Abstract
Background
The immunopathogenesis of chronic rhinosinusitis (CRS) is largely unknown, but it is thought that different inflammatory profiles are responsible for the different CRS subtypes. 25-hydroxyvitamin-D (25-VD3) has been shown to alter inflammatory mediators in other disease processes and 25VD3 deficiency is associated with CRS with nasal polyps (CRSwNP), but it is unknown if 25VD3 levels impacts local inflammation in CRS. This study investigated the correlation between plasma 25-VD3 and sinonasal mucus MCP-1, RANTES and bFGF levels in patients with CRS.
Methods
Study subjects undergoing endoscopic sinus surgery (ESS) for CRS were prospectively enrolled from January 2012-August 2014. Control subjects included patients undergoing ESS for non-inflammatory pathology. Blood and sinonasal mucus were collected at the time of ESS. Plasma 25-VD3 was measured by ELISA and mucus levels of MCP-1, RANTES, and bFGF by cytometric bead array (CBA).
Results
A total of 57 patients were enrolled and categorized as CRS without nasal polyps (CRSsNP) (n=31), CRSwNP (n=14) and controls (n=12). No significant correlation was found between MCP-1 and 25-VD3. There was a significant negative correlation between 25-VD3 and RANTES (r= −0.612; p=0.026) and bFGF (r= −0.578; p=0.039) in CRSwNP patients; however, there was no significant correlation in CRSsNP patients.
Conclusion
This data suggests that 25-VD3 may play a role in regulation of RANTES and bFGF expression in CRSwNP. This may occur through regulation of nasal polyp fibroblasts or other immune cells. Further investigation is warranted to better elucidate the role of RANTES, bFGF and 25-VD3 in CRSwNP.
Keywords: Sinusitis, Vitamin D, 25-dihydroxyvitamin D3, Fibroblast Growth Factor-2, polyps
INTRODUCTION
Chronic rhinosinusitis (CRS) encompasses a broad group of disorders characterized by persistent inflammation of the nasal mucosa and paranasal sinuses that affects up to 16% of the US population and creates a significant socio-economic burden [1]. While the immunopathogenesis of CRS remains largely unknown, current research has provided better insight into potential etiologies. The current evidence supports two predominant immune phenotypes in CRS that arise secondary to skewing of the T helper (Th) cells. In general, CRS without nasal polyposis (CRSsNP) is Th1-skewed whereas CRS with nasal polyposis (CRSwNP) is Th2-skewed. This skewing impacts expression of local inflammatory mediators, which subsequently act in an autocrine and paracrine fashion to influence the local mucosal inflammatory response that is characteristic of CRS. The mechanism of T cell differentiation is incompletely understood, but is likely regulated by the complex interplay of the local cytokine milieu, activation signals and the inflammatory cell infiltrate. The local inflammatory mediator profile for CRS subtypes is still under investigation, but evidence suggests that CRSsNP and CRSwNP are unique disease entities associated with separate and distinct inflammatory mediator profiles within the sinonasal mucosa or mucus [2,3]. Additionally, these differing mediator profiles may explain the disparate histologic findings and distinct inflammatory cell profiles seen in CRS subtypes.
One recognized regulator of the immune response that may impact immune-mediated diseases is Vitamin D3 (VD3) [4]. VD3 is a naturally occurring steroid hormone that enters circulation either through epidermal transfer or intestinal absorption. Once in the body, VD3 is hydroxylated in the liver to form 25-hydroxyvitamin D3 (25-VD3), the most abundant but largely inactive form of the vitamin. It is later converted to its more active form, 1,25-hydroxy VD3 (1,25-VD3) through a second hydroxylation step that primarily occurs in the kidney. Interestingly, patients with CRSwNP have been shown to have to be VD3 deficient and that deficiency is associated with more severe disease [5,6]. However, the means through which VD3 may impact the immunopathogenesis of the disease remains largely unknown.
A potential mechanism by which VD3 may affect CRS is by altering the local inflammatory profile. This effect has been demonstrated in other inflammatory disease processes [7], but the impact of VD3 on the local inflammatory mediator profile within the sinonasal mucus in patients with CRS has not been investigated. The goal of this present investigational study is to ascertain if there is a significant correlation between systemic levels of 25-VD3 and levels of monocyte chemoattractant protein-1 (MCP-1), Regulated upon Activation Normal T cell Expressed and Secreted (RANTES) and basic fibroblast growth factor (bFGF). These mediators were selected because they are known to be altered in patients with CRS and are reported to be impacted by VD3 [8,9,10]
MATERIALS AND METHODS
Study population
The institutional review boards at Oregon Health & Science University (OHSU, Portland, OR, USA) and the Medical University of South Carolina (MUSC, Charleston, SC, USA) approved the study protocol prior to initiation, and written informed consent was obtained from all patients for sample repositories. Subjects were enrolled between January 2012 and August 2014. Patients were included if they were undergoing endoscopic sinus surgery for chronic rhinosinusitis as defined by the 2007 Multi-Disciplinary Sinusitis Guidelines [1]. The diagnosis of CRSwNP was made by preoperative nasal endoscopic examination and patients with allergic fungal rhinosinusitis or Samter’s triad were included in the CRSwNP group. Control patients were those who were undergoing endoscopic sinus surgery for non-inflammatory reasons including, meningioma, cerebrospinal fluid leak repair or trans-sphenoidal skull base surgery. Patients were excluded if they had taken oral steroids or immunotherapy during the four weeks preceding surgery, or if they had underlying diseases that may influence the local or systemic immune response such as rheumatoid arthritis, immunodeficiency, or cystic fibrosis. Additionally, subjects were excluded from final analysis if specimens were damaged during the aspiration process or if mediator results were found to be outside of the detection limits of the assay. This occurred in one CRSsNP for RANTES and bFGF. Two patients, one control and one CRSwNP, did not have plasma available and therefore do not have 25-VD3 levels.
Collection of Plasma and Sinonasal Mucus
At the time of surgery, two 10mL. blood samples were taken from the patient; one in a vacuum tube containing clot activator for serum and one vacuum tube with an anticoagulant for plasma. Mucus was collected as previously described [11]: briefly, after the induction of anesthesia but prior to the application of any intranasal topical agent or injection, a polyurethane sponge (Greer Labs, Lenoir, NC., USA) was atraumatically placed into the middle meatus under endoscopic guidance. The sponges were left in place for ~5 minutes, removed and placed into a centrifuge chamber. Sponges contaminated with blood were discarded in order to avoid alteration in assay results by potential circulating inflammatory mediators. Following harvest, all samples were immediately placed on ice and transported to the laboratory where they were immediately stored at −80 °C following initial processing for storage. The blood samples were centrifuged at 2,000rpm (500 g) for 15 minutes in order to fully separate the plasma and serum. Plasma and serum were placed in labeled cryovials, snap-frozen in liquid nitrogen and stored at −80°C until time of assay. Mucus samples were centrifuged at 4°C, 3,000rpm (15 cm) for 30 minutes to extract the entire sample from the sponge. These samples were then placed in cryovials, flash-frozen in liquid nitrogen and stored at −80°C until time of assay. Plasma and mucus collected at OHSU were shipped overnight on dry ice to MUSC for assay.
Measurement of Plasma 25-Hydroxy Vitamin D3 and Inflammatory Mediators
We elected to measure 25-VD3 since this form of VD3 is commonly used to determine VD3 status in clinical practice due to its long half-life. Levels of 25VD3 were determined by enzyme-linked immunosorbent assay (ELISA; Alpco Immunoassays, Salem, NH., USA) according to the manufacturer’s instructions. VD3 insufficiency can be defined as <30ng/mL. and deficiency as ≤20ng/mL. as previously described [12]. Inflammatory mediators were measured using commercially available Cytometric Bead Array (CBA) systems (BD Biosciences, San Diego, CA., USA). Kits and reagents were purchased for MCP-1, RANTES and bFGF. Undiluted samples of nasal mucus were added to MultiScreenHTS Assay System bottom-filtered 96-well plate (EMD Millipore, Billerica, MA., USA). The assay was carried out according to manufacturers' instructions, including aspirating well contents with a MultiScreenHTS vacuum manifold (EMD Millipore) set no higher than 10 cm. Hg. The samples were read on a Guava easyCyte 8HT flow cytometer (EMD Millipore) and analysis was performed with FCAP Array Software Version 1.0.1 (BD Biosciences, San Jose, CA., USA).
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 6.0 software (La Jolla, CA., USA). A D'Agostino & Pearson omnibus test was used to determine if continuous variables were normally distributed. A one-way analysis of variance (ANOVA) with post hoc t-test was used for normally distributed data (determination of differences in 25-VD3 levels by diagnosis). A Kruskal-Wallis test with post hoc Dunn's multiple comparisons was to test for significant differences immune mediator levels by diagnosis as these values were not parametrically distributed. Two-sided correlation coefficients were examined using either a Pearson (parametrically distributed data) or Spearman correlation analysis (for nonparametric data).
RESULTS
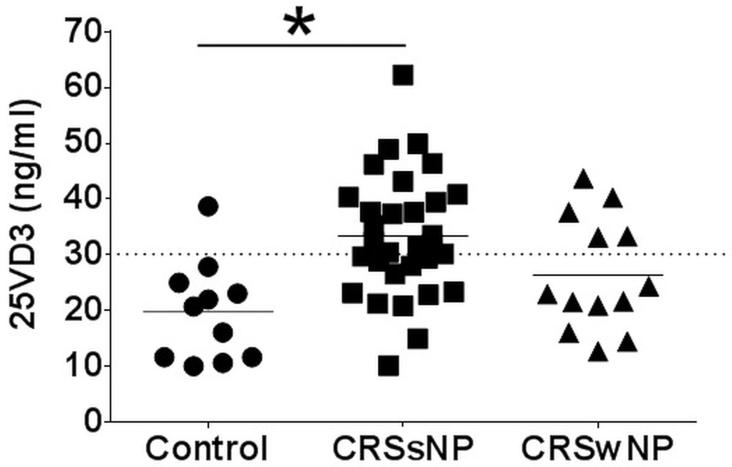
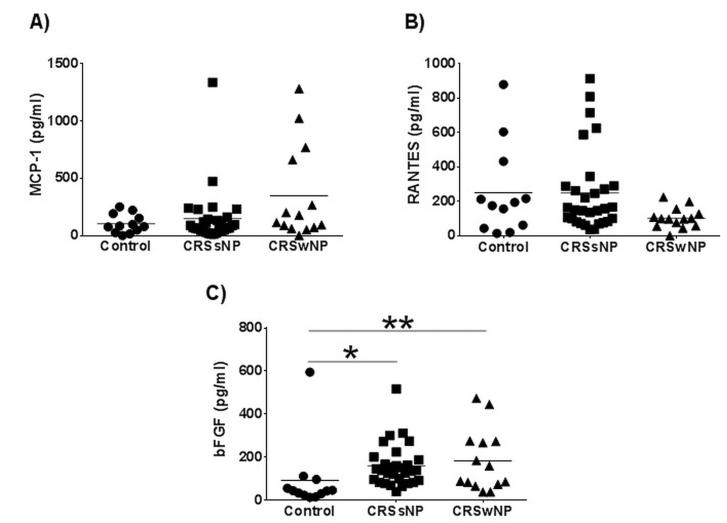
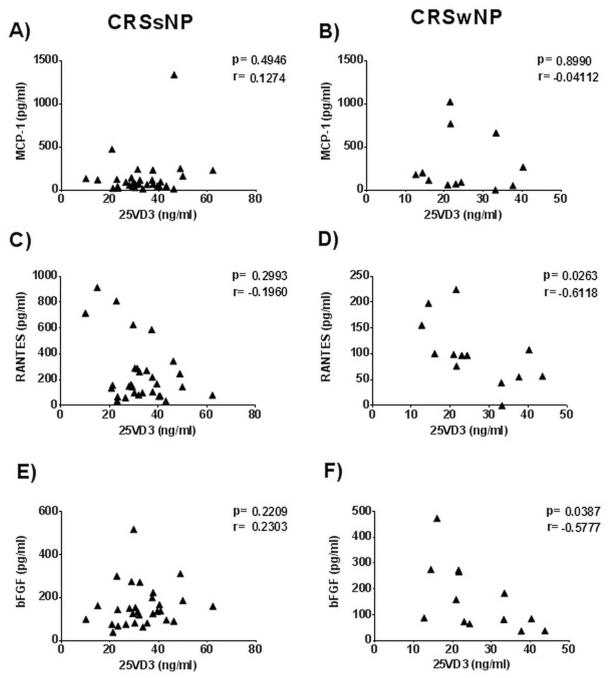
Samples from 57 participants were enrolled and collected from OHSU (n=12) and MUSC (n= 45) consisting of controls (n=12), and participants with both CRSsNP (n=31) and CRSwNP (n=14). There were a total of 26 (45.6%) males and 31 (54.5%) females with a total mean[SD] age of 48.7[18.9] years. Mean 25-VD3 levels were significantly different between control subjects (19.8[8.9]ng/mL.) and CRSsNP (33.4[11.0]ng/mL.; p<0.001) after adjusting for multiple comparisons (Table 1). Mean 25-VD3 levels were not statistically different between CRSsNP and CRSwNP (26.3[10.18] ng/mL.; p=0.055; Figure 1). There were no significant differences between mean MCP-1 levels in any of the control, CRSsNP, or CRSwNP subgroups. Mean levels of RANTES was lower in CRSwNP (102.8[59.8]pg/mL.) compared to both mean levels of controls (250.5[263.3]pg/mL.) and CRSsNP (248.2[238.5]pg/mL.). However none of these average differences reached statistical significance. Control patients had significantly lower mean levels of bFGF when compared to both CRSsNP (p<0.001) and CRSwNP (p=0.013; Figure 2). There was no significant correlation between systemic levels of 25-VD3 and MCP-1 in either CRS subgroup (p≥0.495). Using a Spearman’s correlation, there was a significant negative correlation between 25-VD3 and RANTES in CRSwNP (rs= −0.612; p=0.026) but not in CRSsNP (rs= −0.196; p=0.299; Figure 3). Additionally, there was a significant negative correlation between 25-VD3 and bFGF in CRSwNP patients (rs= −0.578; p=0.039).
Table 1.
Summary of Mean Immune Mediator Levels
| Controls | CRSsNP | CRSwNP | p value | |
|---|---|---|---|---|
| n | 12 | 30 | 14 | |
| bFGF (pg/ml) | 91.9 [161.4] | 159.5 [98.0] | 181.8 [145.2] | 0.002 |
| RANTES (pg/ml) | 250.5 [263.3] | 248.2 [238.5] | 102.8 [59.8] | 0.082 |
| MCP-1 (pg/ml) | 103.1 [82.8] | 147.3 [240.6] | 347.0 [412.8] | 0.184 |
| 25-VD3 (ng/ml) | 19.7 [8.9] | 33.4 [11.0] | 26.3 [10.2] | 0.002 |
Values represent mean[standard deviations]. P-value represents finding from the Kruskall-Wallis test statistic. CRSsNP, chronic rhinosinusitis without nasal polyposis; CRSwNP, chronic rhinosinusitis with nasal polyposis. bFGF, basic fibroblast growth factor; RANTES, regulated upon activation normal T cell expressed and secreted; MCP-1, monocyte chemoattractant protein-1; 25-VD3, 25-hydroxy vitamin D3; Pg, picogram; ml, milliliter.
Figure 1. Mean plasma levels of 25-hydroxy vitamin D3 (25-VD3).
25-VD3 as measured by ELISA from plasma collected at time of endoscopic sinus surgery. Each symbol represents the results of individual patients with values below the dashed line being 25-VD3 deficient. Statistics shown are results of post hoc Dunn’s multiple comparison test between indicated groups; *p<0.01. CRSsNP, chronic rhinosinusitis without nasal polyposis; CRSwNP, chronic rhinosinusitis without nasal polyposis; ng, nanogram; ml, milliliter.
Figure 2. Nasal mucus levels of MCP-1, RANTES and bFGF.
Levels of (A) MPC-1, (B) RANTES and (C) bFGF were measured in nasal mucus by cytometric bead array. Statistics shown are results of post hoc Dunn’s multiple comparison test between indicated groups. Each symbol represents the results of individual patients with values below the embedded line being 25-VD3 deficient. *p<0.01, **p<0.05. CRSsNP, chronic rhinosinusitis without nasal polyposis; CRSwNP, chronic rhinosinusitis without nasal polyposis; pg, picogram; ml, milliliter. bFGF, basic fibroblast growth factor; RANTES, regulated upon activation normal T cell expressed and secreted; MCP-1, monocyte chemoattractant protein-1;
Figure 3. Correlation between plasma 25-VD3 levels and sinonasal mucus levels of inflammatory mediators in CRSsNP (left column) and CRSwNP (right column).
Statistics shown are the results of Spearman correlation (r) analysis for data sets not normally distributed (A, C & E) and Pearson correlation (r) analysis for normally distributed data (B, D & F). CRSsNP, chronic rhinosinusitis without nasal polyposis; CRSwNP, chronic rhinosinusitis without nasal polyposis; pg, picograms; ml, milliliter; bFGF, basic fibroblast growth factor; RANTES, regulated upon activation normal T cell expressed and secreted; MCP-1, monocyte chemoattractant protein-1.
DISCUSSION
VD3 is a known regulator of the immune response and many cells of the immune system possess the vitamin D receptor (VDR) [2]. Additionally, VD3 has been shown to alter the expressed cytokine profile in various immunologic disease processes [13,14]. However, it has not been determined if it affects the inflammation that is central to CRS. Our lab previously demonstrated an association between VD3 insufficiency / deficiency in adult and pediatric patients with CRSwNP and AFRS [3,4]. This association was again demonstrated in this study and was recently corroborated in a study from Taiwan that also noted an inverse correlation between serum levels of 25-VD3 and severity of nasal polyposis [15]. They concluded that VD3 deficiency was associated with worse disease scores and postulated that low levels of VD3 may fail to reduce the level of cytokines released by inflammatory cells and fibroblasts thus contributing to the perpetuation of chronic inflammation. Notably, our control population had the lowest mean 25-VD3. However, this group did not consist of healthy control patients and many had significant risk factors for hypovitaminosis D including: African American ethnicity (50%), obesity (50%), current tobacco abuse, and chronic diseases such as diabetes and chronic obstructive pulmonary disease [12,16,17].
Numerous inflammatory mediators have been shown to be altered in CRS [18]; however, the mediators investigated in this study were selected because there is evidence to support an association between VD3 and their expression [8, 9, 10]. MCP-1, also known as CCL2, is one of the key chemokines that regulates migration and infiltration of monocytes and macrophages to sites of inflammation. These cells are critical to the immune response and are affected by VD3 [19]. Our research group previously demonstrated an increase in MCP-1 in sinus tissue taken from patients with CRSwNP and AFRS when compared to controls [20] and VD3 has been shown to decrease the expression of MCP-1 from peripheral blood monocytes in other disease processes [8]. However, we did not note any correlation between systemic mean levels of 25-VD3 and MCP-1 in CRS participant subgroups or controls. Interestingly, we found a negative correlation between RANTES and 25-VD3 in CRSwNP (p=0.018) but not in CRSsNP (p=0.299). Similarly, there was a negative correlation between plasma levels of 25-VD3 and local levels of bFGF in patients with CRSwNP patients (p=0.010); but, there was no significant correlation in CRSsNP subgroup (p=0.221). This is notable given the different histology of the two disease phenotypes. Nasal polyposis seen in patients of Western descent is marked by an eosinophilic infiltrate, massive tissue edema, proliferation of stromal and epithelial elements and thickening of the basement membrane [21] whereas CRSsNP is characterized by more prominent fibrosis of the extracellular matrix [18] and lacks a profound eosinophilic infiltrate.
Nasal polyps (NP) develop in the setting of chronic inflammation of the nasal mucosa, but the pathogenesis that leads to the proliferation of these benign tissue growths remains unclear. RANTES, also known as CCL5, is a member of the C-C chemokine family and has a variety of functions but is well-described as an eosinophil chemoattractant and activator. In addition to activating and promoting the release of several factors including eosinophilic cationic proteins, major basic proteins, reactive species and lysosomal enzymes from eosinophils, RANTES is able to recruit monocytes, basophils and CD4+ T lymphocytes to the nasal mucosa [22]. Through these mechanisms, RANTES is thought to play a significant role in perpetuating the inflammation seen in CRS and the development of eosinophilic NP [23].
Also known as FGF-2, bFGF is a multifunctional protein that is a member of a family of heparin-binding polypeptide growth factors that are involved in numerous biological activities including cellular proliferation and differentiation, neoangiogenesis and tissue remodeling [24]. Additionally, bFGF is thought to play a significant role in airway remodeling in the setting of chronic inflammation due to its ability to promote migration and/or proliferation of vascular endothelial cells, myofibroblasts and fibroblasts [25]. However, there is a paucity of literature on the role of bFGF and remodeling in chronic sinus disease. Mahfouz et al. [26] noted that bFGF RNA was significantly upregulated in tissue samples taken from antrochoanal polyps when compared to controls and patients with CRSsNP, and in studies specifically looking at CRSwNP, bFGF was elevated at both the mRNA and protein levels when compared to controls[24, 27]. Additionally, both studies comment that bFGF interacting with fibroblasts may also be a key component in polyp development.
The above studies,24,26,27 along with our current investigation, appear to conflict with previous murine studies reported by Sautter et al., in which FGF-2 was down-regulated at one and three weeks in a murine model of CRS [28]. However, there are several limitations to direct comparison of the human and mouse experimental models. In the latter, FGF regulation was determined by real-time reverse transcription polymerase chain reaction (RT-PCR) evaluation of mRNA transcripts, not protein expression. Beyond the first two time points, FGF-2 mRNA expression was no longer statistically significant. Multiple factors are involved in whether or not an RNA transcript is translated into a functional protein in general, and FGF-2 is extensively regulated through post-translational mechanisms as well.
There is evidence to suggest that nasal polyp fibroblasts are responsive to VD3. Topical calcitriol, the active form of VD3, has been shown to inhibit nasal polyp fibroblast proliferation in a dose-dependent fashion [10]. Rostkowska-Nadolska et al. also noted that these antiproliferative effects were maximized when combining calcitriol with budesonide. While this study did not determine a mechanism through which VD3 affects nasal polyp fibroblasts, the authors concluded that VD3 may downregulate VDR-dependent chemokines that play an important role in NP formation. Based on our results, it is possible that the inverse relationship between bFGF and VD3 in patients with CRSwNP could explain these findings. In a similar study from the same group, the administration of topical calcitriol or tacalcitol, a synthetic VD3 analogue, also significantly down-regulated NP fibroblast production of RANTES in a dose-dependent fashion [9]. Again, these effects were augmented by combining VD3 with a topical steroid. There were several drawbacks to the current study including small sample size and challenges with collecting large amounts of mucus. Additionally, our control population did not consist of normal, healthy controls which may provide a better comparison subgroup.
CONCLUSION
The goals of this preliminary study were to determine if there were a potential correlation between systemic levels of 25VD3 and local inflammatory mediators within the sinonasal mucus of patients with CRS. To our knowledge, this is the first study to investigate this relationship. We demonstrated an inverse correlation between plasma levels of 25VD3 and mucus levels of bFGF and RANTES in CRSwNP but not in CRSsNP. This data is consistent with existing literature and gives potential insight into the association between VD3 deficiency and CRSwNP. While this early pilot data is promising, future studies are warranted to further elucidate the relationship between VD3 and CRS.
Acknowledgments
Funding Disclosures: This investigation was partially grant funded by the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA. (R01 DC005805, PI: TLS) and partially grant funded by the American Academy of Otolaryngology-Head and Neck Surgery Foundation / American Rhinologic Society (AAO-HNSF / ARS) Centralized Otolaryngology Research Efforts (CORE, #313516).
Timothy L. Smith, Zachary M. Soler, and Jess C. Mace are funded by the NIDCD. Dr. Smith is also a consultant for Intersect ENT (Palo Alto, CA., USA) which provided no financial support for this investigation. Dr. Schlosser is a consultant for BrainLAB Inc. (Westchester, IL.,USA), Olympus (Center Valley, PA., USA) and has been provided grant support from Optinose (Yardley, PA., USA) and Intersect ENT; none of these provide support for this investigation. Dr. Soler is also consultant for BrainLAB Inc., but no support was provided for this investigation. Dr. Mulligan is funded by a grant from the Flight Attendant Medical Research Institute (ID: 092401) which was not affiliated with this investigation.
Footnotes
Public clinical trial registration: http://www.clinicaltrials.gov/show/NCT01332136 - “Determinants of Medical and Surgical Treatment Outcomes in Chronic Sinusitis”.
Potential Conflict(s) of Interest: There is no relevant conflict of interest or financial disclosure for Drs. Sansoni or Sautter.
Accepted for oral presentation at the 60th annual meeting of the American Rhinologic Society, September 20th, 2014, Orlando, FL., USA
REFERENCES
- 1.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 2.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Bürner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35(9):1186–91. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Bruaene N, C PN, Van Crombruggen K, et al. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy. 2012;42(6):883–90. doi: 10.1111/j.1365-2222.2011.03898.x. [DOI] [PubMed] [Google Scholar]
- 4.Deluca HF, Cantorna MT. Vitamin D: Its role and uses in immunology. FASEB J. 2001;15(14):2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan JK, Bleier BS, O'Connell B, Mulligan RM, Wagner C, Schlosser RJ. Vitamin D3 correlates inversely with systemic dendritic cell numbers and bone erosion in chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Clin Exp Immunol. 2011;164(3):312–320. doi: 10.1111/j.1365-2249.2011.04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulligan JK, White DR, Wang EW, et al. Vitamin D3 deficiency increases sinus mucosa dendritic cells in pediatric chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg. 2012;147(4):773–781. doi: 10.1177/0194599812448852. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal T, Gupta GK, Agrawal DK. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013;43(6):672–83. doi: 10.1111/cea.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvaraj P, Harishankar M, Singh B, Banurekha VV, Jawahar MS. Effect of vitamin D3 on chemokine expression in pulmonary tuberculosis. Cytokine. 2012;60(1):212–219. doi: 10.1016/j.cyto.2012.06.238. [DOI] [PubMed] [Google Scholar]
- 9.Fraczek M, Rostkowska-Nadolska B, Kusmierz D, et al. Vitamin D analogs decrease in vitro secretion of RANTES and enhance the effect of budesonide. Adv Med Sci. 2012;57(2):290–295. doi: 10.2478/v10039-012-0043-5. [DOI] [PubMed] [Google Scholar]
- 10.Rostkowska-Nadolska B, Fraczek M, Gawron W, Latocha M. Influence of vitamin D(3) analogues in combination with budesonide on proliferation of nasal polyp fibroblasts. Acta Biochim Pol. 2009;56(2):235–42. [PubMed] [Google Scholar]
- 11.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013;123(12):E72–8. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med 2007. 2014;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133(4):1748–1754. [PubMed] [Google Scholar]
- 14.Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabry?ová L, et al. The role of 1,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+and IL-10+CD4+T cells. Eur J Immunol. 2012;42(10):2697–2708. doi: 10.1002/eji.201242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LF, Lee CH, Chien CY. Serum 25-hydroxyvitamin D levels are lower in chronic rhinosinusitis with nasal polyposis and are correlated with disease severity in Taiwanese patients. Am J Rhinol Allergy. 2013;27(6):162–165. doi: 10.2500/ajra.2013.27.3948. [DOI] [PubMed] [Google Scholar]
- 16.Gill TK, Hill CL, Shanahan EM, et al. Vitamin D levels in an Australian population. BMC Public Health. 2014;14:1001. doi: 10.1186/1471-2458-14-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(6):e38934. doi: 10.1371/journal.pone.0038934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;3(23):1–298. [PubMed] [Google Scholar]
- 19.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1a,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci. 2001;98(12):6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayers CM, Schlosser RJ, O'Connell BP, et al. Increased presence of dendritic cells and dendritic cell chemokines in the sinus mucosa of chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Int Forum Allergy Rhinol. 2011;1(4):296–302. doi: 10.1002/alr.20046. [DOI] [PubMed] [Google Scholar]
- 21.Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003;3(1):1–6. doi: 10.1097/00130832-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Kuna P, Alam R, Ruta U, Gorski P. RANTES Induces Nasal Mucosal Inflammation Rich in Eosinophils, Basophils, and Lymphocytes In Vivo. Am J Respir Crit Care Med. 1998;157(3):873–879. doi: 10.1164/ajrccm.157.3.9610052. Pt 1. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JE, Bartels J, Gurugh T, et al. The role of RANTES in nasal polyposis. Am J Rhinol. 2005;19(1):15–20. [PubMed] [Google Scholar]
- 24.Kim HJ, Jung HH, Lee SH. Expression of acidic fibroblast growth factor and basic fibroblast growth factor in nasal polyps. Acta Otolaryngol. 2006;126(6):600–605. doi: 10.1080/00016480500452533. [DOI] [PubMed] [Google Scholar]
- 25.Skevaki CL, Psarras S, Volonaki E, et al. Rhinovirus-induced basic fibroblast growth factor release mediates airway remodeling features. Clin Transl Allergy. 2012;2(1):14. doi: 10.1186/2045-7022-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahfouz ME, Elsheikh MN, Ghoname NF. Molecular profile of the antrochoanal polyp: up-regulation of basic fibroblast growth factor and transforming growth factor beta in maxillary sinus mucosa. Am J Rhinol. 2006;20(4):466–470. doi: 10.2500/ajr.2006.20.2894. [DOI] [PubMed] [Google Scholar]
- 27.Norlander T, Westermark A, van Setten G, Valtonen H, Ishizaki H, Pyykkö I. Basic fibroblast growth factor in nasal polyps immunohistochemical and quantitative findings. Rhinology. 2001;39(2):88–92. [PubMed] [Google Scholar]
- 28.Sautter NB, Delaney KL, Hausman FA, Trune DR. Tissue remodeling gene expression in a murine model of chronic rhinosinusitis. Laryngoscope. 2012;122(4):711–717. doi: 10.1002/lary.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]