Abstract
Endometrial stromal and epithelial cell cross talk is known to influence many of the dynamic changes that occur during the menstrual cycle. We modified our previous model and embedded telomerase-immortalized human endometrial stromal cells and Ishikawa adenocarcinoma epithelial cells in a collagen–Matrigel hydrogel to create a tissue-engineered model of the endometrium. Comparisons of single and cocultured cells examined communication between endometrial stromal and epithelial cells, which were cultured with 0 or 10 nmol/L 17β estradiol; conditioned medium was used to look at the production of paracrine factors. Using this model, we were able to identify the changes in interleukin 6 (IL-6) and active matrix metalloproteinase 2, which appear to be due to paracrine signaling and differences in transforming growth factor β1 (TGF-β1) that do not appear to be due to paracrine signaling. Moreover, IL-6, TGF-β1, and DNA content were also affected by the presence of estradiol in many of the tissues. These results indicate that paracrine and endocrine signaling are involved in human endometrial responses and support the use of coculture models to further investigate cell–cell and cell–matrix interactions.
Keywords: coculture, endometrial cells, protease activity, IL-6, proliferative phase
Introduction
Endometrial stromal and epithelial cell interactions undergo phasic changes across the reproductive cycle. Following estradiol and progesterone withdrawal at the onset of menstruation, epithelial cells produce interleukin 1α (IL-1α) and stimulate the secretion of matrix metalloproteinase 1 (MMP-1) by the stromal cells.1,2 Similarly, stromal–epithelial cross talk influences endometrial decidualization, receptivity, and trophoblast attachment.3–5 Communication between stromal and epithelial cells also mediates steroid regulation of MMP-2, -7, and -9 expression6–8 and a myriad of other ovarian steroid effects.9–12 We hypothesized that stromal–epithelial communication might play a key role in regulating cytokine levels and protease activity during the proliferative phase, particularly during reepithelialization of the endometrium.
The most common strategy employed to test stromal–epithelial cell communication is via the use of transwell chamber systems that separate the cell types and allow for paracrine signals to be exchanged.2,5,7,10,12 We wanted to develop a more physiologically relevant model that would allow for contact between cell types and extracellular matrix. Engineered tissues have been shown to yield results that more closely mimic the in vivo condition than traditional monolayer culture.13–18 Tissue engineering replicates in vivo relationships where cells interact with the extracellular matrix and each other. In order to study the stromal–epithelial effects that occur during the proliferative phase of the menstrual cycle, we have modified a hormonally responsive, tissue-engineered human stroma model that we described previously.19 We refined the model by creating a more representative collagen–Matrigel matrix and by the addition of an endometrial epithelial cell line.
The menstrual cycle, including its regenerative proliferative phase, is mostly unique to women and old world primates.20 The endometrial lining must be regenerated cyclically following the localized inflammation and desquamation that occur with each menses. The onset of menstruation is triggered by a significant drop in estrogen and progesterone levels leading to vasoconstriction, leukocyte chemotaxis, MMP activation, and tissue degradation.21 At the cessation of menstruation, leukocyte infiltration abates, protease activity decreases, and the denuded epithelium and stroma reorganize and proliferate to restore a new receptive mucosa.22,23 The proliferative phase is dominated by estradiol that has mitogenic effects on both stromal and epithelial cells24,25; however, the role of estradiol is not completely understood.
In order to study the proliferative phase, we chose to look at the effects of coculture on secretion of the cytokines IL-6 and transforming growth factor β1 (TGF-β1) and protease activity of MMP-2 and -9. Interleukin 6 is an inflammatory cytokine that plays a critical role in wound healing26–29 and is therefore of interest during the regenerative proliferative phase. Estradiol has been shown to downregulate IL-6 in many cell types,30–34 and it may mitigate the inflammation seen during the proliferative phase of the cycle. TGF-β1 is a pleiotropic cytokine that has been shown to affect inflammation, cell proliferation, angiogenesis, and extracellular matrix regulation.35–38 Matrix metalloproteinases are the primary proteinases involved in tissue breakdown during menstruation.21,39–41 We found that MMP-2 activity was elevated following steroid withdrawal in our engineered tissue, with little activity under secretory phase mimetic conditions.19 These cytokines and proteases are regulated throughout the menstrual cycle. During the proliferative phase, changes in production or activation may be influenced by cross talk between the stromal and epithelial cells that are repopulating the endometrium.
Materials and Methods
Materials
Unless otherwise noted, the cell culture reagents were purchased from Cellgro (Manassas, Virginia), chemicals were purchased from Sigma Life Sciences (St Louis, Missouri), and gel electrophoresis and zymography materials from BioRad (Hercules, California). All enzyme-linked immunosorbent assay (ELISA) kits were from R&D Systems (Minneapolis, Minnesota). Bovine dermal collagen I was purchased from MP Biomedicals LLC (Solon, Ohio) and Matrigel from BD Biosciences (Bedford, Massachusetts).
Engineered Tissue Creation and Culture
Human telomerase-immortalized endometrial stromal cells42 were generously donated by Drs. Charles Lockwood and Graciela Krikun (Yale University, New Haven, Connecticut), and Ishikawa endometrial adenocarcinoma cells were the kind gift of Drs. Erlio Gurpide and Fred Schatz (New York University, New York, New York). Frozen aliquots of these cells were thawed and used in this study to provide replenishable and consistent sources of endometrial stromal and epithelial cells, respectively. A tissue-engineered endometrium was created using a modified version of our previously published method.19 Collagen I hydrogels were made as described, yielding a final collagen concentration of 2 mg/mL. In addition, Matrigel was added to the mixture for a final concentration of 0.5 mg/mL. Stromal and epithelial cells were seeded separately or together into the hydrogel solution at concentrations of 2 × 106 cells/mL and 1 × 106 cells/mL, respectively. The hydrogel–cell solution was placed into 12-well dish molds and incubated at 37°C for 1 hour. After the solutions gelled, the disk-shaped hydrogels were transferred to 6-well dishes containing complete culture medium consisting of Dulbecco modified eagle medium (DMEM)–F12 50:50 supplemented with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, l-glutamine, sodium pyruvate, nonessential amino acids, 1% penicillin–streptomycin, and 10% fetal bovine serum. The hydrogels were incubated in the culture medium for 2 days to allow for collagen compaction by the stromal cells; epithelial cells do not compact the gels.
Proliferative Phase Mimetic Tissue Culture Conditions
The contracted gels were washed with phosphate-buffered saline (PBS) and incubated for 3 days in phenol red-free DMEM–F12 50:50 with HEPES buffer, l-glutamine, sodium pyruvate, nonessential amino acids, 50 μg/mL l-ascorbic acid 2-phosphate sesquimagnesium salt, 10% charcoal-stripped fetal calf serum (Hyclone Laboratories, Inc, Logan, Utah), 1% penicillin–streptomycin, 1% sodium pyruvate, and either 0 or 10 nmol/L 17β-estradiol. One half of the medium was changed on day 2. Conditioned media from the 10 nmol/L estradiol cultures were spun down to remove cells and stored at -80°C for use in conditioned medium experiments; estradiol and ascorbic acid were supplemented to account for depletion. Hormone-free controls were cultured in an identical manner minus the addition of estradiol.
Sample Preparation
After 3 days of culture, the medium and hydrogels were prepared and stored for future analysis. Conditioned medium aliquots were kept frozen at -80°C prior to use. Hydrogels were either fixed in 4% paraformaldehyde for imaging or placed in a modified RIPA lysis buffer43 containing 20 mmol/L Tris-HCl, 5 mmol/L EGTA, 150 mmol/L NaCl, 20 mmol/L glycerol-phosphate, 10 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1% Triton X-100, and 0.1% Tween 20 and freshly added 0.1 mmol/L leupeptin. The hydrogels were homogenized via sonication and centrifuged at 14 000g for 10 minutes to remove insoluble material. The supernatant was removed and stored at -80°C. The DNA content of the lysates was determined by Hoechst dye assay with calf thymus DNA serving as the standard curve.19 The protein contents of the homogenates and medium were determined by the BCA method (Thermo Fisher Scientific Inc, Rockford, Illinois).
Immunohistochemistry
After culture, some of the engineered tissue was rinsed in PBS and fixed in 4% paraformaldehyde overnight at room temperature. The tissue was paraffin embedded, and 7-µm sections were cut. The sections were deparaffinized, and antigen retrieval was completed using a combination of proteinase K digestion and citric acid treatment. The sections were incubated overnight at 4°C with the primary antibodies for cytokeratin (Sigma) and vimentin (Cell Signaling Technology Inc, Danvers, Massachusetts). Rhodamine and fluorescein isothiocyanate-conjugated secondary antibodies, respectively, were used for visualization. 4′,6-Diamidino-2-phenylindole (DAPI) was used to stain the DNA. A Zeiss Axioplan wide field microscope (Carl Zeiss Microscopy, Thornwood New York) was used for imaging.
Western Blotting
Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed using 10% polyacrylamide gels; equal protein content was loaded along with a molecular weight ladder. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane and blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) with 0.1% Tween-20. The membranes were incubated overnight with primary antibodies suspended in 5% BSA with TBS and 0.1% Tween-20. Antibodies to vimentin (Cell Signaling) at 1:1000 and cytokeratin (Sigma) at 1:400 dilutions were used. After rinsing with TBS, the membranes were incubated for 1 hour with horse radish peroxidase-labeled secondary antibodies and visualized by chemiluminescent detection using Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare, Pittsburgh, Pennsylvania).
Cytokine Quantitation
Interleukin 6 and TGF-β1 concentrations in the conditioned medium were determined by ELISA. To account for any differences in cell number, we normalized the total cytokine content (concentration × medium volume) to the total DNA content (DNA concentration × lysate volume) of the engineered tissue.
Gelatin Zymography for MMP-2 and -9
Matrix metalloproteinase gelatin zymography was used to compare MMP-2 and MMP-9 activities. Equal amounts of protein from each tissue supernatant were loaded in a 10% gelatin zymogram polyacrylamide gel. After electrophoresis, the gels were rinsed for 30 minutes at room temperature with commercial renaturing buffer and then developing buffer. The gels were incubated at 37°C overnight followed by staining with colloidal blue (Invitrogen, Carlsbad, California).
Statistical Analysis
Data are presented as the mean ± standard error of the mean. Statistical significance was determined via 1-way analysis of variance with post hoc t tests using a 95% confidence interval. Bonferonni corrections were performed for multiple comparisons.
Results
DNA Quantification for Normalization
To understand changes in cell number and to normalize comparisons between cell types and among cocultures, DNA content of the homogenized tissue was measured by Hoechst dye assay (n = 3). Similar DNA contents per cell were justified based on the reports that the telomerase-immortalized stromal cells have a normal female karyotype42 and that even after >20 years in culture, a stable modal karyotype of the Ishikawa adenocarcinoma cell line (44-60 chromosomes) was noted.44 The DNA content per mL was multiplied by the sample volume to determine the total DNA content (Figure 1). This was an important control as endometrial stromal cells contract the engineered tissue, while the epithelial cells do not, resulting in different hydrogel volumes. After culture, there were no discernible differences in DNA content among the different cell combinations in the 0 nmol/L estradiol cultures. Estradiol of 10 nmol/L had a mitogenic effect on the epithelial tissues leading to significant increase in DNA content when compared to the 0 nmol/L estradiol epithelial cell culture. The 10 nmol/L estradiol-treated epithelial cells also had significantly higher DNA content than the 10 nmol/L estradiol stromal only and coculture tissues, which is expected, as these adenocarcinoma cells proliferate more rapidly in monolayer culture than the stromal cells. The final DNA content of the 10 nmol/L estradiol-treated coculture tissues was similar to that of the stromal-only tissues, suggesting a reduction in proliferation of 1 or both of the cell types. To look at potential paracrine effects in the presence of 10 nmol/L estradiol, conditioned medium was used. Culturing the stromal-only tissues in conditioned media from the epithelial cells had no effect on DNA content; there were no significant differences in DNA content compared to any of the other conditions. Culturing epithelial-only tissues in stromal-conditioned medium did lead to a decrease in DNA content similar to that of the coculture tissues, suggesting that paracrine factors are involved.
Figure 1.
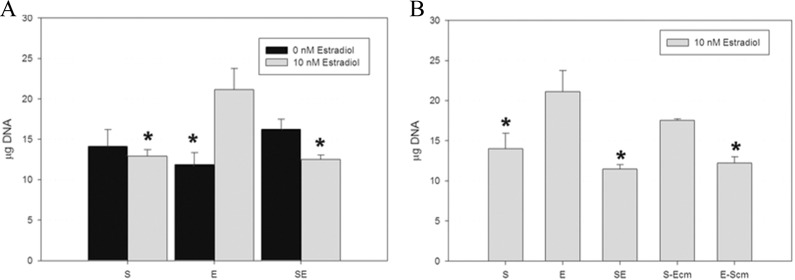
Effects of estradiol on DNA content of engineered tissues. DNA levels were measured using the Hoechst dye assay in cells that were cultured with 0 nmol/L estradiol (black bars) or 10nmol/L estradiol (gray bars, n = 3). A, In the absence of estradiol, there were no significant differences in DNA content between the stromal only (S), epithelial only (E), or cocultured (SE) tissue. In contrast, estradiol had a mitogenic effect on the epithelial cells leading to higher DNA content compared with these cells under 0 nmol/L estradiol conditions as well as compared with estradiol-treated S and SE tissues. B, Conditioned medium was used to look at potential paracrine factors in the estradiol-treated cultures. The addition of epithelial cell conditioned medium to the stromal tissues (S-Ecm) had no significant effect on DNA levels compared to any other conditions. The addition of stromal conditioned medium to the epithelial tissues (E-Scm) resulted in a decrease in DNA content, similar to that of the SE cocultures. * denotes P < .05 compared to epithelial cells cultured with 10 nmol/L estradiol.
Immunohistochemistry, Western Blotting, and Cell Composition
After culture, the tissue was examined by immunohistochemistry and Western blotting to aid in understanding the final composition of the tissue (n = 3). Figure 2A shows an immunostained cross-section through one of the engineered cocultures exposed to 10 nmol/L estradiol. Both cell types were found throughout the tissue, although epithelial cells were found in much lower quantities within the tissue core. Epithelial cells were found more often on the outer layer of the tissue, but as they are not contact inhibited, they do not form a simple monolayer. Although gland-like structures were not observed over the course of the experiments, the peripheral edge of the tissues was both more cellular (as noted by high DAPI staining) and epithelial (as noted by high cytokeratin staining). Western blotting, shown in Figure 2B, demonstrates the characteristic microfilament proteins of the corresponding cell types present in the single cell and cocultured tissues. The effect of estradiol on the cell proliferation is evident by the changes in ratios of vimentin and cytokeratin in the cocultures.
Figure 2.
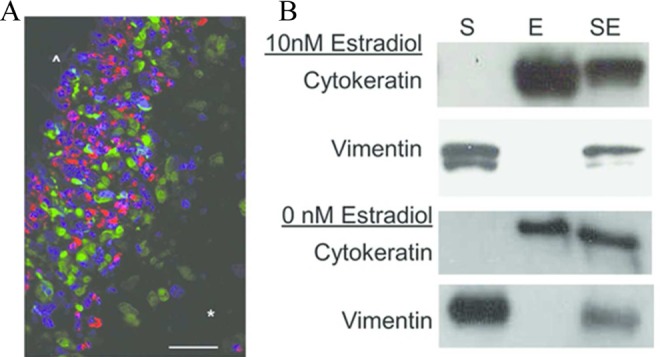
Immunohistochemistry and Western blotting were used to characterize the cellular composition of the cocultured tissues. A, A representative image of a tissue containing both cell types is shown. The scale bar indicates 50 μm. The epithelial cells are depicted in red (cytokeratin), and stromal cells are depicted in green (vimentin). The nuclei are stained blue with DAPI. The cells have proliferated on the outer edge of the tissue (^) leading to a cell dense and highly epithelial region. Within the tissue core (*), the cells are more diffuse, and there is a higher stromal to epithelial cell ratio. B, Western blotting for vimentin and cytokeratin in individual and cocultured (SE) cells allows estimation of the relative contributions of stromal (S) and epithelial (E) components, respectively.
Cytokine and Growth Factor Secretion
The levels of IL-6 and TGF-β1 secreted into the conditioned medium were determined by ELISA (Figure 3, n = 3). In both cultures with 0 and 10 nmol/L estradiol, stromal cells produced significantly higher levels of IL-6 than the epithelial cells, whereas coculture resulted in IL-6 levels similar to those of epithelial-only tissues (Figure 3A). The TGF-β1 was produced by both the stromal and the epithelial cells (Figure 3B); in the presence of 10 nmol/L estradiol, coculture resulted in a synergistic increase in TGF-β1 production.
Figure 3.
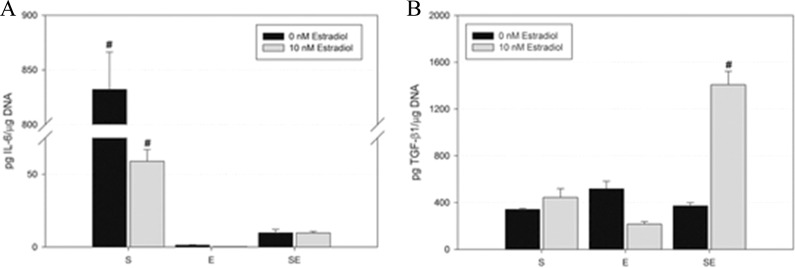
Effects of single cell types and coculture on cytokine and growth factor secretion in engineered tissue. Levels of IL-6 (A) and TGF-β1 (B) were measured in the conditioned medium (n = 3). Stromal tissues (S) produce significantly more IL-6 than epithelial tissues, with significantly more IL-6 production with 0 nmol/L estradiol than 10 nmol/L. Coculture (SE) significantly reduced the amount of IL-6 both with 0 and 10 nmol/L estradiol and increased the amount of TGF-β1 secreted in the presence of 10 nmol/L estradiol. # denotes P < .05 compared to all other conditions. IL-6 indicates interleukin 6; TGF-β1, transforming growth factor β1.
In order to determine whether paracrine factors were involved, stromal-only tissue was incubated with epithelial cell conditioned medium (Figure 4, n = 3). Stromal tissues cultured with epithelial conditioned medium produced significantly less IL-6. Since it was not clear which of the cell types was responsible for the increase in TGF-β1, we looked at the effects of both epithelial and stromal conditioned medium. Conditioned medium from the converse cell type did not result in a significant increase in TGF-β1 levels in either cell type.
Figure 4.
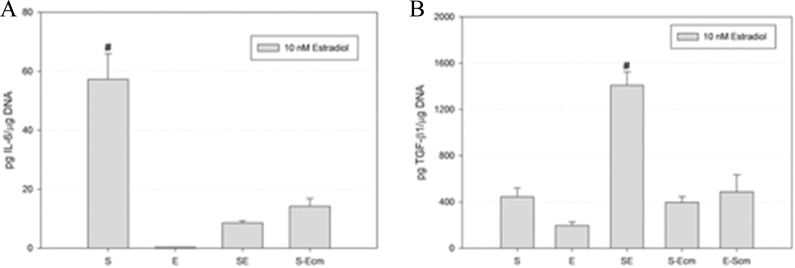
Effects of conditioned medium on cytokine and growth factor secretion in engineered tissue. Conditioned medium was used to explore paracrine factors in the proliferative phase mimetic cultures. Exposure of stromal tissue to epithelial cell conditioned medium (S-Ecm) caused a significant reduction in interleukin 6 (IL-6) and an increase in TGF-β1 relative to stromal tissue alone (S). Epithelial tissue exposed to stromal conditioned medium (E-Scm) did not result in significantly increased TGF-β1 relative to epithelial tissue (E). # denotes P < .05 compared to all other conditions. TGF-β1 indicates transforming growth factor β1.
MMP-2 and -9 Activities
In order to determine differences in the amount and activity of MMP-2 and MMP-9 within the tissue homogenates, traditional gelatin zymography was used (Figure 5, n = 3). Similar trends were seen in the conditioned medium (data not shown). Levels of proMMP-2 were consistent among the different tissue configurations. Mature MMP-2 was found almost exclusively in the stromal tissues. In the cultures both with and without 10 nmol/L estradiol, coculture of the stromal and epithelial cells significantly decreased mature MMP-2 activity (Figure 5A). To determine whether the decrease in activity was primarily due to paracrine signaling and not due to dilution of the stromal cells, the stromal tissue was incubated with epithelial cell conditioned medium. Exposure to the epithelial cell conditioned medium also resulted in a decrease in active MMP-2 (Figure 5B), similar to that found during coculture. The activity of MMP-9 was low and was not detectable in all samples. When MMP-9 activity was seen, it was found exclusively in the epithelial-only tissues (data not shown).
Figure 5.
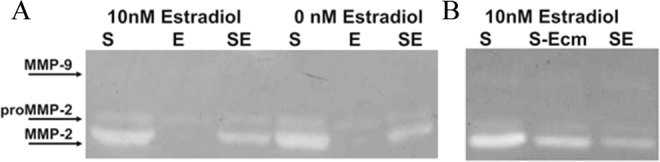
Representative gelatin zymograms are shown (n = 3). A, The effects of 0 or 10 nmol/L estradiol on MMP-2 and MMP-9 in individually or cocultured cells were studied. There were no noticeable differences in band intensity of proMMP-2 among the cultures. Stromal tissue (S) contained active MMP-2, while epithelial cells (E) produced little to no detectable active MMP-2. Reduced amounts of active MMP-2 were found in coculture (SE) relative to stromal tissue (S). B, To understand whether the reduction in activity was due to paracrine effects, stromal tissues were cultured with epithelial cell conditioned medium (S-Ecm). Epithelial cell conditioned medium also caused a reduction in active MMP-2 in stromal cultures. MMP indicates matrix metalloproteinase.
Discussion
The main goal of this work was to develop a more complex model for studying communication between endometrial stromal and epithelial cells. We chose to focus on the proliferative phase, a time of dynamic change in the endometrium when reepithelialization occurs. Our tissue-engineered model allowed the identification of several relevant effects on differential cytokine secretion and protease activity. With respect to stromal function in these 3-dimensional tissues, coculture with epithelial cells resulted in increased secretion of TGF-β1 but decreased IL-6 secretion and MMP-2 activity. These effects were anticipated based on in vivo findings during the proliferative phase, when there is a reduction in inflammation and tissue destruction by proteases. Using conditioned medium, we were able to determine that paracrine factors mediated most of these changes. We also identified changes in cell number that were not influenced by conditioned medium but are likely due to direct cell–cell communication. A limitation of our study is the use of immortalized rather than primary endometrial cells. By eliminating interpatient cell variability and passage limitations of primary cells, these cell lines allowed reliable availability of cells and consistency of responses. However, they may not completely recapitulate the interactions of normal cells. Ishikawa cells have reduced contact inhibition and a higher mitotic rate than the immortalized stromal cells, hence the cell ratios in coculture tissues change over time. Our treatment protocol was optimized to minimize this temporal confounder. Moreover, the selection of Ishikawa cells over other endometrial lines is justified based on their well-differentiated phenotype, steroid responsiveness, and a stable chromosomal complement.44 Moreover, this line has been validated as endometrial epithelium derived, in contrast to others that were recently found to be contaminants from other sources.45 The telomerase immortalized stromal cells42 are a well-characterized nonmalignant cell line that has been used to study decidualization and other progesterone-dependent events.19,46–48 There is some concern regarding the estrogen sensitivity of the cell line, as a previously reported study showed no significant differences between steroid-free and estradiol-treated cultures.49 Nevertheless, these cells have been shown to possess both estrogen receptors α and β,47 and our studies show differences in cytokine secretion and MMP activity induced by the presence of estradiol (10 nmol/L). We believe that the differences we see are influenced by the 3-dimensional culture, as we have previously shown that this model results in more physiologic results than 2-dimensional monolayer culture.19
Endocrine effects were seen in both single cell cultures and in the communication between cells in the cocultures. Estradiol had a mitogenic effect on the epithelial cells, as expected, but there was no significant change in the DNA content of the stromal tissues. This is likely due to the fact that more stromal cells can be found within the collagen–Matrigel matrix than epithelial cells, and collagen I is known to inhibit proliferation.50 Estradiol also resulted in a reduction in IL-6 in the stromal cultures, and a change in the coculture effects in TGF-β1 secretion. Different concentrations of estradiol would likely have different effects on these cytokines.
In terms of coculture effects on cytokine levels, the results are consistent with our hypothesis that cross talk occurring during endometrial reepithelialization reduces the inflammatory and proteolytic environment in the proliferative phase. Although TGF-β1 is a pleiotropic cytokine, it is often found to have anti-inflammatory effects that correlate with the decrease in IL-6. The IL-6 data for the individually cultured cells is also consistent with previously published work, where we showed that primary endometrial stromal cells produced significantly more IL-6 than primary endometrial epithelial cells51; however, our IL-6 results contrast with data published by Chen et al52 using primary endometrial stromal and epithelial cells cultured in a 2-chamber system to look at paracrine signaling. They showed that primary epithelial cells produced more IL-6 than primary stromal cells and that coculture resulted in a reduction in epithelial cell secretion of IL-6. It is possible that differences between our 2 studies reflect our choice of cells, the presence of collagen and Matrigel in our study, or possibly due to factors present in the different types and concentrations of sera in the media. The study by Chen et al used 1% FBS, whereas we used 10% charcoal-stripped serum.
Our study was designed to gain insights into the physiologic changes that occur in endometrial regeneration during the proliferative phase; however, the results have implications for the pathological condition of endometriosis. Coculture of epithelial with stromal cells resulted in alterations in the production of cytokine toward a less inflammatory environment and reduced protease activity. The data support the principle that endometriotic lesions composed predominantly of stromal cells would promote a more inflammatory and invasive phenotype than those containing more epithelial cells. The study of Mai et al53 provides in vivo evidence that an elevated stromal–epithelial cell ratio may play a role in the pathophysiology of endometriosis.
Our results correlate well with what is already known about the physiology of the menstrual cycle and with previous in vitro data, but we must remain reserved in the interpretation of experiments using an adenocarcinoma cell line. We postulate that normal endometrial epithelial cells also secrete anti-inflammatory and antiproteolytic factors, but a limitation of our model is the possible magnitude of effect using adenocarcinoma cells that are highly proliferative and lack contact inhibition. Nevertheless, we have been able to show that this model manifests endocrine and paracrine signaling and offers a new understanding of the cross talk between these cells. Further insights into the effects of and mechanisms behind stromal–epithelial cell communication on cytokine production and protease activity should be afforded by models using primary endometrial cells.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health as part of the Cooperative Research Partnerships to Promote Workforce Diversity in the Reproductive Sciences (U01 HD66439), the Specialized Cooperative Centers Program in Reproduction and Infertility Research (U54 HD55787), and NICHD/NIH (HD55379). Research reported in this publication was also supported in part by the Integrated Cellular Imaging (ICI) Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292.
References
- 1. Pretto CM, Gaide Chevronnay HP, Cornet PB, et al. Production of interleukin-1alpha by human endometrial stromal cells is triggered during menses and dysfunctional bleeding and is induced in culture by epithelial interleukin-1 alpha released upon ovarian steroids withdrawal. J Clin Endocrinol Metab. 2008;93 (10):4126–4134. [DOI] [PubMed] [Google Scholar]
- 2. Singer CF, Marbaix E, Kokorine I, et al. Paracrine stimulation of interstitial collagenase (MMP-1) in the human endometrium by interleukin 1alpha and its dual block by ovarian steroids. Proc Natl Acad Sci USA. 1997;94 (19):10341–10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang H, Bocca S, Anderson S, et al. Sex steroids regulate epithelial–stromal cell cross talk and trophoblast attachment invasion in a three-dimensional human endometrial culture system. Tissue Eng Part C Methods. 2013;19 (9):676–687. [DOI] [PubMed] [Google Scholar]
- 4. Evron A, Goldman S, Shalev E. Effect of primary human endometrial stromal cells on epithelial cell receptivity and protein expression is dependent on menstrual cycle stage. Hum Reprod. 2011;26 (1):176–190. [DOI] [PubMed] [Google Scholar]
- 5. Kim MR, Park DW, Lee JH, et al. Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol Hum Reprod. 2005;11 (11):801–808. [DOI] [PubMed] [Google Scholar]
- 6. Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, Matrisian LM. Stromal–epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci USA. 1994;91 (21):10129–10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruner KL, Rodgers WH, Gold LI, et al. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci USA. 1995;92 (16):7362–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H, Li M, Wang F, et al. Endometriotic epithelial cells induce MMPs expression in endometrial stromal cells via an NFkappaB-dependent pathway. Gynecol Endocrinol. 2010;26 (6):456–467. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Patterson AL, Teixeira JM, Pru JK. Endometrial stromal beta-catenin is required for steroid-dependent mesenchymal–epithelial cross talk and decidualization. Reprod Biol Endocrinol. 2012;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pierro E, Minici F, Alesiani O, et al. Stromal–epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biol Reprod. 2001;64 (3):831–838. [DOI] [PubMed] [Google Scholar]
- 11. Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial–stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28 (1):27–35. [DOI] [PubMed] [Google Scholar]
- 12. Qi XF, Nan ZC, Jin YP, Qu YY, Zhao XJ, Wang AH. Stromal–epithelial interactions modulate the effect of ovarian steroids on goat uterine epithelial cell interleukin-18 release. Domest Anim Endocrinol. 2012;42 (4):210–219. [DOI] [PubMed] [Google Scholar]
- 13. Mohtashami M, Zuniga-Pflucker JC. Three-dimensional architecture of the thymus is required to maintain delta-like expression necessary for inducing T cell development. J Immunol. 2006;176 (2):730–734. [DOI] [PubMed] [Google Scholar]
- 14. Schyschka L, Sanchez JJ, Wang Z, et al. Hepatic 3D cultures but not 2D cultures preserve specific transporter activity for acetaminophen-induced hepatotoxicity. Arch Toxicol. 2013;87 (8):1581–1593. [DOI] [PubMed] [Google Scholar]
- 15. Ghosh S, Spagnoli GC, Martin I, et al. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: a high density oligonucleotide array study. J Cell Physiol. 2005;204 (2):522–531. [DOI] [PubMed] [Google Scholar]
- 16. Gomez-Lechon MJ, Jover R, Donato T, et al. Long-term expression of differentiated functions in hepatocytes cultured in three-dimensional collagen matrix. J Cell Physiol. 1998;177 (4):553–562. [DOI] [PubMed] [Google Scholar]
- 17. Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. J Biol Chem. 1995;270 (45):26794–26801. [DOI] [PubMed] [Google Scholar]
- 18. Kenny PA, Lee GY, Myers CA, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1 (1):84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schutte SC, Taylor RN. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril. 2012;97 (4):997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin RD. The evolution of human reproduction: a primatological perspective. Am J Phys Anthropol. 2007;(suppl 45):59–84. [DOI] [PubMed] [Google Scholar]
- 21. Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy PJ. Menstrual breakdown of human endometrium can be mimicked in vitro and is selectively and reversibly blocked by inhibitors of matrix metalloproteinases. Proc Natl Acad Sci USA. 1996;93 (17):9120–9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salamonsen LA, Woolley DE. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol. 1999;44 (1-2):1–27. [DOI] [PubMed] [Google Scholar]
- 23. Gaide Chevronnay HP, Selvais C, Emonard H, Galant C, Marbaix E, Henriet P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim Biophys Acta. 2012;1824 (1):146–156. [DOI] [PubMed] [Google Scholar]
- 24. Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94 (12):6535–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salama SA, Mohammad MA, Diaz-Arrastia CR, et al. Estradiol-17beta upregulates Pyruvate kinase M2 expression to co-activate estrogen receptor-alpha and to integrate metabolic reprogramming with the mitogenic response in endometrial cells [published online January 28, 2014.]. J Clin Endocrinol Metab. 2014:jc20132639. [DOI] [PubMed] [Google Scholar]
- 26. Wallace A, Cooney TE, Englund R, Lubahn JD. Effects of interleukin-6 ablation on fracture healing in mice. J Orthop Res. 2011;29 (9):1437–1442. [DOI] [PubMed] [Google Scholar]
- 27. Ebihara N, Matsuda A, Nakamura S, Matsuda H, Murakami A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Invest Ophthalmol Vis Sci. 2011;52 (9):8549–8557. [DOI] [PubMed] [Google Scholar]
- 28. Jiang GX, Zhong XY, Cui YF, et al. IL-6/STAT3/TFF3 signaling regulates human biliary epithelial cell migration and wound healing in vitro. Mol Biol Rep. 2010;37 (8):3813–3818. [DOI] [PubMed] [Google Scholar]
- 29. McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184 (12):7219–7228. [DOI] [PubMed] [Google Scholar]
- 30. Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89 (3):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76 (1):36–41. [DOI] [PubMed] [Google Scholar]
- 32. Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128 (2):127–137. [DOI] [PubMed] [Google Scholar]
- 33. Pottratz ST, Bellido T, Mocharla H, Crabb D, Manolagas SC. 17 beta-Estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994;93 (3):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puder JJ, Freda PU, Goland RS, Wardlaw SL. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine responses to endotoxin in women. J Clin Endocrinol Metab. 2001;86 (6):2403–2408. [DOI] [PubMed] [Google Scholar]
- 35. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10 (6):415–424. [DOI] [PubMed] [Google Scholar]
- 36. Han G, Li F, Singh TP, Wolf P, Wang XJ. The pro-inflammatory role of TGFbeta1: a paradox? Int J Biol Sci. 2012;8 (2):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roberts AB, Heine UI, Flanders KC, Sporn MB. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann N Y Acad Sci. 1990;580:225–232. [DOI] [PubMed] [Google Scholar]
- 38. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. [DOI] [PubMed] [Google Scholar]
- 39. Goffin F, Munaut C, Frankenne F, et al. Expression pattern of metalloproteinases and tissue inhibitors of matrix-metalloproteinases in cycling human endometrium. Biol Reprod. 2003;69 (3):976–984. [DOI] [PubMed] [Google Scholar]
- 40. Rigot V, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y. In vivo perimenstrual activation of progelatinase B (proMMP-9) in the human endometrium and its dependence on stromelysin 1 (MMP-3) ex vivo. Biochem J. 2001;358(pt 1):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin JC, Kirk D, Gwatkin RB, Navre M, Cannon P, Giudice LC. Human endometrial matrix metalloproteinase-2, a putative menstrual proteinase. Hormonal regulation in cultured stromal cells and messenger RNA expression during the menstrual cycle. J Clin Invest. 1996;97 (2):438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145 (5):2291–2296. [DOI] [PubMed] [Google Scholar]
- 43. Li WA, Barry ZT, Cohen JD, et al. Detection of femtomole quantities of mature cathepsin K with zymography. Anal Biochem. 2010;401 (1):91–98. [DOI] [PubMed] [Google Scholar]
- 44. Wenger SL, Senft JR, Sargent LM, Bamezai R, Bairwa N, Grant SG. Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci Rep. 2004;24 (6):631–639. [DOI] [PubMed] [Google Scholar]
- 45. Korch C, Spillman MA, Jackson TA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012;127 (1):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laws MJ, Taylor RN, Sidell N, et al. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu Y, Bocca S, Anderson S, et al. Modulation of the expression of the transcription factors T-bet and GATA-3 in immortalized human endometrial stromal cells (HESCs) by sex steroid hormones and cAMP. Reprod Sci. 2013;20 (6):699–709. [DOI] [PubMed] [Google Scholar]
- 48. Logan PC, Ponnampalam AP, Steiner Mitchell MD. Effect of cyclic AMP and estrogen/progesterone on the transcription of DNA methyltransferases during the decidualization of human endometrial stromal cells. Mol Hum Reprod. 2013;19 (5):302–312. [DOI] [PubMed] [Google Scholar]
- 49. Klinkova O, Hansen KA, Winterton E, Mark CJ, Eyster KM, Two-way communication between endometrial stromal cells and monocytes. Reprod Sci. 2010;17 (2):125–136. [DOI] [PubMed] [Google Scholar]
- 50. Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87 (6):1069–1078. [DOI] [PubMed] [Google Scholar]
- 51. Sawatsri S, Desai N, Rock JA, Sidell N. Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril. 2000;73 (5):1012–1019. [DOI] [PubMed] [Google Scholar]
- 52. Chen JC, Erikson DW, Piltonen TT, et al. Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril. 2013;100 (4):1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mai KT, Yazdi HM, Perkins DG, Parks W. Pathogenetic role of the stromal cells in endometriosis and adenomyosis. Histopathology. 1997;30 (5):430–442. [DOI] [PubMed] [Google Scholar]


