Abstract
Growth differentiation factor 9 (GDF9), an oocyte-secreted factor, whose receptors exist in granulosa cells, is involved in follicle progression. Therefore, GDF9 is considered to potentially mediate signals necessary for follicular growth. However, the effect of GDF9 on human granulosa cells is not fully understood. Human immortalized nonluteinized granulosa cell line (HGrC1) which we have previously reported was stimulated with GDF9 and/or follicle-stimulating hormone (FSH). Granulosa cells obtained from in vitro fertilization (IVF) patients were also evaluated with quantitative reverse transcription polymerase chain reaction (RT-PCR). Real-time RT-PCR showed that GDF9 increased messenger RNA (mRNA) levels of enzymes required for cholesterol biosynthesis, such as 3-hydroxy-3-methylglutanyl-CoA synthase 1 (HMGCS1), farnesyl-diphosphate farnesyltransferase 1, squalene epoxidase, lanosterol synthase, and cytochrome P450, family 51, subfamily A, polypeptide 1 (CYP51A1). A greater increase in mRNA levels of HMGCS1 and CYP51A1 was observed by combined treatment with GDF9 and FSH. Clinical samples showed a significant increase in CYP51A1 mRNA in the group of granulosa cells connected with unfertilized oocytes. Our results suggest that GDF9, possibly with FSH, may play significant roles in the regulation of cholesterol biosynthesis and the expression of CYP51A1 which might be a predictor for unfertilization.
Keywords: cholesterol synthesis pathway, CYP51A1, fertilization, GDF9, granulosa cell
Introduction
Bidirectional communication between an oocyte and surrounding somatic cells such as granulosa cells is essential for follicular growth.1 Endocrine, autocrine, and paracrine regulatory factors coordinate the precise development of follicular cells.2 Growth differentiation factor 9 (GDF9) is one of the key factors in signaling from oocytes to granulosa cells. Growth differentiation factor 9 plays a critical role in promoting follicle growth: mice with null mutations in the gdf9 gene are infertile with follicle development arrested at the primary stage.3,4 In folliculogenesis, follicle-stimulating hormone (FSH) is another key factor. Follicle-stimulating hormone induces the proliferation of granulosa cells and upregulates the expression of aromatase and luteinizing hormone receptor in granulosa cells, which are indispensable incidents for follicle growth.5 However, GDF9 and FSH have a wide range of action on granulosa cells and folliculogenesis, which have not been fully revealed.
Growth differentiation factor 9 has been demonstrated to stimulate the cholesterol synthesis pathway in mouse granulosa cells.6 Cholesterol is required to ova before and after fertilization. Some intermediate products of cholesterol biosynthesis produced by granulosa cells are also reported to be involved in oocyte growth. Follicular fluid-derived meiosis-activating sterol (FF-MAS), an intermediary product of cholesterol biosynthesis, was named for its ability to reinitiate meiosis in mammalian oocytes.7,8 Lansterol 14-α demethylase (CYP51A1), which produces FF-MAS through its catalytic activity,9 has been reported to be upregulated by FSH in mouse and porcine granulosa cells.10,11 Therefore, it would be of interest to explore the cholesterol synthesis pathway, especially CYP51A1, in human granulosa cells in association with follicle development and fertilization. However, most of the studies regarding cholesterol biosynthesis and in granulosa cells have been limited in animal experiments because of the limited access to human materials including follicles and granulosa cells.
We therefore established a human immortalized nonluteinized granulosa cell line (HGrC1), which we have previously reported.12 The HGrC1 cells, originally derived from mural granulosa cells, show the expression of FSH receptor and responsiveness to the transforming growth factor (TGF)-β superfamily and FSH, retaining the original granulosa cell character and function. The HGrC1 cells are now being utilized for the gene expression profiling and functional analysis of human granulosa cells. We herein report that the cholesterol biosynthesis pathway discovered in the expression profiling as a screening test is upregulated by GDF9 and FSH in HGrC1 cells. We also quantified the expression levels of enzymes involved in the cholesterol biosynthesis pathway in primary human mural granulosa cells obtained from in vitro fertilization (IVF) patients and compared these levels between fertilized and nonfertilized oocytes.
Materials and Methods
Culture of Immortalized Human Granulosa Cells
The immortalized human granulosa cell line, HGrC1, originally derived from mural granulosa cells, has been proven to possess the characteristics of granulosa cells, including characteristic responses to GDF9 and FSH stimulation. Growth differentiation factor 9-stimulated phosphorylation of SMAD 2/3 was induced in HGrC1 cells. Follicle-stimulating hormone receptor activity was induced with activin and FSH. Stimulation with FSH resulted in increased transcription of aromatase messenger RNA (mRNA) and subsequent elevation in estradiol production.12 The HGrC1 cells were cultured in Dulbecco Modified Eagle Medium (DMEM; Sigma, St Louis, Missouri) containing 10% fetal bovine serum (FCS; Sigma), 100 IU/mL of penicillin, 100 μg/mL of streptomycin, and 25 mg/L of amphotericin B. The HGrC1 cells were seeded onto 6-well multidishes using the Nunclon DELTA Surface (Nunclon, Roskilde, Denmark). The HGrC1 cells were first cultured with 4% charcoal-filtered FCS followed by another 24 hours of serum starvation. The cells were then stimulated for 48 hours with 2 μg/mL of GDF9 and/or 5 IU/mL of FSH.
RNA Extraction, Microarray, and Real-Time Reverse Transcription Polymerase Chain Reaction
RNA was isolated from HGrC1 cells using an RNeasy Mini lit (QIAGEN Inc, Valencia, California) following the manufacture’s protocol. The preliminary transcriptional microarray for screening purpose using GDF9-stimulated HGrC1 cells compared to its nonstimulated control was assessed to confirm the expression and upregulation of cholesterol synthesis-related enzymes before quantitative reverse transcription polymerase chain reaction (RT-PCR). Reverse transcription reactions with 1 μg of total RNA were performed with a first-strand cDNA synthesis kit (ReverTra Ace α; Toyobo Co, Ltd, Osaka, Japan). Thereafter, real-time RT-PCR was performed in 96-well 0.2-mL thin-wall PCR plates using the Thermal Cycler Dice (Takara Bio Inc, Tokyo, Japan) and SYBR Premix Ex Taq II (Takara Bio Inc). The real-time RT-PCR mixture contained 2× SYBR Premix Ex Taq II, 10 μmol/L PCR primers for 3-hydroxy-3-methylglutanyl-CoA synthase 1 (HMGCS1), sterol-C4-methyl oxidase-like (SC4MOL), farnesyl-diphosphate farnesyltransferase 1 (FDFT1), 7-dehydrocholesterol reductase (DHCR7), 3-hydroxyl-3-methylglutanyl-CoA reductase (HMGCR), squalene epoxidase (SQLE), mevalonate kinase (MVK), lanosterol synthase (LSS), cytochrome P450, family 51, subfamily A, polypeptide 1 (CYP51A1), nicotinamide adenine dinucleotide phosphate-dependent steroid dehydrogenase-like (NSDHL), farnesyl diphosphate synthase (FDPS) and 2 μL of complementary DNA in a total volume of 25 μL. The sequences of primers are listed in Table 1. Commercial primers were selected for all genes from Takara Bio Inc (Tokyo, Japan) except for CYP51A1 designed according to Sato et al, which was not available commercially.13 All primers except for HMGCS1 and HMGCR are transintronic. The PCR profile included an initial incubation at 95°C for 30 seconds followed by 40 cycles of denaturation at 95°C for 5 seconds and annealing and extension at 60°C for 30 seconds. Real-time RT-PCR was performed in triplicate for all samples. A single peak was confirmed in the dissociation curve to eliminate the possibility of nonspecific amplification or primer–dimer formation. All PCR products were sequenced and confirmed for appropriate amplification. Quantification was performed by calculating the ratio to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA using the standard curve generated from 10-fold dilution series.
Table 1.
Primer Sets Used for Real-Time RT-PCR.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| HMGCS1 | AAGTCCAGGCCAGCAGTGA | ATATTCACAGCTCCTGAATGTACCA |
| SC4MOL | GCATGGGTGACCATTCGTTTATTAG | CCCACCATGTAAATGTTGAAGCA |
| FDFT1 | AGAATATTGACTTGGCCGTGCAG | GGCAGCCAAAGTGGCAATG |
| DHCR7 | GGAGCTCCACAGCCATGTGA | TTCAGGTACCAGGTTTCGTTCCA |
| HMGCR | CTTGCTTGCCGAGCCTAATGA | ACTAGGCACAGTTCTAGGGCCATTC |
| SQLE | TTGTGATGGGAGTTCAGTACAAGGA | GCCCATCTGCAACAACAGTCA |
| MVK | GGAGCTAATTAACAAGTGGGCCTTC | GTGTTGGTCAGCAGGATCTGGA |
| LSS | GAGGGTTCGCCACCTATGAGA | GAAATACTTAAGCGCCTGCATCAC |
| CYP51A1 | CTACAGTCGCCTGACAACAC | CCACTTTCTCCCCAACTCTC |
| NSDHL | CCTATGCCATGAAACCCATTGAC | ACGAACTTCATCTTGCCGTTCC |
| FDPS | GCATGTATCTACCGCCTGCTGA | TCCAGGGTCTGCCCAATCTC |
Abbreviations: HMGCS1, 3-hydroxy-3-methylglutanyl-CoA synthase 1; SC4MOL, sterol-C4-methyl oxidase-like; FDFT1, farnesyl-diphosphate farnesyltransferase 1; DHCR7, 7-dehydrocholesterol reductase; HMGCR, 3-hydroxyl-3-methylglutanyl-CoA reductase; SQLE, squalene epoxidase; MVK, mevalonate kinase; LSS, lanosterol synthase; CYP51A1, cytochrome P450, family 51, subfamily A, polypeptide 1; NSDHL, NAD(P)-dependent steroid dehydrogenase-like; FDPS, farnesyl diphosphate synthase; RT-PCR, reverse transcription polymerase chain reaction; NAD(P), nicotinamide adenine dinucleotide phosphate.
Immunoblotting for HMGCS and CYP51A1
Cultured HGrC1 cells were lysed in a radioimmunoprecipitation buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% Nonidet P-40, 5 mmol/L EDTA, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1.2% aprotinin, 5 μmol/L leupeptine, 4 μmol/L antipain, 1 mmol/L phenylmethylsulfonylfluoride, and 0.1 mmol/L Na3VO4). The cell lysates were clarified by centrifugation at 13 000g at 4°C for 15 minutes, diluted in 2× sample buffer (125 mmol/L Tris-HCl, pH 6.8, 4% SDS, 10% glycerol, 0.2% bromophenol blue, and 4% 2-mercaptoethanol), resolved by 10% SDS-polyacrylamide gel electrophoresis, and immunoblotted with anti-HMGCS antibody (Abgent, Inc, San Diego, California), anti-CYP51A1 antibody (Epitomics, Burlingame, California), or anti-β-actin antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, California).
Clinical Samples
Ovarian stimulation was performed as described previously.14 Briefly, 150 to 300 IU of urinary or recombinant FSH was administered daily under gonadotropin-releasing hormone antagonist or agonist protocols. When the follicles reached 16 mm or more in mean diameter, a 10 000 IU dose of human chorionic gonadotropin (Gonadotropin; ASKA pharmaceutical, Tokyo, Japan) was administered, and oocyte retrieval was performed 35.5 hours later. Human luteinized mural granulosa cells were collected from follicular fluid obtained via ultrasound-guided transvaginal oocyte retrieval, as described previously.15 Upon aspiration of each follicle, the oocyte obtained was identified with an individual number. At the same time, the amount of follicular fluid aspirated from that follicle was recorded in association with the individual oocyte number. These data represented the size of the follicle from which each oocyte was obtained. The cumulus cell–oocyte complex was isolated from the follicular fluid obtained. Subsequently, all oocytes were evaluated for their maturity by the same embryologist. Oocytes with a first polar body were classified as M2 oocyte.16 The luteinized mural granulosa cells included in each of the follicular fluid aspirated were separated from red and white blood cells using a Percoll gradient (Amersham Biosciences Corp, Piscataway, New Jersey) and were resuspended in DMEM containing 10% FCS, 100 IU/mL of penicillin, 100 μg/mL of streptomycin, and 25 mg/L of amphotericin and l-glutamine.17 These luteinized mural granulosa cells were stored according to each individual oocyte number. The M2 oocytes which subsequently underwent intracytoplasmic sperm injection (ICSI) were clinically followed to identify whether or not they were fertilized. Based on this outcome, the collected mural granulosa cells were retrospectively classified as either granulosa cells that supported fertilized oocytes (ie, granulosa cells connected with fertilized oocytes) or those that supported unfertilized oocytes (ie, granulosa cells connected with unfertilized oocytes). Twenty patients, who gave consent to this study, received ICSI from August 2009 to April 2011 at Nagoya University Hospital and resulted with at least 1 fertilized and 1 unfertilized oocytes, which were both retrieved and received ICSI on the same day, were recruited to this study. The fertilized and unfertilized oocytes were selected in random within each patient. Each accompanying granulosa cells were paired, resulting in 20 pairs of granulosa cells connected with fertilized oocytes and granulosa cells connected with unfertilized oocytes. These 20 pairs were subsequently evaluated for mRNA expression levels of CYP51A1 and SC4MOL by real-time RT-PCR similarly as described previously. This study was approved by the Ethics Committee of Nagoya University Graduate School of Medicine.
Statistical Analysis
The Students t-test was used to evaluate differences in each gene expression levels between GDF9-stimulated and nonstimulated control groups. One-way analysis of variance with the Bonferroni correction was used to determine the significance of difference in each gene expression levels among the 4 groups; control, FSH, GDF9, and GDF9- and FSH-stimulated groups. All statistical analyses were performed using SigmaPlot for Windows version 11.0 (Systat Software Inc, San Jose, California).
Results
Evaluation of Transcription Levels Using Real-Time PCR and Immunoblotting
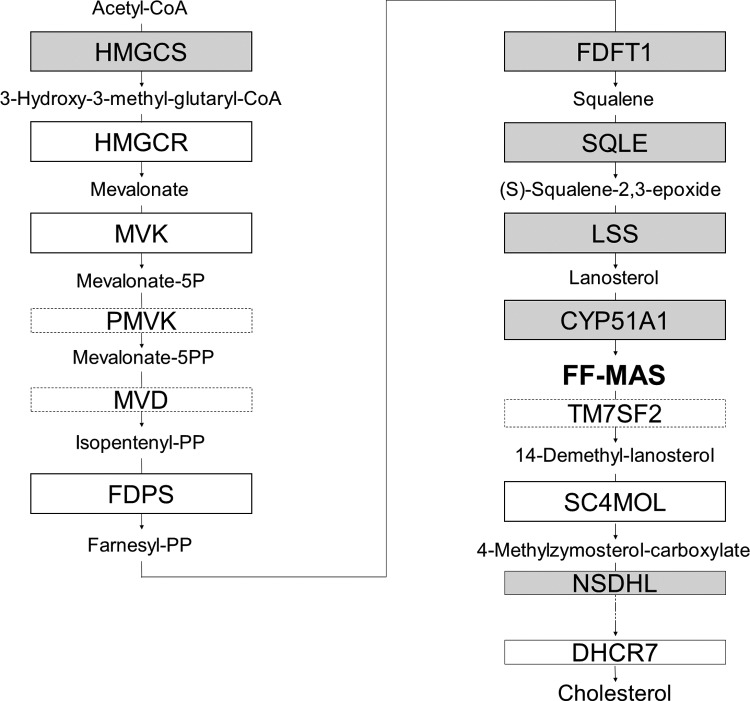
Target genes including enzymes in the cholesterol synthesis pathway were first identified based on a single preliminary screening gene chip experiment (Table 2). To validate this result, 3 sets of RNA obtained from HGrC1 cells independently stimulated with GDF9 with or without FSH for 48 hours were analyzed. A representative result of the 3 sets of real-time RT-PCR is shown in Figure 1. The quantitative changes in the genes were similar in the preliminary microarray and real-time RT-PCR analyses. The cholesterol biosynthesis pathway is responsible for de novo synthesis of cholesterol and many important intermediates. Real-time RT-PCR showed the 6 enzymes associated with cholesterol biosynthesis (HMGCS, FDFT1, SQLE, LSS, CYP51A1, and NSDHL) to be significantly upregulated following GDF9, FSH, or GDF9 + FSH stimulation. Enzymes upstream of CYP51A1 seemed to be particularly upregulated while those downstream seemed less changed. Combined effects of FSH with GDF9 on upregulation were seen in HMGCS and CYP51A1. This upregulation of HMGCS and CYP51A1 under stimulation of GDF9, FSH, or GDF9 + FSH was confirmed by immunoblotting (Figure 2).
Table 2.
Examples of Genes Upregulated in the Microarray Analysis by More Than 2.0-Fold Following GDF9 Stimulation.
| Gene Symbol | Gene Name | Entrez Gene ID | Net Intensity (ratio) |
|---|---|---|---|
| HMGCS1 | 3-Hydroxy-3-methylglutaryl-Coenzyme A synthase 1 | 3157 | 4.380 |
| SC4MOL | Sterol-C4-methyl oxidase-like | 6307 | 4.189 |
| FDFT1 | Farnesyl-diphosphate farnesyltransferase 1 | 2222 | 3.892 |
| DHCR7 | 7-Dehydrocholesterol reductase | 1717 | 3.107 |
| HMGCR | 3-Hydroxy-3-methylglutaryl-Coenzyme A reductase | 3156 | 3.073 |
| SQLE | Squalene epoxidase | 6713 | 2.963 |
| MVK | Mevalonate kinase | 4598 | 2.920 |
| LSS | Lanosterol synthase (2,3-oxidosqualene-lanosterol cyclase) | 4047 | 2.687 |
| CYP51A1 | Cytochrome P450, family 51, subfamily A, polypeptide 1 | 1595 | 2.679 |
| NSDHL | NAD(P) dependent steroid dehydrogenase-like | 50814 | 2.285 |
| FDPS | Farnesyl diphosphate synthase | 2224 | 2.199 |
Abbreviations: HMGCS1, 3-hydroxy-3-methylglutanyl-CoA synthase 1; SC4MOL, sterol-C4-methyl oxidase-like; FDFT1, farnesyl-diphosphate farnesyltransferase 1; DHCR7, 7-dehydrocholesterol reductase; HMGCR, 3-hydroxyl-3-methylglutanyl-CoA reductase; SQLE, squalene epoxidase; MVK, mevalonate kinase; LSS, lanosterol synthase; CYP51A1, cytochrome P450, family 51, subfamily A, polypeptide 1; NSDHL, NAD(P)-dependent steroid dehydrogenase-like; FDPS, farnesyl diphosphate synthase; GDF9, growth differentiation factor 9; NAD(P), nicotinamide adenine dinucleotide phosphate.
Figure 1.
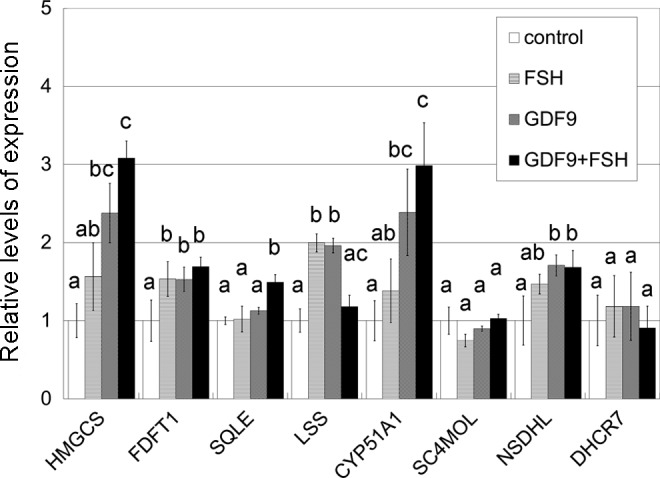
Real-time RT-PCR analysis of mRNA transcripts of HGrC1. HGrC1 was stimulated for 48 hours with 5 IU/mL FSH, 2 μg/mL GDF9, or both. Combined effects on upregulation were seen in HMGCS and CYP51A1. The data are presented as the mean ± SD of 3 independent replicates. Different letters show significant difference (P < .05). RT-PCR indicates reverse transcription polymerase chain reaction; mRNA, messenger RNA; FSH, follicle-stimulating hormone; HGrC1, human immortalized nonluteinized granulosa cell line; GDF9, growth differentiation factor 9; HMGCS, 3-hydroxy-3-methylglutanyl-CoA synthase 1; CYP51A1, cytochrome P450, family 51, subfamily A, polypeptide 1; SD, standard deviation.
Figure 2.
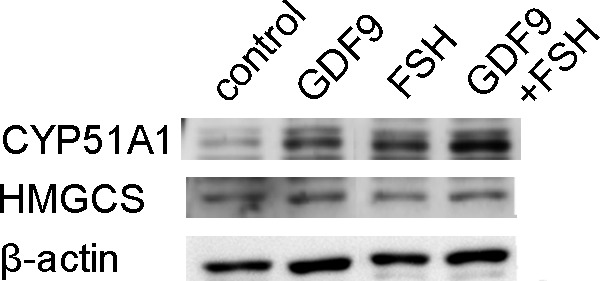
Immunoblotting for CYP51A1 and HMGCS. Representative images of immunoblotting against CYP51A1 (57 kDa), HMGCS (57 kDa), and β-actin (42 kDa) expressed by HGrC1. CYP51A1 was significantly induced by stimulation of cells with GDF9, FSH, or both for 48 hours in HGrC1 cells (upper panel). HMGCS was similarly induced by stimulation of cells with GDF9 or GDF-9 + FSH for 48 hours in HGrC1 cells (middle panel). HMGCS indicates 3-hydroxy-3-methylglutanyl-CoA synthase 1; CYP51A1, cytochrome P450, family 51, subfamily A, polypeptide 1; HGrC1, human immortalized nonluteinized granulosa cell line; GDF9, growth differentiation factor 9; FSH, follicle-stimulating hormone.
The Cholesterol Biosynthesis Pathway in Granulosa Cells Obtained From IVF Patients
The characteristics of the patients and the 20 pairs of granulosa cells connected with fertilized oocytes and unfertilized M2 oocytes are listed in Table 3. Patient age ranged from 31 to 43 years with a median of 37.5 years. The number of oocytes retrieved and fertilization rates ranged from 2 to 17 (median 10) and 10% to 83.3% (median 50.0%), respectively. Patient p was diagnosed with polycystic ovary syndrome according to the Rotterdam criteria.18 Patient j was a poor responder. Patient h had hyperlipidemia. Total sperm number ranged from 0.5 to 69.6 × 106/mL (median 14.6 × 106/mL). Sperm motility ranged from 8.8% to 78.1% (median 43.6%). There was no significant difference in the follicular fluid volume between the fertilized group and the unfertilized group (mean ± standard deviation; 3.755 ± 2.412 versus 2.650 ± 2.201 mL, P = .107, paired t test). Results of the cell line experiment showed that CYP51A1 was significantly upregulated with GDF9 and also showed additive effects with GDF9 and FSH. Because CYP51A1 is known to produce FF-MAS, we speculated that the expression levels of CYP51A1 may play a key role in the fertilization potential of the retrieved oocyte. We therefore studied the expressions of CYP51A1 as well as SC4MOL which exhibits catalytic activity upstream and downstream, respectively, of FF-MAS (Figure 3). Figure 4 shows the gene expression fold increase in the granulosa cells connected with unfertilized oocytes compared with that observed in the granulosa cells connected with unfertilized oocytes. Of 20 pairs, 15 showed increases in the expression of CYP51A1 in the granulosa cells connected with unfertilized oocytes compared to granulosa cells connected with fertilized oocytes. The difference in CYP51A1 levels was statistically significant between the granulosa cells connected with unfertilized oocytes and granulosa cells connected with fertilized oocytes (2.34-fold, 95% confidence interval: 1.35-3.34). No significant increases were seen in the SC4MOL expression.
Table 3.
Characteristics of the Patients and Comparison of “Unfertilized” Granulosa Cells to “Fertilized” Granulosa Cells.
| Patient Age, years | Number of Oocytes, n | Fertilization Rates, % | Follicular Fluid, mL | ||
|---|---|---|---|---|---|
| Fertilized | Unfertilized | ||||
| a | 34 | 12 | 75.0 | 2.0 | 2.0 |
| b | 37 | 9 | 33.3 | 4.0 | 7.0 |
| c | 36 | 10 | 44.4 | 6.5 | 8.0 |
| d | 42 | 10 | 10.0 | 4.3 | 1.5 |
| e | 42 | 15 | 72.7 | 2.0 | 1.0 |
| f | 42 | 6 | 83.3 | 8.0 | 3.0 |
| g | 39 | 8 | 80.0 | 8.0 | 2.0 |
| h | 39 | 8 | 50.0 | 5.5 | 3.0 |
| i | 33 | 10 | 22.2 | 7.0 | 2.0 |
| j | 37 | 2 | 50.0 | 3.0 | 1.5 |
| k | 43 | 6 | 60.0 | 2.3 | 1.5 |
| l | 33 | 4 | 66.7 | 2.5 | 1.0 |
| m | 42 | 10 | 10.0 | 6.0 | 1.0 |
| n | 40 | 7 | 71.4 | 1.0 | 1.0 |
| o | 31 | 16 | 33.3 | 1.0 | 2.0 |
| p | 34 | 17 | 64.7 | 1.5 | 7.5 |
| q | 38 | 17 | 33.3 | 0.5 | 1.0 |
| r | 33 | 10 | 71.4 | 1.5 | 2.0 |
| s | 35 | 16 | 38.5 | 3.5 | 1.8 |
| t | 39 | 7 | 71.4 | 5.0 | 3.2 |
Figure 3.
The cholesterol biosynthesis pathway. Stimulation of HGrC1 cells with GDF9, FSH, or GDF9 + FSH resulted in elevated mRNA levels in 6 enzymes along the cholesterol biosynthesis pathway. Solid line boxes show the enzymes of which mRNA levels were upregulated more than 2.0-fold in the microarray analysis. The enzymes in gray boxes were elevated with GDF9, FSH, or GDF9 + FSH stimulation, while those in white boxes were not changed or analyzed. The enzymes in dotted lined boxes were not evaluated either by microarray or RT-PCR. HGrC1 indicates human immortalized nonluteinized granulosa cell line; GDF9, growth differentiation factor 9; FSH, follicle-stimulating hormone; mRNA, messenger RNA; RT-PCR, reverse transcription polymerase chain reaction.
Figure 4.
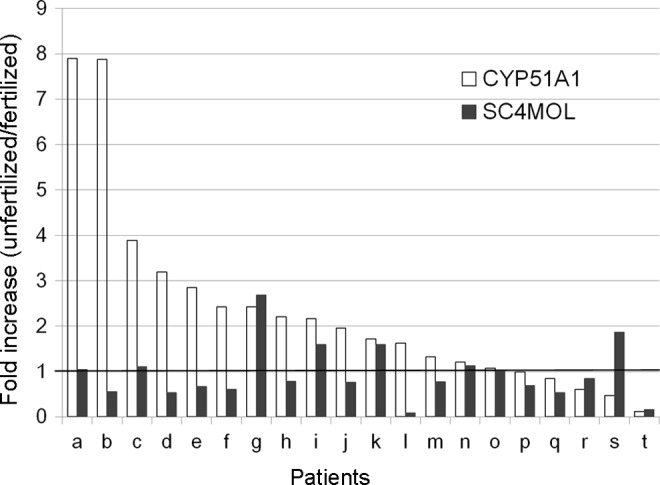
Relative expression levels of CYP51A1 and SC4MOL in granulosa cells. THE x-axis shows each patient (a-t). Fold increase of CYP51A1 and SC4MOL in the granulosa cells connected with unfertilized oocytes compared to the granulosa cells connected with fertilized oocytes was represented by white bars and black bars, respectively. Increase of CYP51A1 in the granulosa cells connected with unfertilized oocytes, not SC4MOL, was statistically significant (P = .004). CYP51A1 indicates cytochrome P450, family 51, subfamily A, polypeptide 1; SC4MOL, sterol-C4-methyl oxidase-like.
Discussion
In the animal research, biosynthesis of cholesterol has been demonstrated to be 1 example of metabolic cooperation between granulosa cells and oocytes.2 At least part of the cholesterol synthesized by granulosa cells has been shown to be transported to oocytes.6 Oocytes may benefit by this “outsourcing” of cholesterol biosynthesis by being protected from potentially harmful catabolic stress, which seems to be an evolutionarily conserved strategy in most animal species.19–22 This cooperative activity in cholesterol metabolism may be essential for the healthy status of both oocytes and granulosa cells, leading to a coordinated development of follicular cells.
Another possible objective for activating the cholesterol biosynthesis pathway is the production of FF-MAS. The FF-MAS, a cholesterol biosynthesis intermediate directly produced by CYP51A1, was first reported in as a meiosis stimulatory molecule in human follicular fluid.23 The ability of MAS to cause resumption of meiosis in oocytes was confirmed by several laboratories.24,25 Despite a large body of evidence supporting the meiosis-inducing capacity of FF-MAS, other reports suggest that FF-MAS might not be a universal mediator of hormone-induced meiotic maturation.24,26,27 The timing of CYP51 expression in the ovary was reported to be incompatible with its role in meiosis.28
However, as long as human folliculogenesis is concerned, it remained unrevealed whether the cholesterol synthesis pathway, FF-MAS and CYP51, is similarly involved in the metabolic cooperation between granulosa cells and oocytes. The HGrC1 cells show efficient responses to regulatory factors including members of the TGF-β superfamily and FSH.12 By utilizing HGrC1 cells, which retain the characteristics of nonluteinized human granulosa cells, we were able to investigate periovulatory granulosa cells in this study. The results of the microarray and real-time PCR analyses demonstrated that stimulation with GDF9 for 48 hours upregulates the transcription of the enzymes required for cholesterol biosynthesis. The cholesterol synthesis pathway and related enzymes are shown in Figure 3. This upregulation seemed more prominent up until CYP51A1. We then examined the additive effects of GDF9 and FSH on HGrC1 cells. We treated HGrC1 with combined stimulation of GDF9 and FSH for 48 hours. The importance of our findings lies in that HMGCS and CYP51A1 were persistently elevated and exhibited combined effects of GDF9 and FSH. Taken together, we speculate that our results showing effects of FSH and GDF9 exclusively in CYP51A1 and HMGCS indicate that the primary role of these upregulations may not only be to synthesize cholesterol but also to produce MAS in the human follicles.
After exploration for the enzymes in cholesterol synthesis pathway with in vitro experiments using the human granulosa cell line, we then looked whether their expression levels may serve as biomarkers of oocyte fertilization potential. The controversial reports over the role of FF-MAS lead us to wonder whether the expression levels of the enzymes upstream and downstream of FF-MAS could correlate with successful fertilization. Sperm concentrations and motility can greatly affect fertilization rates. Therefore, in order to account for the wide range of sperm qualities of our patient group, each granulosa cells connected with fertilized oocytes were paired with granulosa cells connected with unfertilized oocytes that were obtained from the same patient, where both of its oocytes received ICSI on the same day with the same sperm sample. Because the same sperm sample was used in each pair of granulosa cells connected with fertilized and unfertilized oocytes, we consider that the differences in gene expression between these 2 groups are due to the oocyte. The granulosa cells retrieved from IVF patients showed a significant increase in CYP51A1 mRNA transcription in the granulosa cells connected with unfertilized oocytes compared to that observed in the granulosa cells connected with fertilized oocytes. Of 20 pairs, 12 showed increased transcription of CYP51A1 of more than 1.5-fold in the granulosa cells connected with unfertilized oocytes. Because all oocytes recruited in this study were M2, the increase in CYP51A1 expression does not correlate with meiosis resumption. Neither did CYP51A1 expression correlate with follicular size.
Granulosa-derived cholesterol and some intermediate products of cholesterol biosynthesis are reported to be involved in oocyte growth and maturation during follicular development.7,8 Therefore, it seems to be opposite to the expectation that the expression levels of CYP51A1 had a tendency to be higher in the granulosa cells connected with unfertilized oocytes. Baltsen demonstrated that FF-MAS and cholesterol are differently regulated with gonadotropin stimulation in mouse ovaries.28 Based on this result, Baltsen described the speculation that the upregulation of the enzyme coded by CYP51A1 may result from lowered cholesterol availability and therefore, may not be likely to result in the accumulation of FF-MAS.28 Our speculation, similar to Baltsen’s, is that CYP51A1 might be upregulated in a negative feedback effect reflecting lowered cholesterol availability in follicles, which might be implicated in the lowered quality of oocytes. Although we did not examine FF-MAS levels in the follicular fluid, increased CYP51A1 might be a result of insufficient availability of FF-MAS and cholesterol. However, the present study is the first to find that CYP51A1 mRNA expression levels in the accompanying granulosa cells might predict successful oocyte fertilization. Further studies will be required to examine the correlation of CYP51A1 with fertilization and FF-MAS.
In conclusion, the cholesterol biosynthesis pathway was found to be upregulated following GDF9 stimulation in human granulosa cells. Combined effects were observed with GDF9 and FSH. CYP51A1, the key enzyme in producing FF-MAS, was found to be expressed differently between human granulosa cells derived from fertilized and unfertilized oocytes. The cholesterol biosynthesis elevated by GDF9 and FSH may therefore be involved in the achievement of coordinated normal growth of granulosa cells and oocytes and thus leading to the orderly maturation of follicles.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant-in-Aid for Scientific Research 24592468 to Akira Iwase from Japan Society for the Promotion of Science, Japan.
References
- 1. Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. [DOI] [PubMed] [Google Scholar]
- 2. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. [DOI] [PubMed] [Google Scholar]
- 4. Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13(6):1035–1048. [DOI] [PubMed] [Google Scholar]
- 5. Hillier SG, Harlow CR, Shaw HJ, Wickings EJ, Dixson AF, Hodges JK. Cellular aspects of pre-ovulatory folliculogenesis in primate ovaries. Hum Reprod. 1988;3(4):507–511. [DOI] [PubMed] [Google Scholar]
- 6. Su YQ, Sugiura K, Wigglesworth K, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–121. [DOI] [PubMed] [Google Scholar]
- 7. Grondahl C, Ottesen JL, Lessl M, et al. Meiosis-activating sterol promotes resumption of meiosis in mouse oocytes cultured in vitro in contrast to related oxysterols. Biol Reprod. 1998;58(5):1297–302. [DOI] [PubMed] [Google Scholar]
- 8. Rozman D, Cotman M, Frangez R. Lanosterol 14alpha-demethylase and MAS sterols in mammalian gametogenesis. Mol Cell Endocrinol. 2002;187(1-2):179–187. [DOI] [PubMed] [Google Scholar]
- 9. Cavilla JL, Kennedy CR, Baltsen M, Klentzeris LD, Byskov AG, Hartshorne GM. The effects of meiosis activating sterol on in-vitro maturation and fertilization of human oocytes from stimulated and unstimulated ovaries. Hum Reprod. 2001;16(3):547–555. [DOI] [PubMed] [Google Scholar]
- 10. Wang C, Xu B, Zhou B, et al. Reducing CYP51 inhibits follicle-stimulating hormone induced resumption of mouse oocyte meiosis in vitro. J Lipid Res. 2009;50(11):2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin S, Zhang M, Lei L, et al. Meiosis activating sterol (MAS) regulate FSH-induced meiotic resumption of cumulus cell-enclosed porcine oocytes via PKC pathway. Mol Cell Endocrinol. 2006;249(1-2):64–70. [DOI] [PubMed] [Google Scholar]
- 12. Bayasula Iwase A, Kiyono T, et al. Establishment of a human nonluteinized granulosa cell line that transitions from the gonadotropin-independent to the gonadotropin-dependent status. Endocrinology. 2012;153(6):2851–2860. [DOI] [PubMed] [Google Scholar]
- 13. Sato Y, Koshioka S, Kirino Y, et al. Role of dipeptidyl peptidase IV (DPP4) in the development of dyslipidemia: DPP4 contributes to the steroid metabolism pathway. Life Sci. 2011;88(1-2):43–49. [DOI] [PubMed] [Google Scholar]
- 14. Bayasula Iwase A, Kobayashi H, et al. A proteomic analysis of human follicular fluid: comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J Assist Reprod Genet. 2013;30(9):1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwase A, Goto M, Harata T, et al. Insulin attenuates the insulin-like growth factor-I (IGF-I)-Akt pathway, not IGF-I-extracellularly regulated kinase pathway, in luteinized granulosa cells with an increase in PTEN. J Clin Endocrinol Metab. 2009;94(6):2184–2191. [DOI] [PubMed] [Google Scholar]
- 16. Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci. 1988;541:259–274. [DOI] [PubMed] [Google Scholar]
- 17. Quinn MC, McGregor SB, Stanton JL, Hessian PA, Gillett WR, Green DP. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fertil Dev. 2006;18(5):501–508. [DOI] [PubMed] [Google Scholar]
- 18. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 19. Ito Y, Kihara M, Nakamura E, Yonezawa S, Yoshizaki N. Vitellogenin transport and yolk formation in the quail ovary. Zoolog Sci. 2003;20(6):717–726. [DOI] [PubMed] [Google Scholar]
- 20. Monaco ME, Villecco EI, Sanchez SS. Implication of gap junction coupling in amphibian vitellogenin uptake. Zygote. 2007;15(2):149–157. [DOI] [PubMed] [Google Scholar]
- 21. Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–287. [DOI] [PubMed] [Google Scholar]
- 22. Waksmonski SL, Woodruff RI. For uptake of yolk precursors, epithelial cell-oocyte gap junctional communication is required by insects representing six different orders. J Insect Physiol. 2002;48(6):667–675. [DOI] [PubMed] [Google Scholar]
- 23. Byskov AG, Andersen CY, Nordholm L, et al. Chemical structure of sterols that activate oocyte meiosis. Nature. 1995;374(6522):559–362. [DOI] [PubMed] [Google Scholar]
- 24. Downs SM, Ruan B, Schroepfer GJ., Jr Meiosis-activating sterol and the maturation of isolated mouse oocytes. Biol Reprod. 2001;64(1):80–89. [DOI] [PubMed] [Google Scholar]
- 25. Ruan B, Watanabe S, Eppig JJ, et al. Sterols affecting meiosis: novel chemical syntheses and the biological activity and spectral properties of the synthetic sterols. J Lipid Res. 1998;39(10):2005–2020. [PubMed] [Google Scholar]
- 26. Tsafriri A, Popliker M, Nahum R, Beyth Y. Effects of ketoconazole on ovulatory changes in the rat: implications on the role of a meiosis-activating sterol. Mol Hum Reprod. 1998;4(5):483–489. [DOI] [PubMed] [Google Scholar]
- 27. Vaknin KM, Lazar S, Popliker M, Tsafriri A. Role of meiosis-activating sterols in rat oocyte maturation: effects of specific inhibitors and changes in the expression of lanosterol 14alpha-demethylase during the preovulatory period. Biol Reprod. 2001;64(1):299–309. [DOI] [PubMed] [Google Scholar]
- 28. Baltsen M. Gonadotropin-induced accumulation of 4,4-dimethylsterols in mouse ovaries and its temporal relation to meiosis. Biol Reprod. 2001;65(6):1743–1750. [DOI] [PubMed] [Google Scholar]