820–6. doi: 10.1200/JCO.1994.12.4.820. [DOI] [PubMed] [Google Scholar]
- 10.Diez-Campelo M, Perez-Simon JA, Gonzalez-Porras JR, et al. Quality of life assessment in patients undergoing reduced intensity conditioning allogeneic as compared to autologous transplantation: results of a prospective study. Bone Marrow Transplant. 2004;34(8):729–38. doi: 10.1038/sj.bmt.1704646. [DOI] [PubMed] [Google Scholar]
- 11.Prieto JM, Atala J, Blanch J, et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23(25):6063–71. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 12.Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18(7):1539–49. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- 13.Loberiza FR, Jr, Rizzo JD, Bredeson CN, et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20(8):2118–26. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 14.Siston AK, List MA, Daugherty CK, et al. Psychosocial adjustment of patients and caregivers prior to allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;27(11):1181–8. doi: 10.1038/sj.bmt.1703059. [DOI] [PubMed] [Google Scholar]
- 15.Stetz KM, McDonald JC, Compton K. Needs and experiences of family caregivers during marrow transplantation. Oncol Nurs Forum. 1996;23(9):1422–7. [PubMed] [Google Scholar]
- 16.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19(4):357–68. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 18.Santana MJ, Au HJ, Dharma-Wardene M, et al. Health-related quality of life measures in routine clinical care: can FACT-fatigue help to assess the management of fatigue in cancer patients? Int J Technol Assess Health Care. 2009;25(1):90–6. doi: 10.1017/S0266462309090126. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Network NCC. [accessed 3/1/2014];National Comprehensive Cancer Network: Palliative Care Version 2.2013. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf.
- 23.Network NCC. [accessed 3/1/2014];National Comprehensive Cancer Network: Antiemesis Version 1.2014. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
- 24.Network NCC. [accessed 3/1/2014];National Comprehensive Cancer Network: Cancer-Related Fatigue Version 1.2014. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
- 25.El-Jawahri A, Pidala J, Inamoto Y, et al. Impact of Age on Quality of Life, Functional Status, and Survival in Patients with Chronic Graft-Versus-Host Disease. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piazza JR, Charles ST, Almeida DM. Living with chronic health conditions: age differences in affective well-being. J Gerontol B Psychol Sci Soc Sci. 2007;62(6):P313–21. doi: 10.1093/geronb/62.6.p313. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MZ, Rozmus CL, Mendoza TR, et al. Symptoms and quality of life in diverse patients undergoing hematopoietic stem cell transplantation. J Pain Symptom Manage. 2012;44(2):168–80. doi: 10.1016/j.jpainsymman.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.