SUMMARY
The impact of inflammation suppressor pathways on Alzheimer’s disease (AD) evolution remains poorly understood. Human evidence suggests involvement of the cardinal anti-inflammatory cytokine, interleukin-10 (Il10). We crossed the APP/PS1 mouse model of cerebral amyloidosis with a mouse deficient in Il10 (APP/PS1+Il10−/−). Quantitative in silico 3D modeling revealed activated Aβ phagocytic microglia in APP/PS1+Il10−/− mice that restricted cerebral amyloidosis. Genome-wide RNA sequencing of APP/PS1+Il10−/− brains showed selective modulation of innate immune genes that drive neuroinflammation. Il10 deficiency preserved synaptic integrity and mitigated cognitive disturbance in APP/PS1 mice. In vitro knock-down of microglial Il10-Stat3 signaling endorsed Aβ phagocytosis, while exogenous IL-10 had the converse effect. Il10 deficiency also partially overcame inhibition of microglial Aβ uptake by human Apolipoprotein E. Finally, the IL-10 signaling pathway was abnormally elevated in AD patient brains. Our results suggest that ‘re-balancing’ innate immunity by blocking the IL-10 anti-inflammatory response may be therapeutically relevant for AD.
INTRODUCTION
Alzheimer’s Disease (AD), the most common form of dementia in the elderly, is characterized by a triad of pathological features: extracellular amyloid deposits predominantly composed of amyloid-β (Aβ) peptides, intracellular neurofibrillary tangles (NFTs) chiefly comprised of abnormally folded tau protein, and gliosis consisting of reactive microglia and astrocytes surrounding β-amyloid plaques. During the past century, intense focus has been directed toward studying production, aggregation and spreading of β-amyloid plaques and subsequent neurodegeneration (Mucke and Selkoe, 2012). These studies have led to the conclusion that AD pathology is driven by an imbalance between Aβ production and clearance.
Indeed, autosomal-dominant forms of familial Alzheimer’s disease (FAD) are principally linked to mutations affecting β-amyloid precursor protein (β-APP) or Presenilin 1 (PS1) function (De Strooper et al., 2012), leading to amyloidogenic processing of β-APP and accumulation of cerebral amyloid deposits. Nonetheless, the vast majority of patients have the sporadic form of the disease, which likely arises from a combination of poorly defined genetic and environmental risk factors. These factors do not necessarily affect β-APP proteolysis, and it has instead been suggested that dysregulated Aβ clearance–rather than production–is the etiologic driving force in sporadic AD (Mawuenyega et al., 2010). As the resident macrophages of the CNS, microglia are chiefly responsible for phagocytosis and clearance of cellular detritus. Furthermore, numerous studies have validated the ability of microglia to phagocytose Aβ peptides (Grathwohl et al., 2009; Herber et al., 2004; Wilcock et al., 2004; Wyss-Coray et al., 2001). However, mounting evidence suggests that microglia are dysfunctional in the AD brain (Lopes et al., 2008; Streit et al., 2009). While prolonged activation of brain inflammatory processes coordinated by the cerebral innate immune system is now accepted as an AD etiologic event (Wyss-Coray and Mucke, 2002), the role of anti- inflammatory pathways in Aβ clearance and AD pathobiology has been largely overlooked.
Inflammatory responses are kept under control by two key immunoregulatory cytokines: transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) (Li and Flavell, 2008; Mucke and Selkoe, 2012; Strle et al., 2001; Williams et al., 2004; Wyss-Coray and Mucke, 2002). Our laboratory has previously shown that blockade of anti-inflammatory TGF-β-Smad 2/3 signaling in innate immune cells mitigates cerebral amyloidosis and behavioral deficits in the Tg2576 mouse model (Town et al., 2008). These data suggest that the innate immune system can be harnessed to clear Aβ in the context of anti-inflammatory signaling inhibition. Remarkably, cerebral levels of IL-10 were increased in this scenario, in line with the elevated IL-10 signaling observed in reactive glia neighboring β-amyloid plaques in aged Tg2576 mice (Apelt and Schliebs, 2001). Also, a functional polymorphism within the Il10 gene has been linked to increased risk of AD in some (Arosio et al., 2004; Lio et al., 2003; Ma et al., 2005; Vural et al., 2009), but not all populations (Depboylu et al., 2003; Ramos et al., 2006; Scassellati et al., 2004).
IL-10 signaling induced by binding of IL-10 homodimer to its cognate receptor (IL-10R) leads to phosphorylation of associated Janus kinase 1 (Jak1) and downstream phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3). Phosphorylated STAT3 translocates to the nucleus, where it regulates transcription of downstream cytokines and inflammatory genes including SOCS3 (Murray, 2006). To investigate putative involvement of the IL-10 pathway in AD-like pathology, we crossed the Tg(APPswe, PS1ΔE9) mouse model of cerebral amyloidosis with animals deficient in Il10. Genetic disruption of Il10 licensed Aβ phagocytosis by activated microglia and reduced Aβ load in APP/PS1 mouse brains. Transcriptome analysis of brains from APP/PS1+Il10−/− mice by RNA sequencing (RNAseq) revealed modulation of the inflammatory milieu, including select inflammatory and microglial regulatory genes. Finally, Il10 deficiency partially rescued synaptic integrity and behavioral impairment driven by the APP/PS1 transgenes.
RESULTS
Deficiency in Il10 mitigates cerebral amyloidosis in APP/PS1 mice
To assess the role of Il10 in AD-like pathology, we bred Il10−/− mice (Kuhn et al., 1993) to Tg(APPswe,PSEN1ΔE9) animals (referred as APP/PS1 mice in the present study) (Jankowsky et al., 2004; Jankowsky et al., 2001). APP/PS1+Il10−/− and APP/PS1+Il10+/-mice were born at Mendelian ratios and exhibited no anatomical defects or premature death compared to APP/PS1+Il10+/+ mice. IL-10 levels measured in the plasma of 12 month-old mice followed an Il10 allele-dependent expression pattern (pg of IL-10/ml of plasma: APP/PS1+Il10+/+, 19.0 ± 1.1 (n = 6), APP/PS1+Il10+/− (n = 4), 10.8 ± 2.1**; APP/PS1+Il10−/−, 0.20 ± 0.04*** (n = 4); **p>0.01, ***p>0.001 compared to APP/PS1+Il10+/+ mice by one-way ANOVA and Dunnett’s post-hoc test). At 12–13 months of age, APP/PS1+Il10−/− mice manifested significantly reduced amyloid deposition in cingulate cortex (CC), entorhinal cortex (EC) and hippocampus (HC) as measured by thioflavinS histochemistry (Figure 1A-1B; reductions versus APP/PS1+Il10+/+ mice: CC, 74%; EC, 78%; HC, 67%, *** p<0.001; by one-way ANOVA and Dunnett’s post-hoc test). Furthermore, 4G8+ β-amyloid plaques were also significantly reduced in APP/PS1+Il10−/−-compared to APP/PS1+Il10+/+ animals (Figure 1C; CC, 67%; EC, 50%; HC, 70%, *p<0.05, ** p<0.01, *** p<0.001; one-way ANOVA and Dunnett’s post-hoc test). Aβ plaque morphometry was analyzed by blindly assigning plaques to one of three mutually exclusive categories based on maximum diameter. Surprisingly, APP/PS1+Il10−/− mice had modest but statistically significant increases in abundance of small (<25 µm) plaques in the CC and EC versus APP/PS1+Il10+/+ animals (Figure S1A, *p<0.05). Yet, numbers of medium (25–50µm) and large-sized (>50µm) plaques were significantly reduced by 48–74% in the CC, and this effect trended toward significance in the EC and HC (Figure S1B–S1C, *p<0.05; by one-way ANOVA and Fisher’s post-hoc test). In addition to Aβ plaques in brain parenchyma, 86% of AD patients deposit Aβ in cerebral blood vessels; known as cerebral amyloid angiopathy (CAA) (Ellis et al., 1996; Kanekiyo et al., 2012). APP/PS1 mice also develop CAA, and this pathology was significantly reduced by 46–68% in EC and HC (and trended toward significance in CC) of APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice (Figure 1D, *p<0.05, **p<0.01; by one-way ANOVA and Dunnett’s post-hoc test). Interestingly, no evidence for Il10 heterozygous advantage was found for Aβ deposits in brain parenchyma or cerebral vessels (Figure 1A-1D and Figure S1, p>0.05).
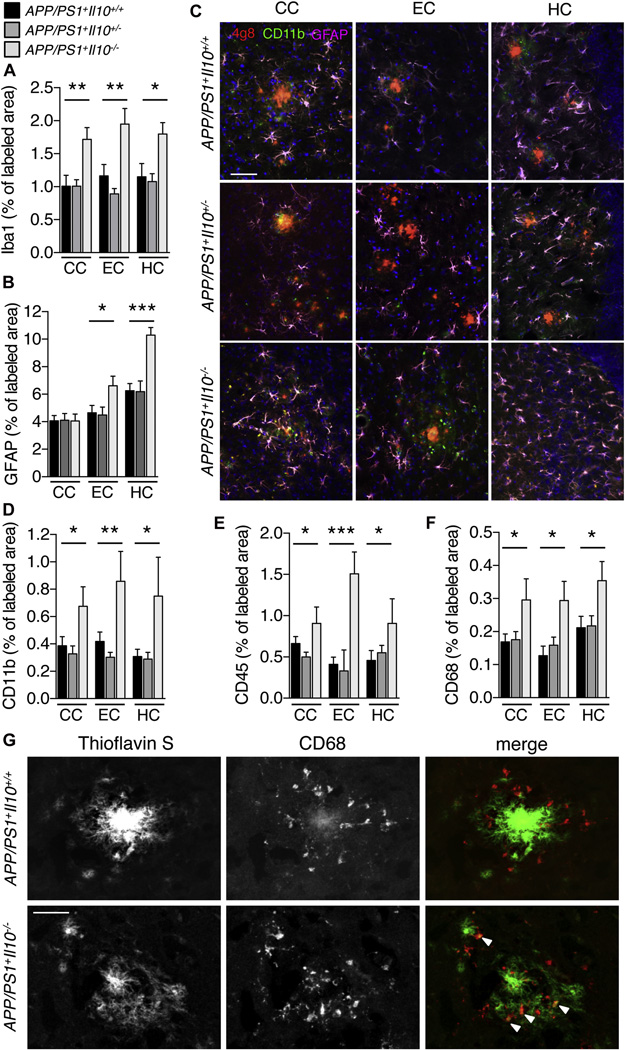
Figure 1. Il10 deficiency reduces cerebral amyloidosis in APP/PS1 mice.
(A) Representative micrographs of amyloid plaques labeled with thioflavinS from the cingulate cortex (CC), entorhinal cortex (EC) and hippocampus (HC) of APP/PS1 mice homozygous, heterozygous or completely deficient for Il10. Scale bar denotes 100 µm. (B, C) Quantitation of thioflavinS (B) and 4G8 (C) labeling.
(D) Semi-quantitative analysis of cerebral amyloid angiopathy severity (CAA score) from thioflavinS-labeled brain sections.
(E–H) ELISA analysis of frontal cortex detergent-soluble (E, F) or guanidine-HCl-extracted (G, H) Aβ1–40 and Aβ1–42 species from mice with the indicated genotypes. For (B–H), data are presented as mean ± SEM for APP/PS1+Il10+/+ (n=10–18), APP/PS1+Il10+/− (n=9–24), and APP/PS1+Il10−/− mice (n=3–10); * p<0.05, ** p<0.01, *** p<0.001.
(I) Western blots of human (h) APP or hPS1 levels in frontal cortex homogenates of APP/PS1 mice with the indicated genotypes. Beta-actin is shown as a loading control.
(J) Quantitation of hAPP or hPS1 protein levels in frontal cortex homogenates from APP/PS1 mice of the indicated genotypes. Expression levels are normalized to β-actin. Data are represented as mean ± SEM for n=6 samples for each group, with APP/PS1+Il10+/+ signal normalized to 100%; non-significant.
(K) Q-PCR analysis of APP and PS1 mRNA levels in frontal cortex from mice with the indicated genotypes. The mRNA levels are normalized to HPRT, and n=6 per group; non-significant. See also Figure S1.
Biochemical analysis revealed striking reductions in both Aβ1–40 and Aβ1–42 abundance in brains of APP/PS1+Il10−/− compared to APP/PS1+Il10+/+ mice. In the detergent-soluble fraction, Aβ1–40 was reduced by 63% and Aβ1–42, by 70% (Figure 1E-1F, *p<0.05, **p<0.01; by one-way ANOVA and Dunnett’s post-hoc test). Additionally, after re-extraction of the detergent-insoluble pellet in the chaotropic agent, guanidine-HCl, Aβ1–40 was lowered by 79% and Aβ1–42, by 85% in APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice (Figure 1G-1H, *p<0.05; by one-way ANOVA and Dunnett’s post-hoc test). Interestingly, detergent-soluble cerebral Aβ1–40 and Aβ1–42 abundance was decreased by 35–42% in APP/PS1+Il10+/- mice, although this trend did not reach statistical significance (Figure 1E-1F, p>0.05; by one-way ANOVA and Dunnett’s post-hoc test).
To rule out the possibility of an effect on cerebral amyloidosis due to altered APPSwe or PS1Δe9 transgene expression, Western blot and quantitative real-time reverse transcriptase PCR (qPCR) analyses were performed on protein and RNA extracted from frontal cortex of all three groups of mice. No between-groups differences were found on PS1 or APP protein or mRNA levels (Figure 1I-1K). To determine if Il10 deficiency altered APP metabolism, amyloidogenic C99 fragments were detected in frontal cortex brain extracts (n = 5–6 for each mouse group) by Western blot, but remained unmodified (quantitation of C99 band intensity normalized to holo-APP and β-actin: APP/PS1+Il10+/+, 99.5±7.8; APP/PS1+Il10+/−, 96.8±7.3; APP/PS1+Il10−/−, 87.9±5.9, p>0.05; by one-way ANOVA and Dunnett’s post-hoc test). Finally, to address the possibility of Aβ peptide efflux from brain to blood, we assayed plasma levels of Aβ1–40 and Aβ1–42, but did not detect significant differences (pg of Aβ1–40/mL of plasma: APP/PS1+Il10+/+, 594.3 ± 92.8; APP/PS1+Il10+/−, 649.4 ± 90.3; APP/PS1+Il10−/−, 752.3 ± 55.4; pg of Aβ1–42/mL of plasma: APP/PS1+Il10+/+, 220.2 ± 60.9; APP/PS1+Il10+/−, 372.8 ± 62.5; APP/PS1+Il10−/−, 294.4 ± 39.2, p>0.05; by one-way ANOVA and Dunnett’s post-hoc test).
Il10 deficiency activates innate immunity in brains of APP/PS1 mice
Within the CNS, IL-10 is mainly produced by astrocytes and microglia (Ledeboer et al., 2002), the latter being brain-resident innate immune cells that are centrally positioned to phagocytose and clear Aβ (Aguzzi et al., 2013; Guillot-Sestier and Town, 2013). To confirm the cellular source of cerebral IL-10 in our experimental animals, Il10 mRNA levels were analyzed by qPCR in CD11b+ and CD11b− cellular fractions isolated from brain single cell suspensions (Figure S2A). The CD11b+ cell fraction highly expressed established microglial markers (i.e., Iba1, Cx3cr1, Csf1r, Itgb5; Figure S2A) (Butovsky et al., 2014), while the CD11b− cell fraction expressed astrocytic and neuronal markers (i.e., S100b, Map2). Interestingly, the CD11b+ fraction was largely enriched in Il10r mRNA compared to CD11b− cells, and Il10r expression was strikingly increased in microglia from APP/PS1+ animals (Figure S2A). Finally, Il10 mRNA levels were markedly increased in microglia from APP/PS1+Il10+/+ brains, suggesting that IL-10 is produced by CD11b+ microglia and likely participates in autocrine signaling via IL-10R in brains of APP/PS1+ mice. To investigate the effect of Il10 deficiency on neuroinflammation in response to Aβ deposition, coronal sections from 12–13 month-old mouse brains were immunostained for ionized calcium-binding receptor 1 (Iba1) (Ahmed et al., 2007), and data showed 54–69% significantly increased signal in APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice (Figure 2A, *p<0.05, **p<0.01; by one-way ANOVA and Dunnett’s post-hoc test). Interestingly, in non-transgenic animals, Il10 deficiency did not modify Iba1+ immunoreactivity (% of labeled area: CC: Il10+/+, 1.35± 0.16; Il10−/−, 1.58± 0.19; EC: Il10+/+, 1.03± 0.15; Il10−/−, 1.44± 0.16; HC: Il10+/+, 2.30± 0.43; Il10−/−, 2.06± 0.23; n=6 per genotype, by t-test), indicating that Il10 deficiency selectively modified microglial phenotype in the context of the APP/PS1 transgenes. A similar pattern of statistically significant results (from 55–64%) was observed for glial fibrillary acidic protein (GFAP)+ reactive astrocytes in EC and HC (Figure 2B-2C, *p<0.05, ***p<0.001; by one-way ANOVA and Dunnett’s post-hoc test). Remarkably, APP/PS1+Il10−/− mice that still had remaining β-amyloid plaques demonstrated statistically significant 74–143% increased CD11b+ activated microglial burden (Townsend et al., 2005) in close vicinity of Aβ deposits in the CC, EC, and HC (Figure 2C-2D, *p<0.05, **p<0.01; by one-way ANOVA and Dunnett’s post-hoc test). Further evidence of 59–266% significantly increased microglial activation in APP/PS1+Il10−/− mice came from CD45 immunostaining data (Figure 2E, *p<0.05, ***p<0.001; by one-way ANOVA and Dunnett’s post-hoc test) (Tan et al., 2000; Zhu et al., 2011). We did not observe histologic evidence of vascular cuffing or presence of round, non-process-bearing CD45 highly-expressing (CD45hi) mononuclear cells (Town et al., 2008), and flow cytometric analysis of single cell suspensions isolated from APP/PS1+Il10+/+ vs. APP/PS1+Il10−/− brains showed no differences on abundance of CD45hi or intermediate-expressing (CD45int) populations (Figure S2B). Interestingly, amyloid plaques in APP/PS1+Il10−/− mice appeared more diffuse than typical dense-cored plaques present in APP/PS1+Il10+/+ mice, and were accompanied by 50–74% increased activated CD68+ microglia (Figure 2F-2G, *p<0.05; by one-way ANOVA and Dunnett’s post-hoc test). In addition, association of microglia with amyloid deposits was significantly enhanced by 34% in APP/PS1+Il10−/− brains compared to APP/PS1+Il10+/+ littermates (p<0,001; by t-test).
Figure 2. Il10 deficient APP/PS1 mice activate cerebral innate immunity.
(A–F) Microgliosis and astrogliosis were quantified in coronal sections labeled with Iba1 (A), GFAP (B), CD11b (D), CD45 (E) or CD68 (F) antibodies in mice with the indicated genotypes. Data are represented as mean ± SEM for APP/PS1+Il10+/+ (n=8–13), APP/PS1+Il10+/− (n=8–16), and APP/PS1+Il10−/− mice (n=3–10); * p<0.05, ** p<0.01, *** p<0.001. (C) Representative micrographs of cingulate cortex (CC), entorhinal cortex (EC) and hippocampus (HC) from APP/PS1 mice homozygous, heterozygous or completely deficient for Il10. Amyloid plaques were labeled using 4G8 antibody, and CD11b+ microglia or GFAP+ astrocytes were found associated with β-amyloid deposits. Scale bar denotes 50 µm.
(G) Representative microphotographs are shown of β-amyloid plaque morphology in cortex of APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice. Amyloid plaques are labeled with thioflavinS while phagocytic microglia are marked by CD68 antibody. White arrows represent CD68+ cells co-localized with amyloid deposits. Scale bar denotes 20 µm. See also Figure S2.
Modified neuroinflammatory profile in APP/PS1 mice deficient for Il10
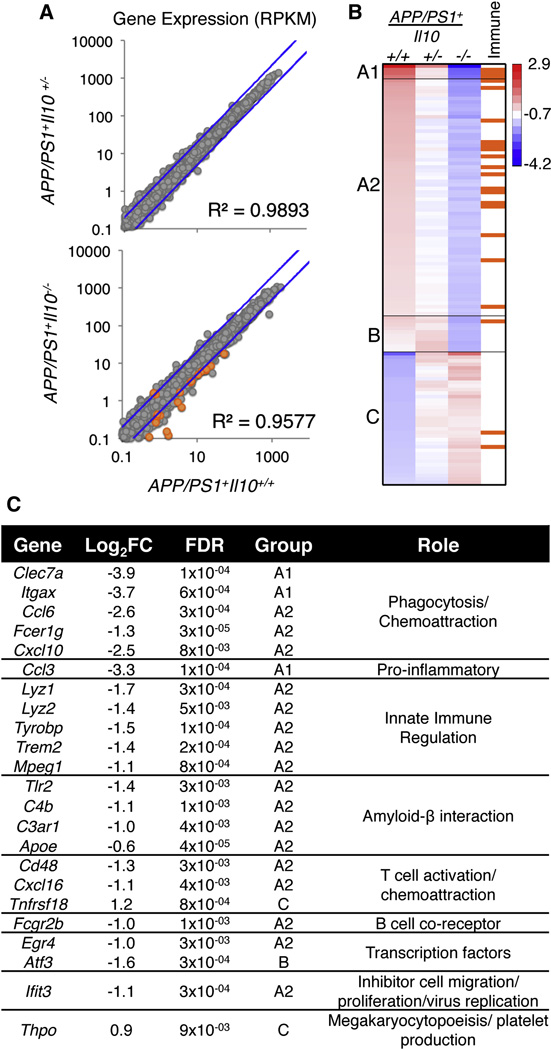
To assess global transcriptional changes in brains of APP/PS1 mice deficient in Il10, RNAseq was performed. Total brain mRNA was isolated from 12 month-old APP/PS1+: Il10+/+, Il10+/−, or Il10−/− animals (n=5 per group). Clustering of individual animal expression profiles resulted in segregation of the two homozygous populations, with heterozygous animals intermixed (Figure S3). Comparative expression of 14,800 detected RefSeq genes (normalized as RPKM; average per genotype) revealed expression changes that were Il10 allele-dependent (Figure 3A). Most genes remained unchanged in APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice, with only 117 genes having greater than two-fold differences. Cluster analysis of those 117 genes was performed, resulting in three distinct patterns: A, B and C (Figure 3B). We further defined these groups as A1, A2, B, and C, where group A1 and A2 genes were decreased in APP/PS1+Il10−/− mice and APP/PS1+Il10+/− mice had an intermediate result; group B genes were only decreased in APP/PS1+Il10−/− mice, and group C genes were increased in APP/PS1+Il10−/− mice. When these genes were further interrogated for immune-related function(s), the majority of immune genes fell into groups A1 and A2 (Figure 3B). These genes, corresponding fold changes with associated statistical significance levels, and global function(s) in immune responses are presented in Figure 3C. Interestingly, expression of Apoe (a well-established genetic risk factor for late-onset AD) was reduced in APP/PS1+Il10−/− compared to APP/PS1+Il10+/+ animals, validating microglial qPCR data (see Figure S2A). In general, immune genes with altered expression profiles were responsible for innate immune cell regulation, chemoattraction, amyloid-β interaction and phagocytosis.
Figure 3. Transcriptome analysis of brains from APP/PS1 mice deficient in Il10.
(A) Scatterplot of the average expression per gene (RPKM) of APP/PS1+Il10+/+ (n=5) plotted against APP/PS1+Il10+/− (n=5) or APP/PS1+Il10−/− mice (n=5). The blue line represents a 2-fold change.
(B) A heat map of k-means cluster analysis of log2 transformed expression (RPKM) is shown for 117 genes with 2-fold or greater change. Genes that are immune-related (as reported by the KEGG database) are indicated in orange.
(C) A table of immune- and inflammation-related genes identified from the heat map with log2 (fold change) of APP/PS1+Il10+/+ versus APP/PS1+Il10−/− mice is shown, and false discovery rate is calculated by the edgeR package in Bioconductor. See also Figure S3.
IL-10 retards while Il10 deficiency promotes microglial Aβ phagocytosis
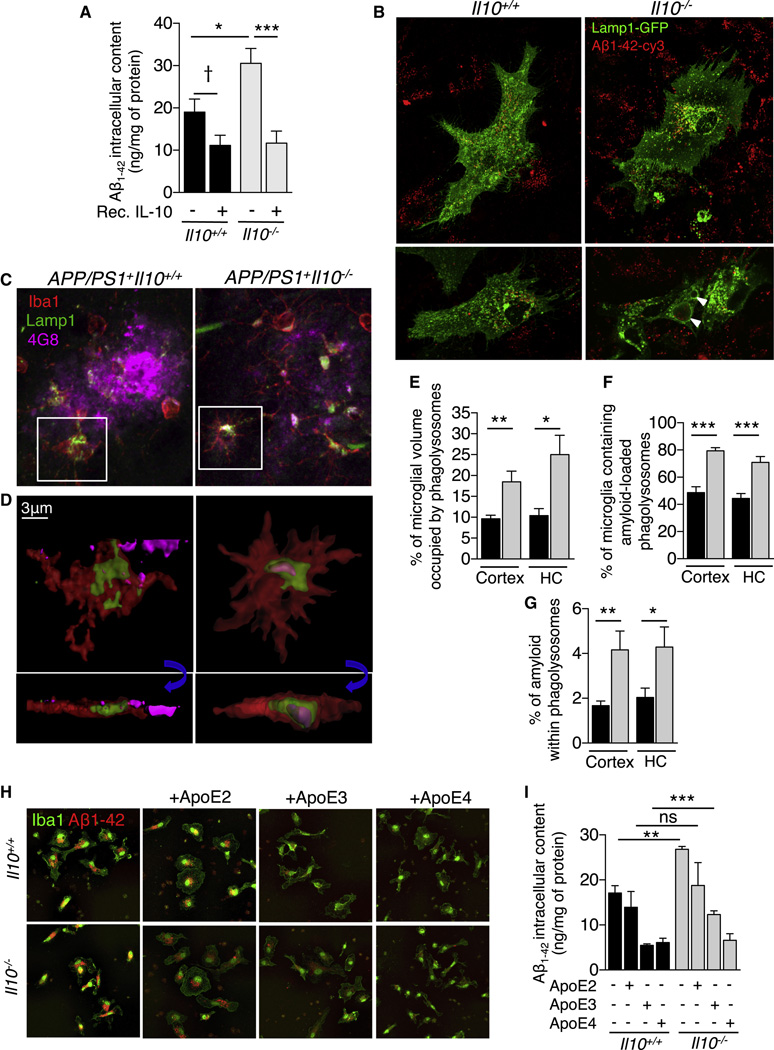
Data described above suggested that Il10 deficiency endorsed a beneficial form of innate immune activation that favored microglial β-amyloid clearance. To directly investigate the effects of IL-10 on microglial Aβ phagocytosis, primary cultures of microglia were established from Il10+/+ or Il10−/− mice, and Aβ1–42 phagocytosis was evaluated. Remarkably, ELISA measurement of Aβ1–42 intracellular content showed recombinant IL-10 treatment to significantly decrease pre-aggregated Aβ1–42 uptake by 41% in Il10+/+ and 62% in Il10−/− mouse primary microglia (Figure 4A, Il10+/+, †p=0.06 and Il10−/−, *** p<0.001; by one-way ANOVA and Sidak’s post-hoc test), and a similar pattern of results was observed in a rat microglial cell line (data not shown). Treatment with recombinant IL-10 also: 1) reduced phagolysosomal CD68 labeling by 49% (Figure S4A, S4B, * p<0.05; by t-test), 2) diminished intracellular Aβ1–42-cy3 signal by 46% (Figure S4C, * p<0.05; by t-test), and 3) induced STAT3 translocation to the nucleus (Figure S4D, S4E, ** p<0.01; by t-test). These data show that IL-10 treatment shifts microglial activation away from Aβ phagocytosis via increasing activation of STAT3.
Figure 4. Il10 deficiency increases microglial β-amyloid phagocytosis.
(A) ELISA analysis of Aβ1–42 intracellular content in cultured Il10+/+ or Il10−/− mouse primary microglia. Cultures were treated for 2 h with recombinant IL-10 before challenge with human synthetic Aβ1–42 microaggregates for 6 h. Data are presented as mean ± SEM of 4 independent experiments carried out in duplicate; †p=0.06, * p<0.05, *** p<0.001.
(B) Representative microphotographs of Lamp1-GFP transfected primary cultures of microglia (Il10+/+ or Il10−/−) challenged with Aβ1–42-cy3 microaggregates. White arrows designate enlarged Lamp1+ phagolysosomes containing Aβ1–42-cy3 in Il10−/− microglia.
(C) Representative microphotographs of amyloid deposits in cortex of APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice. Amyloid deposits are labeled with 4G8, and microglia, with Iba1 and Lamp1.
(D) Three-dimensional reconstruction from confocal image stacks showing 4G8+ Aβ encapsulated within Lamp1+ structures in Iba1+ microglia present in brains of APP/PS1+Il10−/− versus APP/PS1+Il10+/+ mice. Blue arrows indicate rotation of insets in the Z-plane to show presence of Aβ within phagolysosomes.
(E–G) Quantitation of microglial volume occupied by Lamp1+ phagolysosomes (E), percent of Iba1+ microglia containing amyloid-loaded phagolysosomes (F) or 4G8+ amyloid encapsulated in phagolysosomes (G) in the entorhinal cortex and hippocampus (HC) of mice with the indicated genotypes. Data are represented as mean ± SEM for APP/PS1+Il10+/+ (n=4) or APP/PS1+Il10−/− (n=5) mice; * p<0.05, ** p<0.01.
(H) Representative microphotographs of Iba1+ primary cultures of microglia (Il10+/+ or Il10−/−) challenged with Aβ1–42-cy3 microaggregates preincubated with human recombinant ApoE2, ApoE3 or ApoE4.
(I) ELISA analysis of Aβ1–42 intracellular content in cultured mouse primary microglia (Il10+/+ or Il10−/−). Cells were treated for 6 h with human synthetic Aβ1–42 pre-aggregated in presence of recombinant ApoE2, ApoE3 or ApoE4. Data are presented as mean ± SEM of 2 independent experiments carried out in duplicate; ** p<0.01, *** p<0.001. See also Figure S4 and Movies S1 and S2.
In a reciprocal set of experiments, Aβ1–42 uptake was increased by 60% in Il10−/− primary microglia (Figure 4A, Il10+/+ versus Il10−/−, * p<0.05; by one-way ANOVA and Sidak’s post-hoc test). To determine if Stat3 knock-down could phenocopy the effect of Il10 deletion on microglial Aβ phagocytosis, we used a mouse microglial cell line (N9) that responded similarly to mouse primary microglia in terms of IL-10 dependent reduction of Aβ phagocytosis (Figure S4F, * p<0.05; by t-test). Three independent Stat3 knock-down microglial N9 lines were generated via shStat3 lentiviral infection, and STAT3 expression was validated by immunocytochemistry and Western blot (Figure S4G and S4H). Strikingly, reduced STAT3 nuclear translocation occurred with increased Aβ1–42-cy3 within CD68+ lysosomes (Figure S4I) in the same manner as Il10 deletion.
To further investigate this effect, morphology and Aβ phagocytic aptitude of Il10+/+ versus Il10−/− primary microglia were evaluated by live cell imaging. Twenty-four hours after transfection with a Lamp1-GFP construct, cells were challenged with Aβ1–42-cy3 to follow Aβ1–42 uptake into Lamp1+ phagolysosomes. Representative images from supplemental movies S1 and S2 are shown in Figure 4B. Interestingly, Aβ1–42-cy3 was encapsulated within Lamp1+ structures in both Il10+/+ and Il10−/− microglia; however, Aβ-containing phagolysosomes were enlarged in Il10−/− microglia (Figure 4B; see white arrows and supplemental movie S1 and S2).
Strikingly, a similar pattern of results was observed in vivo. APP/PS1+ mice presented Iba1+ microglia containing 4G8+ Aβ encapsulated within Lamp1+ (Figure 4C, 4D) and CD68+ (Figure S4J) phagolysosomes. Yet, APP/PS1+Il10−/− brains had demonstrably increased abundance of Iba1+ microglia that co-stained for Lamp1+ lysosomes and for 4G8+ Aβ (Figure 4C and 4D). Importantly, phagolysosomes within Iba1+ cells were increased by 92% in the cortex and 140% in HC of APP/PS1+IL10−/− versus APP/PS1+Il10+/+ mice (Figure 4E, * p<0.05, ** p<0.01; by t-test). Analysis of plaque-associated microglia revealed an increased proportion of cells containing phagolysosome-encapsulated amyloid in Il10 deficient animals (Figure 4F; cortex, 62%; HC, 60%, *** p<0.001; by t-test). Finally, the total amount of 4G8+ Aβ loaded within Lamp1+ phagolysosomes was significantly augmented in APP/PS1+Il10−/− versus APP/PS1+Il10+/+ brains (Figure 4G; cortex, 148%; HC, 110%, * p<0.05, ** p<0.01; by t-test). Altogether, our results demonstrate that Il10 deficiency enhances microglial amyloid phagocytic function in APP/PS1 mice.
We previously observed by brain RNAseq (Figure 3) and microglial qPCR (Figure S2A) that Apoe expression was reduced in brains of APP/PS1+Il10−/− mice. To determine if human ApoE isoforms (E2, E3, E4) acted as molecular chaperones to bind Aβ and alter microglial phagocytosis, we pre-incubated Cy3-labeled (Figure 4H) or unlabeled (Figure 4I) human recombinant Aβ1–42 with human recombinant ApoE2, ApoE3 or ApoE4. Il10+/+ and Il10−/− primary microglial cultures were then treated with this mixture. Human ApoE drastically reduced Aβ uptake by microglia in an isoform-specific manner (E4>E3>E2), mirroring the well-established ApoE-human AD risk relationship (Figure 4H). This was confirmed by ELISA quantitation of Aβ uptake in a parallel set of experiments using unlabeled Aβ (Figure 4I, ** p<0.01, *** p<0.001; by one-way ANOVA and post-hoc t-test). Strikingly, human ApoE isoform-dependent reduction of microglial Aβ phagocytosis was significantly rescued by Il10 deficiency in the case of ApoE3.
Il10 deficiency preserves synaptic integrity in APP/PS1 mice
A key challenge for immunomodulatory strategies that promote cerebral amyloid clearance is avoidance of bystander neuronal injury due to neuroinflammation (Guillot-Sestier and Town, 2013; Town et al., 2005). To determine whether synaptic health was impacted by Il10 deficiency in APP/PS1 mice, coronal brain sections from 12 month-old mice were stained with an antibody directed against synaptophysin. As predicted, synaptophysin puncta density was reduced by 37–40% in APP/PS1+Il10+/+ versus APP/PS1−Il10+/+ littermates, both in hippocampus and in cerebral cortex (Figure 5A–C; *p<0.05, **p<0.01, by one-way ANOVA and Dunnett’s post-hoc test). Strikingly however, APP/PS1+Il10−/− mice had hippocampal and cortical synaptophysin labeling density that was restored to that of APP/PS1− littermates (Figure 5A–C). Western blot analysis of cortical protein extracts confirmed our immunohistochemical observations (Figure 5D), and densitometric analysis disclosed reduced synaptophysin abundance in brains of APP/PS1+Il10+/+ and APP/PS1+Il10+/− mice compared to non-transgenic controls (APP/PS1−Il10+/+, n=6, normalized to 1 versus APP/PS1+Il10+/+, 0.52 ± 0.20*, n=6; and APP/PS1−Il10+/−, 1.07 ± 0.33, n=6 versus APP/PS1+Il10+/−, 0.65 ± 0.33*, n=8; *p<0.05 by one-way ANOVA and Sidak’s post-hoc test). Remarkably, Il10 deficiency in APP/PS1+ mice normalized synaptophysin abundance to non-transgenic animals (APP/PS1+Il10−/−, 0.72 ± 0.12, n=5 versus APP/PS1−Il10−/−, 0.97 ± 0.30, n=3; not significantly different by one-way ANOVA and Sidak’s post-hoc test). The beneficial effect of Il10 loss on synaptophysin abundance was APP/PS1 transgene-dependent, because synaptophysin levels were not affected in Il10 heterozygotes or Il10 deficient mice that lacked the APP/PS1 transgenes (Figure 5D; p>0.05, by one-way ANOVA and Dunnett’s post-hoc test).
Figure 5. Preservation of synaptic integrity in Il10 deficient APP/PS1 mice.
(A, B) Representative microphotographs of synaptophysin labeling in hippocampus (A) or cortex (B) of mice with the indicated genotypes. Lower panels are 3.5x higher magnification of the upper images. Scale bars denote (A) 87 µm or (B), 25 µm.
(C) Quantitation of synaptophysin labeling in hippocampus and cortex is shown as mean ± SEM (n=4 per group); * p< 0.05, ** p<0.01.
(D) Western blot of synaptophysin levels in frontal cortex homogenates of mice with the indicated genotypes. Beta-actin served as a loading control.
Deficiency in Il10 mitigates APP/PS1 transgene-associated behavioral impairment
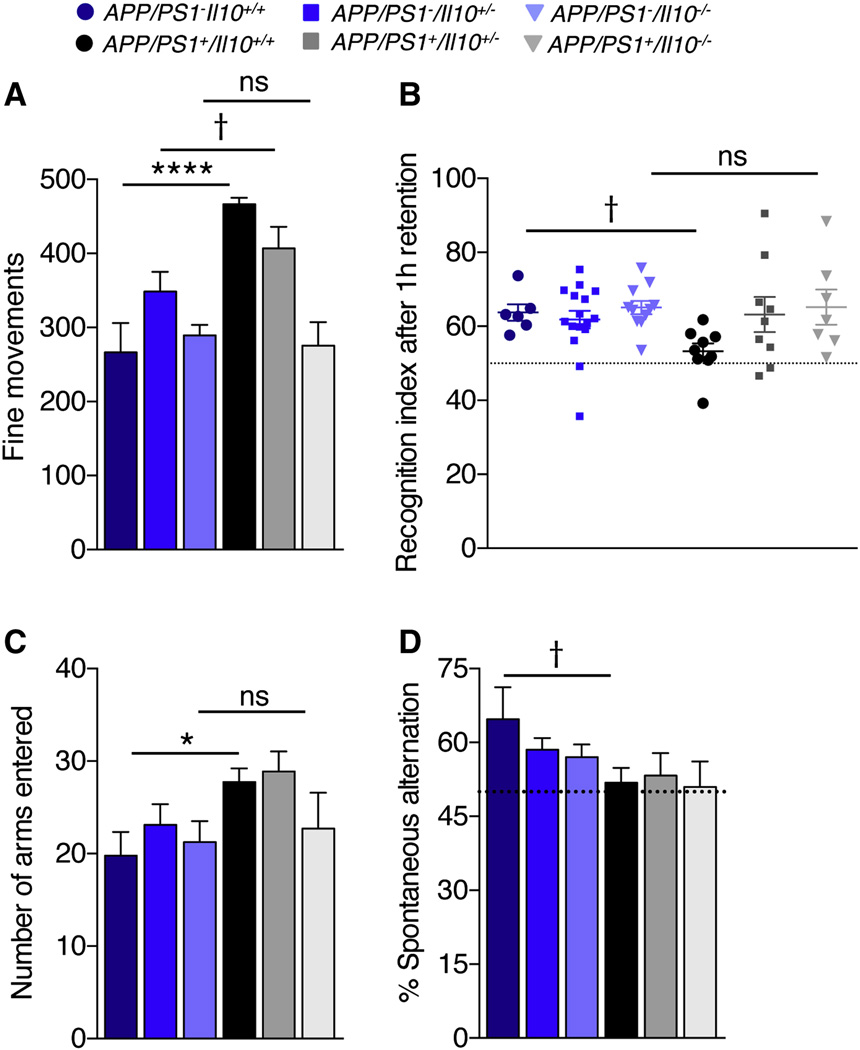
To evaluate functional consequences of preserved synaptic health in APP/PS1+Il10−/− mice, all six groups of littermates were cognitively evaluated. Prior to behavioral testing, mice were subjected to neurological screening to assess auditory, visual, and olfactory acuity, and response to a tactile stimulus. Additionally, coordination, balance and grip strength were tested. Mice performed equally well for each of the neurological screening tests (data not shown), and so all animals were included in subsequent behavioral assays. Locomotion and spontaneous activity were tested in an open field. Yet, no significant differences between any of the six groups were observed when considering rearing or time spent in the center of the field (Figure S5A), indicating that subsequent behavioral results were not distorted by variation in anxiety between-genotypes. However, when considering fine movements (i.e., grooming, exploration on four limbs and sniffing) and total activity, APP/PS1+Il10+/+ mice were hyperactive versus controls, a behavioral phenotype that may result from cortical and hippocampal injury leading to disinhibition (Town et al., 2008). Strikingly, APP/PS1+Il10−/− mice had complete mitigation of hyperactivity (Figure 6A and Figure S5A; † p<0.1, *p<0.05, ****p<0.0001; by one-way ANOVA and Fisher’s LSD post-hoc test).
Figure 6. Il10 deficiency partially restores cognitive function in APP/PS1 mice.
(A) Spontaneous activity was tested in the open field over a 30 min period. Bars represent fine movements of the mice.
(B) Evaluation of episodic memory in the novel object recognition test after 1 h of retention. Plots represent the recognition index.
(C, D) Determination of spontaneous alternation in the Y-maze. Bars represent number of arms entered (C) or percent spontaneous alternation (D).
Data are represented as mean ± SEM for Il10+/+ (n=5–6), Il10+/− (n=15–16), Il10−/− (n=8–11), APP/PS1+Il10+/+ (n=8–10), APP/PS1+Il10+/− (n=8–9) and APP/PS1+Il10−/− mice (n=7); Compared to APP/PS1 groups: †p≤0.1, *p≤0.05, ****p<0.0001. See also Figure S5.
Next, learning and episodic memory were assessed in the novel object recognition test, which is dependent on hippocampal and cortical function (Hammond et al., 2004). If mice remember a previously-encountered object compared to a novel object, they tend to preferentially explore the new object more than the familiar one. As expected, after a 1h retention period, APP/PS1+Il10+/+ mice trended toward lower preference for the novel object than controls (Figure 6B; † p=0.07; by one-way ANOVA and Fisher’s LSD post-hoc test). Strikingly, defective novel object recognition was completely remediated by Il10 deficiency, and partial amelioration of this behavioral defect was observed in APP/PS1+Il10+/− mice (Figure 6B). A similar trend of results occurred after 24h of retention (Figure S5B). Importantly, neither short-term (1h) nor long-term (24h) novel object memory were affected by Il10 deficiency in non-transgenic controls (Figure 6B and Figure S5B; p>0.05, by one-way ANOVA and Fisher’s LSD or Sidak’s post-hoc tests, respectively).
Spatial working memory was evaluated by spontaneous alternation in the Y-maze (Deacon et al., 2002). Similar to the open-field test, APP/PS1+Il10+/+ mice were hyperactive compared to control littermates, as operationalized by total number of arm entries. Again, this behavioral phenotype was completely mitigated by Il10 deficiency (Figure 6C, *p≤0.05; by one-way ANOVA and Fisher’s LSD post-hoc test). As expected, APP/PS1+Il10+/+ mouse % spontaneous alternation trended toward less frequent than controls, and Il10 deficiency did not modify this effect (Figure 6D, † p=0.07; by one-way ANOVA and Fisher’s LSD post-hoc test). As an important control, deficiency in Il10 did not alter spontaneous alternation in control littermates lacking the APP/PS1 transgene (Figure 6D, p>0.05; by one-way ANOVA and Fisher’s LSD post-hoc test).
Finally, mice were tested for hippocampus-dependent spatial reference learning and memory in the Barnes maze (O’Leary and Brown, 2009). During the training phase, all of the mouse groups demonstrated reduced latency to escape with successive acquisition trials; with the exception of APP/PS1+Il10−/− mice, which completed training in two distinct phases. During the 6 first trials, acquisition of the escape hole location was faster than the other groups, but APP/PS1+Il10−/− mice spent more time searching the escape box during the rest of the training (Figure S5C, *p<0.05; by one-way ANOVA and Fisher’s LSD post-hoc test). In the probe trial, latency to escape the maze was increased in APP/PS1+Il10+/+ mice compared to non-transgenic controls. However, complete Il10 deficiency did not significantly restore APP/PS1 behavioral deficit in this task. Surprisingly though, APP/PS1+Il10+/− mice performed significantly better than APP/PS1+Il10+/+ animals in the probe trial (Figure S5D, *p<0.05; by one-way ANOVA and Fisher’s LSD post-hoc test). During the reversal phase of the test, no differences in acquisition of the new escape box location were observed between the six groups (Figure S5E, p<0.05; by one-way ANOVA and Fisher’s LSD post-hoc test). No statistically significant gender differences were found for any of the behavioral paradigms, and so males and females were considered together in all behavioral analyses.
IL-10 signaling is elevated in AD patient brains
Finally, we sought to evaluate IL-10 signaling in post-mortem samples from AD patient brains versus age-matched, non-demented controls. Hippocampal sections were stained for IL-10 receptor alpha chain (IL-10Rα) and microtubule-associated protein 2 (MAP2, a neuronal marker). Interestingly, IL10Rα expression was elevated in AD compared to control brains, and some of these signals could be found co-localized with MAP2+ neurons (Figure 7A; see white arrowheads, and 7B, **p<0.01; by student’s t-test). Furthermore, phospho-Jak1, a key downstream effector kinase of the IL-10 pathway, was elevated in AD brains in close proximity to thioflavinS+ amyloid plaques (Figure 7C and 7D, *p<0.05; by student’s t-test). Western blot analyses of hippocampal protein extracts from a separate cohort confirmed our immunohistochemical observations, with densitometry disclosing increases of: 6.5-fold in IL10Rα (Figure 7E, *p<0.05), 2.3-fold in Jak1 (Figure 7F, †p=0.09), 2.6-fold in phospho-Jak1 (Figure 7G, *p<0.05), 4.2-fold in STAT3 (Figure 7H, *p<0.05), 1.6-fold in phospho-STAT3 (Figure 7I, †p=0.1) and 1.9-fold in SOCS3 (Figure 7J, †p=0.07) abundance (all by student’s t-test). Taken together, these data indicate elevated IL-10 signaling in AD patient versus age-matched control brains. Interestingly, we also observed increased Il10 and Il10r mRNA levels in microglia isolated from brains of APP/PS1+ mice.
Figure 7. IL-10 signaling is elevated in AD patient brains.
(A) Representative microphotographs of IL10Rα (red) and MAP-2 (green) labeling in hippocampal sections of AD patients and age-matched, non-demented control subjects.
(B) Quantitation of IL10Rα immunoreactivity in AD (n=6) and control (n=3) brain sections; data are presented as mean ± SEM of labeled area for 3 optical sections per subject; ** p<0.01.
(C) Representative microphotographs of thioflavinS+ amyloid plaques (green), MAP-2 (blue) and phospho-Jak1 (pJak1, red) signals in hippocampal sections of AD patients and age-matched, non-demented controls.
(D) Quantitation of pJak1 levels in AD (n=6) and control (n=3) brain sections; data are presented as mean ± SEM of labeled area for 3 optical sections per subject; * p<0.05.
(E–J) Quantification and representative Western blots of IL10Rα (E), Jak1 (F), pJak1 (G), STAT3 (H), pSTAT3 (I) and SOCS (J) in hippocampal homogenates of AD patients and age-matched, non-demented controls. Expression levels are normalized to β-tubulin. Data are represented as mean ± SEM for controls (n=6) and AD (n=8) patients; †p<0.1, * p<0.05.
DISCUSSION
While once regarded as epiphenomenon, the impact of the cerebral innate immune response on AD pathology has become a topic of intense interest (Gandy and Heppner, 2013; Guillot-Sestier and Town, 2013; Weitz and Town, 2012). This has prompted the need for a deeper understanding of which innate immune pathways are dysregulated in the context of the disease. While pro-inflammatory cytokines have received attention in this regard, the concept that deregulated anti-inflammatory cytokines may be deleterious in AD has been largely overlooked. While several studies have shown that Il10 polymorphism is associated with late onset AD (Arosio et al., 2004; Lio et al., 2003; Ma et al., 2005; Vural et al., 2009), almost nothing is known regarding the putative role of IL-10 in evolution of disease pathology.
To address this knowledge gap, we generated APP/PS1 mice deficient for Il10 and evaluated AD-like pathology and cognitive impairment. Results showed strikingly reduced cerebral amyloid pathology in these animals, and remaining plaques were associated with activated microglia. Interestingly, plaques in APP/PS1+Il10−/− mice had a “moth-eaten” morphology, similar to observations made in brains of AD patients or APP transgenic mice after Aβ1–42 immunization (Bard et al., 2000; Nicoll et al., 2006; Nicoll et al., 2003; Schenk et al., 1999; Zotova et al., 2011). Importantly, CD68+ phagocytic microglial cells were observed invading moth-eaten plaques in APP/PS1+Il10−/− brains. Recently, Krabbe and colleagues showed that microglial cells fail to reduce Aβ burden in transgenic mouse models of AD due to impaired mobility and phagocytic capacity (Krabbe et al., 2013). Microglial “paralysis” may be owed to increasing Aβ burden with disease progression, as shown by others in vitro and in vivo (Korotzer et al., 1993; Krabbe et al., 2013; Michelucci et al., 2009). Alternatively, it has been hypothesized that microglial senescence in the aging brain could be responsible for reduced capacity of these cells to clear cerebral amyloid (Lopes et al., 2008; Miller and Streit, 2007; Streit et al., 2009). The results we report here show that stimulation of microglia by recombinant IL-10 induces nuclear translocation of the downstream signal transducer STAT3 and reduces Aβ phagocytosis, whereas Il10 deficiency or inhibition of STAT3 increases Aβ uptake by cultured microglia. Additionally, Il10 deficiency increases microglial activation and promotes Aβ uptake into Lamp1+ and CD68+ phagolysosomes in vivo. In this regard, Il10 deficiency in APP/PS1 mice seems to restore physiologic ability to phagocytose Aβ. These findings dovetail with previous studies from our laboratory and others, showing that induction of a pro-inflammatory activation state endorses cerebral amyloid clearance (Chakrabarty et al., 2010a; Chakrabarty et al., 2011; Chakrabarty et al., 2010b; Shaftel et al., 2007; Town et al., 2008). We did not observe histological evidence of brain-infiltrating peripheral mononuclear phagocytes in APP/PS1+/Il10−/− mice (i.e., vascular cuffing or presence of round, non-process-bearing leukocytes) as we previously reported in a different innate immune paradigm (Town et al., 2008). Furthermore, Il10 deficiency did not modify abundance of CD45hi or CD45int mononuclear phagocytes in APP/PS1/Il10+/+ vs. APP/PS1/Il10−/− brains, suggesting that brain-resident microglia are likely the major population responsible for amyloid clearance. However, direct experiments aimed at firmly delineating the role of peripheral vs. central phagocytes in clearance of Aβ are warranted.
The study of global transcriptome changes in brains of APP/PS1 mice via RNAseq demonstrates that Il10 deficiency modifies cerebral innate immunity. During the analysis, we considered classical markers for M2-like (TGF-β, Ym-1, Fizz) and M1-like (TNF-α, IL-1β, IL-6) innate immune activation states. However, we did not detect differential expression of these targets. Yet, Clec7a expression was strongly decreased in APP/PS1+Il10−/− mice, suggesting polarization of microglial activation away from the M2 state. Depending on the type of stimulation, microglia demonstrate remarkable plasticity and often respond with a mixed activation phenotype (Ghassabeh et al., 2006; Town et al., 2005); therefore, we have previously suggested that M1 or M2 define boundaries of a more broad microglial activation continuum (Town et al., 2005). Nonetheless, our data reveal global changes in genes that regulate innate immune activation, inflammation and phagocytosis. Interestingly, genes up-regulated in brains of patients with late onset AD such as Tyrobp, Trem2 and C4b (Brouwers et al., 2012; McGeer et al., 1989; Zhang et al., 2013) were decreased in brains of Il10 deficient APP/PS1 mice. Along similar lines, previous studies have shown that TLR2 and C4b bind Aβ and trigger microglial activation (Richard et al., 2008) and Aβ fibril formation (Sjolander et al., 2012; Trouw et al., 2008), and APP/PS1+Il10−/− brains had decreased expression of both genes. Strikingly, Chakrabarty and coworkers have demonstrated that adeno-associated viral expression of Il10 in brains of APP transgenic mice leads to age-dependent up-regulation of Cxcl10, Tlr2, C4b and C3ar1 transcripts (Chakrabarty et al., 2015). These global gene expression results corroborate our data showing that Il10 deficiency restores microglial functionality that is compromised in APP/PS1 transgenic mice. Of particular interest, Apoe was differentially expressed in APP/PS1+Il10−/− mice as shown by brain RNAseq (Log2FC= −0.6, FDR= 4×10−5) and by microglial qPCR. In vitro, recombinant human ApoE3 and ApoE4 drastically reduced Aβ uptake by microglia, while ApoE2 had no effect, mirroring the well-established ApoE-human AD risk relationship. Strikingly, Il10 deficiency partially rescued human ApoE3-associated reduction of Aβ uptake compared to Il10+/+ microglia, but was unable to recover the deleterious effect of human ApoE4. Again, this tracks well with ApoE4 increased risk for human AD.
But does remodeling of cerebral amyloid in APP/PS1+Il10−/− mice come at the cost of bystander injury to neurons? This question is especially pertinent because we and others have shown that gliosis can potentially be toxic to neurons in the context of AD (Maezawa et al., 2011; Meda et al., 1995; Tan et al., 1999). Given changes in immune gene expression profile associated with Il10 deficiency and mitigation of cerebral amyloid load, we examined synaptic health in APP/PS1+Il10−/− animals. Synaptophysin density was reduced in hippocampus and cortex of APP/PS1 mice compared to non-transgenic controls, as reported in transgenic mouse models of cerebral amyloidosis and in AD patients (Buttini et al., 2005; Imbimbo et al., 2010; Tampellini et al., 2010; Ubhi et al., 2010). Interestingly, synaptophysin loss in APP/PS1 mice was almost completely restored by Il10 deficiency, indicating that innate immune activation associated with amyloid clearance in APP/PS1+Il10−/− mice preserved synaptic integrity.
Behavioral analyses were performed to determine whether maintenance of synaptic health in APP/PS1+Il10−/− mice translated to better cognitive function. Importantly, in non-transgenic control groups, Il10 deficiency did not alter anxiety, learning or memory. On the other hand, APP/PS1 mice were hyperactive, likely resulting from disinhibition associated with hippocampal or cortical damage (Hsiao et al., 1996; Town et al., 2008). Additionally, novel object recognition and spatial working memory were defective in APP/PS1 mice, as previously reported (Hooijmans et al., 2009; O’Leary and Brown, 2009; Webster et al., 2013). Most importantly, Il10 deficiency mitigated APP/PS1 transgene-associated hyperactivity in both the open-field and Y-maze tasks, and both short- and long-term novel object recognition were completely restored. However, spatial memory deficits were not rescued in APP/PS1+Il10−/− mice, although spatial reference memory was completely mitigated in APP/PS1+Il10+/− animals. This latter finding is significant because eventual clinical therapeutic targeting of IL-10 would likely never achieve 100% inhibition. These results indicate that Il10 deficiency mitigates a subset of defective cognitive functions in APP/PS1 mice.
To address the possibility that we were simply studying iatrogenic events not related to human AD, we investigated IL-10 signaling in AD patients versus cognitively healthy, age-matched controls. Data showed that expression of the cognate IL-10 receptor, IL10Rα, was elevated in AD patient brains compared to age-matched, non- demented individuals. Phosphorylated (activated) Jak1 was correspondingly increased in cells surrounding amyloid plaques in AD specimens, and protein levels of IL-10 receptor and downstream effectors were elevated in AD hippocampal homogenates. Collectively, these results indicate abnormally increased IL-10 signaling in AD patient brains. These results corroborate and extend the observations of other groups that reported increased levels of IL-10 in serum and brain extracts from AD patients (Angelopoulos et al., 2008; Culpan et al., 2006; Loewenbrueck et al., 2010). Furthermore, we noted that Il10 as well as IL10r mRNA levels were increased in microglia extracted from APP/PS1+Il10+/+ mouse brains, suggesting an autocrine signaling mechanism associated with increased cerebral amyloidosis, a finding that is in line with the IL-10 immunoreactive cells observed in close vicinity to β-amyloid deposits in 13-month-old Tg2576 mice (Apelt and Schliebs, 2001). Since we show that recombinant IL-10 treatment inhibits Aβ uptake by cultured microglia, elevated IL-10 signaling in AD patient brains and APP/PS1+ mice may hinder the physiological ability of microglia to phagocytose and clear cerebral amyloid.
Altogether, our findings suggest that genetic blockade of Il10 promotes a beneficial form of cerebral innate immunity. Il10 blockade enables cerebral Aβ clearance via two independent mechanisms: 1) reducing IL-10/STAT3 signaling to enhance microglial phagocytic activity, and 2) decreasing microglial Apoe expression, thereby mitigating ApoE-Aβ binding and detrimental reduction of Aβ phagocytosis. Importantly, our data are consistent with recent results showing that forced Il10 expression in brains of APP transgenic mice leads to increased Aβ accumulation and worsening of behavioral deficits (Chakrabarty et al., 2015). Therefore, modulating IL-10 signaling alters the microglial activation footprint and Aβ phagocytosis. Collectively, these results suggest that re-balancing cerebral innate immunity and promoting beneficial neuroinflammation may be more efficacious than generalized anti-inflammatory therapy for AD.
EXPERIMENTAL PROCEDURES
Please see Supplemental Experimental Procedures for detailed methods on immunochemistry, primary microglia isolation, flow cytometry, cell culture, transfection and viral infection, live cell imaging, Aβ uptake quantitation, Western blots, ELISA and MSD technology, RNAseq and Q-PCR, and behavioral experiments.
Human brain samples
Frozen human brain tissue used for Western blotting was obtained from the Alzheimer’s Disease Research Center (ADRC, NIA AG05142) Neuropathology Core (3 female and 5 male AD patient hippocampal samples, 51–100 years old, and 4 female and 2 male control hippocampal samples, 74–93 years old). For IHC, paraffin-embedded 10-µm-thick sections from the hippocampus of 6 AD patients (3 females and 3 males, 84–87 years old) and 3 age-matched non-demented control subjects were obtained from Dr. Serguei Bannykh, director of the Department of Neuropathology at Cedars-Sinai Medical Center.
Animals
Tg(APPswe,PSEN1ΔE9) transgenic mice (referred to as APP/PS1 in this report; B6.Cg-Tg(APPswe,PSEN1dE9)85Dbo/Mmjax MMRRC, stock #034832) (Jankowsky et al., 2004) were bred with Il10 knockout mice (Kuhn et al., 1993) (B6.129P2-Il10tm1Cgn/J, stock #002251). Both mouse strains are on the C57BL/6 background and were obtained from the Jackson Laboratory. All mice were housed under standard conditions with free access to food and water, and all animal experiments were performed in strict accordance with National Institutes of Health guidelines and recommendations from the Association for Assessment and Accreditation of Laboratory Animal Care International.
Western Blot Analysis, ELISA, and Immunohistochemistry
Mice were perfused with ice-cold phosphate-buffered saline (PBS) and brains were extracted and quartered according to our previously published methods (Tan et al., 2002; Town et al., 2008). The anterior two quarters were snap-frozen and posterior quarters were fixed in 4% paraformaldehyde overnight for subsequent agarose or paraffin embedding.
Three-dimensional reconstruction of confocal images
Confocal image stacks (acquired at 60× magnification) of amyloid deposits-associated microglia were converted to 3D images with the surface-rendering feature of Imaris BitPlane software (version 7.6.1).
RNAseq Gene Expression Analysis
Strand-specific libraries were generated with 1 µg of input RNA using the TruSeq Stranded mRNA Sample Prep Kit (Illumina) on an Illumnia HiSeq 2000. Gene classes were generated with Cluster3 by applying k-means clustering to mean-centered log2(RPKM) expression values (de Hoon et al., 2004). Classification of a gene as immune-related was based on KEGG pathway annotation (www.genome.jp/keg). The RNAseq data has been deposited under NCBI BioProject accession number PRJNA219136.
Behavioral Analyses
Behavioral experiments were conducted with age-matched littermates from 12–13 months of age, inclusive of the following six genotypes: Il10+/+, Il10+/−, Il10−/−, APP/PS1+Il10+/+, APP/PS1+Il10+/−, or APP/PS1+Il10−/−. All experiments were done blind with respect to the genotype of the mice. After neurological screening, behavioral tests were conducted in increasing order of difficulty and stress ranging from open field testing, novel object recognition, the Y-maze task, and the Barnes maze. For each test independently, mice that did not perform the exercise were excluded from the analysis.
Statistical Analysis
GraphPad Prism software, version 6.0. was used for all statistics. Multiple group comparisons were performed by one-way analysis of variance followed by Dunnett’s, Sidak’s or Fisher’s LSD post-hoc tests. Otherwise, student’s t-test was performed. For each behavioral test, possible gender differences within each group were statistically evaluated by analysis of variance, followed by Sidak’s multiple comparison test. In all cases, p≤0.05 was considered to be statistically significant. All data are presented as means ± SEM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Serguei Bannykh for human brain sections, Dr. Jean-Philippe Vit for assistance with behavioral testing (Cedars Sinai Medical Center, Los Angeles, CA, USA), Dr. Carol A. Miller from the Alzheimer’s Disease Research Center for frozen human brain tissue (University of Southern California, Los Angeles, CA, USA), and Dr. Eliezer Masliah (University of California, San Diego, CA, USA) for assistance with the synaptophysin immunostaining protocol. We sincerely thank Dr. Tara Weitz (USC Zilkha Neurgenetic Institute, Los Angeles, CA, USA), Drs. Todd Golde and Paramita Chakrabarty (Center for Translational Research in Neurodegenerative Disease, University of Florida, Gainesville, FL, USA) and Dr. Pritam Das (Mayo Clinic, Jacksonville, FL, USA) for helpful discussion. We thank Alexander Vesling for technical help with primary microglial cells. We thank the UCLA Neuroscience Genomics Core (Los Angeles, CA, USA) for assistance with RNAseq. This work was supported by the National Institute on Aging (5R00AG029726-04 and 3R00AG029726-04S1, to T.T.), the National Institute on Neurologic Disorders and Stroke (1R01NS076794-01, to T.T.), an Alzheimer’s Association Zenith Fellows Award (ZEN-10-174633, to T.T.), and an American Federation of Aging Research/Ellison Medical Foundation Julie Martin Mid-Career Award in Aging Research (M11472, to T.T.). Finally, we are grateful for startup funds from the Zilkha Neurogenetic Institute, which made this work possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
M.V.G.S. performed primary microglia, qPCR and behavioral experiments. K.R.D. performed RNaseq and sh viral infections. M.V.G.S., D.G., K.R.D., K.R.Z., B.P.L, and T.T. performed immunostaining, Western blots, and ELISA. M.V.G.S. and D.G. performed in vivo 3D modeling and quantitations, live cell imaging and flow cytometry. J.R. maintained the mouse colony. K.R.D. and D.G. equally contributed to this work. M.V.G.S. and T.T. wrote the manuscript. T.T. directed the project.
REFERENCES
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- Angelopoulos P, Agouridaki H, Vaiopoulos H, Siskou E, Doutsou K, Costa V, Baloyiannis SI. Cytokines in Alzheimer’s disease and vascular dementia. Int J Neurosci. 2008;118:1659–1672. doi: 10.1080/00207450701392068. [DOI] [PubMed] [Google Scholar]
- Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro-and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. doi: 10.1016/s0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- Arosio B, Trabattoni D, Galimberti L, Bucciarelli P, Fasano F, Calabresi C, Cazzullo CL, Vergani C, Annoni G, Clerici M. Interleukin-10 and interleukin-6 gene polymorphisms as risk factors for Alzheimer’s disease. Neurobiol Aging. 2004;25:1009–1015. doi: 10.1016/j.neurobiolaging.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Brouwers N, Van Cauwenberghe C, Engelborghs S, Lambert JC, Bettens K, Le Bastard N, Pasquier F, Montoya AG, Peeters K, Mattheijssens M, et al. Alzheimer risk associated with a copy number variation in the complement receptor 1 increasing C3b/C4b binding sites. Mol Psychiatry. 2012;17:223–233. doi: 10.1038/mp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nature Neuroscience. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Johnson-Wood K, Khan K, Seubert P, Freedman S, Schenk D, et al. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Ceballos-Diaz C, Beccard A, Janus C, Dickson D, Golde TE, Das P. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol. 2010a;184:5333–5343. doi: 10.4049/jimmunol.0903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine TNFalpha results in attenuation of amyloid deposition in vivo. Mol Neurodegener. 2011;6:16. doi: 10.1186/1750-1326-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010b;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, DiNunno N, Rosario AM, Cruz PE, Verbeeck C, Sacino A, Nix S, Janus C, Price ND, Das P, Golde TE. IL-10 Alters Immunoproteostasis in APP mice, Increasing Plaque Burden and Worsening Cognitive Behavior. Neuron. 2015 doi: 10.1016/j.neuron.2014.11.020. xx, xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpan D, Prince JA, Matthews S, Palmer L, Hughes A, Love S, Kehoe PG, Wilcock GK. Neither sequence variation in the IL-10 gene promoter nor presence of IL-10 protein in the cerebral cortex is associated with Alzheimer’s disease. Neurosci Lett. 2006;408:141–145. doi: 10.1016/j.neulet.2006.08.068. [DOI] [PubMed] [Google Scholar]
- de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and gamma-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Du Y, Muller U, Kurz A, Zimmer R, Riemenschneider M, Gasser T, Oertel WH, Klockgether T, Dodel RC. Lack of association of interleukin-10 promoter region polymorphisms with Alzheimer’s disease. Neurosci Lett. 2003;342:132–134. doi: 10.1016/s0304-3940(03)00231-3. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- Gandy S, Heppner FL. Microglia as dynamic and essential components of the amyloid hypothesis. Neuron. 2013;78:575–577. doi: 10.1016/j.neuron.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabeh GH, De Baetselier P, Brys L, Noel W, Van Ginderachter JA, Meerschaut S, Beschin A, Brombacher F, Raes G. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–583. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, et al. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot-Sestier MV, Town T. Innate Immunity in Alzheimer’s Disease: A Complex Affair. CNS Neurol Disord Drug Targets. 2013 doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Herber DL, Roth LM, Wilson D, Wilson N, Mason JE, Morgan D, Gordon MN. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Kiliaan AJ. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis. 2009;33:482–498. doi: 10.1016/j.nbd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Giardino L, Sivilia S, Giuliani A, Gusciglio M, Pietrini V, Del Giudice E, D’Arrigo A, Leon A, Villetti G, et al. CHF5074, a novel gamma-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20:159–173. doi: 10.3233/JAD-2010-1366. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci. 2012;32:16458–16465. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotzer AR, Pike CJ, Cotman CW. beta-Amyloid peptides induce degeneration of cultured rat microglia. Brain Res. 1993;624:121–125. doi: 10.1016/0006-8993(93)90068-x. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H, Heppner FL. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Breve JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, Tilders FJ, Van Dam AM. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci. 2002;16:1175–1185. doi: 10.1046/j.1460-9568.2002.02200.x. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Lio D, Licastro F, Scola L, Chiappelli M, Grimaldi LM, Crivello A, Colonna-Romano G, Candore G, Franceschi C, Caruso C. Interleukin-10 promoter polymorphism in sporadic Alzheimer’s disease. Genes Immun. 2003;4:234–238. doi: 10.1038/sj.gene.6363964. [DOI] [PubMed] [Google Scholar]
- Loewenbrueck KF, Tigno-Aranjuez JT, Boehm BO, Lehmann PV, Tary-Lehmann M. Th1 responses to beta-amyloid in young humans convert to regulatory IL-10 responses in Down syndrome and Alzheimer’s disease. Neurobiol Aging. 2010;31:1732–1742. doi: 10.1016/j.neurobiolaging.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia. 2008;56:1048–1060. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Ma SL, Tang NL, Lam LC, Chiu HF. The association between promoter polymorphism of the interleukin-10 gene and Alzheimer’s disease. Neurobiol Aging. 2005;26:1005–1010. doi: 10.1016/j.neurobiolaging.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Zimin PI, Wulff H, Jin LW. Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J Biol Chem. 2011;286:3693–3706. doi: 10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett. 1989;107:341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, et al. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- O’Leary TP, Brown RE. Visuo-spatial learning and memory deficits on the Barnes maze in the 16-month-old APPswe/PS1dE9 mouse model of Alzheimer’s disease. Behav Brain Res. 2009;201:120–127. doi: 10.1016/j.bbr.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Ramos EM, Lin MT, Larson EB, Maezawa I, Tseng LH, Edwards KL, Schellenberg GD, Hansen JA, Kukull WA, Jin LW. Tumor necrosis factor alpha and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Arch Neurol. 2006;63:1165–1169. doi: 10.1001/archneur.63.8.1165. [DOI] [PubMed] [Google Scholar]
- Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scassellati C, Zanardini R, Squitti R, Bocchio-Chiavetto L, Bonvicini C, Binetti G, Zanetti O, Cassetta E, Gennarelli M. Promoter haplotypes of interleukin-10 gene and sporadic Alzheimer’s disease. Neurosci Lett. 2004;356:119–122. doi: 10.1016/j.neulet.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolander J, Westermark GT, Renstrom E, Blom AM. Islet amyloid polypeptide triggers limited complement activation and binds complement inhibitor C4b–binding protein, which enhances fibril formation. J Biol Chem. 2012;287:10824–10833. doi: 10.1074/jbc.M111.244285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci. 2010;30:14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Mori T, Wu Y, Saxe M, Crawford F, Mullan M. CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J Neurosci. 2000;20:7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- Trouw LA, Nielsen HM, Minthon L, Londos E, Landberg G, Veerhuis R, Janciauskiene S, Blom AM. C4b–binding protein in Alzheimer’s disease: binding to Abeta1-42 and to dead cells. Mol Immunol. 2008;45:3649–3660. doi: 10.1016/j.molimm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Peng K, Lessig S, Estrella J, Adame A, Galasko D, Salmon DP, Hansen LA, Kawas CH, Masliah E. Neuropathology of dementia with Lewy bodies in advanced age: a comparison with Alzheimer disease. Neurosci Lett. 2010;485:222–227. doi: 10.1016/j.neulet.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural P, Degirmencioglu S, Parildar-Karpuzoglu H, Dogru-Abbasoglu S, Hanagasi HA, Karadag B, Gurvit H, Emre M, Uysal M. The combinations of TNFalpha-308 and IL-6 −174 or IL-10 −1082 genes polymorphisms suggest an association with susceptibility to sporadic late-onset Alzheimer’s disease. Acta Neurol Scand. 2009;120:396–401. doi: 10.1111/j.1600-0404.2009.01230.x. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Van Eldik LJ. Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimers Res Ther. 2013;5:28. doi: 10.1186/alzrt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz TM, Town T. Microglia in Alzheimer’s Disease: It’s All About Context. Int J Alzheimers Dis. 2012;2012:314185. doi: 10.1155/2012/314185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol Dis. 2004;15:11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation--a continuing puzzle. Immunology. 2004;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, et al. CD45 deficiency drives amyloid-beta peptide oligomers and neuronal loss in Alzheimer’s disease mice. J Neurosci. 2011;31:1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotova E, Holmes C, Johnston D, Neal JW, Nicoll JA, Boche D. Microglial alterations in human Alzheimer’s disease following Abeta42 immunization. Neuropathol Appl Neurobiol. 2011;37:513–524. doi: 10.1111/j.1365-2990.2010.01156.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.